Alpha Lipoic-Acid Potentiates Ex Vivo Expansion of Human Steady-State Peripheral Blood Hematopoietic Primitive Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. CD34pos Isolation from Steady State Peripheral Blood
2.2. Cell Culture
2.3. Immunophenotyping
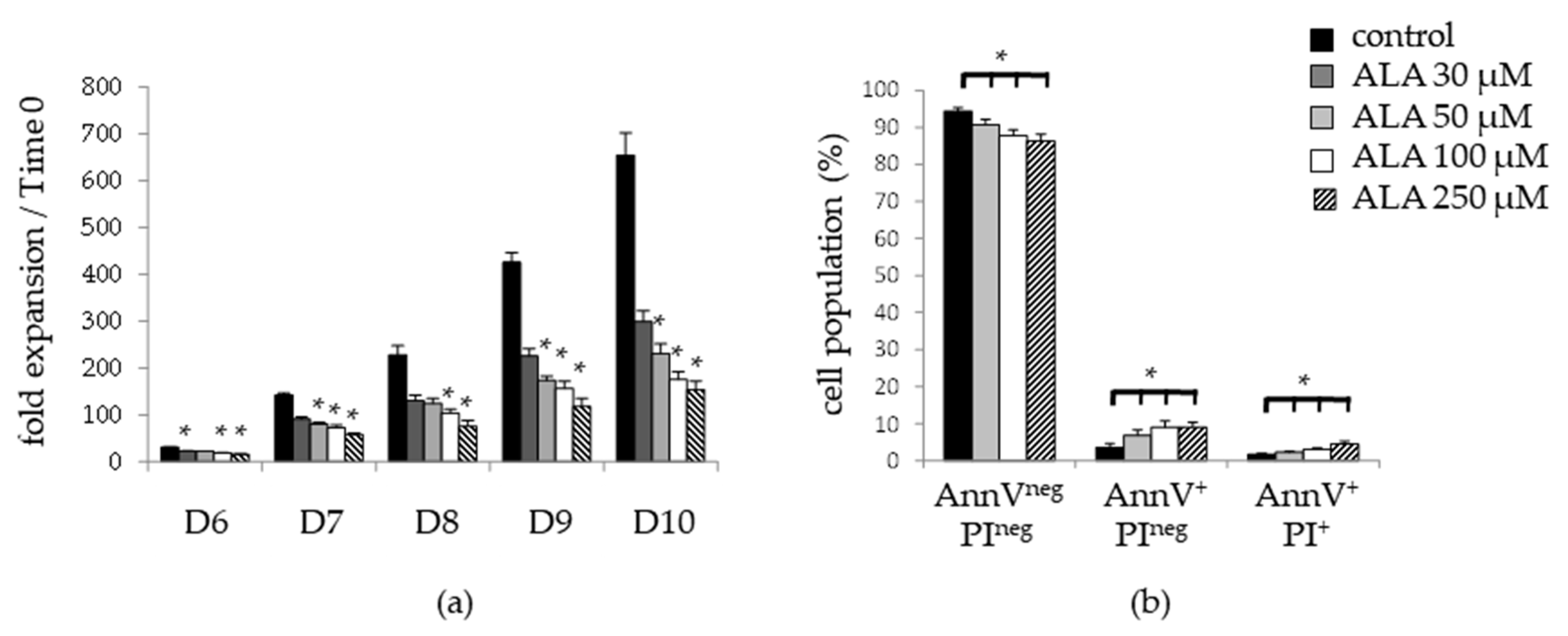
2.4. Apoptosis
2.5. Mice
2.6. SCID-Repopulating Cells (SRC) Limiting Dilution Assay
2.7. Proliferation Analysis
2.8. Cell Cycle Analysis
2.9. Mitochondrial and Cytoplasmic ROS Level Analysis
2.10. GSH and GSSG Determination
2.11. Statistical Analysis
3. Results
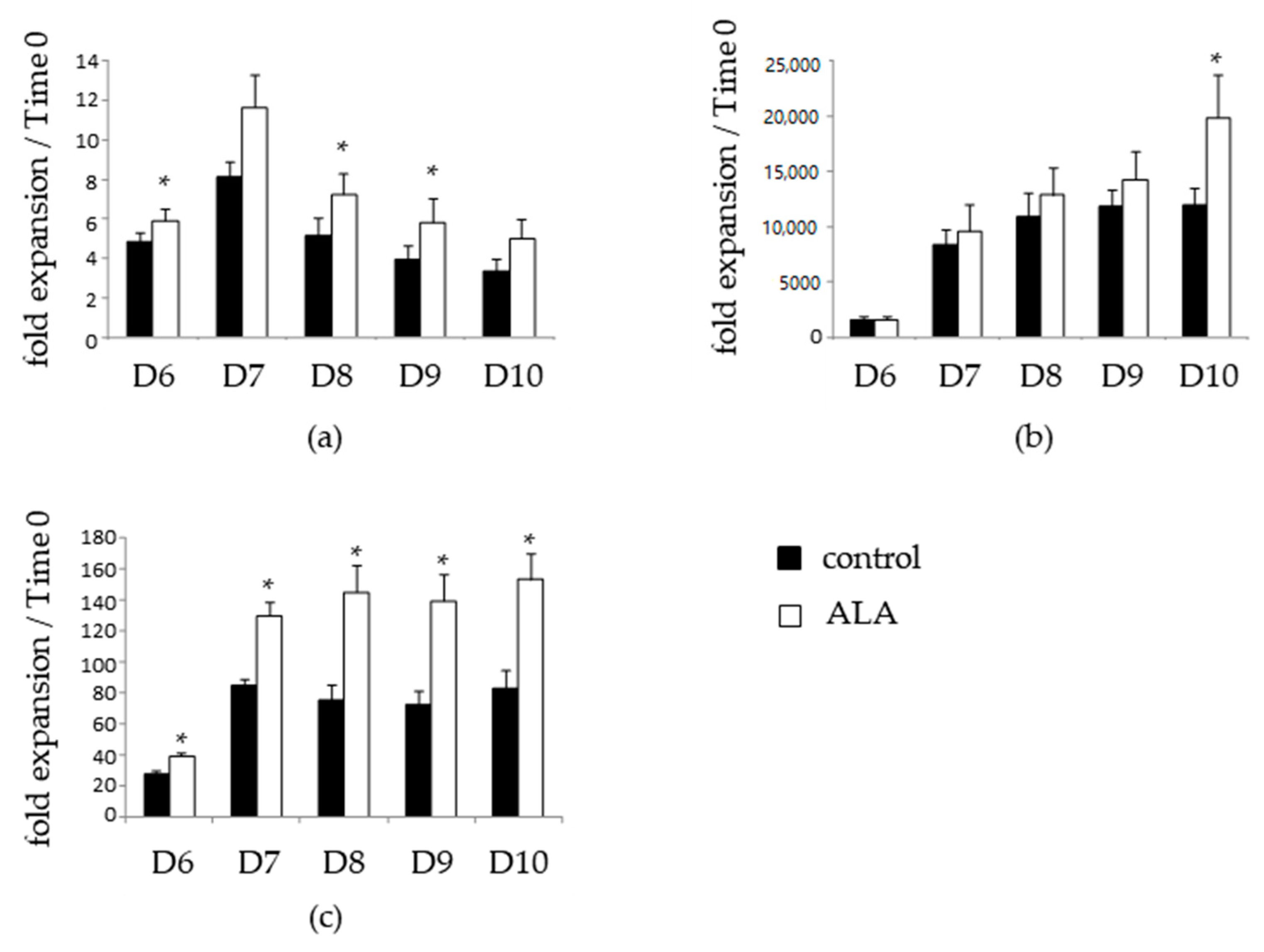
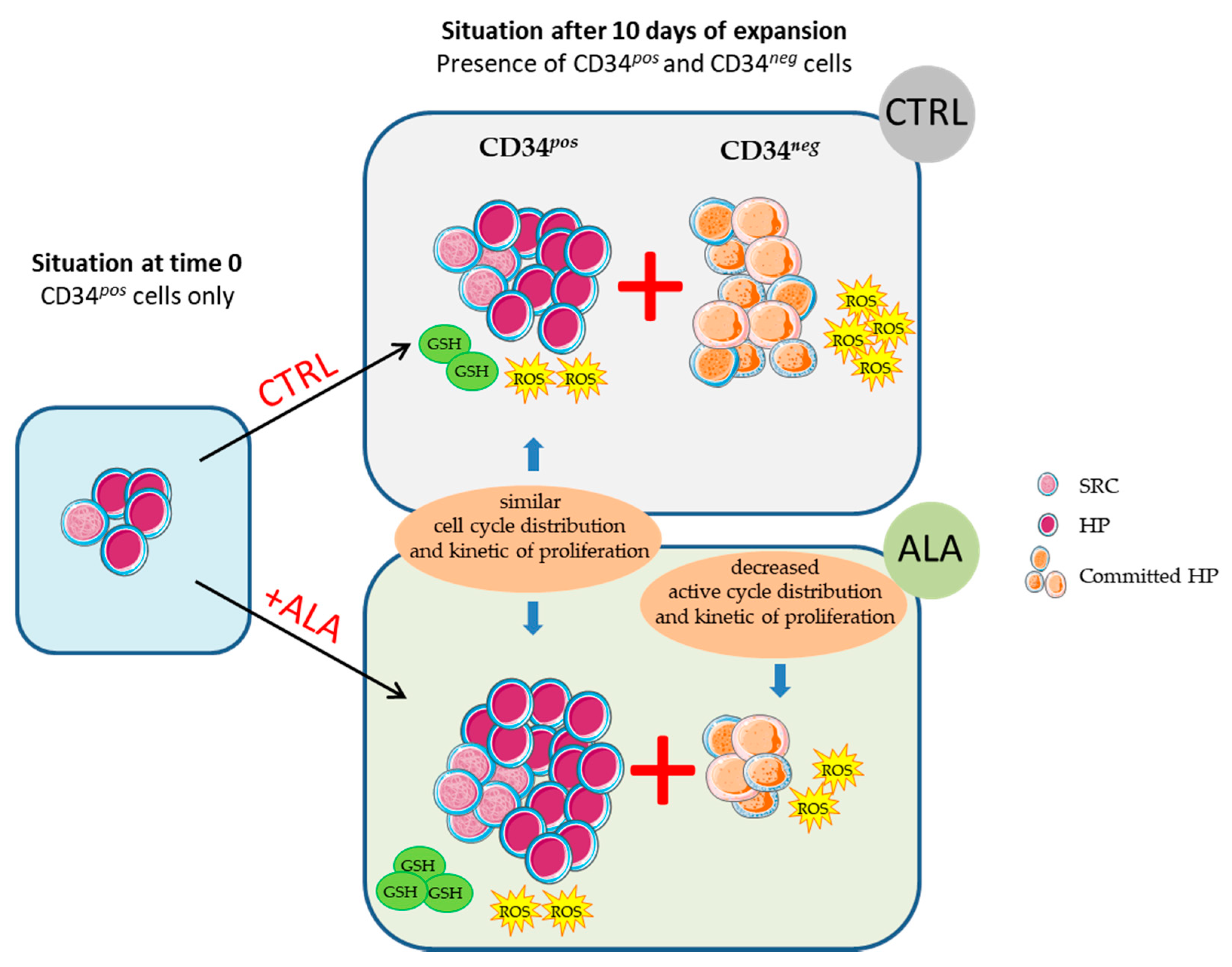
3.1. ALA Allows Expansion of Phenotypically-Defined Primitive Cells
3.2. ALA Increases SRC Frequency after 10 Days of Culture
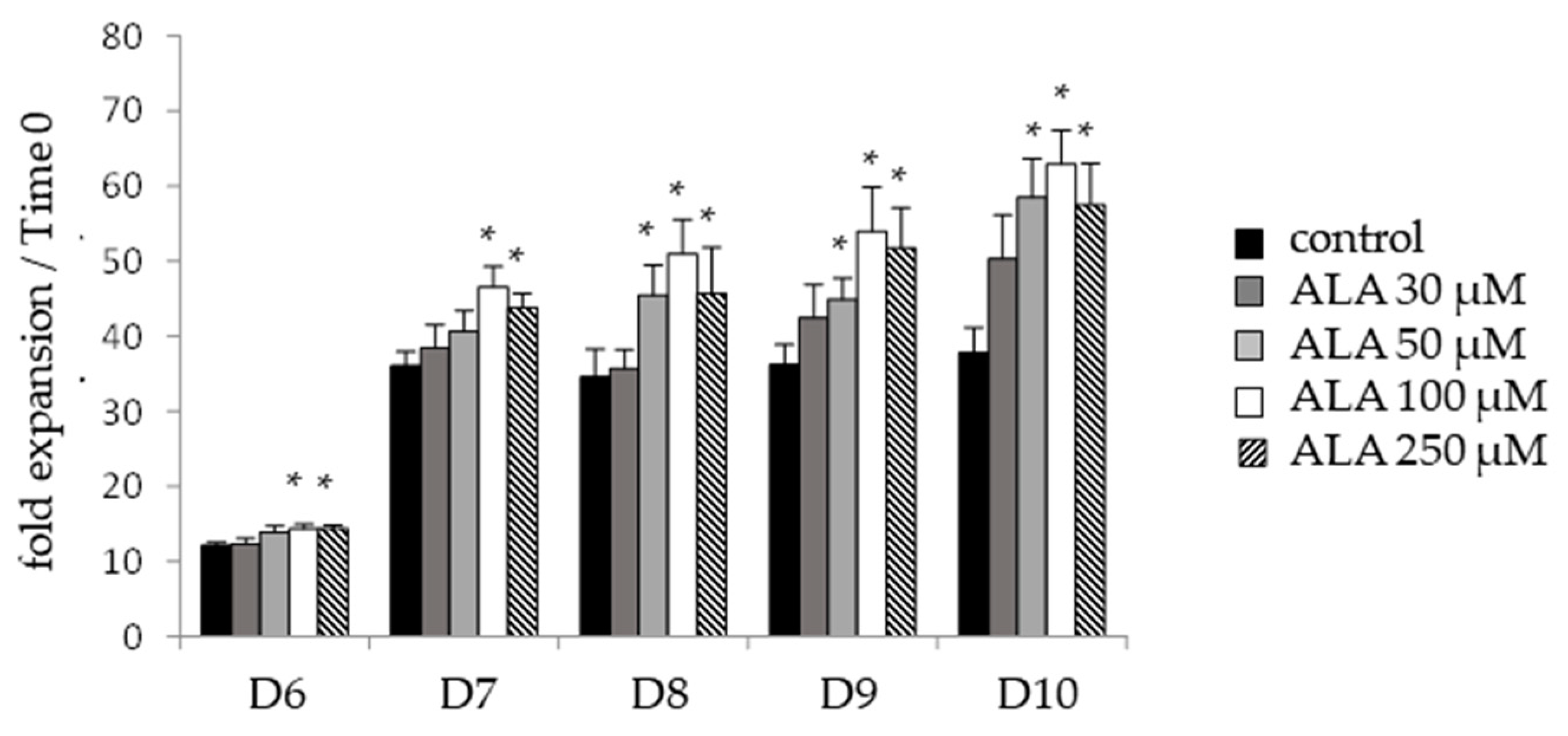
3.3. ALA Modifies Proliferation and Cell Cycle Distribution of CD34neg Population but Not of CD34pos
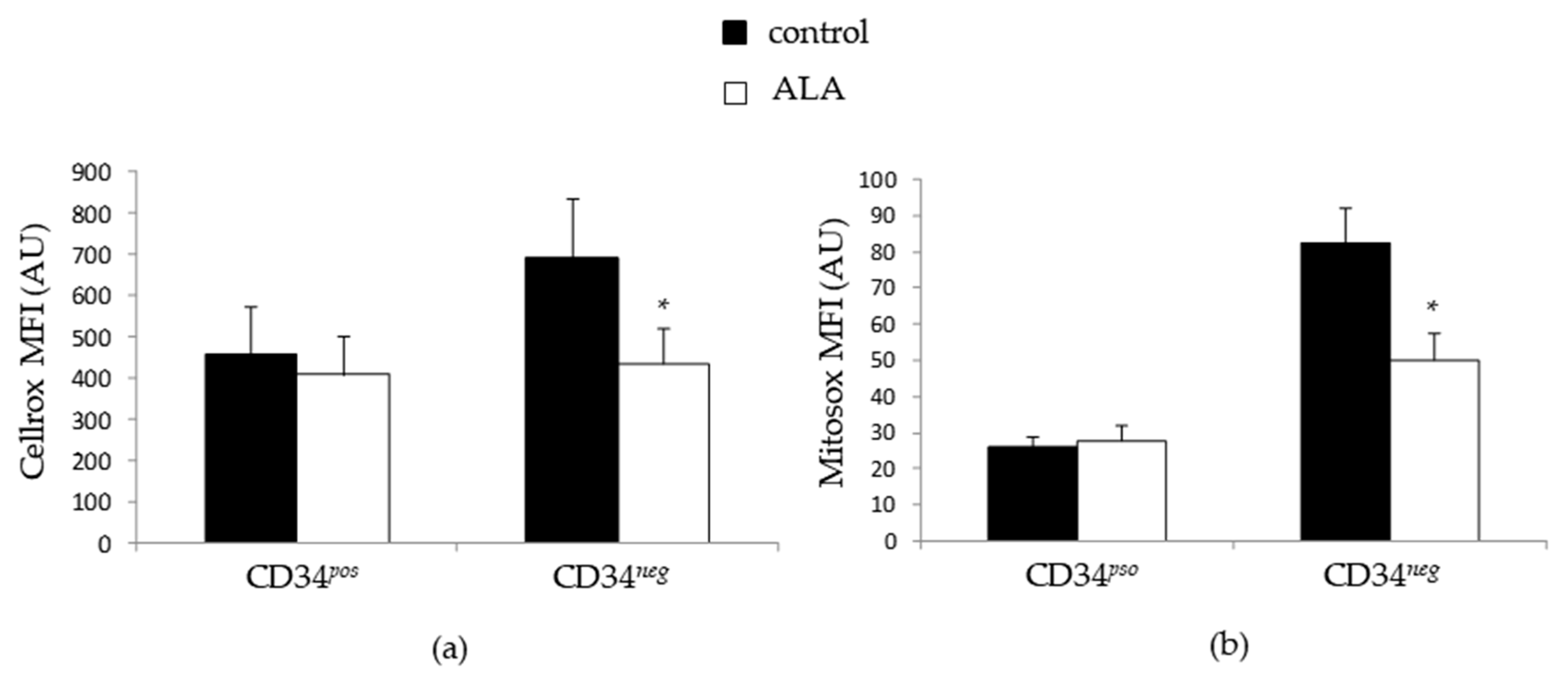
3.4. ALA Doest Not Modify Intracellularros Status in CD34pos Cells but Increases Their GSH Content
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kessinger, A.; Armitage, J.O.; Landmark, J.D.; Weisenburger, D.D. Reconstitution of Human Hematopoietic Function with Autologous Cryopreserved Circulating Stem Cells. Exp. Hematol. 1986, 14, 192–196. [Google Scholar] [PubMed]
- Duhrsen, U.; Villeval, J.; Boyd, J.; Kannourakis, G.; Morstyn, G.; Metcalf, D. Effects of Recombinant Human Granulocyte Colony-Stimulating Factor on Hematopoietic Progenitor Cells in Cancer Patients. Blood 1988, 72, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.; Elias, A.; Schnipper, L.; Cannistra, S.; Antman, K.; Griffin, J. Granulocyte-Macrophage Colony Stimulating Factor Expands the Circulating Haemopoietic Progenitor Cell Compartment in Man. Lancet 1988, 331, 1194–1198. [Google Scholar] [CrossRef]
