Mitochondrial Oxidative Stress and Cell Death in Podocytopathies
Abstract
1. Introduction
2. Spectrum of Podocytopathies
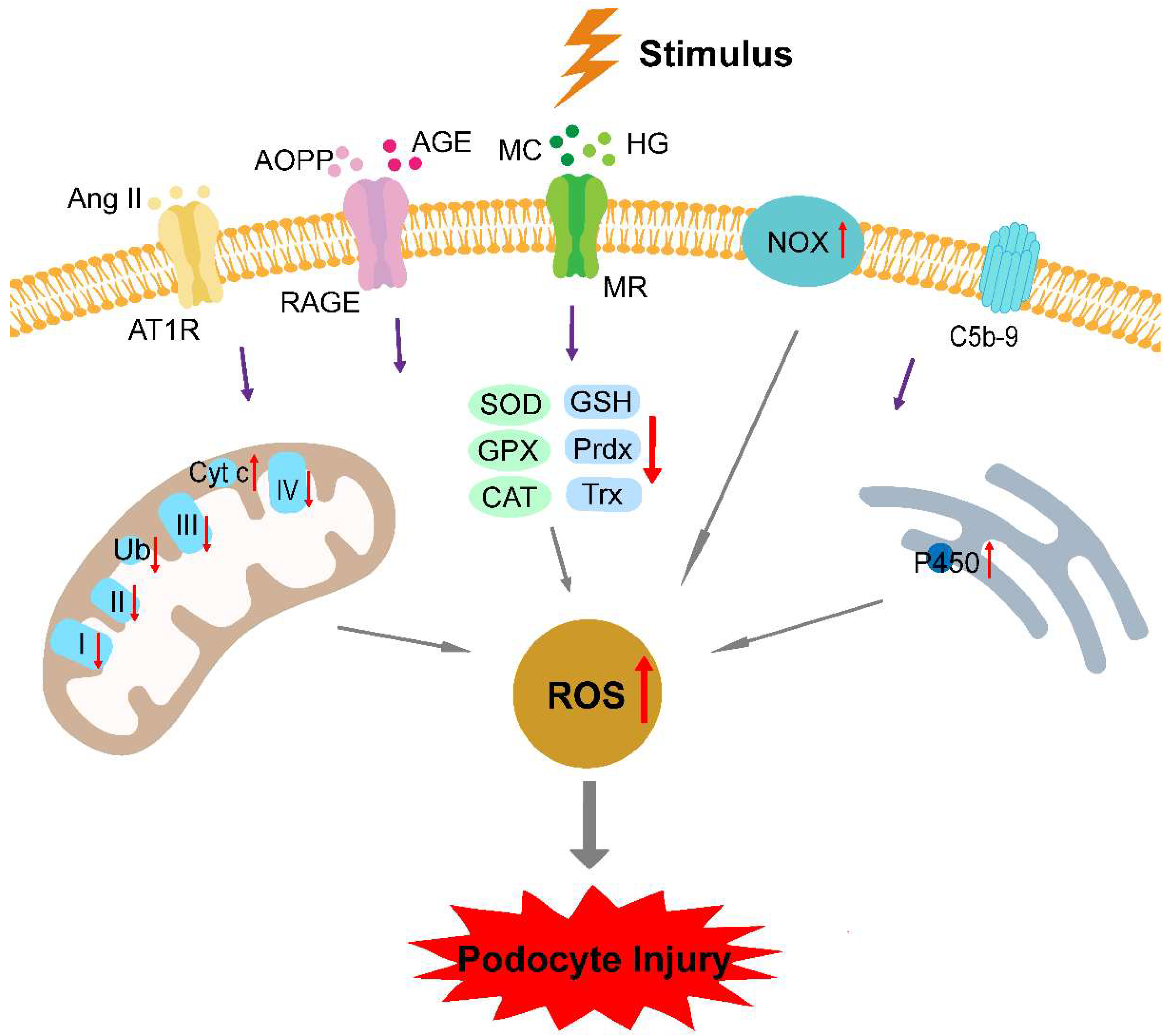
3. Oxidative Stress in Podocytopathies
3.1. Elevated ROS Production
3.2. Deficient Antioxidant Defense Systems
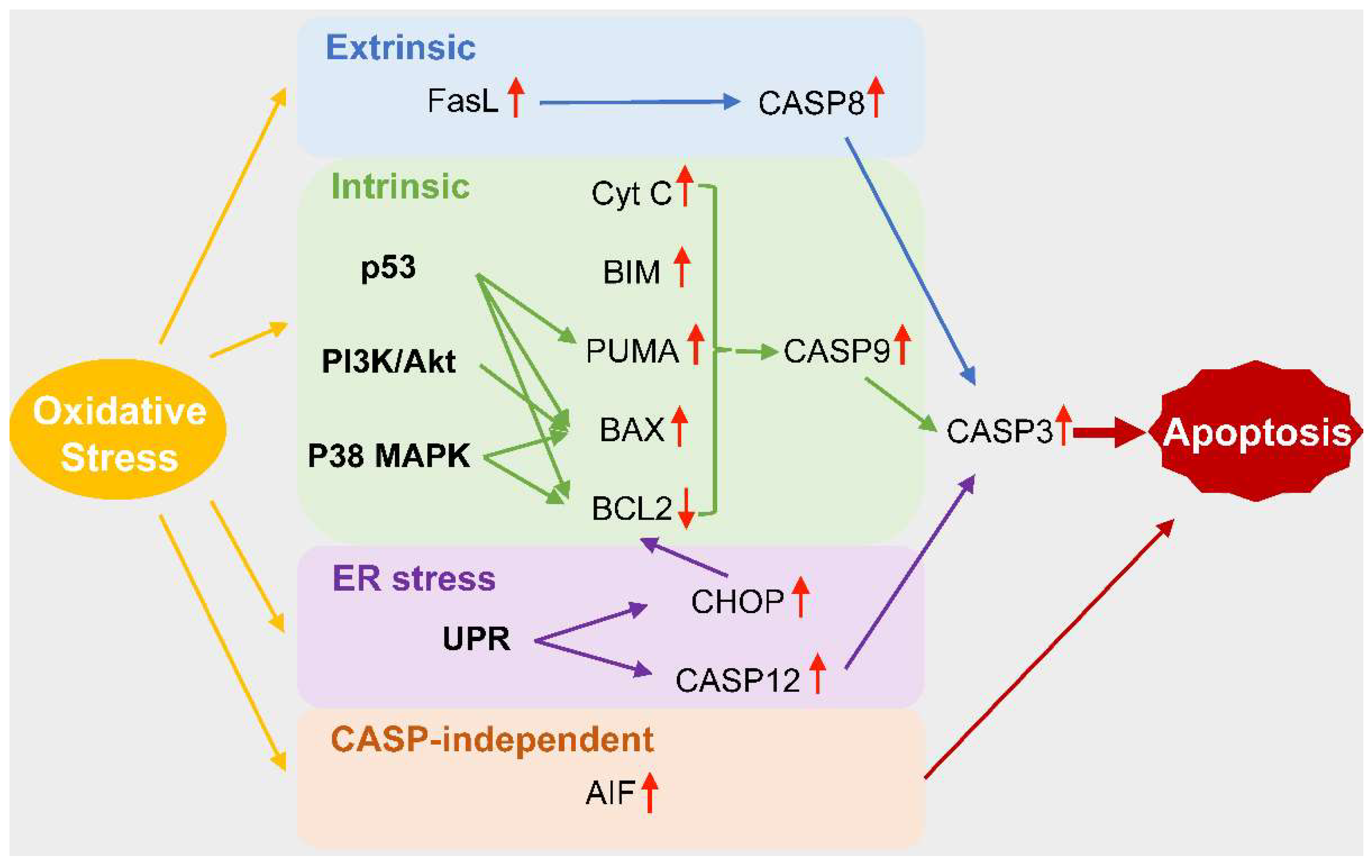
4. Oxidative Stress and Cell Death in Podocytopathies
5. Therapeutic Implications
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kopp, J.B.; Anders, H.J.; Susztak, K.; Podesta, M.A.; Remuzzi, G.; Hildebrandt, F.; Romagnani, P. Podocytopathies. Nat. Rev. Dis. Primers 2020, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, R.C. The Spectrum of Podocytopathies: A Unifying View of Glomerular Diseases. Kidney Int. 2007, 71, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.V.; Petermann, A.T.; Durvasula, R.V.; Shankland, S.J. Podocyte Proliferation and Differentiation in Glomerular Disease: Role of Cell-Cycle Regulatory Proteins. Nephrol. Dial. Transplant. 2003, 18 (Suppl. 6), vi8–vi13. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Mitochondrial Oxidative Stress: Implications for Cell Death. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 143–183. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Hagiwara, M.; Yamagata, K.; Capaldi, R.A.; Koyama, A. Mitochondrial Dysfunction in Focal Segmental Glomerulosclerosis of Puromycin Aminonucleoside Nephrosis. Kidney Int. 2006, 69, 1146–1152. [Google Scholar] [CrossRef]
- Pei, Y.; Xu, Y.; Ruan, J.; Rong, L.; Jiang, M.; Mo, Y.; Jiang, X. Plasma Oxidative Stress Level of IgA Nephropathy in Children and the Effect of Early Intervention with Angiotensin-Converting Enzyme Inhibitors. J. Renin Angiotensin Aldosterone Syst. 2016, 17, 1470320316647240. [Google Scholar] [CrossRef]
- Susztak, K.; Raff, A.C.; Schiffer, M.; Bottinger, E.P. Glucose-Induced Reactive Oxygen Species Cause Apoptosis of Podocytes and Podocyte Depletion at the Onset of Diabetic Nephropathy. Diabetes 2006, 55, 225–233. [Google Scholar] [CrossRef]
- Solin, M.L.; Pitkanen, S.; Taanman, J.W.; Holthofer, H. Mitochondrial Dysfunction in Congenital Nephrotic Syndrome. Lab Investig. 2000, 80, 1227–1232. [Google Scholar] [CrossRef]
- Marshall, C.B.; Pippin, J.W.; Krofft, R.D.; Shankland, S.J. Puromycin Aminonucleoside Induces Oxidant-Dependent DNA Damage in Podocytes in Vitro and in Vivo. Kidney Int. 2006, 70, 1962–1973. [Google Scholar] [CrossRef]
- Vogtlander, N.P.; Tamboer, W.P.; Bakker, M.A.; Campbell, K.P.; van der Vlag, J.; Berden, J.H. Reactive Oxygen Species Deglycosilate Glomerular Alpha-Dystroglycan. Kidney Int. 2006, 69, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.H.; Hoffmann, S.; Endlich, N.; Velic, A.; Schwab, A.; Weide, T.; Schlatter, E.; Pavenstadt, H. Mechanisms of Angiotensin II Signaling on Cytoskeleton of Podocytes. J. Mol. Med. 2008, 86, 1379–1394. [Google Scholar] [CrossRef]
- Ryter, S.W.; Kim, H.P.; Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M. Mechanisms of Cell Death in Oxidative Stress. Antioxid. Redox Signal. 2007, 9, 49–89. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Braun, F.; Becker, J.U.; Brinkkoetter, P.T. Live or Let Die: Is There Any Cell Death in Podocytes? Semin. Nephrol. 2016, 36, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Lan, X.; Wen, H.; Lederman, R.; Chawla, A.; Attia, M.; Bongu, R.P.; Husain, M.; Mikulak, J.; Saleem, M.A.; et al. HIV Promotes NLRP3 Inflammasome Complex Activation in Murine HIV-Associated Nephropathy. Am. J. Pathol. 2016, 186, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, Y.; Hu, J.E.; Ding, Y.; Shen, Y.; Xu, H.; Chen, H.; Wu, N. Sp1-Mediated Upregulation of Prdx6 Expression Prevents Podocyte Injury in Diabetic Nephropathy via Mitigation of Oxidative Stress and Ferroptosis. Life Sci. 2021, 278, 119529. [Google Scholar] [CrossRef]
- Liu, T.; Chen, X.M.; Sun, J.Y.; Jiang, X.S.; Wu, Y.; Yang, S.; Huang, H.Z.; Ruan, X.Z.; Du, X.G. Palmitic Acid-Induced Podocyte Apoptosis via the Reactive Oxygen Species-Dependent Mitochondrial Pathway. Kidney Blood Press Res. 2018, 43, 206–219. [Google Scholar] [CrossRef]
- Chen, Z.; Wan, X.; Hou, Q.; Shi, S.; Wang, L.; Chen, P.; Zhu, X.; Zeng, C.; Qin, W.; Zhou, W.; et al. GADD45B Mediates Podocyte Injury in Zebrafish by Activating the ROS-GADD45B-P38 Pathway. Cell Death Dis. 2016, 7, e2068. [Google Scholar] [CrossRef]
- Hinkes, B.; Wiggins, R.C.; Gbadegesin, R.; Vlangos, C.N.; Seelow, D.; Nürnberg, G.; Garg, P.; Verma, R.; Chaib, H.; Hoskins, B.E.; et al. Positional Cloning Uncovers Mutations in PLCE1 Responsible for A Nephrotic Syndrome Variant that May Be Reversible. Nat. Genet. 2006, 38, 1397–1405. [Google Scholar] [CrossRef]
- Yang, Y.; Jeanpierre, C.; Dressler, G.R.; Lacoste, M.; Niaudet, P.; Gubler, M.C. WT1 and PAX-2 Podocyte Expression in Denys-Drash Syndrome and Isolated Diffuse Mesangial Sclerosis. Am. J. Pathol. 1999, 154, 181–192. [Google Scholar] [CrossRef]
- Abd ElHafeez, S.; Bolignano, D.; D’Arrigo, G.; Dounousi, E.; Tripepi, G.; Zoccali, C. Prevalence and Burden of Chronic Kidney Disease among the General Population and High-Risk Groups in Africa: A Systematic Review. BMJ Open 2018, 8, e015069. [Google Scholar] [CrossRef] [PubMed]
- Udani, S.; Lazich, I.; Bakris, G.L. Epidemiology of Hypertensive Kidney Disease. Nat. Rev. Nephrol. 2011, 7, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gubler, M.C.; Beaufils, H. Dysregulation of Podocyte Phenotype in Idiopathic Collapsing Glomerulopathy and HIV-Associated Nephropathy. Nephron 2002, 91, 416–423. [Google Scholar] [CrossRef]
