Adenosine-Metabolizing Enzymes, Adenosine Kinase and Adenosine Deaminase, in Cancer
Abstract
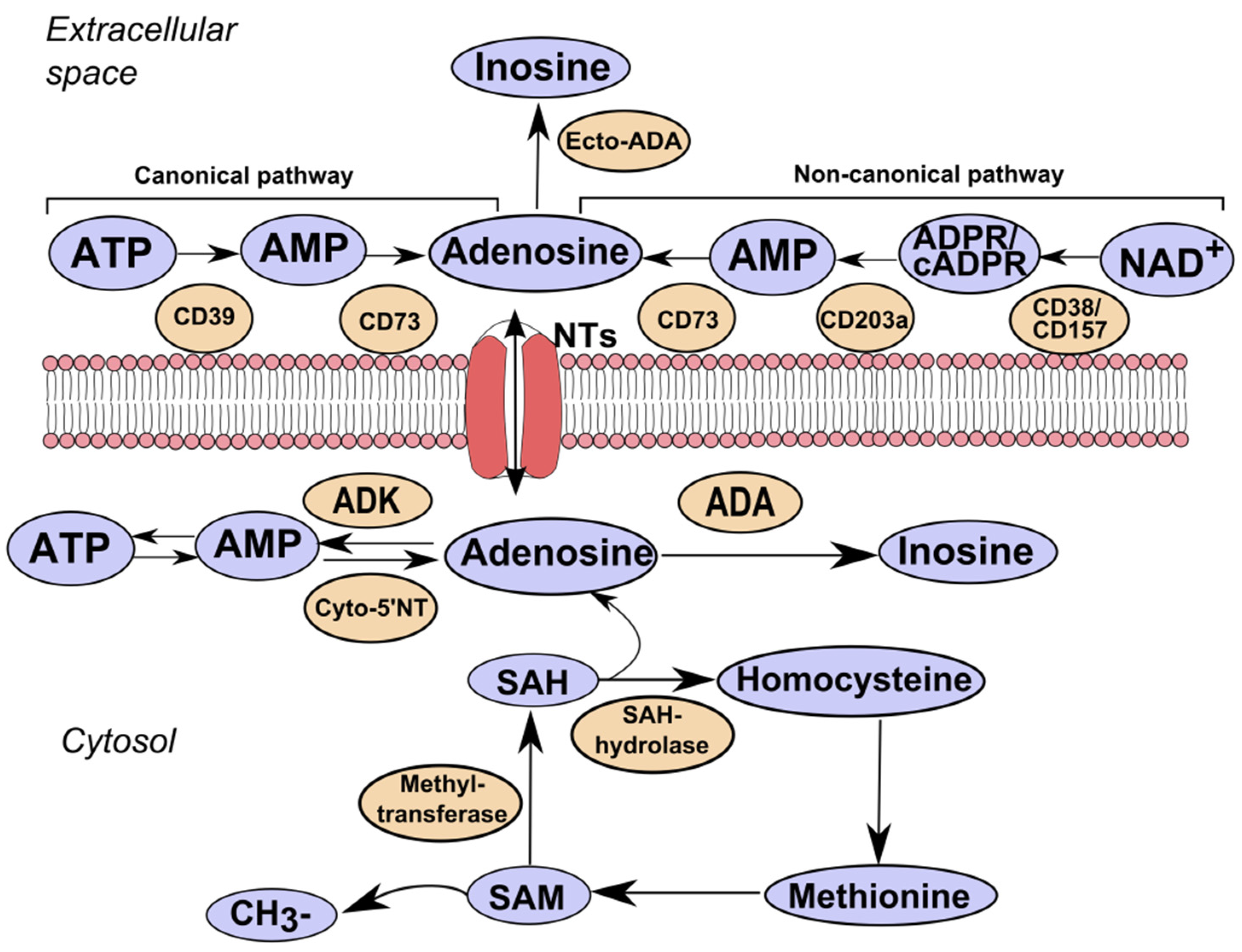
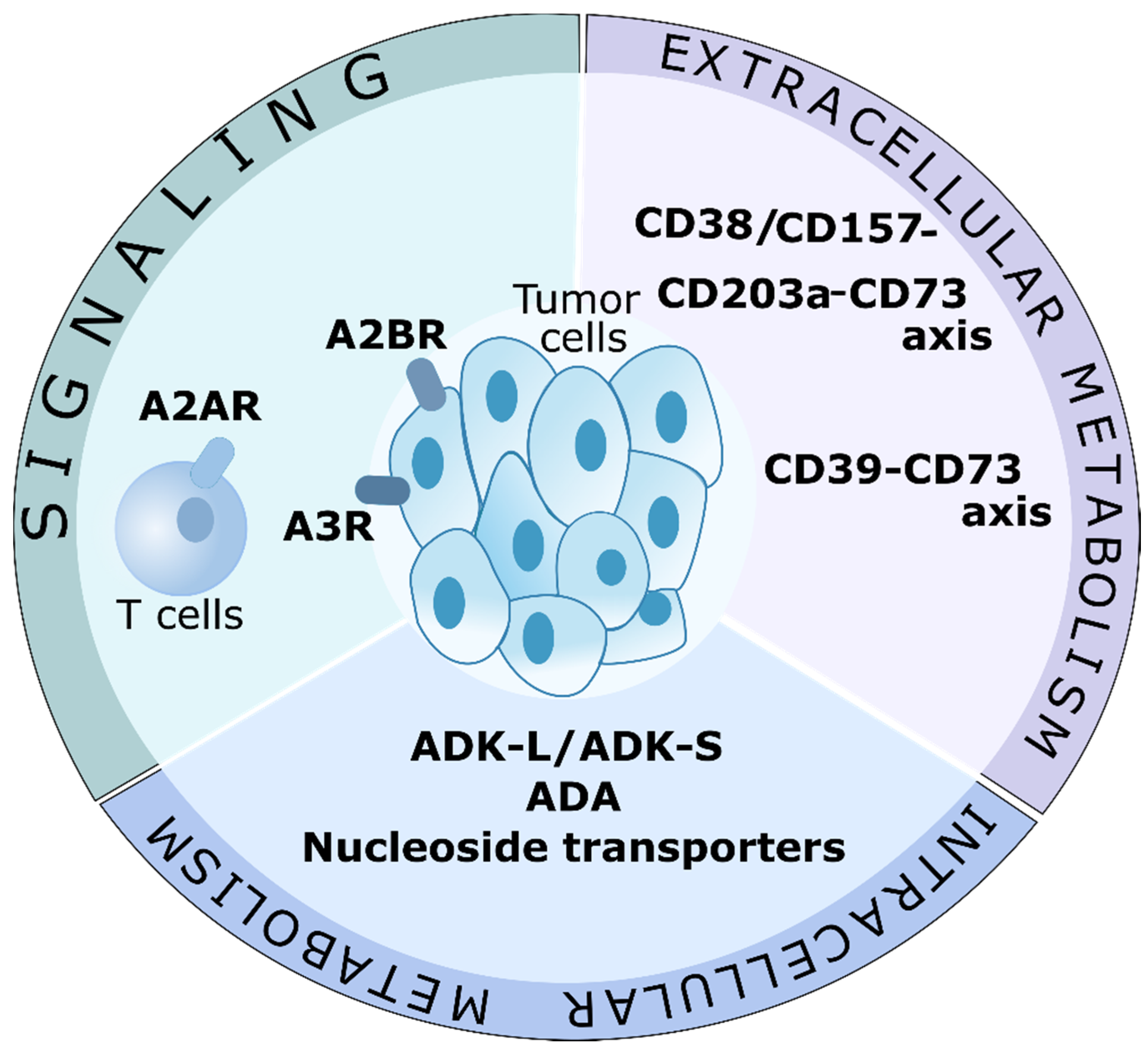
1. Introduction
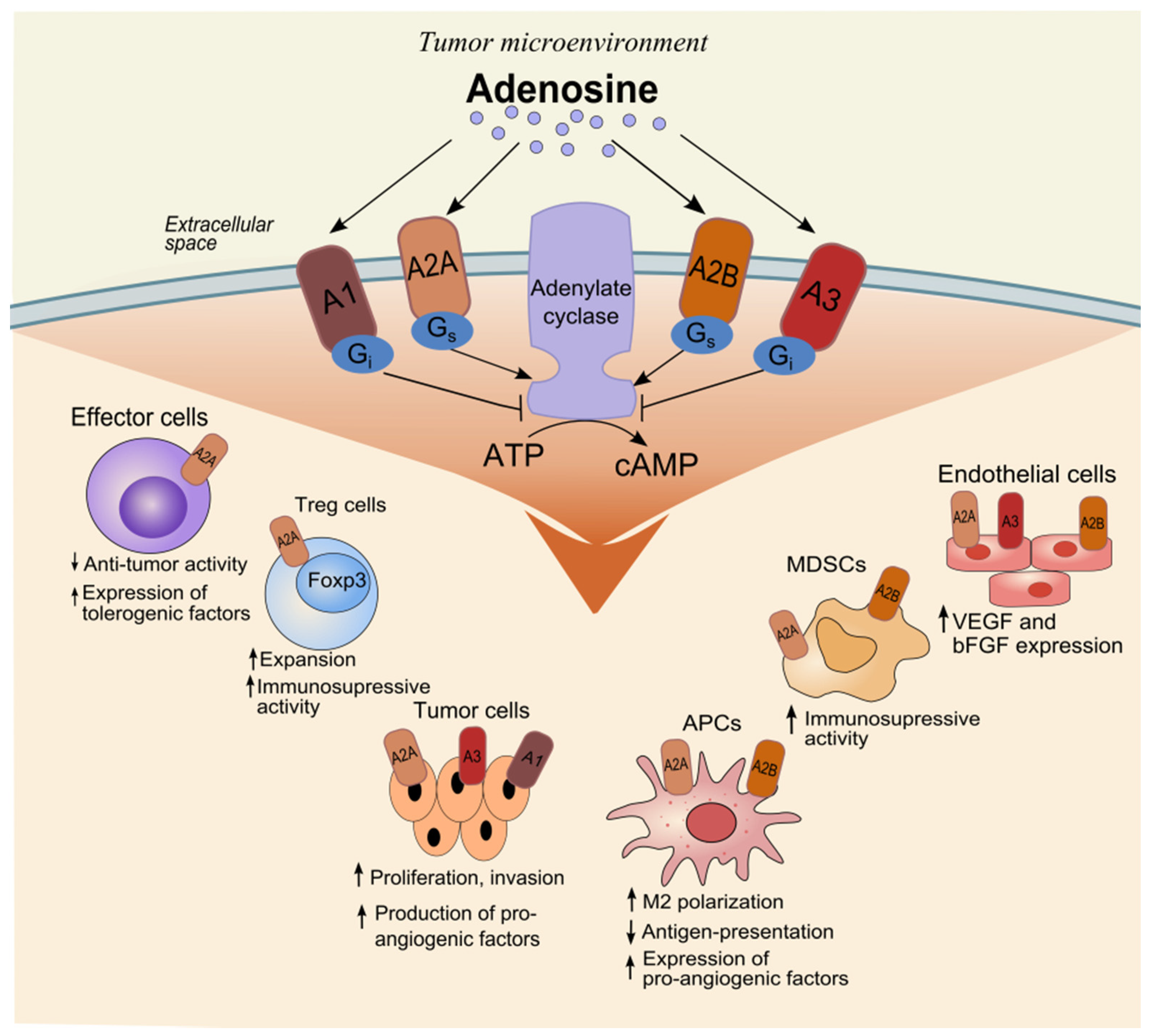
2. The Effect of Adenosine on Cells in the Tumor Microenvironment
3. Adenosine Kinase
3.1. Biological Significance of ADK
3.2. The Role of ADK in Cancer
4. Adenosine Deaminase
4.1. The biological Significance of Adenosine Deaminase
4.2. The Role of ADA in Cancer
5. Targeting ADK and ADA in the Cancer Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H. A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Curr. Oncol. 2020, 27, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Baghbani, E.; Noorolyai, S.; Shanehbandi, D.; Mokhtarzadeh, A.; Aghebati-Maleki, L.; Shahgoli, V.K.; Brunetti, O.; Rahmani, S.; Shadbad, M.A.; Baghbanzadeh, A.; et al. Regulation of Immune Responses through CD39 and CD73 in Cancer: Novel Checkpoints. Life Sci. 2021, 282, 119826. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Fornai, M.; Blandizzi, C.; Pacher, P.; Haskó, G. Adenosine Signaling and the Immune System: When a Lot Could Be Too Much. Immunol. Lett. 2019, 205, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Churov, A.; Zhulai, G. Targeting Adenosine and Regulatory T Cells in Cancer Immunotherapy. Hum. Immunol. 2021, 82, 270–278. [Google Scholar] [CrossRef]
- Boison, D.; Yegutkin, G.G. Adenosine Metabolism: Emerging Concepts for Cancer Therapy. Cancer Cell 2019, 36, 582–596. [Google Scholar] [CrossRef]
- Shevchenko, I.; Mathes, A.; Groth, C.; Karakhanova, S.; Müller, V.; Utikal, J.; Werner, J.; Bazhin, A.V.; Umansky, V. Enhanced Expression of CD39 and CD73 on T Cells in the Regulation of Anti-Tumor Immune Responses. OncoImmunology 2020, 9, 1744946. [Google Scholar] [CrossRef]
- Zhulai, G.A.; Churov, A.V.; Oleinik, E.K.; Romanov, A.A.; Semakova, V.M.; Oleinik, V.M. Activation of CD4+CD39+ T Cells in Colorectal Canser. Bull. Russ. State Med. Univ. 2018, 3, 47–53. [Google Scholar] [CrossRef]
- Cai, X.-Y.; Ni, X.-C.; Yi, Y.; He, H.-W.; Wang, J.-X.; Fu, Y.-P.; Sun, J.; Zhou, J.; Cheng, Y.-F.; Jin, J.-J.; et al. Overexpression of CD39 in Hepatocellular Carcinoma Is an Independent Indicator of Poor Outcome after Radical Resection. Medicine 2016, 95, e4989. [Google Scholar] [CrossRef]
- Koivisto, M.K.; Tervahartiala, M.; Kenessey, I.; Jalkanen, S.; Boström, P.J.; Salmi, M. Cell-Type-Specific CD73 Expression Is an Independent Prognostic Factor in Bladder Cancer. Carcinogenesis 2019, 40, 84–92. [Google Scholar] [CrossRef]
- Wang, J.; Matosevic, S. NT5E/CD73 as Correlative Factor of Patient Survival and Natural Killer Cell Infiltration in Glioblastoma. J. Clin. Med. 2019, 8, 1526. [Google Scholar] [CrossRef]
- Loi, S.; Pommey, S.; Haibe-Kains, B.; Beavis, P.A.; Darcy, P.K.; Smyth, M.J.; Stagg, J. CD73 Promotes Anthracycline Resistance and Poor Prognosis in Triple Negative Breast Cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 11091–11096. [Google Scholar] [CrossRef] [PubMed]
- Horenstein, A.L.; Chillemi, A.; Zaccarello, G.; Bruzzone, S.; Quarona, V.; Zito, A.; Serra, S.; Malavasi, F. A CD38/CD203a/CD73 Ectoenzymatic Pathway Independent of CD39 Drives a Novel Adenosinergic Loop in Human T Lymphocytes. OncoImmunology 2013, 2, e26246. [Google Scholar] [CrossRef] [PubMed]
- Losenkova, K.; Zuccarini, M.; Karikoski, M.; Laurila, J.; Boison, D.; Jalkanen, S.; Yegutkin, G.G. Compartmentalization of Adenosine Metabolism in Cancer Cells and Its Modulation during Acute Hypoxia. J. Cell Sci. 2020, 133, jcs241463. [Google Scholar] [CrossRef]
- Morandi, F.; Morandi, B.; Horenstein, A.L.; Chillemi, A.; Quarona, V.; Zaccarello, G.; Carrega, P.; Ferlazzo, G.; Mingari, M.C.; Moretta, L.; et al. A Non-Canonical Adenosinergic Pathway Led by CD38 in Human Melanoma Cells Induces Suppression of T Cell Proliferation. Oncotarget 2015, 6, 25602–25618. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Gupta, R.S. Adenosine Kinase and Ribokinase—The RK Family of Proteins. Cell. Mol. Life Sci. 2008, 65, 2875–2896. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Saboury, A.A.; Haertlé, T. Adenosine Deaminase Inhibition. Int. J. Biol. Macromol. 2019, 141, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Gupta, R.S. Adenosine Metabolism, Adenosine Kinase, and Evolution. In Adenosine; Masino, S., Boison, D., Eds.; Springer: New York, NY, USA, 2013; pp. 23–54. [Google Scholar] [CrossRef]
- dos Santos-Rodrigues, A.; Grañé-Boladeras, N.; Bicket, A.; Coe, I.R. Nucleoside Transporters in the Purinome. Neurochem. Int. 2014, 73, 229–237. [Google Scholar] [CrossRef]
- Hajizadeh, F.; Okoye, I.; Esmaily, M.; Ghasemi Chaleshtari, M.; Masjedi, A.; Azizi, G.; Irandoust, M.; Ghalamfarsa, G.; Jadidi-Niaragh, F. Hypoxia Inducible Factors in the Tumor Microenvironment as Therapeutic Targets of Cancer Stem Cells. Life Sci. 2019, 237, 116952. [Google Scholar] [CrossRef]
- Spychala, J.; Kitajewski, J. Wnt and β-Catenin Signaling Target the Expression of Ecto-5′-Nucleotidase and Increase Extracellular Adenosine Generation. Exp. Cell Res. 2004, 296, 99–108. [Google Scholar] [CrossRef]
