Microtube Array Membrane Hollow Fiber Assay (MTAM-HFA)—An Accurate and Rapid Potential Companion Diagnostic and Pharmacological Interrogation Solution for Cancer Immunotherapy (PD-1/PD-L1)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrospinning of the Polysufone (PSF) Microtube Array Membrane (MTAM) and Microstructural Characterization
2.2. Cell Culture Preparation
2.3. Isolation of Peripheral Mononuclear Cells (PBMCs) from Whole Blood
2.4. T Cell Activation and Flow Cytometry
2.5. In Vitro and In Vivo Studies
2.6. Patient Primary Cancer Biopsy Samples and PBMC Collection
2.7. The Microtube Array Membrane (MTAM)-Hollow Fiber Assay (HFA) Anticancer Drug Screening
2.8. Response Evaluation Criteria in Solid Tumors (RECIST)
3. Results
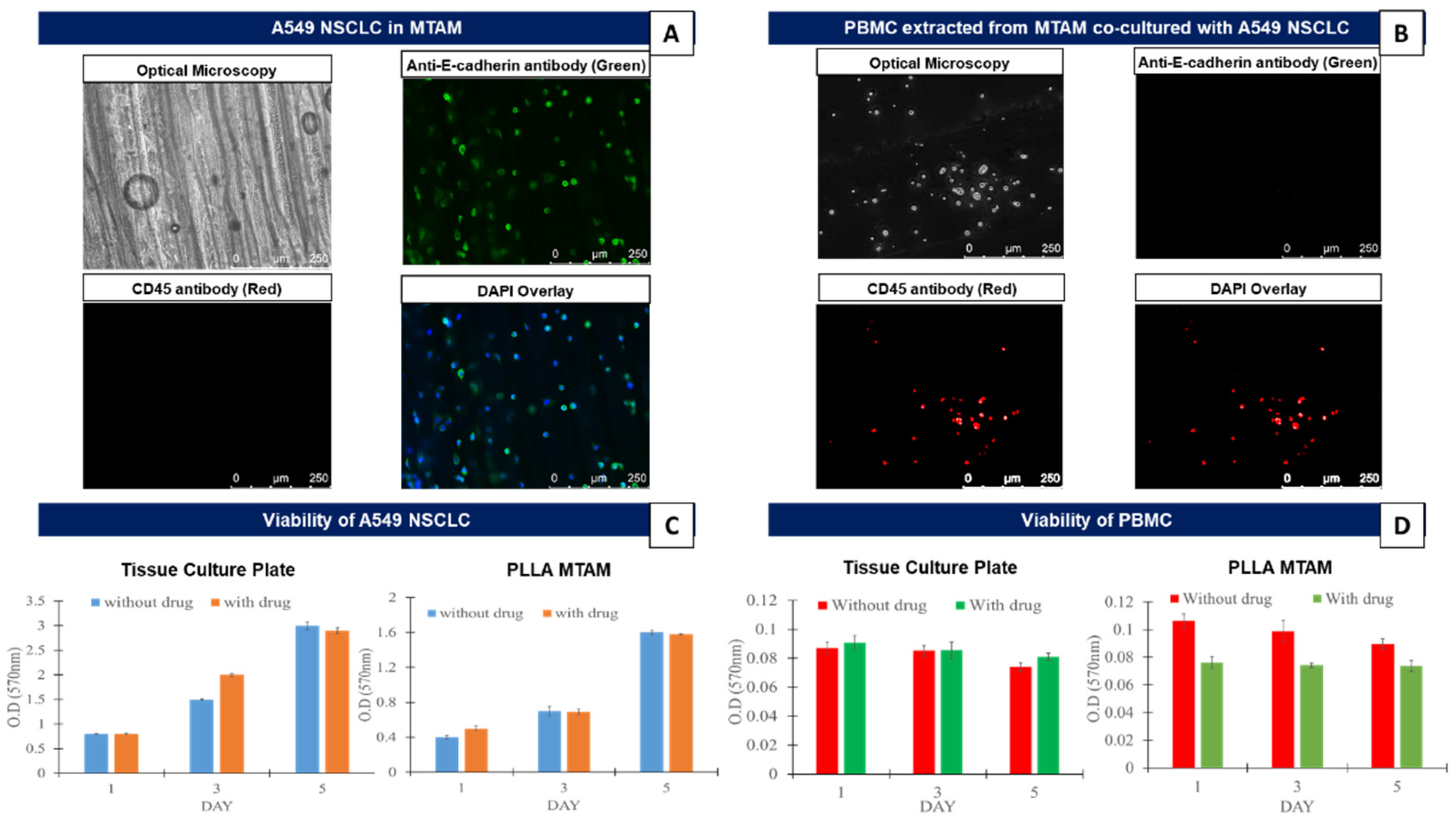
3.1. In Vitro Comparison of the Mono-Cultured A549 Lung Cancer Cell Line and PBMCs in TCPs and MTAMs
3.2. In Vitro Assay of the Co-Cultured A549 Cancer Cell Line and PBMCs Cultured in TCPs and MTAMs
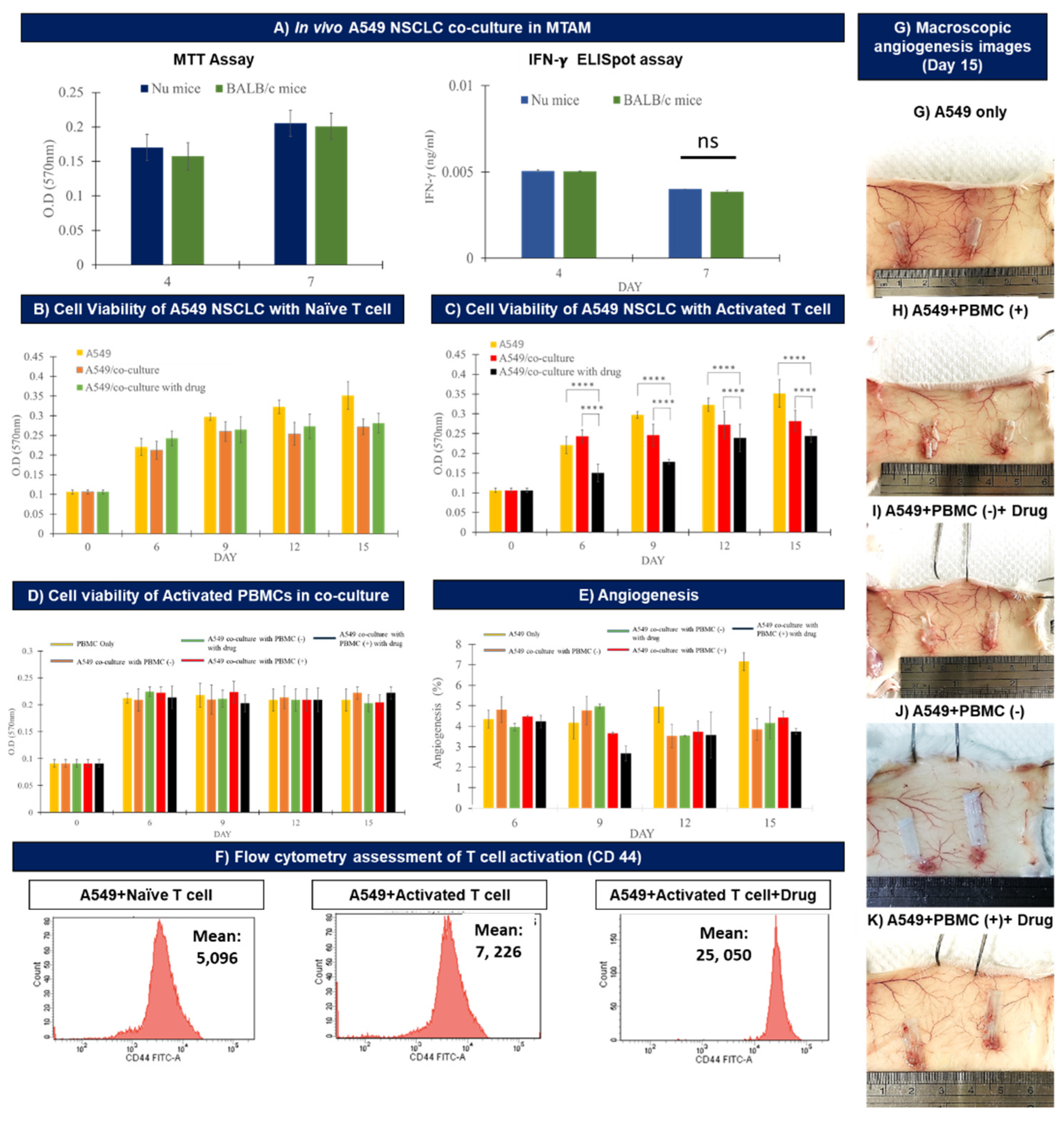
3.3. In Vivo Assay of the Co-cultured A549 Cancer Cell Line and PBMCs Cultured within MTAMs
3.4. Demonstration of MTAM-HFA (Anti-PD1) in the Clinical Setting
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, L.; Wei, R.; Lin, Y.; Kwok, H.F. Clinical and recent patents applications of PD-1/PD-L1 targeting immunotherapy in cancer treatment—current progress, strategy, and future perspective. Front. Immunol. 2020, 11, 1508. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.; Neftelino, S.T.; Hodge, J.P.; Oliva, C.; Campbell, J.R.; Yu, J.X. Combinations take centre stage in PD1/PDL1 inhibitor clinical trials. Nat. Rev. Drug Discov. 2021, 20, 168–170. [Google Scholar] [CrossRef]
- Gellrich, F.F.; Schmitz, M.; Beissert, S.; Meier, F. Anti-PD-1 and Novel Combinations in the Treatment of Melanoma—An Update. J. Clin. Med. 2020, 9, 223. [Google Scholar] [CrossRef]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- Carretero-González, A.; Lora, D.; Ghanem, I.; Zugazagoitia, J.; Castellano, D.; Sepúlveda, J.M.; de Velasco, G. Analysis of response rate with ANTI PD1/PD-L1 monoclonal antibodies in advanced solid tumors: A meta-analysis of randomized clinical trials. Oncotarget 2018, 9, 8706. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, L.; Goldinger, S.M.; Hassel, J.C.; Meier, F.; Tietze, J.K.; Forschner, A.; Weishaupt, C.; Leverkus, M.; Wahl, R.; Dietrich, U.; et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur. J. Cancer 2016, 60, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, L.; Forschner, A.; Loquai, C.; Goldinger, S.M.; Zimmer, L.; Ugurel, S.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.; Göppner, D.; et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer 2016, 60, 190–209. [Google Scholar] [CrossRef]