- Markowitz, G.S.; Appel, G.B.; Fine, P.L.; Fenves, A.Z.; Loon, N.R.; Jagannath, S.; Kuhn, J.A.; Dratch, A.D.; D’Agati, V.D. Collapsing Focal Segmental Glomerulosclerosis Following Treatment with High-Dose Pamidronate. J. Am. Soc. Nephrol. 2001, 12, 1164–1172. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Y.; Wu, Y.; Liu, Q.; Feng, J.; Gu, X.; Xiong, Y.; Fan, Q.; Ye, J. Smad3/Nox4-Mediated Mitochondrial Dysfunction Plays A Crucial Role in Puromycin Aminonucleoside-Induced Podocyte Damage. Cell Signal. 2014, 26, 2979–2991. [Google Scholar] [CrossRef]
- Galvan, D.L.; Badal, S.S.; Long, J.; Chang, B.H.; Schumacker, P.T.; Overbeek, P.A.; Danesh, F.R. Real-Time in Vivo Mitochondrial Redox Assessment Confirms Enhanced Mitochondrial Reactive Oxygen Species in Diabetic Nephropathy. Kidney Int. 2017, 92, 1282–1287. [Google Scholar] [CrossRef]
- Gao, N.; Zhang, Y.; Lei, L.; Li, L.; Cao, P.; Zhao, X.; Lin, L.; Xu, R. Low Doses of Folic Acid Can Reduce Hyperhomocysteinemia-Induced Glomerular Injury in Spontaneously Hypertensive Rats. Hypertens. Res. 2020, 43, 1182–1191. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial Formation of Reactive Oxygen Species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Sarewicz, M.; Osyczka, A. Electronic Connection Between the Quinone and Cytochrome C Redox Pools and Its Role in Regulation of Mitochondrial Electron Transport and Redox Signaling. Physiol. Rev. 2015, 95, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bigler, S.A.; Henegar, J.R.; Baliga, R. Cytochrome P450 2B1 Mediates Oxidant Injury in Puromycin-Induced Nephrotic Syndrome. Kidney Int. 2002, 62, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Kitiyakara, C.; Chabrashvili, T.; Chen, Y.; Blau, J.; Karber, A.; Aslam, S.; Welch, W.J.; Wilcox, C.S. Salt Intake, Oxidative Stress, and Renal Expression of NADPH Oxidase and Superoxide Dismutase. J. Am. Soc. Nephrol. 2003, 14, 2775–2782. [Google Scholar] [CrossRef]
- Tong, J.; Jin, Y.; Weng, Q.; Yu, S.; Hussain, H.M.J.; Ren, H.; Xu, J.; Zhang, W.; Li, X.; Wang, W.; et al. Glomerular Transcriptome Profiles in Focal Glomerulosclerosis: New Genes and Pathways for Steroid Resistance. Am. J. Nephrol. 2020, 51, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Sato, T.; Rodriguez-Iturbe, B.; Vaziri, N.D. Role of Intrarenal Angiotensin System Activation, Oxidative Stress, Inflammation, and Impaired Nuclear Factor-Erythroid-2-Related Factor 2 Activity in The Progression of Focal Glomerulosclerosis. J. Pharmacol. Exp. Ther. 2011, 337, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Gomez, I.G.; Wada, Y.; Roach, A.; Mahad, D.; Duffield, J.S. Deletion of The Mitochondrial Complex-IV Cofactor Heme A:Farnesyltransferase Causes Focal Segmental Glomerulosclerosis and Interferon Response. Am. J. Pathol. 2018, 188, 2745–2762. [Google Scholar] [CrossRef]
- Ashraf, S.; Gee, H.Y.; Woerner, S.; Xie, L.X.; Vega-Warner, V.; Lovric, S.; Fang, H.; Song, X.; Cattran, D.C.; Avila-Casado, C.; et al. ADCK4 Mutations Promote Steroid-Resistant Nephrotic Syndrome through CoQ10 Biosynthesis Disruption. J. Clin. Investig. 2013, 123, 5179–5189. [Google Scholar] [CrossRef]
- Song, C.C.; Hong, Q.; Geng, X.D.; Wang, X.; Wang, S.Q.; Cui, S.Y.; Guo, M.D.; Li, O.; Cai, G.Y.; Chen, X.M.; et al. New Mutation of Coenzyme Q10 Monooxygenase 6 Causing Podocyte Injury in A Focal Segmental Glomerulosclerosis Patient. Chin. Med. J. 2018, 131, 2666–2675. [Google Scholar] [CrossRef]
- Liu, H.; Baliga, M.; Bigler, S.A.; Baliga, R. Role of Cytochrome P450 2B1 in Puromycin Aminonucleoside-Induced Cytotoxicity to Glomerular Epithelial Cells. Nephron Exp. Nephrol. 2003, 94, e17–e24. [Google Scholar] [CrossRef]
- Xie, K.; Zhu, M.; Xiang, P.; Chen, X.; Kasimumali, A.; Lu, R.; Wang, Q.; Mou, S.; Ni, Z.; Gu, L.; et al. Protein Kinase A/Creb Signaling Prevents Adriamycin-Induced Podocyte Apoptosis via Upregulation of Mitochondrial Respiratory Chain Complexes. Mol. Cell Biol. 2018, 38, e00181-17. [Google Scholar] [CrossRef] [PubMed]
- Holthofer, H.; Kretzler, M.; Haltia, A.; Solin, M.L.; Taanman, J.W.; Schagger, H.; Kriz, W.; Kerjaschki, D.; Schlondorff, D. Altered Gene Expression and Functions of Mitochondria in Human Nephrotic Syndrome. FASEB J. 1999, 13, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Solin, M.L.; Ahola, H.; Haltia, A.; Ursini, F.; Montine, T.; Roveri, A.; Kerjaschki, D.; Holthofer, H. Lipid Peroxidation in Human Proteinuric Disease. Kidney Int. 2001, 59, 481–487. [Google Scholar] [CrossRef][Green Version]
- Yu, B.C.; Cho, N.-J.; Park, S.; Kim, H.; Choi, S.J.; Kim, J.K.; Hwang, S.D.; Gil, H.-W.; Lee, E.Y.; Jeon, J.S.; et al. IgA Nephropathy Is Associated with Elevated Urinary Mitochondrial DNA Copy Numbers. Sci. Rep. 2019, 9, 16068. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Elimam, H.; Cybulsky, A.V. Complement-Mediated Cellular Injury. Semin. Nephrol. 2013, 33, 586–601. [Google Scholar] [CrossRef] [PubMed]
- Nangaku, M.; Shankland, S.J.; Couser, W.G. Cellular Response to Injury in Membranous Nephropathy. J. Am. Soc. Nephrol. 2005, 16, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Qin, W.S.; Zeng, C.H.; Zheng, C.X.; Hong, Y.M.; Lu, Y.Z.; Li, L.S.; Liu, Z.H. Triptolide Reduces Proteinuria in Experimental Membranous Nephropathy and Protects Against C5b-9-Induced Podocyte Injury In Vitro. Kidney Int. 2010, 77, 974–988. [Google Scholar] [CrossRef]