- Haas, R.; Ho, A.D.; Bredthauer, U.; Cayeux, S.; Egerer, G.; Knauf, W.; Hunstein, W. Successful Autologous Transplantation of Blood Stem Cells Mobilized with Recombinant Human Granulocyte-Macrophage Colony-Stimulating Factor. Exp. Hematol. 1990, 18, 94–98. [Google Scholar] [PubMed]
- Pulsipher, M.A.; Chitphakdithai, P.; Miller, J.P.; Logan, B.R.; King, R.J.; Rizzo, J.D.; Leitman, S.F.; Anderlini, P.; Haagenson, M.D.; Kurian, S.; et al. Adverse Events among 2408 Unrelated Donors of Peripheral Blood Stem Cells: Results of a Prospective Trial from the National Marrow Donor Program. Blood 2009, 113, 3604–3611. [Google Scholar] [CrossRef] [PubMed]
- Udomsakdi, C.; Lansdorp, P.M.; Hogge, D.E.; Reid, D.S.; Eaves, A.C.; Eaves, C.J. Characterization of Primitive Hematopoietic Cells in Normal Human Peripheral Blood. Blood 1992, 80, 2513–2521. [Google Scholar] [CrossRef]
- Hirayama, F.; Yamaguchi, M.; Yano, M.; Yasui, K.; Horie, Y.; Matsumoto, K.; Nagao, N.; Ikebuchi, K.; Azuma, H.; Ikeda, H.; et al. Spontaneous and Rapid Reexpression of Functional CXCR4 by Human Steady-State Peripheral Blood CD34+ Cells. Int. J. Hematol. 2003, 78, 48–55. [Google Scholar] [CrossRef]
- Brunet de la Grange, P.; Vlaski, M.; Duchez, P.; Chevaleyre, J.; Lapostolle, V.; Boiron, J.-M.; Praloran, V.; Ivanovic, Z. Long-Term Repopulating Hematopoietic Stem Cells and “Side Population” in Human Steady State Peripheral Blood. Stem Cell Res. 2013, 11, 625–633. [Google Scholar] [CrossRef][Green Version]
