The Role of Membrane Lipids in Light-Activation of Drosophila TRP Channels
Abstract
1. Introduction
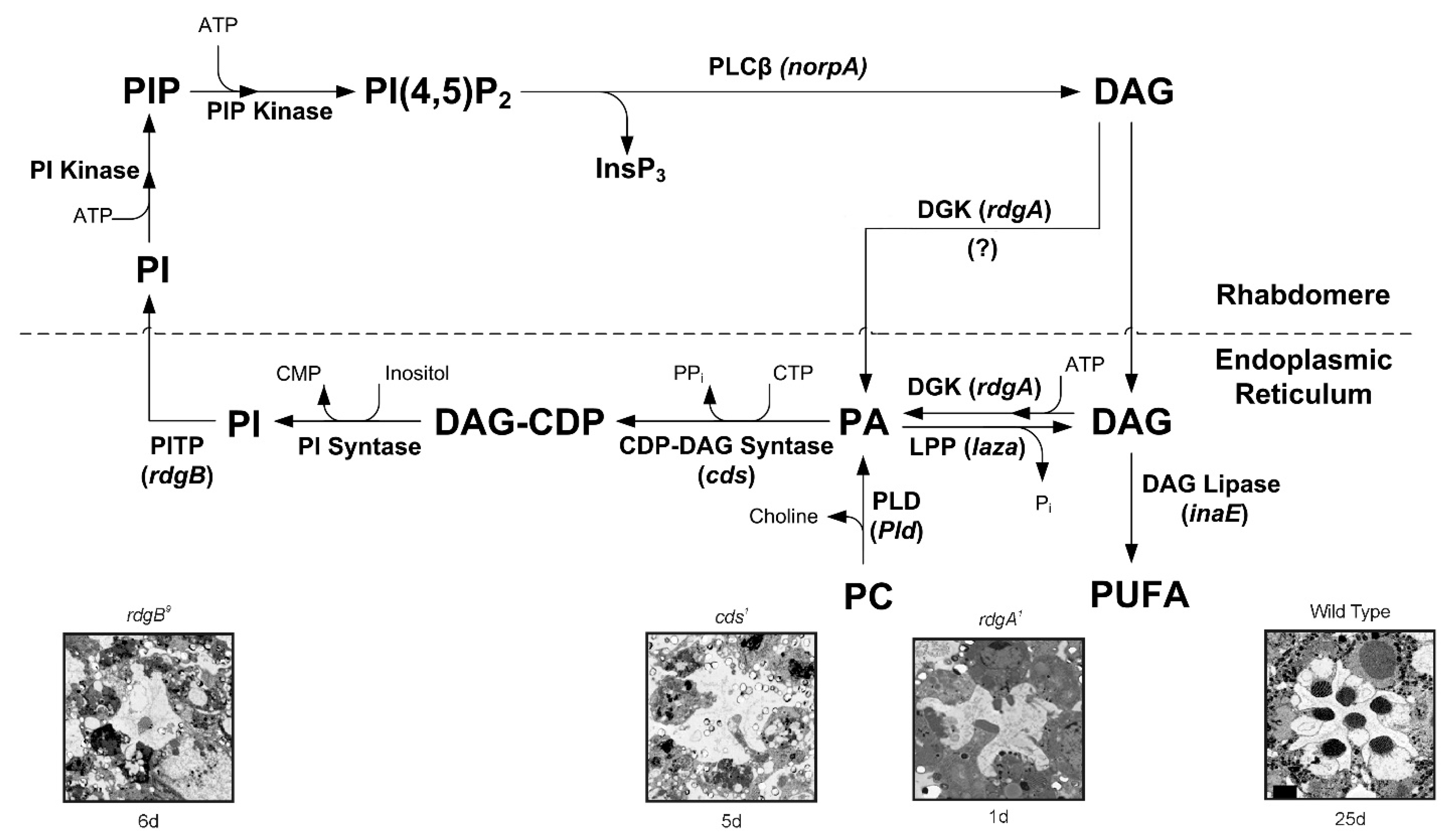
2. The Involvement of the Inositol-Lipid Signaling in TRP Channel Activation
3. Lipid Composition of the Drosophila Head/Retina and the Effects of Its Modification
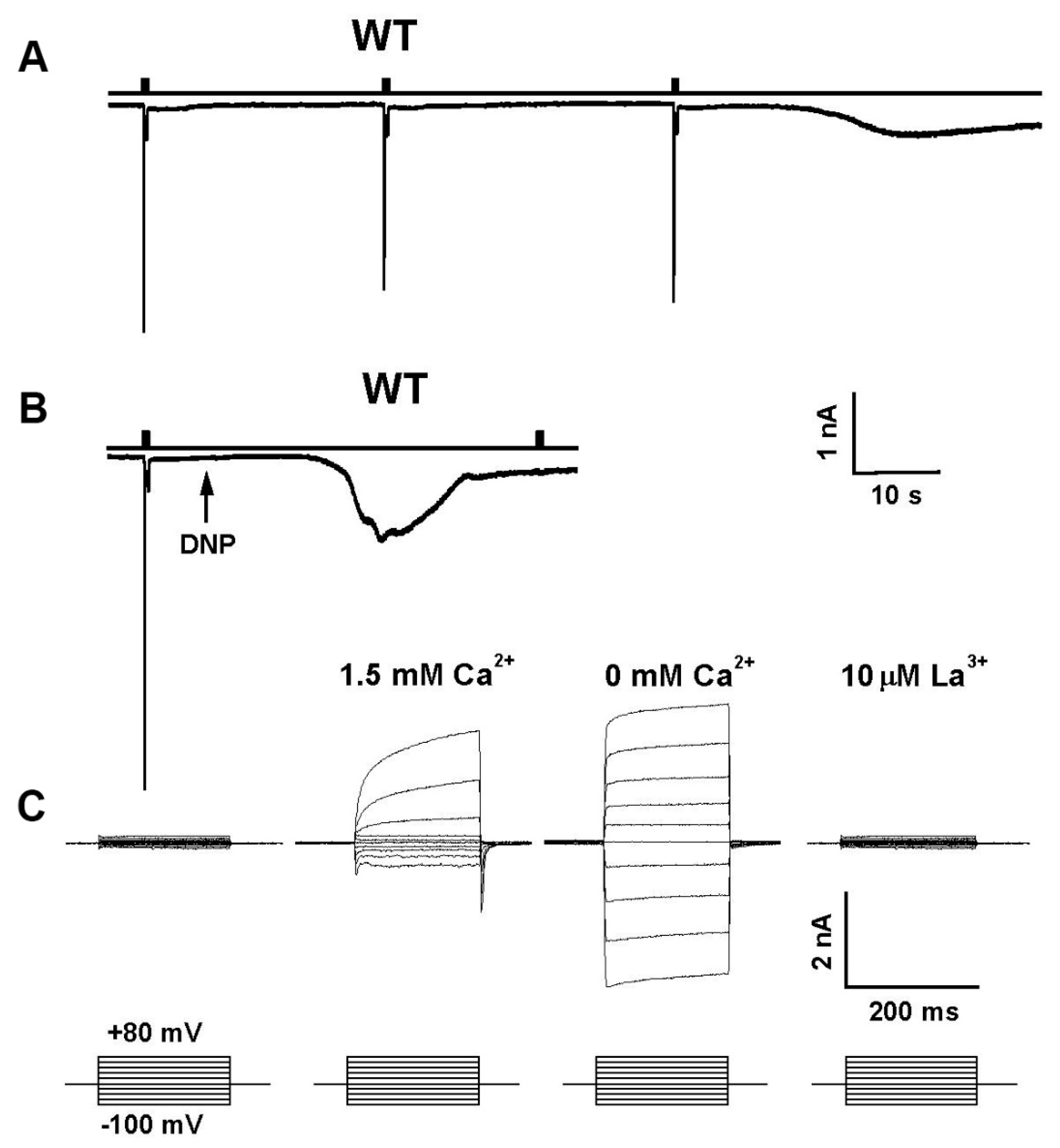
4. Evidence for Lipids Action as Second Messengers
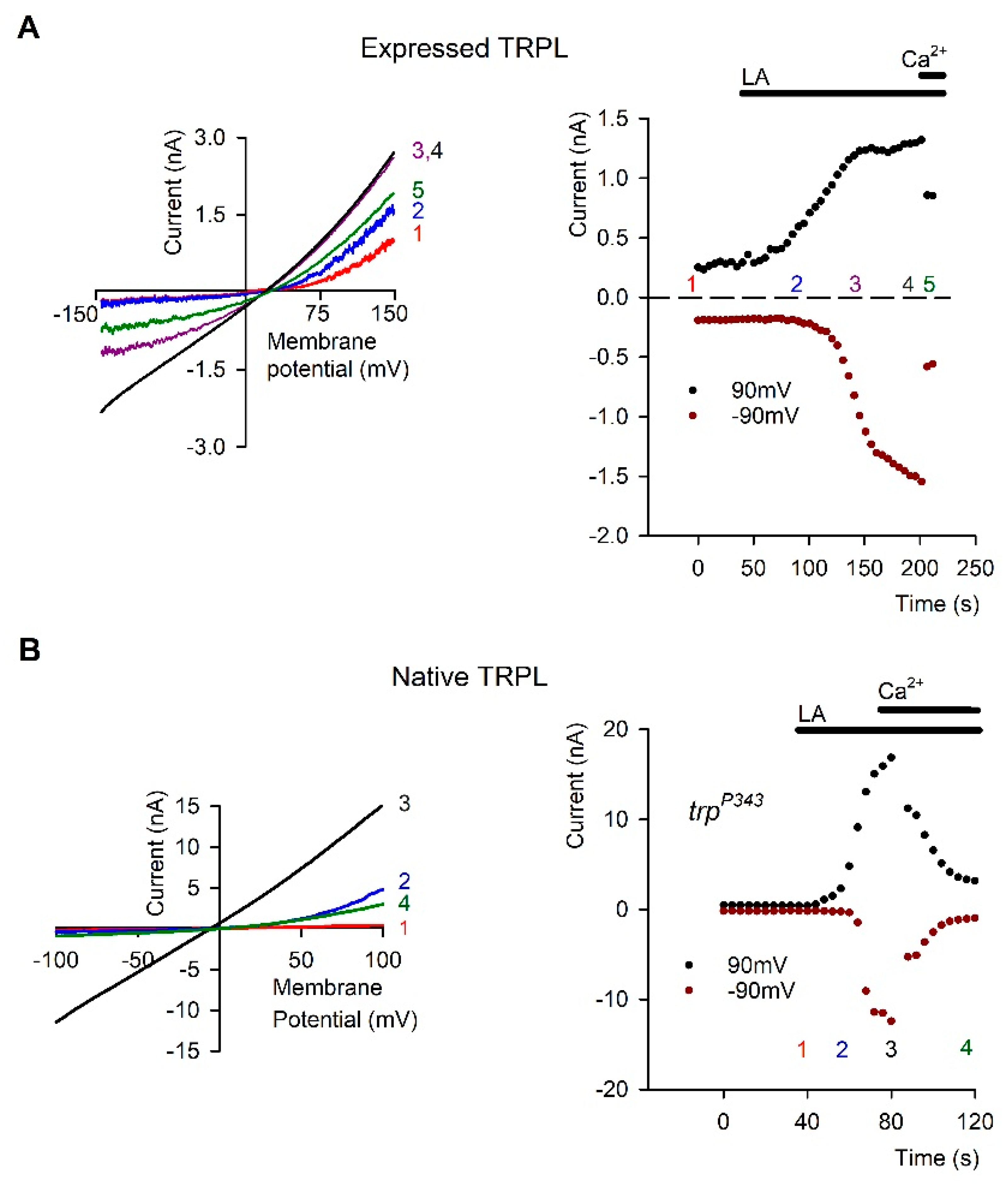
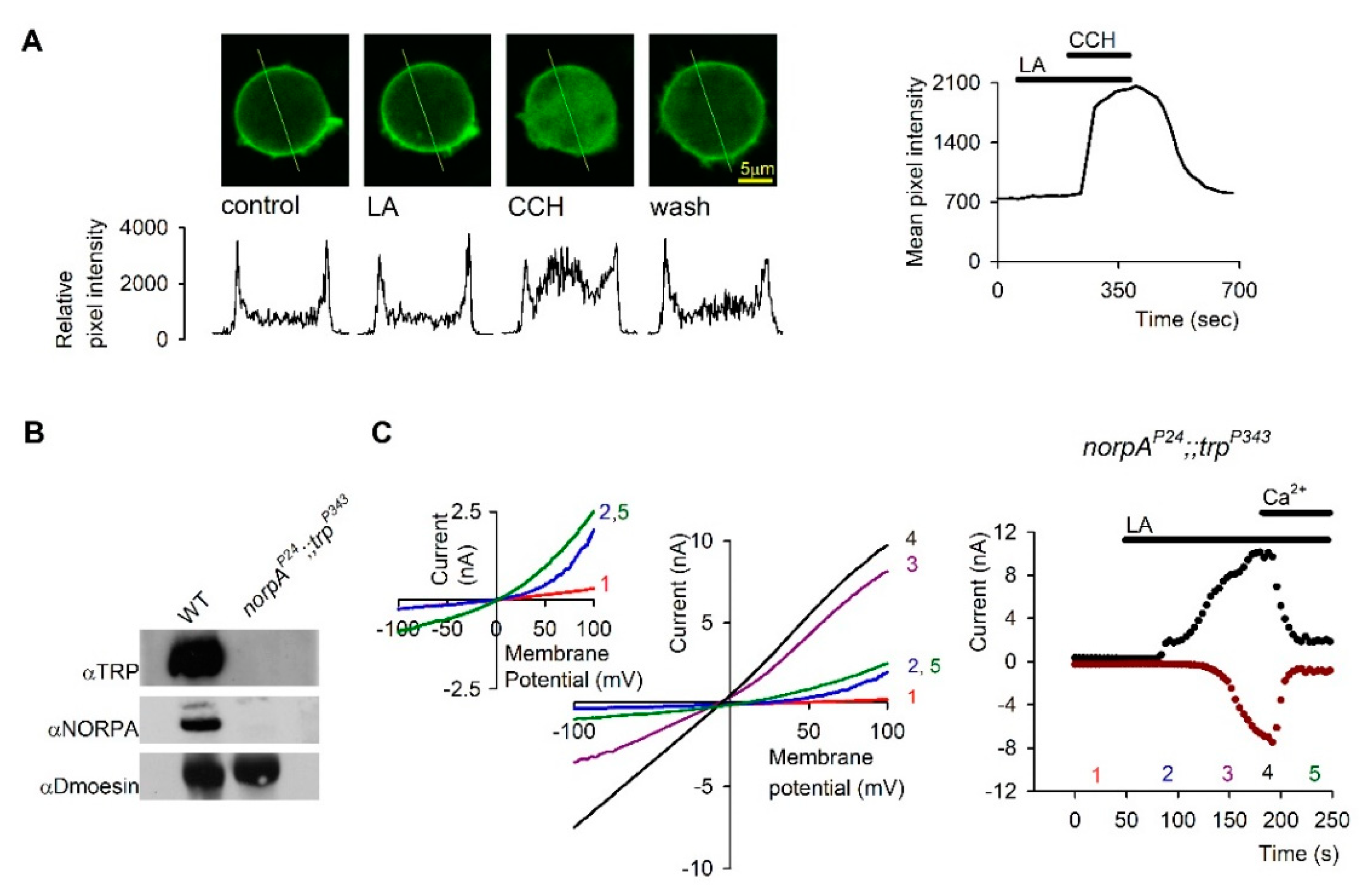
5. PUFAs Activation of TRP and TRPL Channels in the Dark
6. Photomechanical Gating of the TRP/TRPL Channels
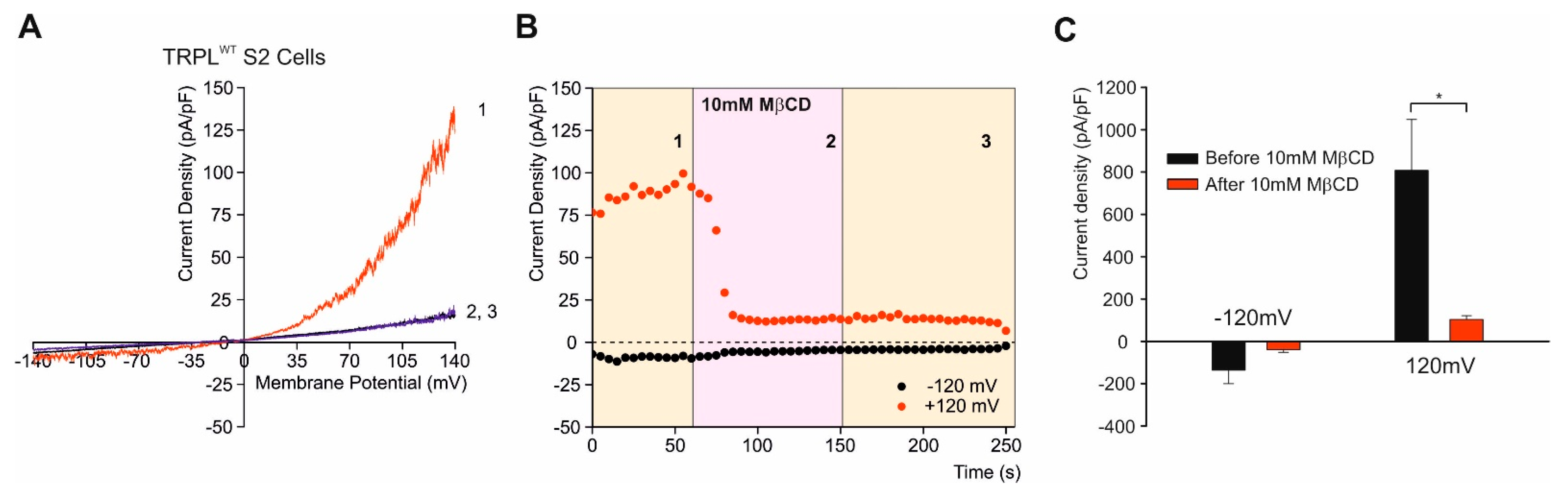
7. Lipid Rafts and Modulation of TRPL Channel Activity by Cholesterol
8. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Damann, N.; Voets, T.; Nilius, B. TRPs in our senses. Curr. Biol. 2008, 18, R880–R889. [Google Scholar] [CrossRef] [PubMed]
- Hardie, R.C.; Raghu, P. Visual transduction in Drosophila. Nature 2001, 413, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Katz, B.; Minke, B. Drosophila photoreceptors and signaling mechanisms. Front. Cell. Neurosci. 2009, 3, 2. [Google Scholar] [CrossRef]
- Kiselyov, K.; Patterson, R.L. The integrative function of TRPC channels. Front. Biosci. 2009, 14, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Minke, B.; Parnas, M. Insights on TRP channels from in vivo studies in Drosophila. Annu. Rev. Physiol. 2006, 68, 649–684. [Google Scholar] [CrossRef]
- Barbera, N.; Ayee, M.A.A.; Akpa, B.S.; Levitan, I. Molecular Dynamics Simulations of Kir2.2 Interactions with an Ensemble of Cholesterol Molecules. Biophys. J. 2018, 115, 1264–1280. [Google Scholar] [CrossRef]
- Yoshioka, T.; Inoue, H.; Hotta, Y. Defective phospholipid metabolism in the retinular cell membrane of norpA (no receptor potential) visual transduction mutants of Drosophila. Biochem. Biophys. Res. Commun. 1983, 111, 567–573. [Google Scholar] [CrossRef]
- Yoshioka, T.; Inoue, H.; Hotta, Y. Absence of phosphatidylinositol phosphodiesterase in the head of a Drosophila visual mutant, norpA (no receptor potential A). J. Biochem. Tokyo 1985, 97, 1251–1254. [Google Scholar] [CrossRef]
- Inoue, H.; Yoshioka, T.; Hotta, Y. Membrane-associated phospholipase C of Drosophila retina. J. Biochem. Tokyo 1988, 103, 91–94. [Google Scholar] [CrossRef]
- Devary, O.; Heichal, O.; Blumenfeld, A.; Cassel, D.; Suss, E.; Barash, S.; Rubinstein, C.T.; Minke, B.; Selinger, Z. Coupling of photoexcited rhodopsin to inositol phospholipid hydrolysis in fly photoreceptors. Proc. Natl. Acad. Sci. USA 1987, 84, 6939–6943. [Google Scholar] [CrossRef]
- Deland, M.C.; Pak, W.L. Reversibly temperature sensitive phototransduction mutant of Drosophila melanogaster. Nat. New Biol. 1973, 244, 184–186. [Google Scholar] [CrossRef]
- Selinger, Z.; Minke, B. Inositol lipid cascade of vision studied in mutant flies. Cold Spring Harb. Symp. Quant. Biol. 1988, 53 Pt 1, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Bloomquist, B.T.; Shortridge, R.D.; Schneuwly, S.; Perdew, M.; Montell, C.; Steller, H.; Rubin, G.; Pak, W.L. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell 1988, 54, 723–733. [Google Scholar] [CrossRef]
- Kohn, E.; Katz, B.; Yasin, B.; Peters, M.; Rhodes, E.; Zaguri, R.; Weiss, S.; Minke, B. Functional Cooperation between the IP3 Receptor and Phospholipase C Secures the High Sensitivity to Light of Drosophila Photoreceptors In Vivo. J. Neurosci. 2015, 35, 2530–2546. [Google Scholar] [CrossRef] [PubMed]