- Yang, J.; Hu, L. Immunomodulators targeting the PD-1/PD-L1 protein-protein interaction: From antibodies to small molecules. Med. Res. Rev. 2019, 39, 265–301. [Google Scholar] [CrossRef]
- Bai, R.; Lv, Z.; Xu, D.; Cui, J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark. Res. 2020, 8, 1–17. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Rouquette, I.; Taranchon-Clermont, E.; Gilhodes, J.; Bluthgen, M.-V.; Perallon, R.; Chalabreysse, L.; De Muret, A.; Hofman, V.; Marx, A.; Parrens, M.; et al. Immune biomarkers in thymic epithelial tumors: Expression patterns, prognostic value and comparison of diagnostic tests for PD-L1. Biomark. Res. 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.J.; Lizotte, P.; Cavanaugh, M.; Kuo, F.C.; Shivdasani, P.; Frieden, A.; Chau, N.G.; Schoenfeld, J.D.; Lorch, J.H.; Uppaluri, R.; et al. Frameshift events predict anti-PD-1/L1 response in head and neck cancer. JCI Insight 2018, 3, e98811. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.-E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef]
- Sacher, A.G.; Gandhi, L. Biomarkers for the Clinical Use of PD-1/PD-L1 Inhibitors in Non–Small-Cell Lung Cancer: A Review. JAMA Oncol. 2016, 2, 1217–1222. [Google Scholar] [CrossRef]
- Nishino, M.; Ramaiya, N.H.; Hatabu, H.; Hodi, F.S. Monitoring immune-checkpoint blockade: Response evaluation and biomarker development. Nat. Rev. Clin. Oncol. 2017, 14, 655–668. [Google Scholar] [CrossRef]
- Hong, L.; Negrao, M.V.; Dibaj, S.S.; Chen, R.; Reuben, A.; Bohac, J.M.; Liu, X.; Skoulidis, F.; Gay, C.M.; Cascone, T.; et al. Programmed death-ligand 1 heterogeneity and its impact on benefit from immune checkpoint inhibitors in NSCLC. J. Thorac. Oncol. 2020, 15, 1449–1459. [Google Scholar] [CrossRef]
- McGrail, D.; Pilié, P.; Rashid, N.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef]
- Georgiadis, A.; Durham, J.N.; Keefer, L.A.; Bartlett, B.R.; Zielonka, M.; Murphy, D.; White, J.R.; Lu, S.; Verner, E.L.; Ruan, F.; et al. Noninvasive Detection of Microsatellite Instability and High Tumor Mutation Burden in Cancer Patients Treated with PD-1 Blockade. Clin. Cancer Res. 2019, 25, 7024–7034. [Google Scholar] [CrossRef]
