Identification of Regions Involved in the Physical Interaction between Melanocortin Receptor Accessory Protein 2 and Prokineticin Receptor 2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Expression Constructs
2.2. Western Blot Assay
2.3. Ligand Production
2.4. Yeast Culture and Transformation
2.5. Preparation of Yeast Membrane Proteins
2.6. Yeast Membrane Proteins Cross-Linking by Bis-Sulfosuccinimidyl-Suberate (BS3)
2.7. The Co-Precipitation of Yeast Membrane Extracts
2.8. Glutathione S-Transferase (GST) Pull-Down
2.9. The Expression and Purification of the Recombinant Carboxy-Terminal Domain and Δ131-MRAP2 in E. coli
2.10. Blue Native PAGE
2.11. CHO-PKR2 Cell Culture, Transfection, and Stimulation
2.12. Adipose Tissue and Hypothalamus Explants
2.13. Real-Time PCR
2.14. Data Analysis
3. Results
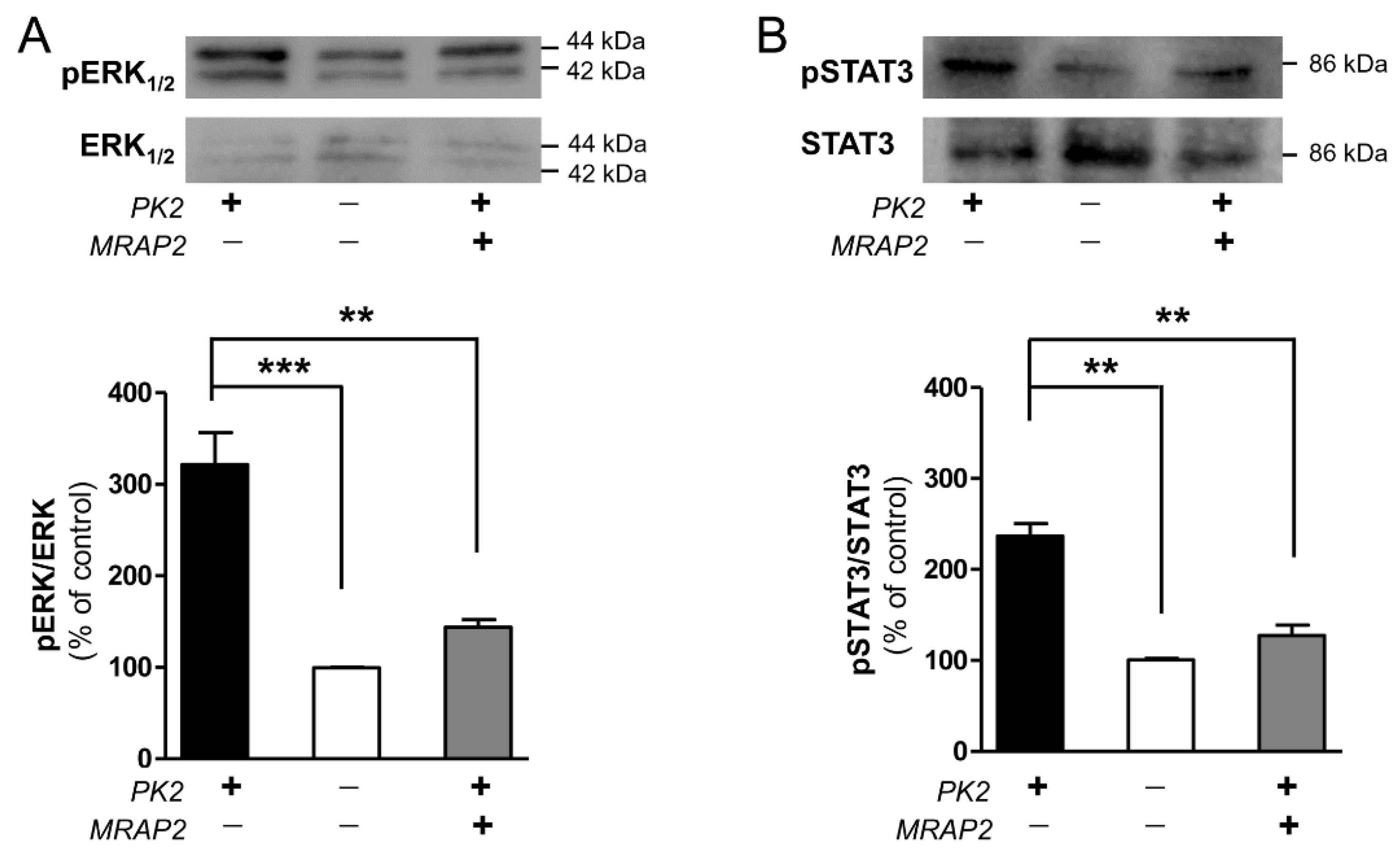
3.1. MRAP2 Inhibits PKRs Gi Coupling
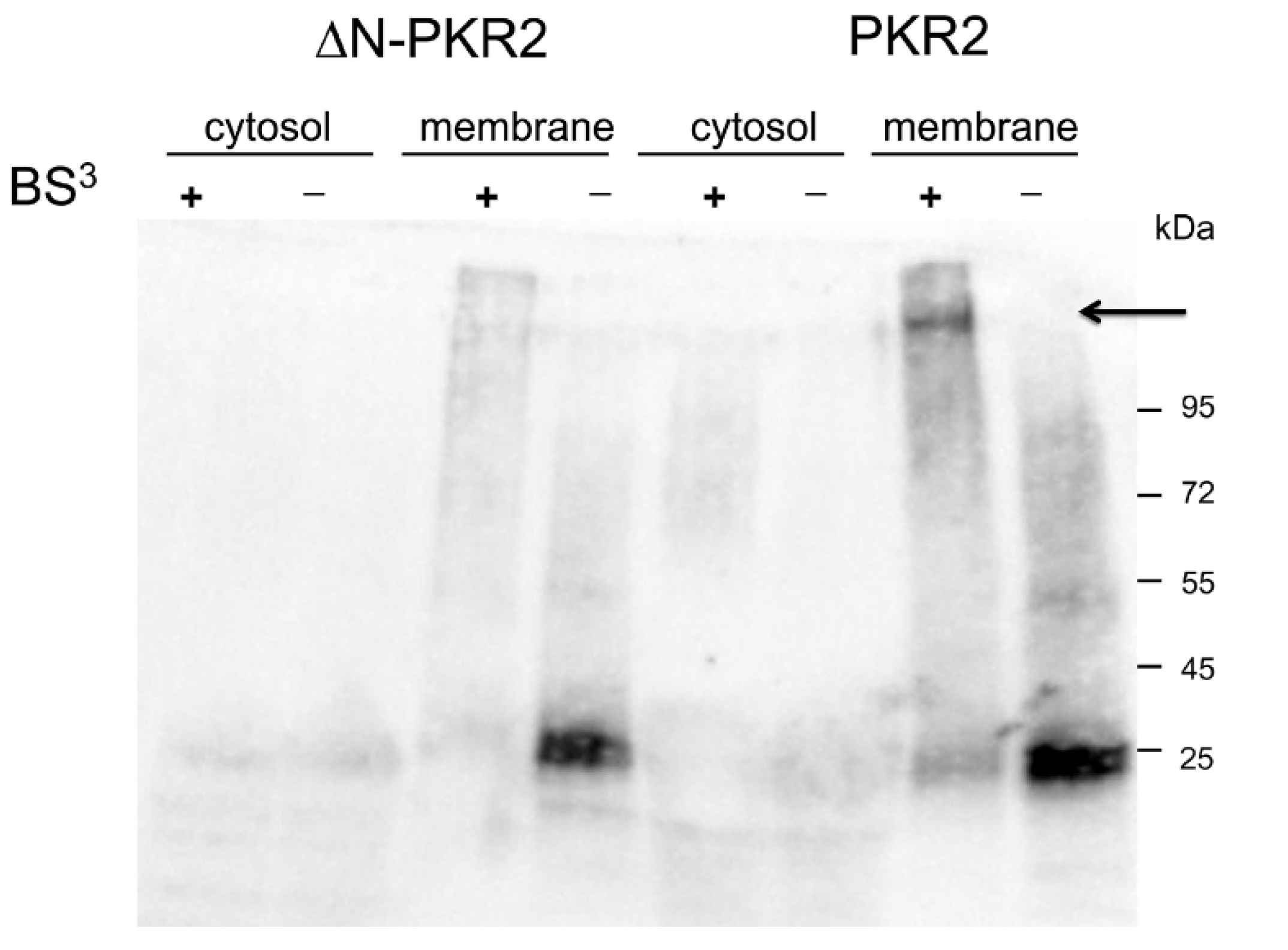
3.2. Identification of PKR2 Region Involved in MRAP2 Binding in S. cerevisiae
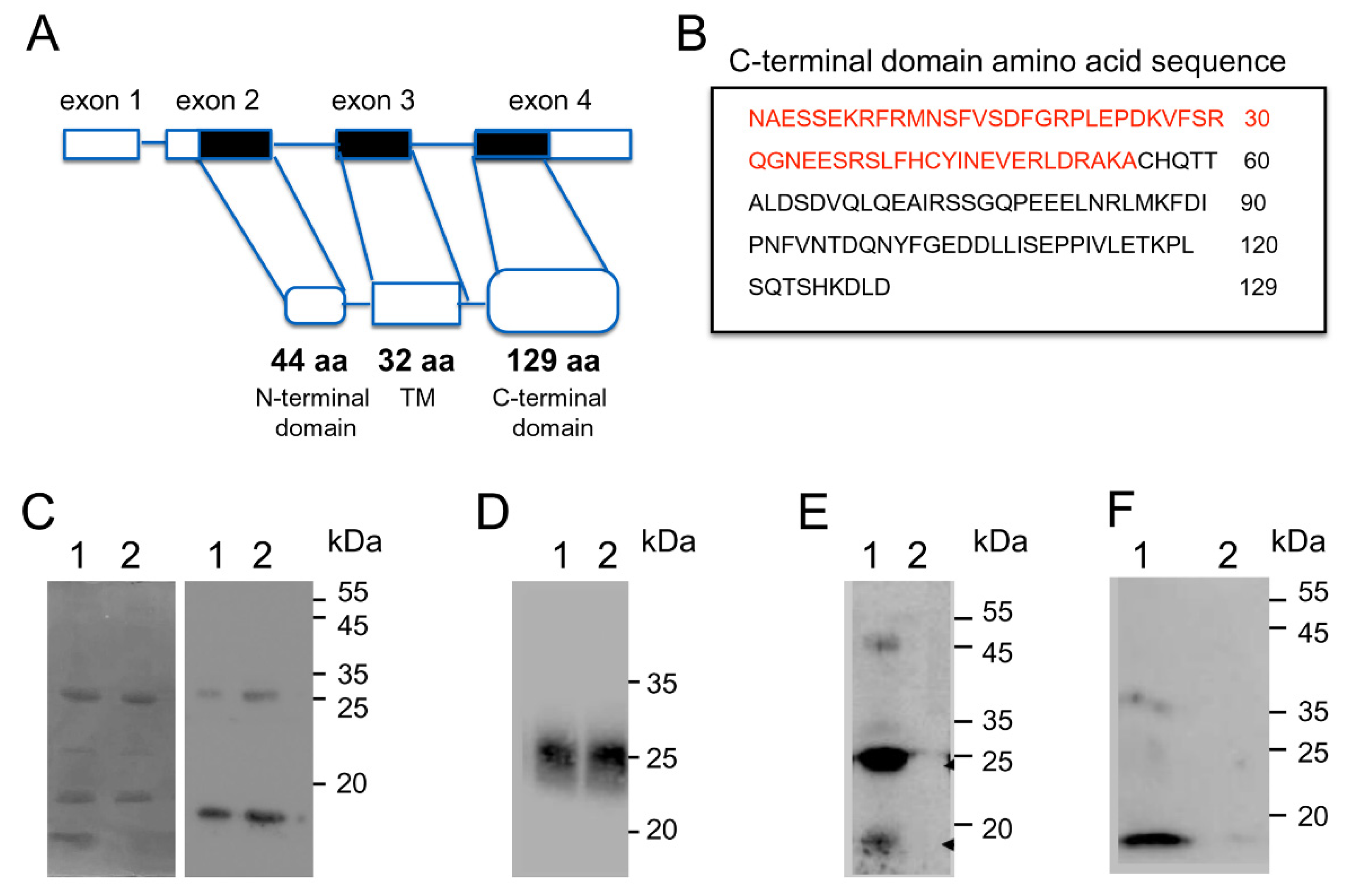
3.3. Biochemical Analysis of the Interaction between the C-Terminal Region of MRAP2 and Prokineticin Receptor 2
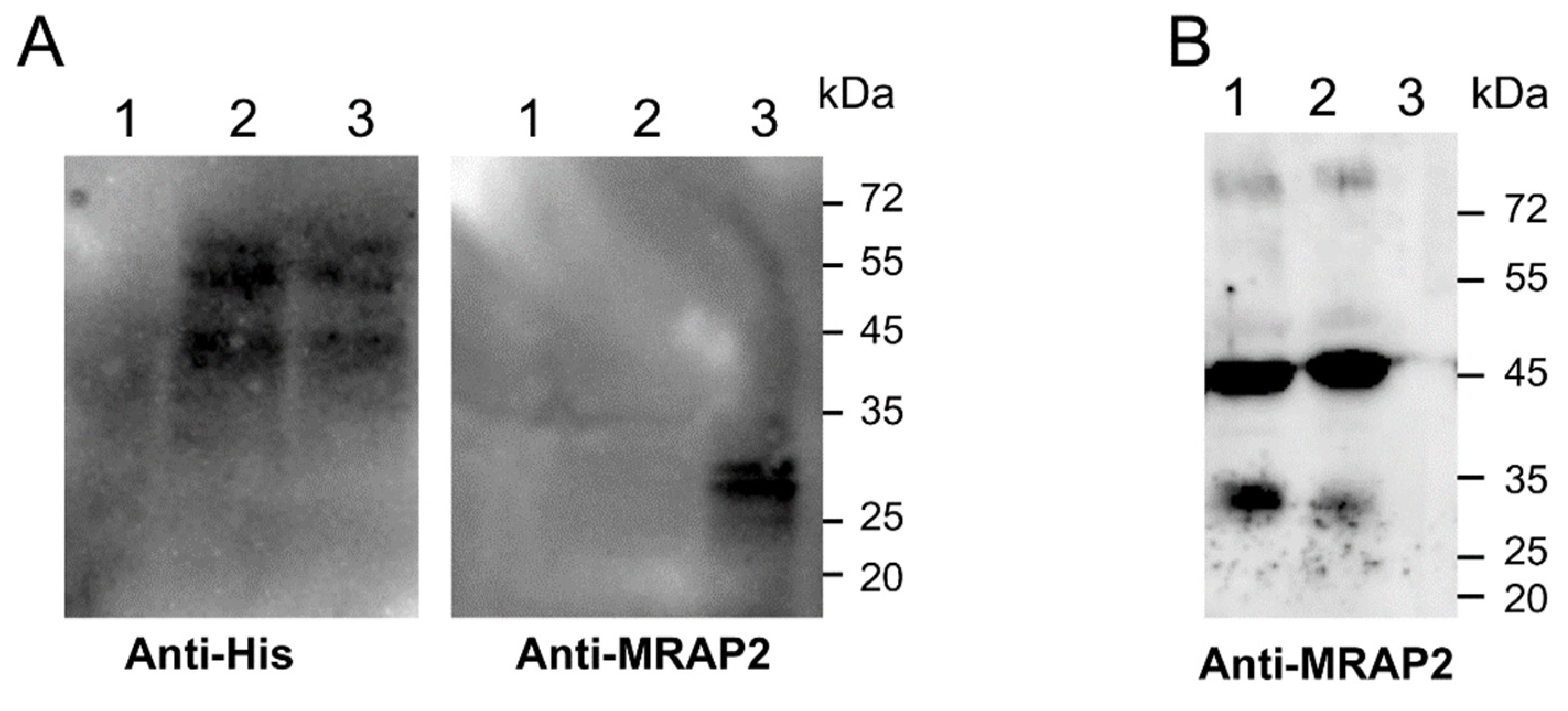
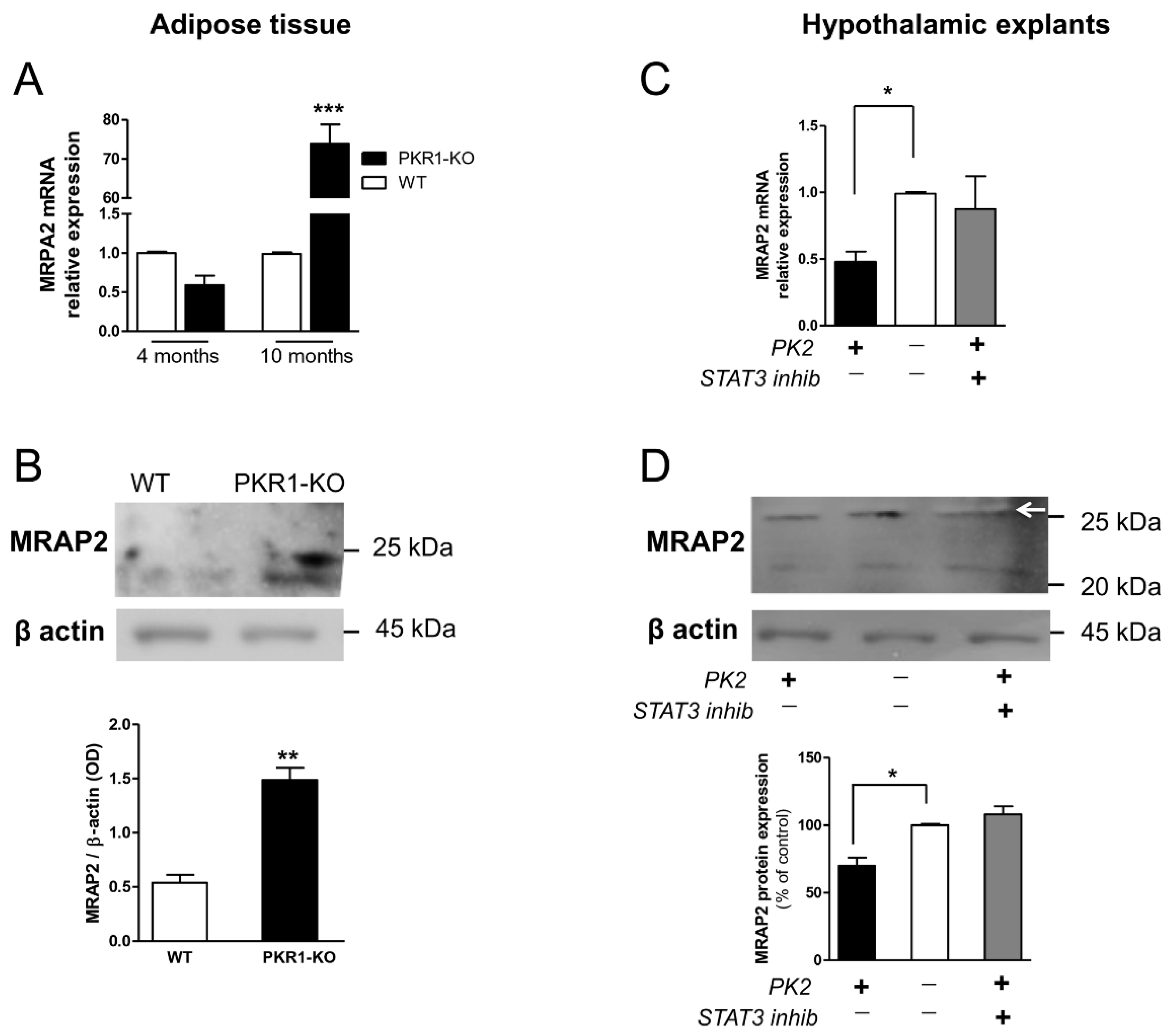