- Pearn, M.T.; Randall, L.L.; Shortridge, R.D.; Burg, M.G.; Pak, W.L. Molecular, biochemical, and electrophysiological characterization of Drosohpila norpA mutannts. J. Biol. Chem. 1996, 271, 4937–4945. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.; Bar, Y.M.; Cohen-Ben, A.H.; Goldstein, R.E.; Paroush, Z.; Selinger, Z.; Minke, B. Phospholipase C and termination of G-protein-mediated signalling in vivo. Nat. Cell Biol. 2000, 2, 296–301. [Google Scholar] [CrossRef]
- Waldo, G.L.; Ricks, T.K.; Hicks, S.N.; Cheever, M.L.; Kawano, T.; Tsuboi, K.; Wang, X.; Montell, C.; Kozasa, T.; Sondek, J.; et al. Kinetic Scaffolding Mediated by a Phospholipase C-{beta} and Gq Signaling Complex. Science 2010, 330, 974–980. [Google Scholar] [CrossRef]
- Yeandle, S.; Spiegler, J.B. Light-evoked and spontaneous discrete waves in the ventral nerve photoreceptor of Limulus. J. Gen. Physiol. 1973, 61, 552–571. [Google Scholar] [CrossRef]
- Rhee, S.G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 2001, 70, 281–312. [Google Scholar] [CrossRef]
- Essen, L.O.; Perisic, O.; Cheung, R.; Katan, M.; Williams, R.L. Crystal structure of a mammalian phosphoinositide-specific phospholipase C delta. Nature 1996, 380, 595–602. [Google Scholar] [CrossRef]
- Essen, L.O.; Perisic, O.; Katan, M.; Wu, Y.; Roberts, M.F.; Williams, R.L. Structural mapping of the catalytic mechanism for a mammalian phosphoinositide-specific phospholipase C. Biochemistry 1997, 36, 1704–1718. [Google Scholar] [CrossRef] [PubMed]
- Running Deer, J.L.; Hurley, J.B.; Yarfitz, S.L. G protein control of Drosophila photoreceptor phospholipase C. J. Biol. Chem. 1995, 270, 12623–12628. [Google Scholar] [CrossRef] [PubMed]
- Hardie, R.C. Inhibition of phospholipase C activity in Drosophila photoreceptors by 1,2-bis(2-aminophenoxy)ethane N,N,N′,N′-tetraacetic acid (BAPTA) and di-bromo BAPTA. Cell Calcium 2005, 38, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Minke, B.; Agam, K. TRP gating is linked to the metabolic state and maintenance of the Drosophila photoreceptor cells. Cell Calcium 2003, 33, 395–408. [Google Scholar] [CrossRef]
- Oberwinkler, J.; Stavenga, D.G. Calcium transients in the rhabdomeres of dark- and light-adapted fly photoreceptor cells. J. Neurosci. 2000, 20, 1701–1709. [Google Scholar] [CrossRef]
- Postma, M.; Oberwinkler, J.; Stavenga, D.G. Does Ca2+ reach millimolar concentrations after single photon absorption in Drosophila photoreceptor microvilli? Biophys. J. 1999, 77, 1811–1823. [Google Scholar] [CrossRef][Green Version]
- Gu, Y.; Oberwinkler, J.; Postma, M.; Hardie, R.C. Mechanisms of light adaptation in Drosophila photoreceptors. Curr. Biol. 2005, 15, 1228–1234. [Google Scholar] [CrossRef]
- Hardie, R.C.; Raghu, P.; Moore, S.; Juusola, M.; Baines, R.A.; Sweeney, S.T. Calcium influx via TRP channels is required to maintain PIP 2 levels in Drosophila photoreceptors. Neuron 2001, 30, 149–159. [Google Scholar] [CrossRef]
- Katz, B.; Minke, B. Phospholipase C-Mediated Suppression of Dark Noise Enables Single-Photon Detection in Drosophila Photoreceptors. J. Neurosci. 2012, 32, 2722–2733. [Google Scholar] [CrossRef]
- Cook, B.; Minke, B. TRP and calcium stores in Drosophila phototransduction. Cell Calcium 1999, 25, 161–171. [Google Scholar] [CrossRef]
- Hardie, R.C.; Minke, B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 1992, 8, 643–651. [Google Scholar] [CrossRef]
- Lu, C.; Vihtelic, T.S.; Hyde, D.R.; Li, T. A neuronal-specific mammalian homolog of the Drosophila retinal degeneration B gene with expression restricted to the retina and dentate gyrus. J. Neurosci. 1999, 19, 7317–7325. [Google Scholar] [CrossRef] [PubMed]