- Liu, H.; Tian, N.; Arany, I.; Bigler, S.A.; Waxman, D.J.; Shah, S.V.; Baliga, R. Cytochrome P450 2B1 Mediates Complement-Dependent Sublytic Injury in A Model of Membranous Nephropathy. J. Biol. Chem. 2010, 285, 40901–40910. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, H.; Miao, X.; Xu, J.; Yang, R.; Zhao, L.; Liu, J.; Yang, L.; Gao, F.; Zhang, W.; et al. Nestin Protects Podocyte from Injury in Lupus Nephritis by Mitophagy and Oxidative Stress. Cell Death Dis. 2020, 11, 319. [Google Scholar] [CrossRef]
- Qi, Y.Y.; Zhou, X.J.; Cheng, F.J.; Hou, P.; Ren, Y.L.; Wang, S.X.; Zhao, M.H.; Yang, L.; Martinez, J.; Zhang, H. Increased Autophagy Is Cytoprotective Against Podocyte Injury Induced by Antibody and Interferon-Alpha in Lupus Nephritis. Ann. Rheum. Dis. 2018, 77, 1799–1809. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.; Boutary, S.; Braych, K.; Sabra, R.; Massaad, C.; Hamdy, A.; Rashid, A.; Moodad, S.; Block, K.; Gorin, Y.; et al. mTORC2 Signaling Regulates Nox4-Induced Podocyte Depletion in Diabetes. Antioxid. Redox Signal. 2016, 25, 703–719. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.A.; Gorin, Y.; Fagg, B.M.; Maalouf, R.; Barnes, J.L.; Block, K.; Abboud, H.E. Mechanisms of Podocyte Injury in Diabetes: Role of Cytochrome P450 and NADPH Oxidases. Diabetes 2009, 58, 1201–1211. [Google Scholar] [CrossRef]
- Habibi, J.; Aroor, A.R.; Das, N.A.; Manrique-Acevedo, C.M.; Johnson, M.S.; Hayden, M.R.; Nistala, R.; Wiedmeyer, C.; Chandrasekar, B.; DeMarco, V.G. The Combination of A Neprilysin Inhibitor (Sacubitril) and Angiotensin-II Receptor Blocker (Valsartan) Attenuates Glomerular and Tubular Injury in the Zucker Obese Rat. Cardiovasc Diabetol. 2019, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Jha, J.C.; Gray, S.P.; Barit, D.; Okabe, J.; El-Osta, A.; Namikoshi, T.; Thallas-Bonke, V.; Wingler, K.; Szyndralewiez, C.; Heitz, F.; et al. Genetic Targeting or Pharmacologic Inhibition of NADPH Oxidase Nox4 Provides Renoprotection in Long-Term Diabetic Nephropathy. J. Am. Soc. Nephrol. 2014, 25, 1237–1254. [Google Scholar] [CrossRef]
- Holterman, C.E.; Thibodeau, J.F.; Towaij, C.; Gutsol, A.; Montezano, A.C.; Parks, R.J.; Cooper, M.E.; Touyz, R.M.; Kennedy, C.R. Nephropathy and Elevated BP in Mice with Podocyte-Specific NADPH Oxidase 5 Expression. J. Am. Soc. Nephrol. 2014, 25, 784–797. [Google Scholar] [CrossRef]
- Shao, X.; Zhang, X.; Hu, J.; Gao, T.; Chen, J.; Xu, C.; Wei, C. Dopamine 1 Receptor Activation Protects Mouse Diabetic Podocytes Injury via Regulating the PKA/NOX-5/P38 MAPK Axis. Exp. Cell Res. 2020, 388, 111849. [Google Scholar] [CrossRef]
- Toyonaga, J.; Tsuruya, K.; Ikeda, H.; Noguchi, H.; Yotsueda, H.; Fujisaki, K.; Hirakawa, M.; Taniguchi, M.; Masutani, K.; Iida, M. Spironolactone Inhibits Hyperglycemia-Induced Podocyte Injury by Attenuating ROS Production. Nephrol. Dial. Transplant. 2011, 26, 2475–2484. [Google Scholar] [CrossRef]
- Zhang, T.; Chi, Y.; Kang, Y.; Lu, H.; Niu, H.; Liu, W.; Li, Y. Resveratrol Ameliorates Podocyte Damage in Diabetic Mice via SIRT1/PGC-1alpha Mediated Attenuation of Mitochondrial Oxidative Stress. J. Cell Physiol. 2019, 234, 5033–5043. [Google Scholar] [CrossRef]
- Bock, F.; Shahzad, K.; Wang, H.; Stoyanov, S.; Wolter, J.; Dong, W.; Pelicci, P.G.; Kashif, M.; Ranjan, S.; Schmidt, S.; et al. Activated Protein C Ameliorates Diabetic Nephropathy by Epigenetically Inhibiting the Redox Enzyme P66shc. Proc. Natl. Acad. Sci. USA 2013, 110, 648–653. [Google Scholar] [CrossRef]
- Zheng, D.; Tao, M.; Liang, X.; Li, Y.; Jin, J.; He, Q. P66shc Regulates Podocyte Autophagy in High Glucose Environment Through the Notch-PTEN-PI3k/Akt/mTOR Pathway. Histol. Histopathol. 2020, 35, 405–415. [Google Scholar] [CrossRef]
- Lambeth, J.D. Nox Enzymes and the Biology of Reactive Oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Che, G.; Gao, H.; Hu, Q.; Xie, H.; Zhang, Y. Angiotensin II Promotes Podocyte Injury by Activating Arf6-Erk1/2-Nox4 Signaling Pathway. PLoS ONE 2020, 15, e0229747. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Nagase, M.; Yoshida, S.; Kawachi, H.; Fujita, T. Podocyte as the Target for Aldosterone: Roles of Oxidative Stress and Sgk1. Hypertension 2007, 49, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Nagase, M.; Fujita, T. Aldosterone and Glomerular Podocyte Injury. Clin. Exp. Nephrol. 2008, 12, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Abais, J.M.; Xia, M.; Li, G.; Gehr, T.W.; Boini, K.M.; Li, P.L. Contribution of Endogenously Produced Reactive Oxygen Species to the Activation of Podocyte NLRP3 Inflammasomes in Hyperhomocysteinemia. Free Radic. Biol. Med. 2014, 67, 211–220. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, X.; Lu, M.; Wu, Q.; Yuan, Q.; Hu, C.; Miao, J.; Zhang, Y.; Li, H.; Hou, F.F.; et al. Wnt/Beta-Catenin Links Oxidative Stress to Podocyte Injury and Proteinuria. Kidney Int. 2019, 95, 830–845. [Google Scholar] [CrossRef]
- Rong, G.; Tang, X.; Guo, T.; Duan, N.; Wang, Y.; Yang, L.; Zhang, J.; Liang, X. Advanced Oxidation Protein Products Induce Apoptosis in Podocytes Through Induction of Endoplasmic Reticulum Stress. J. Physiol. Biochem 2015, 71, 455–470. [Google Scholar] [CrossRef]
- Zhou, L.L.; Hou, F.F.; Wang, G.B.; Yang, F.; Xie, D.; Wang, Y.P.; Tian, J.W. Accumulation of Advanced Oxidation Protein Products Induces Podocyte Apoptosis and Deletion Through Nadph-Dependent Mechanisms. Kidney Int. 2009, 76, 1148–1160. [Google Scholar] [CrossRef]
- Das, R.; Xu, S.; Nguyen, T.T.; Quan, X.; Choi, S.K.; Kim, S.J.; Lee, E.Y.; Cha, S.K.; Park, K.S. Transforming Growth Factor Beta1-Induced Apoptosis in Podocytes via the Extracellular Signal-Regulated Kinase-Mammalian Target of Rapamycin Complex 1-NADPH Oxidase 4 Axis. J. Biol. Chem. 2015, 290, 30830–30842. [Google Scholar] [CrossRef]