3.4. Modulation of MRAP2 Expression by the Prokineticin System
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hinkle, P.M.; Sebag, J.A. Structure and function of the melanocortin2 receptor accessory protein (MRAP). Mol. Cell. Endocrinol. 2009, 300, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asai, M.; Ramachandrappa, S.; Joachim, M.; Shen, Y.; Zhang, R.; Nuthalapati, N.; Ramanathan, V.; Strochlic, D.E.; Ferket, P.; Linhart, K.; et al. Loss of Function of the Melanocortin 2 Receptor Accessory Protein 2 Is Associated with Mammalian Obesity. Science 2013, 341, 275–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berruien, N.N.; Smith, C.L. Emerging roles of melanocortin receptor accessory proteins (MRAP and MRAP2) in physiology and pathophysiology. Gene 2020, 757, 144949. [Google Scholar] [CrossRef] [PubMed]
- Chaly, A.L.; Srisai, L.; E Gardner, E.; A Sebag, J. The Melanocortin Receptor Accessory Protein 2 promotes food intake through inhibition of the Prokineticin Receptor-1. eLife 2016, 5, e12397. [Google Scholar] [CrossRef] [Green Version]
- Rouault, A.A.; Lee, A.A.; Sebag, J.A. Regions of MRAP2 required for the inhibition of orexin and prokineticin receptor signaling. Biochim. Biophys. Acta 2017, 1864, 2322–2329. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, R.; Miele, R. Prokineticin-Receptor Network: Mechanisms of Regulation. Life 2022, 12, 172. [Google Scholar] [CrossRef]
- Negri, L.; Ferrara, N. The Prokineticins: Neuromodulators and Mediators of Inflammation and Myeloid Cell-Dependent Angiogenesis. Physiol. Rev. 2018, 98, 1055–1082. [Google Scholar] [CrossRef] [Green Version]
- Maftei, D.; Lattanzi, R.; Vincenzi, M.; Squillace, S.; Fullone, M.R.; Miele, R. The balance of concentration between Prokineticin 2β and Prokineticin 2 modulates the food intake by STAT3 signaling. BBA Adv. 2021, 1, 100028. [Google Scholar] [CrossRef]
- Szatkowski, C.; Vallet, J.; Dormishian, M.; Messaddeq, N.; Valet, P.; Boulberdaa, M.; Metzger, D.; Chambon, P.; Nebigil, C.G. Prokineticin Receptor 1 as a Novel Suppressor of Preadipocyte Proliferation and Differentiation to Control Obesity. PLoS ONE 2013, 8, e81175. [Google Scholar] [CrossRef] [Green Version]
- Webb, T.; Chan, L.; Cooray, S.N.; Cheetham, M.; Chapple, P.; Clark, A.J.L. Distinct Melanocortin 2 Receptor Accessory Protein Domains Are Required for Melanocortin 2 Receptor Interaction and Promotion of Receptor Trafficking. Endocrinology 2009, 150, 