- Milligan, S.C.; Alb, J.G.; Elagina, R.B.; Bankaitis, V.A.; Hyde, D.R. The phosphatidyl inositol transfer protein domain of Drosophila retinal degeneration B protein is essential for photoreceptor cell survival and recovery from light stimulation. J. Cell Biol. 1997, 139, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Minke, B.; Selinger, Z. Inositol lipid pathway in fly photoreceptors: Excitation, calcium mobilization and retinal degeneration. In Progress in Retinal Research; Osborne, N.A., Chader, G.J., Eds.; Pergamon Press Oxford: Oxford, UK, 1991; pp. 99–124. [Google Scholar]
- Hardie, R.C. Calcium signalling: Setting store by calcium channels. Curr. Biol. 1996, 6, 1371–1373. [Google Scholar] [CrossRef]
- Zhang, I.; Hu, H. Store-Operated Calcium Channels in Physiological and Pathological States of the Nervous System. Front. Cell. Neurosci. 2020, 14, 600758. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W.B.; Heller, S. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Hardie, R.C. Excitation of Drosophila photoreceptors by BAPTA and ionomycin: Evidence for capacitative Ca2+ entry? Cell Calcium 1996, 20, 315–327. [Google Scholar] [CrossRef]
- Raghu, P.; Colley, N.J.; Webel, R.; James, T.; Hasan, G.; Danin, M.; Selinger, Z.; Hardie, R.C. Normal phototransduction in Drosophila photoreceptors lacking an InsP 3 receptor gene. Mol. Cell. Neurosci. 2000, 15, 429–445. [Google Scholar] [CrossRef]
- Acharya, J.K.; Jalink, K.; Hardy, R.W.; Hartenstein, V.; Zuker, C.S. InsP 3 receptor is essential for growth and differentiation but not for vision in Drosophila. Neuron 1997, 18, 881–887. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chen, Z.; Oliva, M.K.; Luo, J.; Collier, S.; Montell, C.; Hardie, R.C. Rapid Release of Ca2+ from Endoplasmic Reticulum Mediated by Na+/Ca2+ Exchange. J. Neurosci. 2020, 40, 12. [Google Scholar] [CrossRef]
- Hardie, R.C. Regulation of Drosophila TRP channels by lipid messengers. Novartis. Found. Symp. 2004, 258, 160–167. [Google Scholar]
- Katz, B.; Minke, B. The Drosophila light-activated TRP and TRPL channels—Targets of the phosphoinositide signaling cascade. Prog. Retin. Eye Res. 2018, 66, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, B.; Groschner, K. Reprint of "Mechanisms of lipid regulation and lipid gating in TRPC channels". Cell Calcium 2016, 60, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Stark, W.S.; Lin, T.N.; Brackhahn, D.; Christianson, J.S.; Sun, G.Y. Fatty acids in the lipids of Drosophila heads: Effects of visual mutants, carotenoid deprivation and dietary fatty acids. Lipids 1993, 28, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Stark, W.S.; Lin, T.N.; Brackhahn, D.; Christianson, J.S.; Sun, G.Y. Phospholipids in Drosophila heads: Effects of visual mutants and phototransduction manipulations. Lipids 1993, 28, 23–28. [Google Scholar] [CrossRef]
- Eroglu, C.; Brugger, B.; Wieland, F.; Sinning, I. Glutamate-binding affinity of Drosophila metabotropic glutamate receptor is modulated by association with lipid rafts. Proc. Natl. Acad. Sci. USA 2003, 100, 10219–10224. [Google Scholar] [CrossRef]
- Rietveld, A.; Neutz, S.; Simons, K.; Eaton, S. Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophila raft lipid microdomains. J. Biol. Chem. 1999, 274, 12049–12054. [Google Scholar] [CrossRef] [PubMed]
- Randall, A.S.; Liu, C.H.; Chu, B.; Zhang, Q.; Dongre, S.A.; Juusola, M.; Franze, K.; Wakelam, M.J.; Hardie, R.C. Speed and sensitivity of phototransduction in Drosophila depend on degree of saturation of membrane phospholipids. J. Neurosci. 2015, 35, 2731–2746. [Google Scholar] [CrossRef]