- Das, R.; Xu, S.; Quan, X.; Nguyen, T.T.; Kong, I.D.; Chung, C.H.; Lee, E.Y.; Cha, S.K.; Park, K.S. Upregulation of Mitochondrial Nox4 Mediates TGF-Beta-Induced Apoptosis in Cultured Mouse Podocytes. Am. J. Physiol. Renal Physiol. 2014, 306, F155–F167. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Guh, J.Y.; Lai, Y.H. Alterations of Glomerular and Extracellular Glutathione Peroxidase Levels in Patients and Rats with Focal Segmental Glomerulosclerosis. J. Lab Clin. Med. 2001, 137, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Gasser, D.L.; Winkler, C.A.; Peng, M.; An, P.; McKenzie, L.M.; Kirk, G.D.; Shi, Y.; Xie, L.X.; Marbois, B.N.; Clarke, C.F.; et al. Focal Segmental Glomerulosclerosis Is Associated with A PDSS2 Haplotype and, Independently, with A Decreased Content of Coenzyme Q10. Am. J. Physiol. Renal Physiol. 2013, 305, F1228–F1238. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.R.; Bonventre, J.V.; Karnovsky, M.J. A Role for Oxygen Free Radicals in Aminonucleoside Nephrosis. Kidney Int. 1986, 29, 478–483. [Google Scholar] [CrossRef]
- Fujii, Y.; Matsumura, H.; Yamazaki, S.; Shirasu, A.; Nakakura, H.; Ogihara, T.; Ashida, A. Efficacy of A Mitochondrion-Targeting Agent for Reducing the Level of Urinary Protein in Rats with Puromycin Aminonucleoside-Induced Minimal-Change Nephrotic Syndrome. PLoS ONE 2020, 15, e0227414. [Google Scholar] [CrossRef]
- Deman, A.; Ceyssens, B.; Pauwels, M.; Zhang, J.; Houte, K.V.; Verbeelen, D.; Van den Branden, C. Altered Antioxidant Defence in A Mouse Adriamycin Model of Glomerulosclerosis. Nephrol. Dial. Transplant. 2001, 16, 147–150. [Google Scholar] [CrossRef]
- Takiue, K.; Sugiyama, H.; Inoue, T.; Morinaga, H.; Kikumoto, Y.; Kitagawa, M.; Kitamura, S.; Maeshima, Y.; Wang, D.-H.; Masuoka, N.; et al. Acatalasemic Mice Are Mildly Susceptible to Adriamycin Nephropathy and Exhibit Increased Albuminuria and Glomerulosclerosis. BMC Nephrol. 2012, 13, 14. [Google Scholar] [CrossRef]
- Van den Branden, C.; Deman, A.; Ceyssens, B.; Pauwels, M.; Empsen, C.; Verbeelen, D. Vitamin E Protects Renal Antioxidant Enzymes and Attenuates Glomerulosclerosis in Adriamycin-Treated Rats. Nephron 2002, 91, 129–133. [Google Scholar] [CrossRef]
- Prunotto, M.; Carnevali, M.L.; Candiano, G.; Murtas, C.; Bruschi, M.; Corradini, E.; Trivelli, A.; Magnasco, A.; Petretto, A.; Santucci, L.; et al. Autoimmunity in Membranous Nephropathy Targets Aldose Reductase and SOD2. J. Am. Soc. Nephrol. 2010, 21, 507–519. [Google Scholar] [CrossRef]
- Chan, J.C.; Mahan, J.D.; Trachtman, H.; Scheinman, J.; Flynn, J.T.; Alon, U.S.; Lande, M.B.; Weiss, R.A.; Norkus, E.P. Vitamin E Therapy in IgA Nephropathy: A Double-Blind, Placebo-Controlled Study. Pediatr. Nephrol. 2003, 18, 1015–1019. [Google Scholar] [CrossRef]
- Dos Santos, M.; Poletti, P.T.; Favero, G.; Stacchiotti, A.; Bonomini, F.; Montanari, C.C.; Bona, S.R.; Marroni, N.P.; Rezzani, R.; Veronese, F.V. Protective Effects of Quercetin Treatment in A Pristane-Induced Mouse Model of Lupus Nephritis. Autoimmunity 2018, 51, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Morigi, M.; Perico, L.; Corna, D.; Locatelli, M.; Cassis, P.; Carminati, C.E.; Bolognini, S.; Zoja, C.; Remuzzi, G.; Benigni, A.; et al. C3a Receptor Blockade Protects Podocytes from Injury in Diabetic Nephropathy. JCI Insight 2020, 5, e131849. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.Q.; Tang, L.; Gao, Y.B.; Wang, Y.F.; Meng, Y.; Shen, C.; Shen, Z.L.; Liu, Z.Q.; Zhao, W.J.; Liu, W.J. Effect of Baoshenfang Formula on Podocyte Injury via Inhibiting the NOX-4/ROS/P38 Pathway in Diabetic Nephropathy. J. Diabetes Res. 2019, 2019, 2981705. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.K.; Harris, R.C. EGR Receptor Deletion in Podocytes Attenuates Diabetic Nephropathy. J. Am. Soc. Nephrol. 2015, 26, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Poirier, B.; Lannaud-Bournoville, M.; Conti, M.; Bazin, R.; Michel, O.; Bariety, J.; Chevalier, J.; Myara, I. Oxidative Stress Occurs in Absence of Hyperglycaemia and Inflammation in the Onset of Kidney Lesions in Normotensive Obese Rats. Nephrol. Dial. Transplant. 2000, 15, 467–476. [Google Scholar] [CrossRef]
- Shah, A.; Xia, L.; Masson, E.A.; Gui, C.; Momen, A.; Shikatani, E.A.; Husain, M.; Quaggin, S.; John, R.; Fantus, I.G. Thioredoxin-Interacting Protein Deficiency Protects Against Diabetic Nephropathy. J. Am. Soc. Nephrol. 2015, 26, 2963–2977. [Google Scholar] [CrossRef]
- Wood, Z.A.; Poole, L.B.; Karplus, P.A. Peroxiredoxin Evolution and the Regulation of Hydrogen Peroxide Signaling. Science 2003, 300, 650–653. [Google Scholar] [CrossRef]
- Hsu, H.H.; Hoffmann, S.; Di Marco, G.S.; Endlich, N.; Peter-Katalinic, J.; Weide, T.; Pavenstadt, H. Downregulation of the Antioxidant Protein Peroxiredoxin 2 Contributes to Angiotensin II-Mediated Podocyte Apoptosis. Kidney Int. 2011, 80, 959–969. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Liu, Y.; Yang, Y.; Xu, J.; Dai, D.; Yan, C.; Li, X.; Tang, R.; Yu, C.; Ren, H. Antioxidative Stress Effects of Salvia Przewalskii Extract in Experimentally Injured Podocytes. Nephron 2016, 134, 253–271. [Google Scholar] [CrossRef]