- Niemelä, J.; Henttinen, T.; Yegutkin, G.G.; Airas, L.; Kujari, A.-M.; Rajala, P.; Jalkanen, S. IFN-α Induced Adenosine Production on the Endothelium: A Mechanism Mediated by CD73 (Ecto-5′-Nucleotidase) Up-Regulation. J. Immunol. 2004, 172, 1646–1653. [Google Scholar] [CrossRef]
- Schuler, P.J.; Saze, Z.; Hong, C.-S.; Muller, L.; Gillespie, D.G.; Cheng, D.; Harasymczuk, M.; Mandapathil, M.; Lang, S.; Jackson, E.K.; et al. Human CD4+CD39+ Regulatory T Cells Produce Adenosine upon Co-Expression of Surface CD73 or Contact with CD73+ Exosomes or CD73+ Cells. Clin. Exp. Immunol. 2014, 177, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A. A Metabolic Immune Checkpoint: Adenosine in Tumor Microenvironment. Front. Immunol. 2016, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Raskovalova, T.; Lokshin, A.; Huang, X.; Jackson, E.K.; Gorelik, E. Adenosine-Mediated Inhibition of Cytotoxic Activity and Cytokine Production by IL-2/NKp46-Activated NK Cells: Involvement of Protein Kinase A Isozyme I (PKA I). Immunol. Res. 2006, 36, 91–100. [Google Scholar] [CrossRef]
- Häusler, S.F.M.; Montalbán del Barrio, I.; Strohschein, J.; Anoop Chandran, P.; Engel, J.B.; Hönig, A.; Ossadnik, M.; Horn, E.; Fischer, B.; Krockenberger, M.; et al. Ectonucleotidases CD39 and CD73 on OvCA Cells Are Potent Adenosine-Generating Enzymes Responsible for Adenosine Receptor 2A-Dependent Suppression of T Cell Function and NK Cell Cytotoxicity. Cancer Immunol. Immunother. 2011, 60, 1405–1418. [Google Scholar] [CrossRef]
- Raskovalova, T.; Lokshin, A.; Huang, X.; Su, Y.; Mandic, M.; Zarour, H.M.; Jackson, E.K.; Gorelik, E. Inhibition of Cytokine Production and Cytotoxic Activity of Human Antimelanoma Specific CD8+ and CD4+ T Lymphocytes by Adenosine-Protein Kinase A Type I Signaling. Cancer Res. 2007, 67, 5949–5956. [Google Scholar] [CrossRef]
- Ma, S.-R.; Deng, W.-W.; Liu, J.-F.; Mao, L.; Yu, G.-T.; Bu, L.-L.; Kulkarni, A.B.; Zhang, W.-F.; Sun, Z.-J. Blockade of Adenosine A2A Receptor Enhances CD8+ T Cells Response and Decreases Regulatory T Cells in Head and Neck Squamous Cell Carcinoma. Mol. Cancer 2017, 16, 99. [Google Scholar] [CrossRef]
- Mediavilla-Varela, M.; Luddy, K.; Noyes, D.; Khalil, F.K.; Neuger, A.M.; Soliman, H.; Antonia, S.J. Antagonism of Adenosine A2A Receptor Expressed by Lung Adenocarcinoma Tumor Cells and Cancer Associated Fibroblasts Inhibits Their Growth. Cancer Biol. Ther. 2013, 14, 860–868. [Google Scholar] [CrossRef]
- Cekic, C.; Day, Y.-J.; Sag, D.; Linden, J. Myeloid Expression of Adenosine A2A Receptor Suppresses T and NK Cell Responses in the Solid Tumor Microenvironment. Cancer Res. 2014, 74, 7250–7259. [Google Scholar] [CrossRef]
- Ohta, A.; Kini, R.; Ohta, A.; Subramanian, M.; Madasu, M.; Sitkovsky, M. The Development and Immunosuppressive Functions of CD4+ CD25+ FoxP3+ Regulatory T Cells Are under Influence of the Adenosine-A2A Adenosine Receptor Pathway. Front. Immunol. 2012, 3, 190. [Google Scholar] [CrossRef]
- Sorrentino, C.; Miele, L.; Porta, A.; Pinto, A.; Morello, S. Myeloid-Derived Suppressor Cells Contribute to A2B Adenosine Receptor-Induced VEGF Production and Angiogenesis in a Mouse Melanoma Model. Oncotarget 2015, 6, 27478–27489. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A. Hypoxia-Driven Adenosine Accumulation: A Crucial Microenvironmental Factor Promoting Tumor Progression. In Oxygen Transport to Tissue XXXVII; Elwell, C.E., Leung, T.S., Harrison, D.K., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2016; Volume 876, pp. 177–183. [Google Scholar] [CrossRef]
- Mittal, D.; Sinha, D.; Barkauskas, D.; Young, A.; Kalimutho, M.; Stannard, K.; Caramia, F.; Haibe-Kains, B.; Stagg, J.; Khanna, K.K.; et al. Adenosine 2B Receptor Expression on Cancer Cells Promotes Metastasis. Cancer Res. 2016, 76, 4372–4382. [Google Scholar] [CrossRef] [PubMed]
- Gessi, S.; Bencivenni, S.; Battistello, E.; Vincenzi, F.; Colotta, V.; Catarzi, D.; Varano, F.; Merighi, S.; Borea, P.A.; Varani, K. Inhibition of A2A Adenosine Receptor Signaling in Cancer Cells Proliferation by the Novel Antagonist TP455. Front. Pharmacol. 2017, 8, 888. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chu, X.; Deng, F.; Tong, L.; Tong, G.; Yi, Y.; Liu, J.; Tang, J.; Tang, Y.; Xia, Y.; et al. The Adenosine A2b Receptor Promotes Tumor Progression of Bladder Urothelial Carcinoma by Enhancing MAPK Signaling Pathway. Oncotarget 2017, 8, 48755–48768. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Guo, G.; Huang, L.; Deng, L.; Chang, C.-S.; Achyut, B.R.; Canning, M.; Xu, N.; Arbab, A.S.; Bollag, R.J.; et al. CD73 on Cancer-Associated Fibroblasts Enhanced by the A2B-Mediated Feedforward Circuit Enforces an Immune Checkpoint. Nat Commun. 2020, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Rotondo, J.C.; Lanzillotti, C.; Campione, G.; Martini, F.; Tognon, M. Cancer Biology and Molecular Genetics of A3 Adenosine Receptor. Oncogene 2022, 41, 301–308. [Google Scholar] [CrossRef]
