Pheromone Receptor Knock-Out Affects Pheromone Detection and Brain Structure in a Moth
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Rearing, Generation of SlitOR5 Mutants
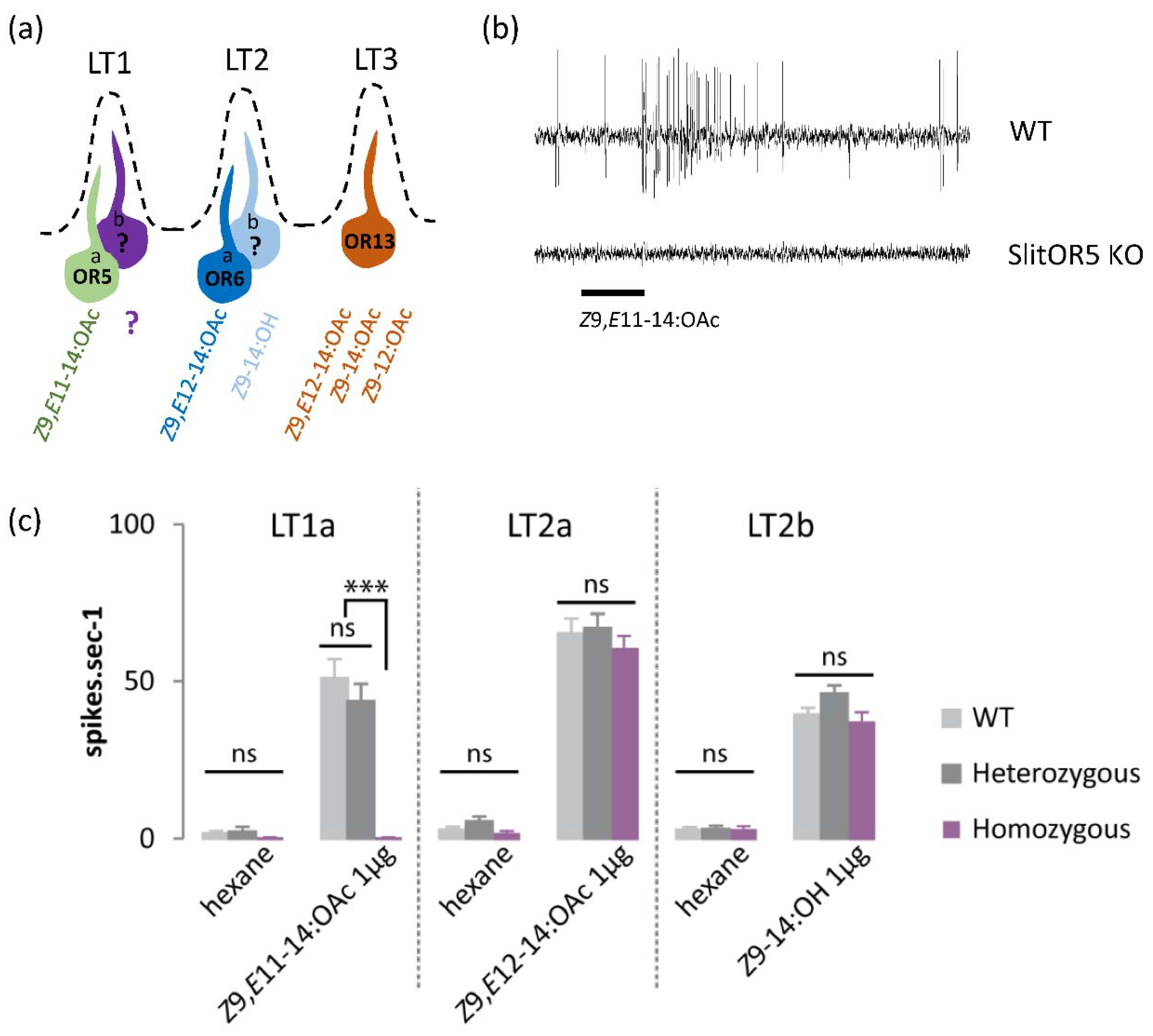
2.2. Single-Sensillum Recordings
2.3. Brain Dissection and Immunostaining
2.4. Confocal Microscopy
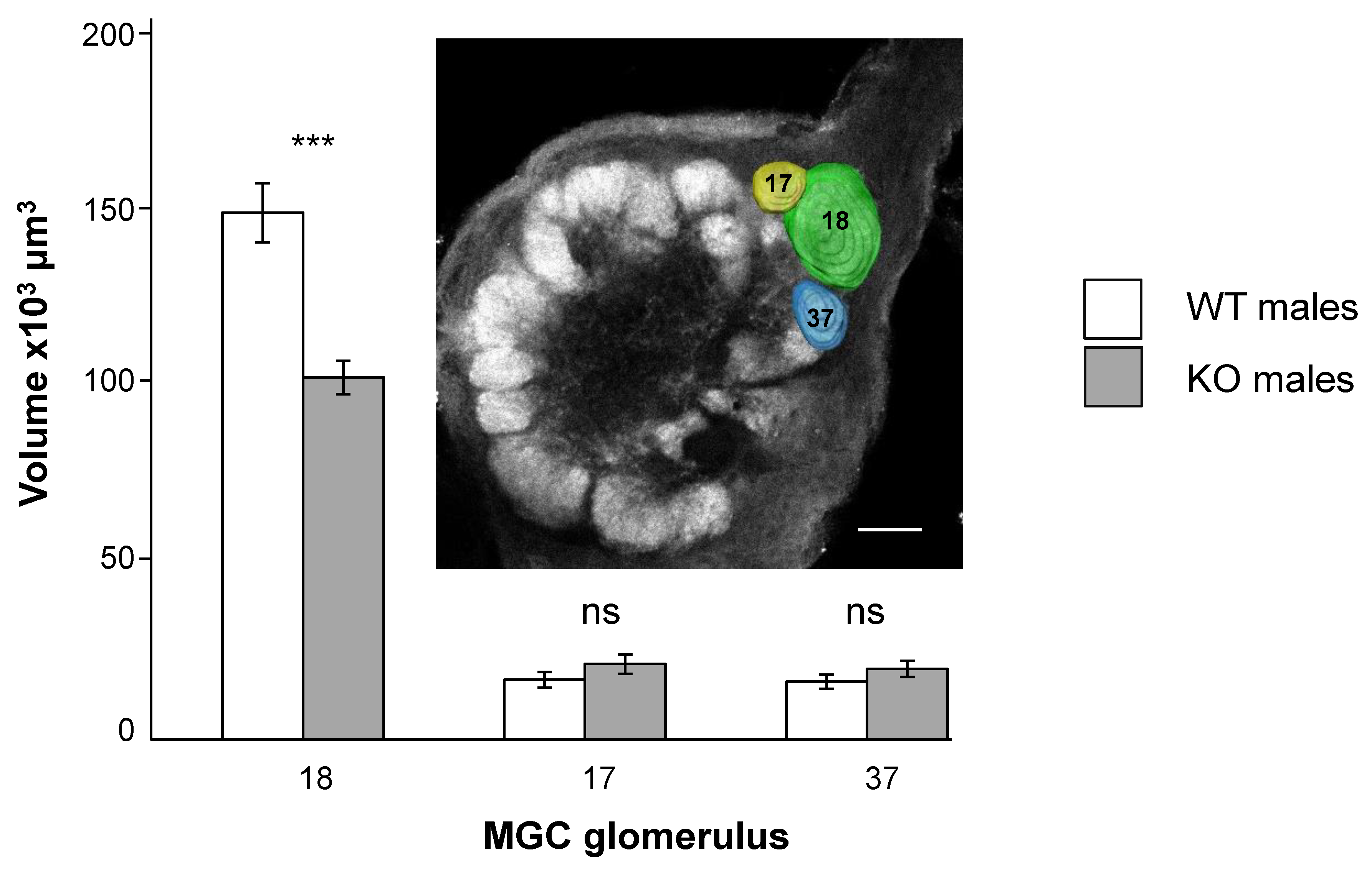
2.5. Reconstructions and Volume Measurements
2.6. Statistical Analysis
3. Results
3.1. SlitOR5 Knock-Out Specifically Abolishes the Responses of LT1a Neurons to Z9,E11-14:OAc
3.2. SlitOR5 Knock-Out Modifies the Size of the Cumulus in the Macroglomerular Complex
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wyatt, T.D. Pheromones and Animal Behavior: Chemical Signals and Signatures, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Jacquin-Joly, E.; Groot, A.T. Pheromones, Insects. In Encyclopedia of Reproduction; Skinner, M.K., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 6, pp. 465–471. [Google Scholar]
- Koutroumpa, F.A.; Jacquin-Joly, E. Sex in the night: Fatty acid-derived sex pheromones and corresponding membrane pheromone receptors in insects. Biochimie 2014, 107, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kaissling, K.E. Pheromone Reception in Insects: The Example of Silk Moths. In Neurobiology of Chemical Communication; Mucignat-Caretta, C., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2014. [Google Scholar]
- Fleischer, J.; Krieger, J. Insect Pheromone Receptors—Key Elements in Sensing Intraspecific Chemical Signals. Front. Cell. Neurosci. 2018, 12, 425. [Google Scholar] [CrossRef] [PubMed]