- Zheng, S.; Carlson, E.C.; Yang, L.; Kralik, P.M.; Huang, Y.; Epstein, P.N. Podocyte-Specific Overexpression of the Antioxidant Metallothionein Reduces Diabetic Nephropathy. J. Am. Soc. Nephrol. 2008, 19, 2077–2085. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host Cell Death and Inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of Cell Death: The Calcium-Apoptosis Link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Tharaux, P.L.; Huber, T.B. How Many Ways Can A Podocyte Die? Semin. Nephrol. 2012, 32, 394–404. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, S.; Lee, S.W.; Lee, J.H.; Lee, E.S.; Kim, M.; Kim, Y.; Kang, J.S.; Chung, C.H.; Moon, J.S.; et al. RIPK3 Contributes to Lyso-Gb3-Induced Podocyte Death. Cells 2021, 10, 245. [Google Scholar] [CrossRef]
- Meyer, T.W.; Bennett, P.H.; Nelson, R.G. Podocyte Number Predicts Long-Term Urinary Albumin Excretion in Pima Indians with Type II Diabetes and Microalbuminuria. Diabetologia 1999, 42, 1341–1344. [Google Scholar] [CrossRef]
- Nagata, S. Apoptosis and Clearance of Apoptotic Cells. Annu. Rev. Immunol. 2018, 36, 489–517. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of Apoptosis by the Bcl-2 Protein Family: Implications for Physiology and Therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Dickens, L.S.; Powley, I.R.; Hughes, M.A.; MacFarlane, M. The ‘Complexities’ of Life and Death: Death Receptor Signalling Platforms. Experimental. Cell Res. 2012, 318, 1269–1277. [Google Scholar] [CrossRef]
- Xiang, X.Y.; Liu, T.; Wu, Y.; Jiang, X.S.; He, J.L.; Chen, X.M.; Du, X.G. Berberine Alleviates Palmitic Acid-Induced Podocyte Apoptosis by Reducing Reactive Oxygen Species-Mediated Endoplasmic Reticulum Stress. Mol. Med. Rep. 2021, 23. [Google Scholar] [CrossRef]
- Chuang, P.Y.; Yu, Q.; Fang, W.; Uribarri, J.; He, J.C. Advanced Glycation Endproducts Induce Podocyte Apoptosis by Activation of the FOXO4 Transcription Factor. Kidney Int. 2007, 72, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hitomi, H.; Diah, S.; Deguchi, K.; Mori, H.; Masaki, T.; Nakano, D.; Kobori, H.; Nishiyama, A. Roles of Na(+)/H(+) Exchanger Type 1 and Intracellular pH in Angiotensin II-Induced Reactive Oxygen Species Generation and Podocyte Apoptosis. J. Pharmacol. Sci. 2013, 122, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Ye, W.; Li, S.; Wang, H.; Ding, X. Icariin Modulates Mitochondrial Function and Apoptosis in High Glucose-Induced Glomerular Podocytes Through G Protein-Coupled Estrogen Receptors. Mol. Cell Endocrinol. 2018, 473, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, W.; Xiao, J.; Zhang, Y.; Chen, Y.; Luo, C.; Huang, Q.; Peng, F.; Gong, W.; Li, S.; et al. FOXO3a Accumulation and Activation Accelerate Oxidative Stress-Induced Podocyte Injury. FASEB J. 2020, 34, 13300–13316. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.; Ley, S.C. Mitogen-Activated Protein Kinases in Innate Immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Gao, Y.; Zhao, W.; Zou, D.; Zhu, Z.; Wu, X.; Tian, N.; Wang, X.; Liu, J.; Tong, Y. Effect of Tongxinluo on Podocyte Apoptosis via Inhibition of Oxidative Stress and P38 Pathway in Diabetic Rats. Evid. Based Complement. Altern. Med. 2016, 2016, 5957423. [Google Scholar] [CrossRef]
- Cantley, L.C. The Phosphoinositide 3-Kinase Pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef]
- Cui, F.Q.; Wang, Y.F.; Gao, Y.B.; Meng, Y.; Cai, Z.; Shen, C.; Liu, Z.Q.; Jiang, X.C.; Zhao, W.J. Effects of BSF on Podocyte Apoptosis via Regulating the Ros-Mediated PI3K/AKT Pathway in DN. J. Diabetes Res. 2019, 2019, 9512406. [Google Scholar] [CrossRef]
- Vousden, K.H.; Prives, C. Blinded by the Light: The Growing Complexity of P53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef]
- Eid, A.A.; Ford, B.M.; Block, K.; Kasinath, B.S.; Gorin, Y.; Ghosh-Choudhury, G.; Barnes, J.L.; Abboud, H.E. AMP-Activated Protein Kinase (AMPK) Negatively Regulates Nox4-Dependent Activation of P53 and Epithelial Cell Apoptosis in Diabetes. J. Biol. Chem. 2010, 285, 37503–37512. [Google Scholar] [CrossRef]
- Li, Z.; Deng, W.; Cao, A.; Zang, Y.; Wang, Y.; Wang, H.; Wang, L.; Peng, W. Huangqi Decoction Inhibits Hyperglycemia-Induced Podocyte Apoptosis by Down-Regulated Nox4/P53/Bax Signaling In Vitro and In Vivo. Am. J. Transl. Res. 2019, 11, 3195–3212. [Google Scholar] [PubMed]
- Zhou, L.L.; Cao, W.; Xie, C.; Tian, J.; Zhou, Z.; Zhou, Q.; Zhu, P.; Li, A.; Liu, Y.; Miyata, T.; et al. The Receptor of Advanced Glycation End Products Plays A Central Role in Advanced Oxidation Protein Products-Induced Podocyte Apoptosis. Kidney Int. 2012, 82, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Oakes, S.A.; Papa, F.R. The Role of Endoplasmic Reticulum Stress in Human Pathology. Annu. Rev. Pathol. 2015, 10, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Susin, S.A.; Lorenzo, H.K.; Zamzami, N.; Marzo, I.; Snow, B.E.; Brothers, G.M.; Mangion, J.; Jacotot, E.; Costantini, P.; Loeffler, M.; et al. Molecular Characterization of Mitochondrial Apoptosis-Inducing Factor. Nature 1999, 397, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular Mechanisms and Health Implications. Cell Res. 2020, 32, 107–125. [Google Scholar] [CrossRef]
- Guo, C.; Fu, R.; Zhou, M.; Wang, S.; Huang, Y.; Hu, H.; Zhao, J.; Gaskin, F.; Yang, N.; Fu, S.M. Pathogenesis of Lupus Nephritis: RIP3 Dependent Necroptosis and NLRP3 Inflammasome Activation. J. Autoimmun. 2019, 103, 102286. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, H.; Hu, Y.; Fang, Y.; Qi, C.; Huang, J.; Cai, X.; Wu, H.; Ding, X.; Zhang, Z. High Glucose-Induced Apoptosis and Necroptosis in Podocytes Is Regulated by UCHL1 via RIPK1/RIPK3 Pathway. Exp. Cell Res. 2019, 382, 111463. [Google Scholar] [CrossRef]