720–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsango, S.; Bonaccorsi di Patti, M.C.; Barra, D.; Miele, R. Evidence that prokineticin receptor 2 exists as a dimer in vivo. Cell Mol. Life Sci. 2011, 68, 2919–2929. [Google Scholar] [CrossRef] [PubMed]
- Sposini, S.; Caltabiano, G.; Hanyaloglu, A.; Miele, R. Identification of transmembrane domains that regulate spatial arrangements and activity of prokineticin receptor 2 dimers. Mol. Cell. Endocrinol. 2015, 399, 362–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lattanzi, R.; Maftei, D.; Vincenzi, M.; Fullone, M.R.; Miele, R. Identification and Characterization of a New Splicing Variant of Prokineticin 2. Life 2022, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Ausubel, F.; Kingston, R.; Moore, D.; Seidman, J.; Smith, J.; Struhl, K. Current Protocols in Molecular Biology; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Lattanzi, R.; Maftei, D.; Fullone, M.R.; Miele, R. Trypanosoma cruzi trans-sialidase induces STAT3 and ERK activation by prokineticin receptor 2 binding. Cell Biochem. Funct. 2020, 39, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Wittig, I.; Beckhaus, T.; Wumaier, Z.; Karas, M.; Schägger, H. Mass Mass estimation of native proteins by blue native electrophoresis: Principles and practical hints. Mol. Cell. Proteom. 2010, 9, 2149–2161. [Google Scholar] [CrossRef] [Green Version]