- Lapostolle, V.; Chevaleyre, J.; Duchez, P.; Rodriguez, L.; Vlaski-Lafarge, M.; Sandvig, I.; Brunet de la Grange, P.; Ivanovic, Z. Repopulating Hematopoietic Stem Cells from Steady-State Blood before and after Ex Vivo Culture Are Enriched in the CD34+ CD133+ CXCR4 low Fraction. Haematologica 2018, 103, 1604–1615. [Google Scholar] [CrossRef]
- Bourdieu, A.; Avalon, M.; Lapostolle, V.; Ismail, S.; Mombled, M.; Debeissat, C.; Guérinet, M.; Duchez, P.; Chevaleyre, J.; Vlaski-Lafarge, M.; et al. Steady State Peripheral Blood Provides Cells with Functional and Metabolic Characteristics of Real Hematopoietic Stem Cells. J. Cell. Physiol. 2018, 233, 338–349. [Google Scholar] [CrossRef]
- Zimran, E.; Papa, L.; Hoffman, R. Ex Vivo Expansion of Hematopoietic Stem Cells: Finally Transitioning from the Lab to the Clinic. Blood Rev. 2021, 50, 100853. [Google Scholar] [CrossRef] [PubMed]
- Simsek, T.; Kocabas, F.; Zheng, J.; DeBerardinis, R.J.; Mahmoud, A.I.; Olson, E.N.; Schneider, J.W.; Zhang, C.C.; Sadek, H.A. The Distinct Metabolic Profile of Hematopoietic Stem Cells Reflects Their Location in a Hypoxic Niche. Cell Stem Cell 2010, 7, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Vannini, N.; Girotra, M.; Naveiras, O.; Nikitin, G.; Campos, V.; Giger, S.; Roch, A.; Auwerx, J.; Lutolf, M.P. Specification of Haematopoietic Stem Cell Fate via Modulation of Mitochondrial Activity. Nat. Commun. 2016, 7, 13125. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R.; et al. Direct Measurement of Local Oxygen Concentration in the Bone Marrow of Live Animals. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef]