- Julius, D. From peppers to peppermints: Natural products as probes of the pain pathway. Harvey Lect. 2005, 101, 89–115. [Google Scholar]
- Berridge, M.J. Inositol trisphosphate and calcium signalling. Nature 1993, 361, 315–325. [Google Scholar] [CrossRef]
- Raghu, P.; Yadav, S.; Mallampati, N.B. Lipid signaling in Drosophila photoreceptors. Biochim. Biophys. Acta 2012, 1821, 1154–1165. [Google Scholar] [CrossRef]
- Masai, I.; Okazaki, A.; Hosoya, T.; Hotta, Y. Drosophila retinal degeneration A gene encodes an eye-specific diacylglycerol kinase with cysteine-rich zinc-finger motifs and ankyrin repeats. Proc. Natl. Acad. Sci. USA 1993, 90, 11157–11161. [Google Scholar] [CrossRef] [PubMed]
- Masai, I.; Suzuki, E.; Yoon, C.S.; Kohyama, A.; Hotta, Y. Immunolocalization of Drosophila eye-specific diacylgylcerol kinase, rdgA, which is essential for the maintenance of the photoreceptor. J. Neurobiol. 1997, 32, 695–706. [Google Scholar] [CrossRef]
- Wu, L.; Niemeyer, B.; Colley, N.; Socolich, M.; Zuker, C.S. Regulation of PLC-mediated signalling in vivo by CDP-diacylglycerol synthase. Nature 1995, 373, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Vihtelic, T.S.; Hyde, D.R.; O’Tousa, J.E. Isolation and characterization of the Drosophila retinal degeneration B (rdgB) gene. Genetics 1991, 127, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, S.; Raghu, P. Topological organisation of the phosphatidylinositol 4,5-bisphosphate-phospholipase C resynthesis cycle: PITPs bridge the ER-PM gap. Biochem. J. 2016, 473, 4289–4310. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, S.; Garner, K.; Yadav, S.; Gomez-Espinoza, E.; Raghu, P. RdgBα reciprocally transfers PA and PI at ER-PM contact sites to maintain PI(4,5)P2 homoeostasis during phospholipase C signalling in Drosophila photoreceptors. Biochem. Soc. Trans. 2016, 44, 286–292. [Google Scholar] [CrossRef]
- Raghu, P.; Basak, B.; Krishnan, H. Emerging perspectives on multidomain phosphatidylinositol transfer proteins. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158984. [Google Scholar] [CrossRef]
- Garcia-Murillas, I.; Pettitt, T.; Macdonald, E.; Okkenhaug, H.; Georgiev, P.; Trivedi, D.; Hassan, B.; Wakelam, M.; Raghu, P. lazaro encodes a lipid phosphate phosphohydrolase that regulates phosphatidylinositol turnover during Drosophila phototransduction. Neuron 2006, 49, 533–546. [Google Scholar] [CrossRef]
- Kwon, Y.; Montell, C. Dependence on the Lazaro phosphatidic acid phosphatase for the maximum light response. Curr. Biol. 2006, 16, 723–729. [Google Scholar] [CrossRef]
- LaLonde, M.M.; Janssens, H.; Rosenbaum, E.; Choi, S.Y.; Gergen, J.P.; Colley, N.J.; Stark, W.S.; Frohman, M.A. Regulation of phototransduction responsiveness and retinal degeneration by a phospholipase D-generated signaling lipid. J. Cell Biol. 2005, 169, 471–479. [Google Scholar] [CrossRef]
- Thakur, R.; Panda, A.; Coessens, E.; Raj, N.; Yadav, S.; Balakrishnan, S.; Zhang, Q.; Georgiev, P.; Basak, B.; Pasricha, R.; et al. Phospholipase D activity couples plasma membrane endocytosis with retromer dependent recycling. Elife 2016, 5, e18515. [Google Scholar] [CrossRef] [PubMed]
- Raghu, P.; Usher, K.; Jonas, S.; Chyb, S.; Polyanovsky, A.; Hardie, R.C. Constitutive activity of the light-sensitive channels TRP and TRPL in the Drosophila diacylglycerol kinase mutant, rdgA. Neuron 2000, 26, 169–179. [Google Scholar] [CrossRef]