- Novitskiy, S.V.; Ryzhov, S.; Zaynagetdinov, R.; Goldstein, A.E.; Huang, Y.; Tikhomirov, O.Y.; Blackburn, M.R.; Biaggioni, I.; Carbone, D.P.; Feoktistov, I.; et al. Adenosine Receptors in Regulation of Dendritic Cell Differentiation and Function. Blood 2008, 112, 1822–1831. [Google Scholar] [CrossRef]
- Chen, S.; Akdemir, I.; Fan, J.; Linden, J.; Zhang, B.; Cekic, C. The Expression of Adenosine A2B Receptor on Antigen-Presenting Cells Suppresses CD8+ T-Cell Responses and Promotes Tumor Growth. Cancer Immunol. Res. 2020, 8, 1064–1074. [Google Scholar] [CrossRef]
- Boison, D. Adenosine Kinase: Exploitation for Therapeutic Gain. Pharmacol. Rev. 2013, 65, 906–943. [Google Scholar] [CrossRef]
- Murugan, M.; Fedele, D.; Millner, D.; Alharfoush, E.; Vegunta, G.; Boison, D. Adenosine Kinase: An Epigenetic Modulator in Development and Disease. Neurochem. Int. 2021, 147, 105054. [Google Scholar] [CrossRef]
- Boison, D.; Scheurer, L.; Zumsteg, V.; Rulicke, T.; Litynski, P.; Fowler, B.; Brandner, S.; Mohler, H. Neonatal Hepatic Steatosis by Disruption of the Adenosine Kinase Gene. Proc. Natl. Acad. Sci. USA 2002, 99, 6985–6990. [Google Scholar] [CrossRef]
- Bjursell, M.K.; Blom, H.J.; Cayuela, J.A.; Engvall, M.L.; Lesko, N.; Balasubramaniam, S.; Brandberg, G.; Halldin, M.; Falkenberg, M.; Jakobs, C.; et al. Adenosine Kinase Deficiency Disrupts the Methionine Cycle and Causes Hypermethioninemia, Encephalopathy, and Abnormal Liver Function. Am. J. Hum. Genet. 2011, 89, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Staufner, C.; Lindner, M.; Dionisi-Vici, C.; Freisinger, P.; Dobbelaere, D.; Douillard, C.; Makhseed, N.; Straub, B.K.; Kahrizi, K.; Ballhausen, D.; et al. Adenosine Kinase Deficiency: Expanding the Clinical Spectrum and Evaluating Therapeutic Options. J. Inherit. Metab. Dis. 2016, 39, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Weltha, L.; Reemmer, J.; Boison, D. The Role of Adenosine in Epilepsy. Brain Res. Bull. 2019, 151, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.V.C.; Bourc’his, D. The Diverse Roles of DNA Methylation in Mammalian Development and Disease. Nat. Rev. Mol. Cell Biol. 2019, 20, 590–607. [Google Scholar] [CrossRef]
- Sahin, B.; Kansy, J.W.; Nairn, A.C.; Spychala, J.; Ealick, S.E.; Fienberg, A.A.; Greene, R.W.; Bibb, J.A. Molecular Characterization of Recombinant Mouse Adenosine Kinase and Evaluation as a Target for Protein Phosphorylation: Recombinant Mouse AK as a Protein Phosphorylation Target. Eur. J. Biochem. 2004, 271, 3547–3555. [Google Scholar] [CrossRef]
- Cui, X.A.; Agarwal, T.; Singh, B.; Gupta, R.S. Molecular Characterization of Chinese Hamster Cells Mutants Affected in Adenosine Kinase and Showing Novel Genetic and Biochemical Characteristics. BMC Biochem. 2011, 12, 22. [Google Scholar] [CrossRef]
- Cui, X.A.; Singh, B.; Park, J.; Gupta, R.S. Subcellular Localization of Adenosine Kinase in Mammalian Cells: The Long Isoform of AdK Is Localized in the Nucleus. Biochem. Biophys. Res. Commun. 2009, 388, 46–50. [Google Scholar] [CrossRef]
- Williams-Karnesky, R.L.; Sandau, U.S.; Lusardi, T.A.; Lytle, N.K.; Farrell, J.M.; Pritchard, E.M.; Kaplan, D.L.; Boison, D. Epigenetic Changes Induced by Adenosine Augmentation Therapy Prevent Epileptogenesis. J. Clin. Investig. 2013, 123, 3552–3563. [Google Scholar] [CrossRef]
- Kiese, K.; Jablonski, J.; Boison, D.; Kobow, K. Dynamic Regulation of the Adenosine Kinase Gene during Early Postnatal Brain Development and Maturation. Front. Mol. Neurosci. 2016, 9, 99. [Google Scholar] [CrossRef]
- Gebril, H.M.; Rose, R.M.; Gesese, R.; Emond, M.P.; Huo, Y.; Aronica, E.; Boison, D. Adenosine Kinase Inhibition Promotes Proliferation of Neural Stem Cells after Traumatic Brain Injury. Brain Commun. 2020, 2, fcaa017. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, N.; Zhang, G.; Sauve, A.A. NRH Salvage and Conversion to NAD+ Requires NRH Kinase Activity by Adenosine Kinase. Nat. Metab. 2020, 2, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Bjorness, T.; Greene, R. Adenosine and Sleep. Curr. Neuropharmacol. 2009, 7, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Palchykova, S.; Winsky-Sommerer, R.; Shen, H.-Y.; Boison, D.; Gerling, A.; Tobler, I. Manipulation of Adenosine Kinase Affects Sleep Regulation in Mice. J. Neurosci. 2010, 30, 13157–13165. [Google Scholar] [CrossRef] [PubMed]
- Bjorness, T.E.; Dale, N.; Mettlach, G.; Sonneborn, A.; Sahin, B.; Fienberg, A.A.; Yanagisawa, M.; Bibb, J.A.; Greene, R.W. An Adenosine-Mediated Glial-Neuronal Circuit for Homeostatic Sleep. J. Neurosci. 2016, 36, 3709–3721. [Google Scholar] [CrossRef]
- Li, T.; Ren, G.; Lusardi, T.; Wilz, A.; Lan, J.Q.; Iwasato, T.; Itohara, S.; Simon, R.P.; Boison, D. Adenosine Kinase Is a Target for the Prediction and Prevention of Epileptogenesis in Mice. J. Clin. Investig. 2008, 118, JCI33737. [Google Scholar] [CrossRef]
- Annes, J.P.; Ryu, J.H.; Lam, K.; Carolan, P.J.; Utz, K.; Hollister-Lock, J.; Arvanites, A.C.; Rubin, L.L.; Weir, G.; Melton, D.A. Adenosine Kinase Inhibition Selectively Promotes Rodent and Porcine Islet β-Cell Replication. Proc. Natl. Acad. Sci. USA 2012, 109, 3915–3920. [Google Scholar] [CrossRef]