- Montagné, M.; Wanner, K.W.; Jacquin-Joly, E. Olfactory genomics within the Lepidoptera. In Insect Pheromone Biochemistry and Molecular Biology, 2nd, ed.; Blomquist, G.J., Vogt, R.G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 469–505. [Google Scholar]
- Larsson, M.C.; Domingos, A.I.; Jones, W.D.; Chiappe, M.E.; Amrein, H.; Vosshall, L.B. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 2004, 43, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Sakurai, T.; Nishioka, T.; Touhara, K. Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science 2005, 307, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Pellegrino, M.; Nakagawa, T.; Vosshall, L.B.; Touhara, K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 2008, 452, 1002–1006. [Google Scholar] [CrossRef]
- Wicher, D.; Schafer, R.; Bauernfeind, R.; Stensmyr, M.C.; Heller, R.; Heinemann, S.H.; Hansson, B.S. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 2008, 452, 1007–1011. [Google Scholar] [CrossRef]
- Hansson, B.S. Antennal lobe projection patterns of pheromone-specific olfactory receptor neurons in moths. In Insect Pheromone Research: New Directions; Cardé, R.T., Minks, A.K., Eds.; Springer: Boston, MA, USA, 1997; pp. 164–183. [Google Scholar]
- Rospars, J.-P. Structure and development of the insect antennodeutocerebral system. Int. J. Insect Morphol. Embryol. 1988, 17, 243–294. [Google Scholar] [CrossRef]
- Anton, S.; Rossler, W. Plasticity and modulation of olfactory circuits in insects. Cell Tissue Res. 2021, 383, 149–164. [Google Scholar] [CrossRef]