- Hardie, R.C. Regulation of trp channels via lipid second messengers. Annu. Rev. Physiol 2003, 65, 735–759. [Google Scholar] [CrossRef] [PubMed]
- Storch, U.; Mederos Y Schnitzler, M.; Gudermann, T. A greasy business: Identification of a diacylglycerol binding site in human TRPC5 channels by cryo-EM. Cell Calcium 2021, 97, 102414. [Google Scholar] [CrossRef]
- Song, K.; Wei, M.; Guo, W.; Quan, L.; Kang, Y.; Wu, J.X.; Chen, L. Structural basis for human TRPC5 channel inhibition by two distinct inhibitors. Elife 2021, 10, e63429. [Google Scholar] [CrossRef]
- Agam, K.; von-Campenhausen, M.; Levy, S.; Ben-Ami, H.C.; Cook, B.; Kirschfeld, K.; Minke, B. Metabolic stress reversibly activates the Drosophila light-sensitive channels TRP and TRPL in vivo. J. Neurosci. 2000, 20, 5748–5755. [Google Scholar] [CrossRef] [PubMed]
- Hardie, R.C.; Martin, F.; Chyb, S.; Raghu, P. Rescue of light responses in the Drosophila “null1” phospholipase C mutant, norpAP24 by diacylglycerol kinase mutant, rdgA and by metabolic inhibition. J. Biol. Chem. 2003, 278, 18851–18858. [Google Scholar] [CrossRef]
- Hardie, R.C.; Gu, Y.; Martin, F.; Sweeney, S.T.; Raghu, P. In vivo light-induced and basal phospholipase C activity in Drosophila photoreceptors measured with genetically targeted phosphatidylinositol 4,5-bisphosphate-sensitive ion channels (Kir2.1). J. Biol. Chem. 2004, 279, 47773–47782. [Google Scholar] [CrossRef]
- Hardie, R.C.; Minke, B. Spontaneous activation of light-sensitive channels in Drosophila photoreceptors. J. Gen. Physiol. 1994, 103, 389–407. [Google Scholar] [CrossRef]
- Chu, B.; Liu, C.H.; Sengupta, S.; Gupta, A.; Raghu, P.; Hardie, R.C. Common mechanisms regulating dark noise and quantum bump amplification in Drosophila photoreceptors. J. Neurophysiol. 2013, 109, 2044–2055. [Google Scholar] [CrossRef][Green Version]
- Lev, S.; Katz, B.; Tzarfaty, V.; Minke, B. Signal-dependent hydrolysis of phosphatidylinositol 4,5-bisphosphate without activation of phospholipase C: Implications on gating of Drosophila TRPL (transient receptor potential-like) channel. J. Biol. Chem. 2012, 287, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.; Muñoz, Y.; Peña-Cortés, H.; Giavalisco, P.; Bacigalupo, J. Diacylglycerol activates the light-dependent channel TRP in the photosensitive microvilli of Drosophila melanogaster photoreceptors. J. Neurosci. 2014, 34, 6679–6686. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.; Bacigalupo, J. Unitary recordings of TRP and TRPL channels from isolated Drosophila retinal photoreceptor rhabdomeres: Activation by light and lipids. J. Neurophysiol. 2009, 101, 2372–2379. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.; Delgado, M.G.; Bastin-Héline, L.; Glavic, A.; O’Day, P.M.; Bacigalupo, J. Light-Induced Opening of the TRP Channel in Isolated Membrane Patches Excised from Photosensitive Microvilli from Drosophila Photoreceptors. Neuroscience 2019, 396, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Chyb, S.; Raghu, P.; Hardie, R.C. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature 1999, 397, 255–259. [Google Scholar] [CrossRef]
- Parnas, M.; Katz, B.; Lev, S.; Tzarfaty, V.; Dadon, D.; Gordon-Shaag, A.; Metzner, H.; Yaka, R.; Minke, B. Membrane lipid modulations remove divalent open channel block from TRP-like and NMDA channels. J. Neurosci. 2009, 29, 2371–2383. [Google Scholar] [CrossRef]
- Minke, B.; Cook, B. TRP channel proteins and signal transduction. Physiol. Rev. 2002, 82, 429–472. [Google Scholar] [CrossRef]
- Xu, X.Z.S.; Li, H.S.; Guggino, W.B.; Montell, C. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell 1997, 89, 1155–1164. [Google Scholar] [CrossRef]