- Wang, Q.-X.; Qu, C.-H.; Gao, Y.-H.; Ding, P.-R.; Yun, J.-P.; Xie, D.; Cai, M.-Y. The degree of microsatellite instability predicts response to PD-1 blockade immunotherapy in mismatch repair-deficient/microsatellite instability-high colorectal cancers. Exp. Hematol. Oncol. 2021, 10, 2. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Randon, G.; Di Bartolomeo, M.; Luciani, A.; Chao, J.; Smyth, E.; Petrelli, F. Predictive role of microsatellite instability for PD-1 blockade in patients with advanced gastric cancer: A meta-analysis of randomized clinical trials. ESMO Open 2021, 6, 100036. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sandhu, J.; Ouyang, C.; Ye, J.; Lee, P.P.; Fakih, M. Clinical response to immunotherapy targeting programmed cell death receptor 1/programmed cell death ligand 1 in patients with treatment-resistant microsatellite stable colorectal cancer with and without liver metastases. JAMA Netw. Open 2021, 4, e2118416. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Luo, H.; Huang, L.; Luo, H.; Zhu, X. Microsatellite instability: A review of what the oncologist should know. Cancer Cell Int. 2020, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Nivolumab plus Ipilimumab Achieves Responses in dMMR/MSI-H Tumors. Cancer Discov. 2018, 8, 263. [CrossRef]
- Rizzo, A.; Ricci, A.D.; Gadaleta-Caldarola, G. MSI-H/dMMR and cancer immunotherapy: Current state and future implications. Exp. Rev. Precis. Med. Drug Dev. 2021, 6, 345–347. [Google Scholar] [CrossRef]
- Jørgensen, J.T. An update on companion and complementary diagnostic assays for PD-1/PD-L1 checkpoint inhibitors in NSCLC. Exp. Rev. Mol. Diagn. 2021, 21, 445–454. [Google Scholar] [CrossRef]
- Bravaccini, S.; Ulivi, P. What’s the best modality for patient selection for predicting response to PD-1/PD-L1 inhibitors? Transl. Lung Cancer Res. 2020, 9, 158–159. [Google Scholar] [CrossRef]
- Chang, E.; Pelosof, L.; Lemery, S.; Gong, Y.; Goldberg, K.B.; Farrell, A.T.; Keegan, P.; Veeraraghavan, J.; Wei, G.; Blumenthal, G.M.; et al. Systematic review of PD-1/PD-L1 inhibitors in oncology: From personalized medicine to public health. Oncologist 2021, 26, e1786–e1799. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Huang, W.-T.; Chew, C.H.; Lai, J.-K.; Tu, S.-H.; Wei, P.-L.; Lee, K.-Y.; Lai, G.-M.; Chen, C.-C. Electrospun Polylactic Acid (PLLA) Microtube Array Membrane (MTAM)—An Advanced Substrate for Anticancer Drug Screening. Materials 2019, 12, 569. [Google Scholar] [CrossRef]