- Navarro, G.; Abdolazimi, Y.; Zhao, Z.; Xu, H.; Lee, S.; Armstrong, N.A.; Annes, J.P. Genetic Disruption of Adenosine Kinase in Mouse Pancreatic β-Cells Protects Against High-Fat Diet–Induced Glucose Intolerance. Diabetes 2017, 66, 1928–1938. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Yan, S.; Cao, K.; Zeng, X.; Zhou, Y.; Liu, Z.; Yang, Q.; Pan, Y.; Wang, X.; et al. Adenosine Kinase Is Critical for Neointima Formation after Vascular Injury by Inducing Aberrant DNA Hypermethylation. Cardiovasc. Res. 2021, 117, 561–575. [Google Scholar] [CrossRef]
- Zhang, M.; Zeng, X.; Yang, Q.; Xu, J.; Liu, Z.; Zhou, Y.; Cao, Y.; Zhang, X.; An, X.; Xu, Y.; et al. Ablation of Myeloid ADK (Adenosine Kinase) Epigenetically Suppresses Atherosclerosis in ApoE−/− (Apolipoprotein E Deficient) Mice. Arter. Thromb. Vasc. Biol. 2018, 38, 2780–2792. [Google Scholar] [CrossRef]
- Shamloo, B.; Kumar, N.; Owen, R.H.; Reemmer, J.; Ost, J.; Perkins, R.S.; Shen, H.-Y. Dysregulation of Adenosine Kinase Isoforms in Breast Cancer. Oncotarget 2019, 10, 7238–7250. [Google Scholar] [CrossRef]
- Ohta, A.; Gorelik, E.; Prasad, S.J.; Ronchese, F.; Lukashev, D.; Wong, M.K.K.; Huang, X.; Caldwell, S.; Liu, K.; Smith, P.; et al. A2A Adenosine Receptor Protects Tumors from Antitumor T Cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13132–13137. [Google Scholar] [CrossRef] [PubMed]
- Sitkovsky, M.V.; Hatfield, S.; Abbott, R.; Belikoff, B.; Lukashev, D.; Ohta, A. Hostile, Hypoxia–A2-Adenosinergic Tumor Biology as the Next Barrier to Overcome for Tumor Immunologists. Cancer Immunol. Res. 2014, 2, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Giglioni, S.; Leoncini, R.; Aceto, E.; Chessa, A.; Civitelli, S.; Bernini, A.; Tanzini, G.; Carraro, F.; Pucci, A.; Vannoni, D. Adenosine Kinase Gene Expression in Human Colorectal Cancer. Nucleosides Nucleotides Nucleic Acids 2008, 27, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Vannoni, D.; Bernini, A.; Carlucci, F.; Civitelli, S.; Di Pietro, M.C.; Leoncini, R.; Rosi, F.; Tabucchi, A.; Tanzini, G.; Marinello, E. Enzyme Activities Controlling Adenosine Levels in Normal and Neoplastic Tissues. Med. Oncol. 2004, 21, 187–196. [Google Scholar] [CrossRef]
- Huang, J.; He, Y.; Chen, M.; Du, J.; Li, G.; Li, S.; Liu, W.; Long, X. Adenosine Deaminase and Adenosine Kinase Expression in Human Glioma and Their Correlation with Glioma-Associated Epilepsy. Mol. Med. Rep. 2015, 12, 6509–6516. [Google Scholar] [CrossRef]
- El-Kharrag, R.; Owen, R.; Boison, D. Adenosine Kinase Deficiency Increases Susceptibility to a Carcinogen. J. Caffeine Adenosine Res. 2019, 9, 4–11. [Google Scholar] [CrossRef]
- Wahba, A.E.; Fedele, D.; Gebril, H.; AlHarfoush, E.; Toti, K.S.; Jacobson, K.A.; Boison, D. Adenosine Kinase Expression Determines DNA Methylation in Cancer Cell Lines. ACS Pharmacol. Transl. Sci. 2021, 4, 680–686. [Google Scholar] [CrossRef]
- Morote-Garcia, J.C.; Rosenberger, P.; Nivillac, N.M.I.; Coe, I.R.; Eltzschig, H.K. Hypoxia-Inducible Factor–Dependent Repression of Equilibrative Nucleoside Transporter 2 Attenuates Mucosal Inflammation During Intestinal Hypoxia. Gastroenterology 2009, 136, 607–618. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Yan, S.; Zhou, Y.; Yang, Q.; Pan, Y.; Zeng, X.; An, X.; Liu, Z.; Wang, L.; et al. Intracellular Adenosine Regulates Epigenetic Programming in Endothelial Cells to Promote Angiogenesis. EMBO Mol. Med. 2017, 9, 1263–1278. [Google Scholar] [CrossRef]
- Kaljas, Y.; Liu, C.; Skaldin, M.; Wu, C.; Zhou, Q.; Lu, Y.; Aksentijevich, I.; Zavialov, A.V. Human Adenosine Deaminases ADA1 and ADA2 Bind to Different Subsets of Immune Cells. Cell. Mol. Life Sci. 2017, 74, 555–570. [Google Scholar] [CrossRef]
- Meyts, I.; Aksentijevich, I. Deficiency of Adenosine Deaminase 2 (DADA2): Updates on the Phenotype, Genetics, Pathogenesis, and Treatment. J. Clin. Immunol. 2018, 38, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Canet, J.; Gracia, E.; Lluís, C.; Mallol, J.; Canela, E.I.; Cortés, A.; Casadó, V. Molecular Evidence of Adenosine Deaminase Linking Adenosine A2A Receptor and CD26 Proteins. Front. Pharmacol. 2018, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.; Martinez-Navio, J.M.; Lejeune, M.; Climent, N.; Oliva, H.; Gatell, J.M.; Gallart, T.; Mallol, J.; Lluis, C.; Franco, R. CD26, Adenosine Deaminase, and Adenosine Receptors Mediate Costimulatory Signals in the Immunological Synapse. Proc. Natl. Acad. Sci. USA 2005, 102, 9583–9588. [Google Scholar] [CrossRef] [PubMed]
- Ginés, S.; Mariño, M.; Mallol, J.; Canela, E.I.; Morimoto, C.; Callebaut, C.; Hovanessian, A.; Casadó, V.; Lluis, C.; Franco, R. Regulation of Epithelial and Lymphocyte Cell Adhesion by Adenosine Deaminase–CD26 Interaction. Biochem. J. 2002, 361, 203–209. [Google Scholar] [CrossRef]
- Mandapathil, M.; Szczepanski, M.; Harasymczuk, M.; Ren, J.; Cheng, D.; Jackson, E.K.; Gorelik, E.; Johnson, J.; Lang, S.; Whiteside, T.L. CD26 Expression and Adenosine Deaminase Activity in Regulatory T Cells (Treg) and CD4+ T Effector Cells in Patients with Head and Neck Squamous Cell Carcinoma. OncoImmunology 2012, 1, 659–669. [Google Scholar] [CrossRef]