- Ito, K.; Hirao, A.; Arai, F.; Matsuoka, S.; Takubo, K.; Hamaguchi, I.; Nomiyama, K.; Hosokawa, K.; Sakurada, K.; Nakagata, N.; et al. Regulation of Oxidative Stress by ATM Is Required for Self-Renewal of Haematopoietic Stem Cells. Nature 2004, 431, 997–1002. [Google Scholar] [CrossRef]
- Ito, K.; Hirao, A.; Arai, F.; Takubo, K.; Matsuoka, S.; Miyamoto, K.; Ohmura, M.; Naka, K.; Hosokawa, K.; Ikeda, Y.; et al. Reactive Oxygen Species Act through P38 MAPK to Limit the Lifespan of Hematopoietic Stem Cells. Nat. Med. 2006, 12, 446–451. [Google Scholar] [CrossRef]
- Tothova, Z.; Kollipara, R.; Huntly, B.J.; Lee, B.H.; Castrillon, D.H.; Cullen, D.E.; McDowell, E.P.; Lazo-Kallanian, S.; Williams, I.R.; Sears, C.; et al. FoxOs Are Critical Mediators of Hematopoietic Stem Cell Resistance to Physiologic Oxidative Stress. Cell 2007, 128, 325–339. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Y.; Liu, R.; Ikenoue, T.; Guan, K.-L.; Liu, Y.; Zheng, P. TSC–MTOR Maintains Quiescence and Function of Hematopoietic Stem Cells by Repressing Mitochondrial Biogenesis and Reactive Oxygen Species. J. Exp. Med. 2008, 205, 2397–2408. [Google Scholar] [CrossRef]
- Tsai, J.J.; Dudakov, J.A.; Takahashi, K.; Shieh, J.-H.; Velardi, E.; Holland, A.M.; Singer, N.V.; West, M.L.; Smith, O.M.; Young, L.F.; et al. Nrf2 Regulates Haematopoietic Stem Cell Function. Nat. Cell Biol. 2013, 15, 309–316. [Google Scholar] [CrossRef]
- Gupta, R.; Karpatkin, S.; Basch, R.S. Hematopoiesis and Stem Cell Renewal in Long-Term Bone Marrow Cultures Containing Catalase. Blood 2006, 107, 1837–1846. [Google Scholar] [CrossRef]