- Gasser, A.; Brogi, S.; Urayama, K.; Nishi, T.; Kurose, H.; Tafi, A.; Ribeiro, N.; Désaubry, L.; Nebigil, C.G. Discovery and Cardioprotective Effects of the First Non-Peptide Agonists of the G Protein-Coupled Prokineticin Receptor-1. PLoS ONE 2015, 10, e0121027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lattanzi, R.; Maftei, D.; Negri, L.; Fusco, I.; Miele, R. PK2β ligand, a splice variant of prokineticin 2, is able to modulate and drive signaling through PKR1 receptor. Neuropeptides 2018, 71, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, R.; Miele, R. Versatile Role of Prokineticins and Prokineticin Receptors in Neuroinflammation. Biomedicines 2021, 9, 1648. [Google Scholar] [CrossRef]
- Verdinez, J.A.; Sebag, J.A. Role of N-Linked Glycosylation in PKR2 Trafficking and Signaling. Front. Neurosci. 2021, 15, 1055. [Google Scholar] [CrossRef]
- Rouault, A.A.J.; Rosselli-Murai, L.K.; Hernandez, C.C.; Gimenez, L.E.; Tall, G.G.; Sebag, J.A. The GPCR accessory protein MRAP2 regulates both biased signaling and constitutive activity of the ghrelin receptor GHSR1a. Sci. Signal. 2020, 13, eaax4569. [Google Scholar] [CrossRef] [Green Version]