- Naval-Macabuhay, I.; Casanova, V.; Navarro, G.; García, F.; León, A.; Miralles, L.; Rovira, C.; Martinez-Navio, J.M.; Gallart, T.; Mallol, J.; et al. Adenosine Deaminase Regulates Treg Expression in Autologous T Cell-Dendritic Cell Cocultures from Patients Infected with HIV-1. J. Leukoc. Biol. 2016, 99, 349–359. [Google Scholar] [CrossRef]
- Tardif, V.; Muir, R.; Cubas, R.; Chakhtoura, M.; Wilkinson, P.; Metcalf, T.; Herro, R.; Haddad, E.K. Adenosine Deaminase-1 Delineates Human Follicular Helper T Cell Function and Is Altered with HIV. Nat. Commun. 2019, 10, 823. [Google Scholar] [CrossRef]
- Zavialov, A.V.; Yu, X.; Spillmann, D.; Lauvau, G.; Zavialov, A.V. Structural Basis for the Growth Factor Activity of Human Adenosine Deaminase ADA2. J. Biol. Chem. 2010, 285, 12367–12377. [Google Scholar] [CrossRef]
- Zavialov, A.V.; Gracia, E.; Glaichenhaus, N.; Franco, R.; Zavialov, A.V.; Lauvau, G. Human Adenosine Deaminase 2 Induces Differentiation of Monocytes into Macrophages and Stimulates Proliferation of T Helper Cells and Macrophages. J. Leukoc. Biol. 2010, 88, 279–290. [Google Scholar] [CrossRef]
- Valdés, L.; San José, E.; Alvarez, D.; Valle, J.M. Adenosine Deaminase (ADA) Isoenzyme Analysis in Pleural Effusions: Diagnostic Role, and Relevance to the Origin of Increased ADA in Tuberculous Pleurisy. Eur. Respir. J. 1996, 9, 747–751. [Google Scholar] [CrossRef]
- Tsuboi, I.; Sagawa, K.; Shichijo, S.; Yokoyama, M.M.; Ou, D.W.; Wiederhold, M.D. Adenosine Deaminase Isoenzyme Levels in Patients with Human T-Cell Lymphotropic Virus Type 1 and Human Immunodeficiency Virus Type 1 Infections. Clin. Diagn. Lab. Immunol. 1995, 2, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Hitoglou, S.; Hatzistilianou, M.; Gougoustamou, D.; Athanassiadou, F.; Kotsis, A.; Catriu, D. Adenosine Deaminase Activity and Its Isoenzyme Pattern in Patients with Juvenile Rheumatoid Arthritis and Systemic Lupus Erythematosus. Clin. Rheumatol. 2001, 20, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Delemarre, E.M.; van Hoorn, L.; Bossink, A.W.J.; Drylewicz, J.; Joosten, S.A.; Ottenhoff, T.H.M.; Akkerman, O.W.; Goletti, D.; Petruccioli, E.; Navarra, A.; et al. Serum Biomarker Profile Including CCL1, CXCL10, VEGF, and Adenosine Deaminase Activity Distinguishes Active from Remotely Acquired Latent Tuberculosis. Front. Immunol. 2021, 12, 725447. [Google Scholar] [CrossRef] [PubMed]
- Zavialov, A.V.; Engström, Å. Human ADA2 Belongs to a New Family of Growth Factors with Adenosine Deaminase Activity. Biochem. J. 2005, 391, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Caorsi, R.; Penco, F.; Schena, F.; Gattorno, M. Monogenic Polyarteritis: The Lesson of ADA2 Deficiency. Pediatr. Rheumatol. 2016, 14, 51. [Google Scholar] [CrossRef][Green Version]
- Zhou, Q.; Yang, D.; Ombrello, A.K.; Zavialov, A.V.; Toro, C.; Zavialov, A.V.; Stone, D.L.; Chae, J.J.; Rosenzweig, S.D.; Bishop, K.; et al. Early-Onset Stroke and Vasculopathy Associated with Mutations in ADA2. N. Engl. J. Med. 2014, 370, 911–920. [Google Scholar] [CrossRef]
- Navon Elkan, P.; Pierce, S.B.; Segel, R.; Walsh, T.; Barash, J.; Padeh, S.; Zlotogorski, A.; Berkun, Y.; Press, J.J.; Mukamel, M.; et al. Mutant Adenosine Deaminase 2 in a Polyarteritis Nodosa Vasculopathy. N. Engl. J. Med. 2014, 370, 921–931. [Google Scholar] [CrossRef]
- Gaspar, H.B.; Aiuti, A.; Porta, F.; Candotti, F.; Hershfield, M.S.; Notarangelo, L.D. How I Treat ADA Deficiency. Blood 2009, 114, 3524–3532. [Google Scholar] [CrossRef]
- Rostampour, F.; Biglari, M.; Vaisi-Raygani, A.; Salimi, S.; Tavilani, H. Adenosine Deaminase Activity in Fertile and Infertile Men: Adenosine Deaminase and Male Infertility. Andrologia 2012, 44, 586–589. [Google Scholar] [CrossRef]
- Fattahi, A.; Khodadadi, I.; Amiri, I.; Latifi, Z.; Ghorbani, M.; Tavilani, H. The Role of G22 A Adenosine Deaminase 1 Gene Polymorphism and the Activities of ADA Isoenzymes in Fertile and Infertile Men. Urology 2015, 86, 730–734. [Google Scholar] [CrossRef]
- Odumade, O.A.; Plotkin, A.L.; Pak, J.; Idoko, O.T.; Pettengill, M.A.; Kollmann, T.R.; Ozonoff, A.; Kampmann, B.; Levy, O.; Smolen, K.K. Plasma Adenosine Deaminase (ADA)-1 and -2 Demonstrate Robust Ontogeny Across the First Four Months of Human Life. Front. Immunol. 2021, 12, 578700. [Google Scholar] [CrossRef] [PubMed]
- Bowers, S.M.; Gibson, K.M.; Cabral, D.A.; Brown, K.L. Adenosine Deaminase 2 Activity Negatively Correlates with Age during Childhood. Pediatr. Rheumatol. 2020, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Durak, I.; Cetin, R.; Canbolat, O.; Cetin, D.; Yurtarslani, Z.; Ünal, A. Adenosine Deaminase, 5′-Nucleotidase, Guanase and Cytidine Deaminase Activities in Gastric Tissues from Patients with Gastric Cancer. Cancer Lett. 1994, 84, 199–202. [Google Scholar] [CrossRef]
- Durak, I.; Örmeci, N.; Akyol, Ö.; Canbolat, O.; Kavutçu, M.; Bülbül, M. Adenosine Deaminase, 5′-Nucleotidase, Xanthine Oxidase, Superoxide Dismutase, and Catalase Activities in Gastric Juices from Patients with Gastric Cancer, Ulcer, and Atrophic Gastritis. Digest. Dis. Sci. 1994, 39, 721–728. [Google Scholar] [CrossRef]