- Li, Y.; He, M.; Zhang, W.; Yang, M.; Ding, Y.; Xu, S.; Gu, J.; Li, Y.; Yin, J.; Gao, Y. Antioxidant Small Molecule Compound Chrysin Promotes the Self-Renewal of Hematopoietic Stem Cells. Front. Pharmacol. 2020, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Fayez, A.M.; Zakaria, S.; Moustafa, D. Alpha Lipoic Acid Exerts Antioxidant Effect via Nrf2/HO-1 Pathway Activation and Suppresses Hepatic Stellate Cells Activation Induced by Methotrexate in Rats. Biomed. Pharmacother. 2018, 105, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, S.-Y.; Yang, K.-J.; Kim, Y.; Lee, D.; Lee, M.H.; Kim, D.-K. α-Lipoic Acid Prevents against Cisplatin Cytotoxicity via Activation of the NRF2/HO-1 Antioxidant Pathway. PLoS ONE 2019, 14, e0226769. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Li, S.; Bai, J.; Zhang, Q.; Han, Z. A-Lipoic Acid Protects against Microcystin-LR Induced Hepatotoxicity through Regeneration of Glutathione via Activation of Nrf2. Environ. Toxicol. 2020, 35, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhou, X.; Zhang, J.; Wang, J.; Xie, H.; Wu, Z. α-Lipoic Acid Protects against the Cytotoxicity and Oxidative Stress Induced by Cadmium in HepG2 Cells through Regeneration of Glutathione by Glutathione Reductase via Nrf2/ARE Signaling Pathway. Environ. Toxicol. Pharmacol. 2016, 45, 274–281. [Google Scholar] [CrossRef]
- Song, G.; Liu, Z.; Wang, L.; Shi, R.; Chu, C.; Xiang, M.; Tian, Q.; Liu, X. Protective Effects of Lipoic Acid against Acrylamide-Induced Neurotoxicity: Involvement of Mitochondrial Energy Metabolism and Autophagy. Food Funct. 2017, 8, 4657–4667. [Google Scholar] [CrossRef]
- Dong, Y.; Bai, J.; Zhang, Y.; Zhou, Y.; Pan, X.; Li, X.; Zhou, Q.; Chen, Y.; Lai, M.; Mao, B.; et al. Alpha Lipoic Acid Promotes Development of Hematopoietic Progenitors Derived from Human Embryonic Stem Cells by Antagonizing ROS Signals. J. Leukoc. Biol. 2020, 108, 1711–1725. [Google Scholar] [CrossRef]