- Chen, V.; Bruno, A.E.; Britt, L.L.; Hernandez, C.C.; Gimenez, L.E.; Peisley, A.; Cone, R.D.; Millhauser, G.L. Membrane orientation and oligomerization of the melanocortin receptor accessory protein 2. J. Biol. Chem. 2020, 295, 16370–16379. [Google Scholar] [CrossRef] [PubMed]
- Dormishian, M.; Turkeri, G.; Urayama, K.; Nguyen, T.L.; Boulberdaa, M.; Messaddeq, N.; Renault, G.; Henrion, D.; Nebigil, C.G. Prokineticin Receptor-1 Is a New Regulator of Endothelial Insulin Uptake and Capillary Formation to Control Insulin Sensitivity and Cardiovascular and Kidney Functions. J. Am. Heart Assoc. 2013, 2, e000411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.S.; Kim, Y.-J.; Cho, S.Y.; Lee, T.R.; Kim, S.H. Transcriptional activation of melanocortin 2 receptor accessory protein by PPARγ in adipocytes. Biochem. Biophys. Res. Commun. 2013, 439, 401–406. [Google Scholar] [CrossRef] [PubMed]
| Oligonucleotide | Sequence |
|---|---|
| MRAP2 BamHI up | 5′-AAG GAT CCA TGTCCGCCCAGAGG-3′ |
| MRAP2 EcoRI dw, | 5′-AAGAATTCTTAAACCTTATCGTC-3′ |
| T70 BamHI | 5′-GGATCCACCAAGACAGGAGCCCCA-3′ |
| GAPDH fw | 5′-GCC AAG GCT GTG GGC AAG GT-3′ |