- Lan, X.; Jhaveri, A.; Cheng, K.; Wen, H.; Saleem, M.A.; Mathieson, P.W.; Mikulak, J.; Aviram, S.; Malhotra, A.; Skorecki, K.; et al. APOL1 Risk Variants Enhance Podocyte Necrosis through Compromising Lysosomal Membrane Permeability. Am. J. Physiol. Renal. Physiol. 2014, 307, F326–F336. [Google Scholar] [CrossRef]
- Pak, O.; Scheibe, S.; Esfandiary, A.; Gierhardt, M.; Sydykov, A.; Logan, A.; Fysikopoulos, A.; Veit, F.; Hecker, M.; Kroschel, F.; et al. Impact of the Mitochondria-Targeted Antioxidant MitoQ on Hypoxia-Induced Pulmonary Hypertension. Eur. Respir. J. 2018, 51, 1701024. [Google Scholar] [CrossRef]
- Dare, A.J.; Bolton, E.A.; Pettigrew, G.J.; Bradley, J.A.; Saeb-Parsy, K.; Murphy, M.P. Protection Against Renal Ischemia-Reperfusion Injury in Vivo by the Mitochondria Targeted Antioxidant MitoQ. Redox. Biol. 2015, 5, 163–168. [Google Scholar] [CrossRef]
- Zhu, Z.; Liang, W.; Chen, Z.; Hu, J.; Feng, J.; Cao, Y.; Ma, Y.; Ding, G. Mitoquinone Protects Podocytes from Angiotensin II-Induced Mitochondrial Dysfunction and Injury via the Keap1-Nrf2 Signaling Pathway. Oxid. Med. Cell Longev. 2021, 2021, 1394486. [Google Scholar] [CrossRef] [PubMed]
- Azuma, J.; Sawamura, A.; Awata, N. Usefulness of Taurine in Chronic Congestive Heart Failure and Its Prospective Application. Jpn. Circ. J. 1992, 56, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Katakawa, M.; Fukuda, N.; Tsunemi, A.; Mori, M.; Maruyama, T.; Matsumoto, T.; Abe, M.; Yamori, Y. Taurine and Magnesium Supplementation Enhances the Function of Endothelial Progenitor Cells Through Antioxidation in Healthy Men and Spontaneously Hypertensive Rats. Hypertens. Res. 2016, 39, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, Y.; Minami, A.; Harada, N.; Sakamoto, S.; Niwa, Y.; Ohnaka, M. Taurine Improves Insulin Sensitivity in the Otsuka Long-Evans Tokushima Fatty Rat, A Model of Spontaneous Type 2 Diabetes. Am. J. Clin. Nutr. 2000, 71, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Kim, H.W. Effects and Mechanisms of Taurine as A Therapeutic Agent. Biomol. Ther. 2018, 26, 225–241. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Favero, G.; Lavazza, A.; Monsalve, M.; Rodella, L.F.; Rezzani, R. Taurine Supplementation Alleviates Puromycin Aminonucleoside Damage by Modulating Endoplasmic Reticulum Stress and Mitochondrial-Related Apoptosis in Rat Kidney. Nutrients 2018, 10, 689. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Gao, Q.; Jiang, H.; Zhang, S.; Lu, M.; Liu, F.; Xue, X. Taurine Supplementation Reverses Diabetes-Induced Podocytes Injury via Modulation of the Cse/Trpc6 Axis and Improvement of Mitochondrial Function. Nephron 2020, 144, 84–95. [Google Scholar] [CrossRef]
- Ha, H.; Yu, M.R.; Kim, K.H. Melatonin and Taurine Reduce Early Glomerulopathy in Diabetic Rats. Free Radic. Biol. Med. 1999, 26, 944–950. [Google Scholar] [CrossRef]
- Oktem, F.; Ozguner, F.; Yilmaz, H.R.; Uz, E.; Dundar, B. Melatonin Reduces Urinary Excretion of N-Acetyl-Beta-D-Glucosaminidase, Albumin and Renal Oxidative Markers in Diabetic Rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 95–101. [Google Scholar] [CrossRef]
- Ji, Z.Z.; Xu, Y.C. Melatonin Protects Podocytes from Angiotensin II-Induced Injury in An in Vitro Diabetic Nephropathy Model. Mol. Med. Rep. 2016, 14, 920–926. [Google Scholar] [CrossRef]
- Romecin, P.; Atucha, N.M.; Navarro, E.G.; Ortiz, M.C.; Iyu, D.; Rosado, J.A.; Garcia-Estan, J. Role of Homocysteine and Folic Acid on the Altered Calcium Homeostasis of Platelets from Rats with Biliary Cirrhosis. Platelets 2017, 28, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, W.; Zhao, J.; Sun, W.; Yang, Q.; Chen, C.; Xia, P.; Zhu, J.; Zhou, Y.; Huang, G.; et al. Apigenin Ameliorates Doxorubicin-Induced Renal Injury via Inhibition of Oxidative Stress and Inflammation. Biomed Pharm. 2021, 137, 111308. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, Y.; Li, Y.; Huang, M.; Zhao, W. Geniposide Alleviates Diabetic Nephropathy of Mice through AMPK/SIRT1/NF-kappaB Pathway. Eur. J. Pharmacol. 2020, 886, 173449. [Google Scholar] [CrossRef]
- Yang, S.M.; Hua, K.F.; Lin, Y.C.; Chen, A.; Chang, J.M.; Kuoping Chao, L.; Ho, C.L.; Ka, S.M. Citral Is Renoprotective for Focal Segmental Glomerulosclerosis by Inhibiting Oxidative Stress and Apoptosis and Activating Nrf2 Pathway in Mice. PLoS ONE 2013, 8, e74871. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; He, L. Epigallocatechin-3-Gallate Attenuates Adriamycin-Induced Focal Segmental Glomerulosclerosis via Suppression of Oxidant Stress and Apoptosis by Targeting Hypoxia-Inducible Factor-1alpha/ Angiopoietin-Like 4 Pathway. Pharmacology 2019, 103, 303–314. [Google Scholar] [CrossRef]
- Yang, S.-M.; Chan, Y.-L.; Hua, K.-F.; Chang, J.-M.; Chen, H.-L.; Tsai, Y.-J.; Hsu, Y.-J.; Chao, L.K.; Feng-Ling, Y.; Tsai, Y.-L.; et al. Osthole Improves An Accelerated Focal Segmental Glomerulosclerosis Model in the Early Stage by Activating the Nrf2 Antioxidant Pathway and Subsequently Inhibiting NF-kappaB-Mediated COX-2 Expression and Apoptosis. Free Radic. Biol. Med. 2014, 73, 260–269. [Google Scholar] [CrossRef]
- Liu, X.; Cao, W.; Qi, J.; Li, Q.; Zhao, M.; Chen, Z.; Zhu, J.; Huang, Z.; Wu, L.; Zhang, B.; et al. Leonurine Ameliorates Adriamycin-Induced Podocyte Injury via Suppression of Oxidative Stress. Free Radic Res. 2018, 52, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Son, S.S.; Kang, J.S.; Lee, E.Y. Paclitaxel Ameliorates Palmitate-Induced Injury in Mouse Podocytes. Med. Sci. Monit. Basic Res. 2020, 26, e928265. [Google Scholar] [CrossRef]
- Wang, F.; Li, R.; Zhao, L.; Ma, S.; Qin, G. Resveratrol Ameliorates Renal Damage by Inhibiting Oxidative Stress-Mediated Apoptosis of Podocytes in Diabetic Nephropathy. Eur. J. Pharmacol. 2020, 885, 173387. [Google Scholar] [CrossRef]