- Chew, C.; Wu, C.; Chen, C. A novel electrospun Microtube Array Membrane (MTAM) based low cost conceptual tubular Microbial Fuel Cell (MFC). Eur. Polym. J. 2016, 83, 138–147. [Google Scholar] [CrossRef]
- Yang, A.-J.; Marito, S.; Yang, J.J.; Keshari, S.; Chew, C.H.; Chen, C.-C.; Huang, C.-M. A Microtube Array Membrane (MTAM) Encapsulated Live Fermenting Staphylococcus epidermidis as a Skin Probiotic Patch against Cutibacterium acnes. Int. J. Mol. Sci. 2018, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Piscioneri, A.; Curcio, E.; Salerno, S.; Chen, C.-C.; De Bartolo, L. Membrane bioreactor for investigation of neurodegeneration. Mater. Sci. Eng. C 2019, 103, 109793. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Piscioneri, A.; Salerno, S.; Chen, C.-C.; Chew, C.H.; Giorno, L.; Drioli, E.; De Bartolo, L. Microtube array membrane bioreactor promotes neuronal differentiation and orientation. Biofabrication 2017, 9, 025018. [Google Scholar] [CrossRef] [PubMed]
- Chew, C.H.; Cheng, L.; Huang, W.; Wu, Y.M.; Lee, C.; Wu, M.; Chen, C. Ultrahigh packing density next generation microtube array membrane: A novel solution for absorption-based extracorporeal endotoxin removal device. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2903–2911. [Google Scholar] [CrossRef]
- Ou, K.-L.; Chen, C.-S.; Lin, L.-H.; Lu, J.-C.; Shu, Y.-C.; Tseng, W.-C.; Yang, J.-C.; Lee, S.-Y.; Chen, C.-C. Membranes of epitaxial-like packed, super aligned electrospun micron hollow poly (l-lactic acid)(PLLA) fibers. J. Eur. Polym. 2011, 47, 882–892. [Google Scholar] [CrossRef]
- Yang, J.-C.; Lee, S.-Y.; Tseng, W.-C.; Shu, Y.-C.; Lu, J.-C.; Shie, H.-S.; Chen, C.-C. Formation of Highly Aligned, Single-Layered, Hollow Fibrous Assemblies and the Fabrication of Large Pieces of PLLA Membranes. Macromol. Mater. Eng. 2011, 297, 115–122. [Google Scholar] [CrossRef]
- De Falco, G.; Terlizzi, M.; Sirignano, M.; Commodo, M.; D’Anna, A.; Aquino, R.P.; Pinto, A.; Sorrentino, R. Human peripheral blood mononuclear cells (PBMCs) from smokers release higher levels of IL-1-like cytokines after exposure to combustion-generated ultrafine particles. Sci. Rep. 2017, 7, 43016. [Google Scholar] [CrossRef]
- Murakami, T.; Kim, J.; Li, Y.; Green, G.E.; Shikanov, A.; Ono, A. Secondary lymphoid organ fibroblastic reticular cells mediate trans-infection of HIV-1 via CD44-hyaluronan interactions. Nat. Commun. 2018, 9, 2436. [Google Scholar] [CrossRef]