- Pirinççi, N.; Geçit, I.; Güneş, M.; Yuksel, M.; Kaba, M.; Tanık, S.; Demir, H.; Aslan, M. Serum Adenosine Deaminase, Catalase and Carbonic Anhydrase Activities in Patients with Bladder Cancer. Clinics 2012, 67, 1443–1446. [Google Scholar] [CrossRef]
- Durak, İ.; Perk, H.; Kavutçu, M.; Canbolat, O.; Akyol, Ö.; Bedük, Y. Adenosine Deaminase, 5′nucleotidase, Xanthine Oxidase, Superoxide Dismutase, and Catalase Activities in Cancerous and Noncancerous Human Bladder Tissues. Free. Radic. Biol. Med. 1994, 16, 825–831. [Google Scholar] [CrossRef]
- Faisal, A.; Taha, M. Serum Adenosine Deaminase Activity in Iraqi Patients with Breast Cancer on Tamoxifen Therapy. Gaziantep Med. J. 2012, 18, 139. [Google Scholar] [CrossRef]
- Aghaei, M.; Karami-Tehrani, F.; Salami, S.; Atri, M. Diagnostic Value of Adenosine Deaminase Activity in Benign and Malignant Breast Tumors. Arch. Med. Res. 2010, 41, 14–18. [Google Scholar] [CrossRef]
- Pirinççi, N.; Kaya, T.Y.; Kaba, M.; Ozan, T.; Geçit, İ.; Özveren, H.; Eren, H.; Ceylan, K. Serum Adenosine Deaminase, Catalase, and Carbonic Anhydrase Activities in Patients with Renal Cell Carcinoma. Redox Rep. 2017, 22, 252–256. [Google Scholar] [CrossRef]
- Kutryb-Zajac, B.; Harasim, G.; Jedrzejewska, A.; Krol, O.; Braczko, A.; Jablonska, P.; Mierzejewska, P.; Zielinski, J.; Slominska, E.M.; Smolenski, R.T. Macrophage-Derived Adenosine Deaminase 2 Correlates with M2 Macrophage Phenotype in Triple Negative Breast Cancer. Int. J. Mol. Sci. 2021, 22, 3764. [Google Scholar] [CrossRef]
- Battisti, V.; Maders, L.D.K.; Bagatini, M.D.; Battisti, I.E.; Bellé, L.P.; Santos, K.F.; Maldonado, P.A.; Thomé, G.R.; Schetinger, M.R.C.; Morsch, V.M. Ectonucleotide Pyrophosphatase/Phosphodiesterase (E-NPP) and Adenosine Deaminase (ADA) Activities in Prostate Cancer Patients: Influence of Gleason Score, Treatment and Bone Metastasis. Biomed. Pharmacother. 2013, 67, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Durak, İ.; Işik, C.Ü.; Canbolat, O.; Akyol, Ö.; Kavutçu, M. Adenosine Deaminase, 5′ Nucleotidase, Xanthine Oxidase, Superoxide Dismutase, and Catalase Activities in Cancerous and Noncancerous Human Laryngeal Tissues. Free. Radic. Biol. Med. 1993, 15, 681–684. [Google Scholar] [CrossRef]
- Theodoraki, M.-N.; Hoffmann, T.K.; Jackson, E.K.; Whiteside, T.L. Exosomes in HNSCC Plasma as Surrogate Markers of Tumour Progression and Immune Competence. Clin. Exp. Immunol. 2018, 194, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Zanini, D.; Manfredi, L.H.; Pelinson, L.P.; Pimentel, V.C.; Cardoso, A.M.; do Carmo Araújo Gonçalves, V.; dos Santos, C.B.; Gutierres, J.M.; Morsch, V.M.; Leal, D.B.R.; et al. ADA Activity Is Decreased in Lymphocytes from Patients with Advanced Stage of Lung Cancer. Med. Oncol. 2019, 36, 78. [Google Scholar] [CrossRef]
- Hocanlı, İ.; Uzer, F.; Çil, B.; Kırhan, İ.; Günak, F. Diagnostic Value of Adenosine Deaminase in Bronchoalveolar Lavage Fluid for Patients with Lung Cancer. Int. J. Clin. Pract. 2021, 75, e14918. [Google Scholar] [CrossRef]
- Wang, T.; Gnanaprakasam, J.N.R.; Chen, X.; Kang, S.; Xu, X.; Sun, H.; Liu, L.; Rodgers, H.; Miller, E.; Cassel, T.A.; et al. Inosine Is an Alternative Carbon Source for CD8+-T-Cell Function under Glucose Restriction. Nat. Metab. 2020, 2, 635–647. [Google Scholar] [CrossRef]
- Wang, L.; Londono, L.M.; Cowell, J.; Saatci, O.; Aras, M.; Ersan, P.G.; Serra, S.; Pei, H.; Clift, R.; Zhao, Q.; et al. Targeting Adenosine with Adenosine Deaminase 2 to Inhibit Growth of Solid Tumors. Cancer Res. 2021, 81, 3319–3332. [Google Scholar] [CrossRef]
- Qu, Y.; Dunn, Z.S.; Chen, X.; MacMullan, M.; Cinay, G.; Wang, H.; Liu, J.; Hu, F.; Wang, P. Adenosine Deaminase 1 Overexpression Enhances the Antitumor Efficacy of Chimeric Antigen Receptor-Engineered T Cells. Hum. Gene Ther. 2021. [Google Scholar] [CrossRef]
- Kreitman, R.J. Hairy Cell Leukemia: Present and Future Directions. Leuk. Lymphoma 2019, 60, 2869–2879. [Google Scholar] [CrossRef]
- Nakajima, Y.; Kanno, T.; Nagaya, T.; Kuribayashi, K.; Nakano, T.; Gotoh, A.; Nishizaki, T. Adenosine Deaminase Inhibitor EHNA Exhibits a Potent Anticancer Effect Against Malignant Pleural Mesothelioma. Cell. Physiol. Biochem. 2015, 35, 51–60. [Google Scholar] [CrossRef]
- Kutryb-Zajac, B.; Koszalka, P.; Mierzejewska, P.; Bulinska, A.; Zabielska, M.A.; Brodzik, K.; Skrzypkowska, A.; Zelazek, L.; Pelikant-Malecka, I.; Slominska, E.M.; et al. Adenosine Deaminase Inhibition Suppresses Progression of 4T1 Murine Breast Cancer by Adenosine Receptor-Dependent Mechanisms. J. Cell. Mol. Med. 2018, 22, 5939–5954. [Google Scholar] [CrossRef]
- Boison, D.; Jarvis, M.F. Adenosine Kinase: A Key Regulator of Purinergic Physiology. Biochem. Pharmacol. 2021, 187, 114321. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Abdalhamid Osman, M.; Sun, Y.; Li, R.; Lin, H.; Zeng, D.; Chen, X.; He, D.; Feng, H.; Yang, Z.; Wang, J.; et al. Deletion of Pancreatic Β-cell Adenosine Kinase Improves Glucose Homeostasis in Young Mice and Ameliorates Streptozotocin-induced Hyperglycaemia. J. Cell. Mol. Med. 2019, 23, 4653–4665. [Google Scholar] [CrossRef] [PubMed]