- Zhang, T.; Chi, Y.; Ren, Y.; Du, C.; Shi, Y.; Li, Y. Resveratrol Reduces Oxidative Stress and Apoptosis in Podocytes via Sir2-Related Enzymes, Sirtuins1 (SIRT1)/Peroxisome Proliferator-Activated Receptor Gamma Co-Activator 1alpha (PGC-1alpha) Axis. Med. Sci. Monit. 2019, 25, 1220–1231. [Google Scholar] [CrossRef]
- Yang, K.; Bai, Y.; Yu, N.; Lu, B.; Han, G.; Yin, C.; Pang, Z. Huidouba Improved Podocyte Injury by Down-Regulating Nox4 Expression in Rats with Diabetic Nephropathy. Front. Pharmacol. 2020, 11, 587995. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, Y.; Zhang, T.; Liu, M.; Chi, Y.; Liu, S.; Shi, Y. Salidroside Reduces High-Glucose-Induced Podocyte Apoptosis and Oxidative Stress via Upregulating Heme Oxygenase-1 (HO-1) Expression. Med. Sci. Monit. 2017, 23, 4067–4076. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.; Shan, Z.; Zhao, Y.; Li, M.; Wang, B.; Zheng, X.; Feng, W. The Protective Effect and Mechanism of Catalpol on High Glucose-Induced Podocyte Injury. BMC Complement. Altern. Med. 2019, 19, 244. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.; Jiang, M.; Fu, Y.; Zhu, Y.; Jiao, N.; Liu, L.; Du, Q.; Wu, H.; Xu, H.; et al. Loganin and Catalpol Exert Cooperative Ameliorating Effects on Podocyte Apoptosis upon Diabetic Nephropathy by Targeting Ages-Rage Signaling. Life Sci. 2020, 252, 117653. [Google Scholar] [CrossRef]
- Gui, D.; Guo, Y.; Wang, F.; Liu, W.; Chen, J.; Chen, Y.; Huang, J.; Wang, N. Astragaloside IV, A Novel Antioxidant, Prevents Glucose-Induced Podocyte Apoptosis in Vitro and In Vivo. PLoS ONE 2012, 7, e39824. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, M.; Qian, L.; Wen, D.; Wu, G. Luteolin Attenuates High Glucose-Induced Podocyte Injury via Suppressing NLRP3 Inflammasome Pathway. Life Sci. 2019, 225, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.K.; Park, S.H.; Kim, Y.H.; Lee, E.J.; Antika, L.D.; Kim, D.Y.; Choi, Y.J.; Kang, Y.H. Chrysin Ameliorates Podocyte Injury and Slit Diaphragm Protein Loss via Inhibition of the PERK-eIF2alpha-ATF-CHOP Pathway in Diabetic Mice. Acta Pharmacol. Sin. 2017, 38, 1129–1140. [Google Scholar] [CrossRef]
- Zhao, K.; Li, Y.; Wang, Z.; Han, N.; Wang, Y. Carnosine Protects Mouse Podocytes from High Glucose Induced Apoptosis Through PI3K/AKT and Nrf2 Pathways. Biomed. Res. Int. 2019, 2019, 4348973. [Google Scholar] [CrossRef]
- Riedl, E.; Pfister, F.; Braunagel, M.; Brinkkötter, P.; Sternik, P.; Deinzer, M.; Bakker, S.J.; Henning, R.H.; Born, J.V.D.; Krämer, B.K.; et al. Carnosine Prevents Apoptosis of Glomerular Cells and Podocyte Loss in STZ Diabetic Rats. Cell Physiol. Biochem. 2011, 28, 279–288. [Google Scholar] [CrossRef]
- Quan, X.; Liu, H.; Ye, D.; Ding, X.; Su, X. Forsythoside A Alleviates High Glucose-Induced Oxidative Stress and Inflammation in Podocytes by Inactivating MAPK Signaling via MMP12 Inhibition. Diabetes Metab. Syndr. Obes. 2021, 14, 1885–1895. [Google Scholar] [CrossRef]
- He, J.Y.; Hong, Q.; Chen, B.X.; Cui, S.Y.; Liu, R.; Cai, G.Y.; Guo, J.; Chen, X.M. Ginsenoside Rb1 Alleviates Diabetic Kidney Podocyte Injury by Inhibiting Aldose Reductase Activity. Acta Pharmacol. Sin. 2022, 43, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Bao, L.; Ren, J.; Li, Y.; Zhang, Z. Grape Seed Procyanidin B2 Protects Podocytes from High Glucose-Induced Mitochondrial Dysfunction and Apoptosis via the AMPK-SIRT1-PGC-1alpha Axis In Vitro. Food Funct. 2016, 7, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.M.; Papadimitriou, A.; Duarte, D.A.; Lopes de Faria, J.M.; Lopes de Faria, J.B. The Use of Green Tea Polyphenols for Treating Residual Albuminuria in Diabetic Nephropathy: A Double-Blind Randomised Clinical Trial. Sci. Rep. 2016, 6, 28282. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Zhao, H.; Liu, X.; Liu, D.; Chen, J.; Li, Z.; Chu, S.; Kou, X.; Liao, S.; Deng, Y.; et al. Protective Effect of Hydroxysafflor Yellow A on Nephropathy by Attenuating Oxidative Stress and Inhibiting Apoptosis in Induced Type 2 Diabetes in Rat. Oxid. Med. Cell Longev. 2020, 2020, 7805393. [Google Scholar] [CrossRef] [PubMed]
- Li, T.X.; Mao, J.H.; Huang, L.; Fu, H.D.; Chen, S.H.; Liu, A.M.; Liang, Y.Q. Beneficial Effects of Huaiqihuang on Hyperglycemia-Induced MPC5 Podocyte Dysfunction through the Suppression of Mitochondrial Dysfunction and Endoplasmic Reticulum Stress. Mol. Med. Rep. 2017, 16, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, S.; Li, H.; Chen, F.; Shi, J. Naringin Ameliorates Diabetic Nephropathy by Inhibiting NADPH Oxidase 4. Eur. J. Pharmacol. 2017, 804, 1–6. [Google Scholar] [CrossRef]
- Xue, W.; Mao, J.; Chen, Q.; Ling, W.; Sun, Y. Mogroside IIIE Alleviates High Glucose-Induced Inflammation, Oxidative Stress and Apoptosis of Podocytes by the Activation of AMPK/SIRT1 Signaling Pathway. Diabetes Metab. Syndr. Obes. 2020, 13, 3821–3830. [Google Scholar] [CrossRef]
- Sohn, E.; Kim, J.; Kim, C.S.; Jo, K.; Kim, J.S. Extract of Rhizoma Polygonum Cuspidatum Reduces Early Renal Podocyte Injury in Streptozotocin-Induced Diabetic Rats and Its Active Compound Emodin Inhibits Methylglyoxalmediated Glycation of Proteins. Mol. Med. Rep. 2015, 12, 5837–5845. [Google Scholar] [CrossRef]
- Li, J.; Wang, B.; Zhou, G.; Yan, X.; Zhang, Y. Tetrahydroxy Stilbene Glucoside Alleviates High Glucose-Induced MPC5 Podocytes Injury through Suppression of NLRP3 Inflammasome. Am. J. Med. Sci. 2018, 355, 588–596. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 23 February 2022).