- Hu, Y.; Smyth, G.K. ELDA: Extreme Limiting Dilution Analysis for Comparing Depleted and Enriched Populations in Stem Cell and Other Assays. J. Immunol. Methods 2009, 347, 70–78. [Google Scholar] [CrossRef]
- Radtke, S.; Görgens, A.; Kordelas, L.; Schmidt, M.; Kimmig, K.R.; Köninger, A.; Horn, P.A.; Giebel, B. CD133 Allows Elaborated Discrimination and Quantification of Haematopoietic Progenitor Subsets in Human Haematopoietic Stem Cell Transplants. Br. J. Haematol. 2015, 169, 868–878. [Google Scholar] [CrossRef]
- Han, D.; Handelman, G.; Marcocci, L.; Sen, C.K.; Roy, S.; Kobuchi, H.; Tritschler, H.J.; Flohé, L.; Packer, L. Lipoic Acid Increases de Novo Synthesis of Cellular Glutathione by Improving Cystine Utilization. BioFactors 1997, 6, 321–338. [Google Scholar] [CrossRef]
- van de Mark, K.; Chen, J.S.; Steliou, K.; Perrine, S.P.; Faller, D.V. α-Lipoic Acid Induces P27Kip-Dependent Cell Cycle Arrest in Non-Transformed Cell Lines and Apoptosis in Tumor Cell Lines. J. Cell. Physiol. 2003, 194, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, Y.; Lv, G.; Lin, Y.; Tang, J.; Lu, J.; Zhang, M.; Liu, W.; Sun, X. α-Lipoic Acid Inhibits Human Lung Cancer Cell Proliferation through Grb2-Mediated EGFR Downregulation. Biochem. Biophys. Res. Commun. 2017, 494, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Lee, B.; Kim, Y.-R.; Kim, M.-A.; Ryu, N.; Jung, D.J.; Kim, U.-K.; Baek, J.-I.; Lee, K.-Y. Evaluating Protective and Therapeutic Effects of Alpha-Lipoic Acid on Cisplatin-Induced Ototoxicity. Cell Death Dis. 2018, 9, 827. [Google Scholar] [CrossRef] [PubMed]