- Gokul, G.; Khosla, S. DNA Methylation and Cancer. In Epigenetics: Development and Disease; Kundu, T.K., Ed.; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2013; Volume 61, pp. 597–625. [Google Scholar] [CrossRef]
- Mohammad, H.P.; Barbash, O.; Creasy, C.L. Targeting Epigenetic Modifications in Cancer Therapy: Erasing the Roadmap to Cancer. Nat. Med. 2019, 25, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Pignataro, G.; Simon, R.P.; Boison, D. Transgenic Overexpression of Adenosine Kinase Aggravates Cell Death in Ischemia. J. Cereb. Blood Flow Metab. 2007, 27, 1–5. [Google Scholar] [CrossRef]
- Zhulai, G.; Oleinik, E. Targeting Regulatory T Cells in Anti-PD-1/PD-L1 Cancer Immunotherapy. Scand. J. Immunol. 2022, 95, e13129. [Google Scholar] [CrossRef]
- Iannone, R.; Miele, L.; Maiolino, P.; Pinto, A.; Morello, S. Adenosine Limits the Therapeutic Effectiveness of Anti-CTLA4 MAb in a Mouse Melanoma Model. Am. J. Cancer Res. 2014, 4, 172–181. [Google Scholar]
- Beavis, P.A.; Henderson, M.A.; Giuffrida, L.; Mills, J.K.; Sek, K.; Cross, R.S.; Davenport, A.J.; John, L.B.; Mardiana, S.; Slaney, C.Y.; et al. Targeting the Adenosine 2A Receptor Enhances Chimeric Antigen Receptor T Cell Efficacy. J. Clin. Investig. 2017, 127, 929–941. [Google Scholar] [CrossRef]
- Leone, R.D.; Sun, I.-M.; Oh, M.-H.; Sun, I.-H.; Wen, J.; Englert, J.; Powell, J.D. Inhibition of the Adenosine A2a Receptor Modulates Expression of T Cell Coinhibitory Receptors and Improves Effector Function for Enhanced Checkpoint Blockade and ACT in Murine Cancer Models. Cancer Immunol. Immunother. 2018, 67, 1271–1284. [Google Scholar] [CrossRef]
- Yang, R.; Elsaadi, S.; Misund, K.; Abdollahi, P.; Vandsemb, E.N.; Moen, S.H.; Kusnierczyk, A.; Slupphaug, G.; Standal, T.; Waage, A.; et al. Conversion of ATP to Adenosine by CD39 and CD73 in Multiple Myeloma Can Be Successfully Targeted Together with Adenosine Receptor A2A Blockade. J. Immunother. Cancer 2020, 8, e000610. [Google Scholar] [CrossRef]
- Yan, J.; Li, X.-Y.; Roman Aguilera, A.; Xiao, C.; Jacoberger-Foissac, C.; Nowlan, B.; Robson, S.C.; Beers, C.; Moesta, A.K.; Geetha, N.; et al. Control of Metastases via Myeloid CD39 and NK Cell Effector Function. Cancer Immunol. Res. 2020, 8, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Ngiow, S.F.; Barkauskas, D.S.; Sult, E.; Hay, C.; Blake, S.J.; Huang, Q.; Liu, J.; Takeda, K.; Teng, M.W.L.; et al. Co-Inhibition of CD73 and A2AR Adenosine Signaling Improves Anti-Tumor Immune Responses. Cancer Cell 2016, 30, 391–403. [Google Scholar] [CrossRef] [PubMed]
| ADK | ADA | |||
|---|---|---|---|---|
| ADK-L (Isoform 1) | ADK-S (Isoform 2) | ADA1 | ADA2 | |
| Gene (chromosome) | ADK (10q22.2) | ADA 20q13.12 | ADA2 22q11.1 | |
| Protein structure | 40.5 kDa monomer, 362-amino acid form | monomer, 345-amino acid form | 40.8 kDa monomer, 363 amino acids | 59 kDa monomer-homodimer, 511 amino acids |
| Tissue specificity | Widely expressed, occurs in large amounts in liver, heart, kidney, lung, pancreas, and spleen | Widely expressed, occurs in large amounts in liver, brain, kidney, lung, and pancreas | Found in all tissues, occurs in large amounts in lymphocytes and intestine | Human adult heart, lung, lymphoblasts, and placenta, fetal lung, liver, and kidney |
| Cell specificity | Ubiquitously, neuronal cells, glial cells | Ubiquitously, lymphocytes, erythrocytes | Myeloid cells | |
| Cellular localization | Intracellular, nucleus | Intracellular, cytoplasm; plasma membrane | Intracellular; extracellular via CD26 | Extracellular, secreted; lysosome |
| Functions | Catalyzes the phosphorylation of adenosine to AMP; Facilitates methylation reactions by the removal of adenosine, the end product of SAM-dependent transmethylation reactions | Catalyzes the phosphorylation of adenosine to AMP, using ATP as a phosphate donor and produces ADP and AMP; Acts as a regulator of concentrations of extracellular adenosine and intracellular adenine nucleotides | Catalyzes the hydrolysis of adenosine to inosine and 2-deoxyadenosine to 2-deoxyinosine; Acts as a positive regulator of T cell coactivation, by binding CD26; Enhances DC immunogenicity; Acts as a positive modulator of A1R and A2AR | May contribute to the degradation of extracellular adenosine Binds to cell surfaces via proteoglycans and may play a role in the regulation of cell proliferation and differentiation, independently of its enzyme activity |
| Km for adenosine | Approx. 1 µM | - | Approx. 37 µM | Approx. 2.25 mM |
| Disease at deficiency | Hypermethioninemia encephalopathy due to adenosine kinase deficiency | T-B-NK- severe combined immunodeficiency | ADA2 deficiency | |
| Cancer/Model | Parameter | Significance | Ref. |
|---|---|---|---|
| PC3 human prostate carcinoma cell line and MDA-MB-231 human breast adenocarcinoma cells | Protein level | The adenosine-ATP catalytic cascade is initiated via ADK-mediated phosphorylation of adenosine into AMP rather than its deamination to inosine | [13] |