- Harigopal, M.; Shin, S.J.; Murray, M.P.; Tickoo, S.K.; Brogi, E.; Rosen, P.P. Aberrant E-cadherin staining patterns in invasive mammary carcinoma. World J. Surg. Oncol. 2005, 3, 73. [Google Scholar] [CrossRef]
- Boehm, U.; Guethlein, L.; Klamp, T.; Ozbek, K.; Schaub, A.; Fütterer, A.; Pfeffer, K.; Howard, J.C. Two families of GTPases dominate the complex cellular response to IFN-γ. J. Immunol. 1998, 161, 6715–6723. [Google Scholar]
- Chew, C.H.; Lee, C.-W.; Huang, W.-T.; Cheng, L.-W.; Chen, A.; Cheng, T.-M.; Liu, Y.-L.; Chen, C.-C. Microtube Array Membrane (MTAM)-Based Encapsulated Cell Therapy for Cancer Treatment. Membranes 2020, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-C.; Lin, L.-H.; Tsen, W.-C.; Shie, H.-S.; Chiu, H.-L.; Yang, T.C.-K.; Chen, C.-C. Permeation of biological compounds through porous poly(l-lactic acid) (PLLA) microtube array membranes (MTAMs). Eur. Polym. J. 2015, 67, 166–173. [Google Scholar] [CrossRef]
- George, S.L.; Xiang, J.; Stapleton, J.T. Clinical isolates of GB virus type C vary in their ability to persist and replicate in peripheral blood mononuclear cell cultures. Virology 2003, 316, 191–201. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhang, D.; Wu, S.; Xu, M.; Zhou, X.; Lu, X.-J.; Ji, J. Resistance to PD-1/PD-L1 blockade cancer immunotherapy: Mechanisms, predictive factors, and future perspectives. Biomark. Res. 2020, 8, 35. [Google Scholar] [CrossRef]
- Ribas, A. Tumor Immunotherapy Directed at PD-1. N. Engl. J. Med. 2012, 366, 2517–2519. [Google Scholar] [CrossRef]
- Xia, A.-L.; Xu, Y.; Lu, X.-J. Cancer immunotherapy: Challenges and clinical applications. J. Med. Genet. 2018, 56, 1–3. [Google Scholar] [CrossRef]
- Dijkstra, K.K.; Cattaneo, C.M.; Weeber, F.; Chalabi, M.; Van De Haar, J.; Fanchi, L.F.; Slagter, M.; Van Der Velden, D.L.; Kaing, S.; Kelderman, S.; et al. Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell 2018, 174, 1586–1598.e12. [Google Scholar] [CrossRef]
- Lee, A.J.; Haworth, C.; Hutchinson, R.M.; Patel, R.; Carter, R.; James, R.F.L. Enhancement of cALL immunogenicity by co-culture with a CD154 expressing 293 cell line. Clin. Exp. Immunol. 2001, 124, 359–368. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, W.; Wang, L.; Xiao, C.; Gong, Y.; Huang, D.; Guo, B.; Li, Q.; Xiang, Y.; Nan, Y. Co-culture of dendritic cells and cytokine-induced killer cells effectively suppresses liver cancer stem cell growth by inhibiting pathways in the immune system. BMC Cancer 2018, 18, 984. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ohaegbulam, K.C.; Assal, A.; Lazar-Molnar, E.; Yao, Y.; Zang, X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol. Med. 2014, 21, 24–33. [Google Scholar] [CrossRef]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concept. 2018, 9, 64–79. [Google Scholar] [CrossRef]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumour cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; Van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef]
- Schumann, J.; Stanko, K.; Schliesser, U.; Appelt, C.; Sawitzki, B. Differences in CD44 surface expression levels and function discriminates IL-17 and IFN-γ producing helper T cells. PLoS ONE 2015, 10, e0132479. [Google Scholar]
- Baaten, B.J.G.; Tinoco, R.; Chen, A.T.; Bradley, L.M. Regulation of Antigen-Experienced T Cells: Lessons from the Quintessential Memory Marker CD44. Front. Immunol. 2012, 3, 23. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-T.; Yun, T.; Chew, C.-H.; Chen, A.; Wei, P.-L.; Lee, K.-Y.; Lee, H.-L.; Feng, P.-H.; Chiou, J.-F.; Chen, C.-M.; et al. Microtube Array Membrane Hollow Fiber Assay (MTAM-HFA)—An Accurate and Rapid Potential Companion Diagnostic and Pharmacological Interrogation Solution for Cancer Immunotherapy (PD-1/PD-L1). Biomolecules 2022, 12, 480. https://doi.org/10.3390/biom12040480
Huang W-T, Yun T, Chew C-H, Chen A, Wei P-L, Lee K-Y, Lee H-L, Feng P-H, Chiou J-F, Chen C-M, et al. Microtube Array Membrane Hollow Fiber Assay (MTAM-HFA)—An Accurate and Rapid Potential Companion Diagnostic and Pharmacological Interrogation Solution for Cancer Immunotherapy (PD-1/PD-L1). Biomolecules. 2022; 12(4):480. https://doi.org/10.3390/biom12040480
Chicago/Turabian StyleHuang, Wan-Ting, Tsao Yun, Chee-Ho Chew, Amanda Chen, Po-Li Wei, Kang-Yun Lee, Hsin-Lun Lee, Po-Hao Feng, Jeng-Fong Chiou, Ching-Mei Chen, and et al. 2022. "Microtube Array Membrane Hollow Fiber Assay (MTAM-HFA)—An Accurate and Rapid Potential Companion Diagnostic and Pharmacological Interrogation Solution for Cancer Immunotherapy (PD-1/PD-L1)" Biomolecules 12, no. 4: 480. https://doi.org/10.3390/biom12040480
APA StyleHuang, W.-T., Yun, T., Chew, C.-H., Chen, A., Wei, P.-L., Lee, K.-Y., Lee, H.-L., Feng, P.-H., Chiou, J.-F., Chen, C.-M., & Chen, C.-C. (2022). Microtube Array Membrane Hollow Fiber Assay (MTAM-HFA)—An Accurate and Rapid Potential Companion Diagnostic and Pharmacological Interrogation Solution for Cancer Immunotherapy (PD-1/PD-L1). Biomolecules, 12(4), 480. https://doi.org/10.3390/biom12040480