- Baeeri, M.; Bahadar, H.; Rahimifard, M.; Navaei-Nigjeh, M.; Khorasani, R.; Rezvanfar, M.A.; Gholami, M.; Abdollahi, M. α-Lipoic Acid Prevents Senescence, Cell Cycle Arrest, and Inflammatory Cues in Fibroblasts by Inhibiting Oxidative Stress. Pharmacol. Res. 2019, 141, 214–223. [Google Scholar] [CrossRef]
- Dozio, E.; Ruscica, M.; Passafaro, L.; Dogliotti, G.; Steffani, L.; Pagani, A.; Demartini, G.; Esposti, D.; Fraschini, F.; Magni, P. The Natural Antioxidant Alpha-Lipoic Acid Induces P27Kip1-Dependent Cell Cycle Arrest and Apoptosis in MCF-7 Human Breast Cancer Cells. Eur. J. Pharmacol. 2010, 641, 29–34. [Google Scholar] [CrossRef]
- Havens, C.G.; Ho, A.; Yoshioka, N.; Dowdy, S.F. Regulation of Late G1 /S Phase Transition and APCCdh1 by Reactive Oxygen Species. Mol. Cell. Biol. 2006, 26, 4701–4711. [Google Scholar] [CrossRef]
- Bigarella, C.L.; Liang, R.; Ghaffari, S. Stem Cells and the Impact of ROS Signaling. Development 2014, 141, 4206–4218. [Google Scholar] [CrossRef]
- Papa, L.; Djedaini, M.; Hoffman, R. Mitochondrial Role in Stemness and Differentiation of Hematopoietic Stem Cells. Stem Cells Int. 2019, 2019, 4067162. [Google Scholar] [CrossRef]
- Tan, D.Q.; Suda, T. Reactive Oxygen Species and Mitochondrial Homeostasis as Regulators of Stem Cell Fate and Function. Antioxid. Redox Signal. 2018, 29, 149–168. [Google Scholar] [CrossRef]
- Fan, J.; Cai, H.; Yang, S.; Yan, L.; Tan, W. Comparison between the Effects of Normoxia and Hypoxia on Antioxidant Enzymes and Glutathione Redox State in Ex Vivo Culture of CD34+ Cells. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 153–158. [Google Scholar] [CrossRef]