- Vaca, L.; Sinkins, W.G.; Hu, Y.; Kunze, D.L.; Schilling, W.P. Activation of recombinant trp by thapsigargin in Sf9 insect cells. Am. J. Physiol. 1994, 267, C1501–C1505. [Google Scholar] [CrossRef]
- Hu, Y.; Vaca, L.; Zhu, X.; Birnbaumer, L.; Kunze, D.L.; Schilling, W.P. Appearance of a novel Ca2+ influx pathway in Sf9 insect cells following expression of the transient receptor potential-like (trpl) protein of Drosophila. Biochem. Biophys. Res. Commun. 1994, 201, 1050–1056. [Google Scholar] [CrossRef]
- Yagodin, S.; Hardie, R.C.; Lansdell, S.J.; Millar, N.S.; Mason, W.T.; Sattelle, D.B. Thapsigargin and receptor-mediated activation of Drosophila TRPL channels stably expressed in a Drosophila S2 cell line. Cell Calcium 1998, 23, 219–228. [Google Scholar] [CrossRef]
- Hardie, R.C.; Reuss, H.; Lansdell, S.J.; Millar, N.S. Functional equivalence of native light-sensitive channels in the Drosophila trp 301 mutant and TRPL cation channels expressed in a stably transfected Drosophila cell line. Cell Calcium 1997, 21, 431–440. [Google Scholar] [CrossRef]
- Parnas, M.; Katz, B.; Minke, B. Open channel block by Ca2+ underlies the voltage dependence of Drosophila TRPL channel. J. Gen. Physiol. 2007, 129, 17–28. [Google Scholar] [CrossRef]
- Estacion, M.; Sinkins, W.G.; Schilling, W.P. Regulation of Drosophila transient receptor potential-like (TrpL) channels by phospholipase C-dependent mechanisms. J. Physiol. 2001, 530, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Harteneck, C.; Obukhov, A.G.; Zobel, A.; Kalkbrenner, F.; Schultz, G. The Drosophila cation channel trpl expressed in insect Sf9 cells is stimulated by agonists of G-protein-coupled receptors. FEBS Lett. 1995, 358, 297–300. [Google Scholar] [CrossRef]
- Hambrecht, J.; Zimmer, S.; Flockerzi, V.; Cavalie, A. Single-channel currents through transient-receptor-potential-like (TRPL) channels. Pflugers Arch. 2000, 440, 418–426. [Google Scholar] [CrossRef]
- Lev, S.; Katz, B.; Minke, B. The activity of the TRP-like channel depends on its expression system. Channels (Austin) 2012, 6, 86–93. [Google Scholar] [CrossRef]
- Grimm, C.; Kraft, R.; Sauerbruch, S.; Schultz, G.; Harteneck, C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J. Biol. Chem. 2003, 278, 21493–21501. [Google Scholar] [CrossRef]
- Leung, H.T.; Tseng-Crank, J.; Kim, E.; Mahapatra, C.; Shino, S.; Zhou, Y.; An, L.; Doerge, R.W.; Pak, W.L. DAG lipase activity is necessary for TRP channel regulation in Drosophila photoreceptors. Neuron 2008, 58, 884–896. [Google Scholar] [CrossRef]
- Lundbaek, J.A.; Collingwood, S.A.; Ingólfsson, H.I.; Kapoor, R.; Andersen, O.S. Lipid bilayer regulation of membrane protein function: Gramicidin channels as molecular force probes. J. R. Soc. Interface 2010, 7, 373–395. [Google Scholar] [CrossRef]
- Hamill, O.P. Twenty odd years of stretch-sensitive channels. Pflugers Arch. 2006, 453, 333–351. [Google Scholar] [CrossRef]
- Spassova, M.A.; Hewavitharana, T.; Xu, W.; Soboloff, J.; Gill, D.L. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc. Natl. Acad. Sci. USA 2006, 103, 16586–16591. [Google Scholar] [CrossRef] [PubMed]
- Hardie, R.C.; Franze, K. Photomechanical responses in Drosophila photoreceptors. Science 2012, 338, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Morenilla-Palao, C.; Pertusa, M.; Meseguer, V.; Cabedo, H.; Viana, F. Lipid raft segregation modulates TRPM8 channel activity. J. Biol. Chem. 2009, 284, 9215–9224. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, Y.; Deng, X.L.; Sun, H.Y.; Li, G.R. Cholesterol down-regulates BK channels stably expressed in HEK 293 cells. PLoS ONE 2013, 8, e79952. [Google Scholar] [CrossRef]