| Breast cancer (n = 46 patients) and breast cancer MDA-MB-231 cell line | Protein level | ADK-L expression was significantly increased in breast cancer tissue; ADK downregulation suppressed proliferation, viability, migration, and invasion of cancer cells | [62] |
| Colorectal cancer (n = 10 patients) | Gene expression | ADK expression is higher in tumor than in healthy tissue | [65] |
| Colorectal cancer (n = 40 patients) | Enzyme activity | Higher in tumor than in healthy tissue (p < 0.01) | [66] |
| Glioma (n = 45 patients) | Gene and protein expression levels | In tumoral and peritumoral tissues, ADK expression was markedly elevated compared with that in control tissues (p < 0.05) | [67] |
| Liver cancer (n = 11 patients) and mouse model of hepatic ADK deficiency | Protein level | ADK in the liver might play a role in determining the liver’s susceptibility to cancer development | [68] |
| HeLa, HepG2, and U373 cancer cell lines | Protein level | HeLa cells combine the highest DNA methylation levels with the highest expression levels of ADK-L; ADK inhibitors significantly reduced global DNA methylation in HeLa cells | [69] |
| Cancer/Model | Parameter | Significance | Ref. |
|---|---|---|---|
| Gastric cancer (n = 15 patients) | Enzyme activity | ADA activity increased in the cancerous tissues (p < 0.0005); there were no significant differences between I-II stages and III-IV stages | [95] |
| Gastric cancer (n = 26 patients) | Enzyme activity and protein level | ADA activity of the cancer gastric juices were lower (p < 0.01) and protein concentrations were higher than in the healthy control group | [96] |
| Bladder cancer (n = 40 patients) | Serum enzyme activity | ADA activity was significantly higher in cancer than in healthy controls | [97] |
| Bladder cancer (n = 36 patients) | Enzyme activity | Increased ADA activity was found in cancerous tissues compared with cancer-free adjacent tissues (p < 0.05) | [98] |
| Breast cancer (n = 160 patients) | Protein level | Level of serum ADA was higher compared with healthy control (p < 0.05); level of ADA was significantly reduced upon tamoxifen treatment (p < 0.05) | [99] |
| Breast cancer (n = 58 patients) | Enzyme activity | The mean values for ADA activity (tissue and serum) of patients with breast cancer were significantly higher than those of the benign breast disease (p < 0.005) and healthy subjects (p < 0.0001) | [100] |
| Breast cancer (n= 19 triple-negative breast cancer) and MDA-MB-231 triple negative breast cancer cells | Enzyme activity | Patients had higher plasma ADA2 activities and lower ADA1/ADA2 ratio at advanced stages of cancer development than in the initial stages; the activity of ADA changes during the interaction of tumor cells with lymphocytes, macrophages, and endothelial cells in vitro contributing to cancer progression. | [102] |
| Colorectal cancer (n = 40 patients) | Enzyme activity | Higher in tumor than in healthy tissue (p < 0.01) | [66] |
| Renal cell cancer (n = 33 patients) | Serum enzyme activity | ADA activity was significantly higher in patients than in the healthy group (p < 0.001) | [101] |
| Prostate cancer (n = 68 patients) | Serum enzyme activity | ADA activity in serum of patients with prostate cancer and patients with bone metastases were significantly decreased (p < 0.05) when compared with the healthy control group | [103] |
| Laryngeal cancer (n = 15 patients) | Enzyme activity | ADA activity was decreased in cancerous tissues when compared with the cancer-free adjacent tissues (p < 0.025) | [104] |
| Head and neck squamous cell carcinomas (n = 14) | Protein level | With progression of the disease, the expression of ADA/CD26 in effector T cells and CD3+ exosomes derived from T cells gets suppressed | [105] |
| Lung cancer (n = 13 patients with advanced stage) | Enzyme activity | Patients with advanced stage of lung cancer exhibited a decrease in ADA activity in both lymphocyte and erythrocyte (p < 0.005) | [106] |
| Lung cancer (n = 43 patients) | Enzyme activity | ADA levels in bronchoalveolar lavage fluids were statistically higher compared with the non-malignant group (p < 0.001) and may be a diagnostic biomarker in lung malignancies | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhulai, G.; Oleinik, E.; Shibaev, M.; Ignatev, K. Adenosine-Metabolizing Enzymes, Adenosine Kinase and Adenosine Deaminase, in Cancer. Biomolecules 2022, 12, 418. https://doi.org/10.3390/biom12030418
Zhulai G, Oleinik E, Shibaev M, Ignatev K. Adenosine-Metabolizing Enzymes, Adenosine Kinase and Adenosine Deaminase, in Cancer. Biomolecules. 2022; 12(3):418. https://doi.org/10.3390/biom12030418
Chicago/Turabian StyleZhulai, Galina, Eugenia Oleinik, Mikhail Shibaev, and Kirill Ignatev. 2022. "Adenosine-Metabolizing Enzymes, Adenosine Kinase and Adenosine Deaminase, in Cancer" Biomolecules 12, no. 3: 418. https://doi.org/10.3390/biom12030418
APA StyleZhulai, G., Oleinik, E., Shibaev, M., & Ignatev, K. (2022). Adenosine-Metabolizing Enzymes, Adenosine Kinase and Adenosine Deaminase, in Cancer. Biomolecules, 12(3), 418. https://doi.org/10.3390/biom12030418