| SRC Frequency (1/…) | Pairwise Test (ELDA Webtool) | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | Lower | Estimate | Upper | Group 1 | Group 2 | Chisq | DF | Pr (>Chisq) |
| Time 0 | 64,247 | 26,736 | 11,126 | Control | Time 0 | 16.6 | 1 | 4.51 × 10−5 |
| Control | 6643 | 3796 | 2169 | ALA | Time 0 | 23.2 | 1 | 1.44 × 10−6 |
| ALA | 4596 | 2485 | 1343 | ALA | Control | 0.972 | 1 | 0.324 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debeissat, C.; Avalon, M.; Huart, M.; Duchez, P.; Rodriguez, L.; Vlaski-Lafarge, M.; Ivanovic, Z.; Brunet de la Grange, P. Alpha Lipoic-Acid Potentiates Ex Vivo Expansion of Human Steady-State Peripheral Blood Hematopoietic Primitive Cells. Biomolecules 2022, 12, 431. https://doi.org/10.3390/biom12030431
Debeissat C, Avalon M, Huart M, Duchez P, Rodriguez L, Vlaski-Lafarge M, Ivanovic Z, Brunet de la Grange P. Alpha Lipoic-Acid Potentiates Ex Vivo Expansion of Human Steady-State Peripheral Blood Hematopoietic Primitive Cells. Biomolecules. 2022; 12(3):431. https://doi.org/10.3390/biom12030431
Chicago/Turabian StyleDebeissat, Christelle, Maryse Avalon, Mathilde Huart, Pascale Duchez, Laura Rodriguez, Marija Vlaski-Lafarge, Zoran Ivanovic, and Philippe Brunet de la Grange. 2022. "Alpha Lipoic-Acid Potentiates Ex Vivo Expansion of Human Steady-State Peripheral Blood Hematopoietic Primitive Cells" Biomolecules 12, no. 3: 431. https://doi.org/10.3390/biom12030431
APA StyleDebeissat, C., Avalon, M., Huart, M., Duchez, P., Rodriguez, L., Vlaski-Lafarge, M., Ivanovic, Z., & Brunet de la Grange, P. (2022). Alpha Lipoic-Acid Potentiates Ex Vivo Expansion of Human Steady-State Peripheral Blood Hematopoietic Primitive Cells. Biomolecules, 12(3), 431. https://doi.org/10.3390/biom12030431