- Romanenko, V.G.; Fang, Y.; Byfield, F.; Travis, A.J.; Vandenberg, C.A.; Rothblat, G.H.; Levitan, I. Cholesterol sensitivity and lipid raft targeting of Kir2.1 channels. Biophys. J. 2004, 87, 3850–3861. [Google Scholar] [CrossRef]
- Shieh, B.H.; Niemeyer, B. A novel protein encoded by the InaD gene regulates recovery of visual transduction in Drosophila. Neuron 1995, 14, 201–210. [Google Scholar] [CrossRef]
- Tsunoda, S.; Sierralta, J.; Sun, Y.; Bodner, R.; Suzuki, E.; Becker, A.; Socolich, M.; Zuker, C.S. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature 1997, 388, 243–249. [Google Scholar] [CrossRef]
- Sanxaridis, P.D.; Cronin, M.A.; Rawat, S.S.; Waro, G.; Acharya, U.; Tsunoda, S. Light-induced recruitment of INAD-signaling complexes to detergent-resistant lipid rafts in Drosophila photoreceptors. Mol. Cell. Neurosci. 2007, 36, 36–46. [Google Scholar] [CrossRef][Green Version]
- Clark, A.J.; Block, K. The absence of sterol synthesis in insects. J. Biol. Chem. 1959, 234, 2578–2582. [Google Scholar] [CrossRef]
- Bos, M.; Burnet, B.; Farrow, R.; Woods, R.A. Development of Drosophila on sterol mutants of the yeast Saccharomyces cerevisiae. Genet. Res. 1976, 28, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.E.; Woodruff, E.A.; Liang, P.; Patten, M.; Broadie, K. Neuronal loss of Drosophila NPC1a causes cholesterol aggregation and age-progressive neurodegeneration. J. Neurosci. 2008, 28, 6569–6582. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, Y.; Irie, T.; Uekama, K.; Fukunaga, K.; Pitha, J. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur. J. Biochem. 1989, 186, 17–22. [Google Scholar] [CrossRef]
- Levitan, I.; Christian, A.E.; Tulenko, T.N.; Rothblat, G.H. Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells. J. Gen. Physiol. 2000, 115, 405–416. [Google Scholar] [CrossRef]
- Christian, A.E.; Haynes, M.P.; Phillips, M.C.; Rothblat, G.H. Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 1997, 38, 2264–2272. [Google Scholar] [CrossRef]
- Kilsdonk, E.P.; Yancey, P.G.; Stoudt, G.W.; Bangerter, F.W.; Johnson, W.J.; Phillips, M.C.; Rothblat, G.H. Cellular cholesterol efflux mediated by cyclodextrins. J. Biol. Chem. 1995, 270, 17250–17256. [Google Scholar] [CrossRef]
- Matthews, D.A.; Bolin, J.T.; Burridge, J.M.; Filman, D.J.; Volz, K.W.; Kaufman, B.T.; Beddell, C.R.; Champness, J.N.; Stammers, D.K.; Kraut, J. Refined crystal structures of Escherichia coli and chicken liver dihydrofolate reductase containing bound trimethoprim. J. Biol. Chem. 1985, 260, 381–391. [Google Scholar] [CrossRef]
- Niu, S.L.; Mitchell, D.C.; Litman, B.J. Manipulation of cholesterol levels in rod disk membranes by methyl-beta-cyclodextrin: Effects on receptor activation. J. Biol. Chem. 2002, 277, 20139–20145. [Google Scholar] [CrossRef]
- Kabouridis, P.S.; Janzen, J.; Magee, A.L.; Ley, S.C. Cholesterol depletion disrupts lipid rafts and modulates the activity of multiple signaling pathways in T lymphocytes. Eur. J. Immunol. 2000, 30, 954–963. [Google Scholar] [CrossRef]
- Predescu, S.A.; Predescu, D.N.; Shimizu, K.; Klein, I.K.; Malik, A.B. Cholesterol-dependent syntaxin-4 and SNAP-23 clustering regulates caveolar fusion with the endothelial plasma membrane. J. Biol. Chem. 2005, 280, 37130–37138. [Google Scholar] [CrossRef]
- Scheiffele, P.; Roth, M.G.; Simons, K. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 1997, 16, 5501–5508. [Google Scholar] [CrossRef]