| GAPDH rv | 5′-TCT CCA GGC GGC ACG TCA GA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fullone, M.R.; Maftei, D.; Vincenzi, M.; Lattanzi, R.; Miele, R. Identification of Regions Involved in the Physical Interaction between Melanocortin Receptor Accessory Protein 2 and Prokineticin Receptor 2. Biomolecules 2022, 12, 474. https://doi.org/10.3390/biom12030474
Fullone MR, Maftei D, Vincenzi M, Lattanzi R, Miele R. Identification of Regions Involved in the Physical Interaction between Melanocortin Receptor Accessory Protein 2 and Prokineticin Receptor 2. Biomolecules. 2022; 12(3):474. https://doi.org/10.3390/biom12030474
Chicago/Turabian StyleFullone, Maria Rosaria, Daniela Maftei, Martina Vincenzi, Roberta Lattanzi, and Rossella Miele. 2022. "Identification of Regions Involved in the Physical Interaction between Melanocortin Receptor Accessory Protein 2 and Prokineticin Receptor 2" Biomolecules 12, no. 3: 474. https://doi.org/10.3390/biom12030474
APA StyleFullone, M. R., Maftei, D., Vincenzi, M., Lattanzi, R., & Miele, R. (2022). Identification of Regions Involved in the Physical Interaction between Melanocortin Receptor Accessory Protein 2 and Prokineticin Receptor 2. Biomolecules, 12(3), 474. https://doi.org/10.3390/biom12030474