| Herbs/Components | Models of Podocytopathies | Anti-Death Mechanisms | Refs. |
|---|---|---|---|
| Salvia przewalskii | PA-induced rats | Anti-apoptosis | [89] |
| Berberine | Palmitic acid-cultured podocytes | Anti-apoptosis | [100] |
| Apigenin | Adriamycin-induced mice | Anti-apoptosis | [132] |
| Citral | Adriamycin-induced rats | Anti-apoptosis | [134] |
| Epigallocatechin-3-Gallate | Adriamycin-induced mice | Anti-apoptosis | [135] |
| Osthole | Adriamycin-induced mice | Anti-apoptosis | [136] |
| Leonurine | Adriamycin-induced mice | Anti-apoptosis | [137] |
| Paclitaxel | Palmitate-cultured podocytes | Anti-apoptosis | [138] |
| Quercetin | Pristane-induced mice | Anti-apoptosis | [81] |
| Resveratrol | db/db mice STZ-induced mice | Anti-apoptosis | [59,139,140] |
| Baoshenfang | KK-Ay mice STZ-induced rats | Anti-apoptosis | [83,108] |
| Icariin | HG-cultured podocytes | Anti-apoptosis | [103] |
| Tongxinluo | STZ-induced rats | Anti-apoptosis | [106] |
| Huangqi | STZ-induced mice | Anti-apoptosis | [111] |
| Geniposide | HFD/STZ-induced mice | Anti-pyroptosis | [133] |
| Huidouba | Unilateral nephrectomy/STZ-induced rats | Anti-apoptosis | [141] |
| Salidroside | HG-cultured podocytes | Anti-apoptosis | [142] |
| Catalpol | KK-Ay/HFD induced mice HG-cultured podocytes AGEs-cultured podocytes | Anti-apoptosis | [143,144] |
| Loganin | KK-Ay/HFD mice AGEs-cultured podocytes | Anti-apoptosis | [144] |
| Astragaloside IV | STZ-induced rats | Anti-apoptosis | [145] |
| Luteolin | HG-cultured podocytes | Anti-apoptosis | [146] |
| Chrysin | db/db mice | Anti-apoptosis | [147] |
| Carnosine | STZ-induced rats HG-cultured podocytes | Anti-apoptosis | [148,149] |
| Forsythoside A | HG-cultured podocytes | Anti-apoptosis | [150] |
| Ginsenoside Rb1 | STZ-induced mice | Anti-apoptosis | [151] |
| Grape seed procyanidin B2 | HG-treated podocytes | Anti-apoptosis | [152] |
| Green tea polyphenols | Diabetic patients’ plasma-cultured podocytes | Anti-apoptosis | [153] |
| Hydroxysafflor yellow A | STZ-induced rats | Anti-apoptosis | [154] |
| Huaiqihuang | HG-treated podocytes | Anti-apoptosis | [155] |
| Naringin | STZ-induced rats | Anti-apoptosis | [156] |
| Mogroside IIIE | HG-treated podocytes | Anti-apoptosis | [157] |
| Rhizoma Polygonum cuspidatum | STZ-induced rats | Anti-apoptosis | [158] |
| Tetrahydroxy stilbene glucoside | HG-treated podocytes | Anti-apoptosis | [159] |
| Therapeutic Targets | Antioxidants | |
| Mitochondrial respiration chain | Complex I | Resveratrol [59,139,140] |
| Complex III | Resveratrol [59,139,140] | |
| Complex IV | Chrysin [147]; Forsythoside A [150] | |
| Cyt C | Resveratrol [59,139,140]; Carnosine [149] | |
| Quinone | MitoQ [121] | |
| NADPH oxidase | Folic Acid [29]; Resveratrol [59,139,140]; Baoshenfang [83,108]; Huangqi [111]; Epigallocatechin-3-Gallate [135]; Paclitaxel [138]; Tongxinluo [106]; Huidouba [141]; Catalpol [143,144]; Loganin [144]; Forsythoside A [150]; Ginsenoside Rb1 [151]; Naringin [156] | |
| Antioxidant defense systems | SOD | Melatonin [129]; Folic Acid [29]; Resveratrol [59,139,140]; Quercetin [81]; Apigenin [132]; Leonurine [137]; Paclitaxel [138]; Geniposide [133]; Catalpol [143,144]; Loganin [144]; Astragaloside IV [145]; Forsythoside A [150]; Grape Seed Procyanidin B2 [152]; Hydroxysafflor Yellow A [154]; Mogroside IIIE [157] |
| CAT | Quercetin [81]; Astragaloside IV [145]; Forsythoside A [150]; Mogroside IIIE [157] | |
| GPX | Osthole [136]; Geniposide [133]; Grape Seed Procyanidin B2 [152]; Naringin [156] | |
| GSH | Apigenin [132] | |
| Therapeutic Targets | Drugs in Clinical Trials | |
|---|---|---|
| Mitochondrial respiration chain | Complex IV | S-equol (NCT02142777), Resveratrol (NCT02123121), etc. |
| Quinone | MitoQ (NCT02364648), Ubiquinol (NCT02847585), Coenzyme Q10 (NCT01163500), etc. | |
| NADPH oxidase | GKT137831 (NCT02010242, NCT03865927), Pioglitazone (NCT03060772), Januvia (NCT00659711), ImmunAge (NCT02332993), Threalose plus polyphenols (NCT04061070), Apocynin (NCT03680404), etc. | |
| Antioxidant defense systems | SOD | GC4711 (NCT03762031), PC-SOD (NCT03995732), APN201 (NCT01513278), rhSOD (NCT00264186), Glisodin (NCT03878433), Melatonin (NCT02463318), etc. |
| CAT | Melatonin (NCT02463318), Oligopin (NCT03260803), etc. | |
| GPX | N-acetylcysteine (NCT00493727), Curcumin (NCT03475017), Rutin (NCT04955145), Melatonin (NCT01858909), etc. | |
| GSH | Glutathione (NCT02948673, NCT02948673), etc. | |
| Other antioxidants | Vitamin E (NCT00384618), Vitamin C (NCT04210453), Vitamin D3 (NCT03931889), L-carnitine (NCT01819701), etc. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.-T.; Wan, C.; Lin, J.-H.; Hammes, H.-P.; Zhang, C. Mitochondrial Oxidative Stress and Cell Death in Podocytopathies. Biomolecules 2022, 12, 403. https://doi.org/10.3390/biom12030403
Zhu Y-T, Wan C, Lin J-H, Hammes H-P, Zhang C. Mitochondrial Oxidative Stress and Cell Death in Podocytopathies. Biomolecules. 2022; 12(3):403. https://doi.org/10.3390/biom12030403
Chicago/Turabian StyleZhu, Yu-Ting, Cheng Wan, Ji-Hong Lin, Hans-Peter Hammes, and Chun Zhang. 2022. "Mitochondrial Oxidative Stress and Cell Death in Podocytopathies" Biomolecules 12, no. 3: 403. https://doi.org/10.3390/biom12030403
APA StyleZhu, Y.-T., Wan, C., Lin, J.-H., Hammes, H.-P., & Zhang, C. (2022). Mitochondrial Oxidative Stress and Cell Death in Podocytopathies. Biomolecules, 12(3), 403. https://doi.org/10.3390/biom12030403