- Harder, T.; Scheiffele, P.; Verkade, P.; Simons, K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 1998, 141, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Rouquette-Jazdanian, A.K.; Pelassy, C.; Breittmayer, J.P.; Aussel, C. Revaluation of the role of cholesterol in stabilizing rafts implicated in T cell receptor signaling. Cell Signal. 2006, 18, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Gaus, K.; Rodriguez, M.; Ruberu, K.R.; Gelissen, I.; Sloane, T.M.; Kritharides, L.; Jessup, W. Domain-specific lipid distribution in macrophage plasma membranes. J. Lipid Res. 2005, 46, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Ottico, E.; Prinetti, A.; Prioni, S.; Giannotta, C.; Basso, L.; Chigorno, V.; Sonnino, S. Dynamics of membrane lipid domains in neuronal cells differentiated in culture. J. Lipid Res. 2003, 44, 2142–2151. [Google Scholar] [CrossRef]
- Tikku, S.; Epshtein, Y.; Collins, H.; Travis, A.J.; Rothblat, G.H.; Levitan, I. Relationship between Kir2.1/Kir2.3 activity and their distributions between cholesterol-rich and cholesterol-poor membrane domains. Am. J. Physiol. Cell Physiol. 2007, 293, C440–C450. [Google Scholar] [CrossRef]
- Peters, M.; Katz, B.; Lev, S.; Zaguri, R.; Gutorov, R.; Minke, B. Depletion of Membrane Cholesterol Suppresses Drosophila Transient Receptor Potential-Like (TRPL) Channel Activity. Curr. Top. Membr. 2017, 80, 233–254. [Google Scholar] [CrossRef]
- Yancey, P.G.; Rodrigueza, W.V.; Kilsdonk, E.P.; Stoudt, G.W.; Johnson, W.J.; Phillips, M.C.; Rothblat, G.H. Cellular cholesterol efflux mediated by cyclodextrins. Demonstration Of kinetic pools and mechanism of efflux. J. Biol. Chem. 1996, 271, 16026–16034. [Google Scholar] [CrossRef]
- Picazo-Juárez, G.; Romero-Suárez, S.; Nieto-Posadas, A.; Llorente, I.; Jara-Oseguera, A.; Briggs, M.; McIntosh, T.J.; Simon, S.A.; Ladrón-de-Guevara, E.; Islas, L.D.; et al. Identification of a binding motif in the S5 helix that confers cholesterol sensitivity to the TRPV1 ion channel. J. Biol. Chem. 2011, 286, 24966–24976. [Google Scholar] [CrossRef] [PubMed]
- Balajthy, A.; Hajdu, P.; Panyi, G.; Varga, Z. Sterol Regulation of Voltage-Gated K. Curr. Top. Membr. 2017, 80, 255–292. [Google Scholar] [CrossRef] [PubMed]
- Lichtenegger, M.; Tiapko, O.; Svobodova, B.; Stockner, T.; Glasnov, T.N.; Schreibmayer, W.; Platzer, D.; de la Cruz, G.G.; Krenn, S.; Schober, R.; et al. An optically controlled probe identifies lipid-gating fenestrations within the TRPC3 channel. Nat. Chem. Biol. 2018, 14, 396–404. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutorov, R.; Katz, B.; Rhodes-Mordov, E.; Zaguri, R.; Brandwine-Shemmer, T.; Minke, B. The Role of Membrane Lipids in Light-Activation of Drosophila TRP Channels. Biomolecules 2022, 12, 382. https://doi.org/10.3390/biom12030382
Gutorov R, Katz B, Rhodes-Mordov E, Zaguri R, Brandwine-Shemmer T, Minke B. The Role of Membrane Lipids in Light-Activation of Drosophila TRP Channels. Biomolecules. 2022; 12(3):382. https://doi.org/10.3390/biom12030382
Chicago/Turabian StyleGutorov, Rita, Ben Katz, Elisheva Rhodes-Mordov, Rachel Zaguri, Tal Brandwine-Shemmer, and Baruch Minke. 2022. "The Role of Membrane Lipids in Light-Activation of Drosophila TRP Channels" Biomolecules 12, no. 3: 382. https://doi.org/10.3390/biom12030382
APA StyleGutorov, R., Katz, B., Rhodes-Mordov, E., Zaguri, R., Brandwine-Shemmer, T., & Minke, B. (2022). The Role of Membrane Lipids in Light-Activation of Drosophila TRP Channels. Biomolecules, 12(3), 382. https://doi.org/10.3390/biom12030382