- Guerrieri, F.; Gemeno, C.; Monsempes, C.; Anton, S.; Jacquin-Joly, E.; Lucas, P.; Devaud, J.M. Experience-dependent modulation of antennal sensitivity and input to antennal lobes in male moths (Spodoptera littoralis) pre-exposed to sex pheromone. J. Exp. Biol. 2012, 215, 2334–2341. [Google Scholar] [CrossRef]
- Mansourian, S.; Fandino, R.A.; Riabinina, O. Progress in the use of genetic methods to study insect behavior outside Drosophila. Curr. Opin. Insect Sci. 2019, 36, 45–56. [Google Scholar] [CrossRef]
- Li, J.J.; Shi, Y.; Wu, J.N.; Li, H.; Smagghe, G.; Liu, T.X. CRISPR/Cas9 in lepidopteran insects: Progress, application and prospects. J. Insect Physiol. 2021, 135, 104325. [Google Scholar] [CrossRef] [PubMed]
- Koutroumpa, F.A.; Monsempes, C.; Francois, M.C.; de Cian, A.; Royer, C.; Concordet, J.P.; Jacquin-Joly, E. Heritable genome editing with CRISPR/Cas9 induces anosmia in a crop pest moth. Sci. Rep. 2016, 6, 29620. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, W.; Zeng, B.; Wang, G.; Hao, D.; Huang, Y. Deletion of the Bombyx mori odorant receptor co-receptor (BmOrco) impairs olfactory sensitivity in silkworms. Insect Biochem. Mol. Biol. 2017, 86, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Fandino, R.A.; Haverkamp, A.; Bisch-Knaden, S.; Zhang, J.; Bucks, S.; Nguyen, T.A.T.; Schroder, K.; Werckenthin, A.; Rybak, J.; Stengl, M.; et al. Mutagenesis of odorant coreceptor Orco fully disrupts foraging but not oviposition behaviors in the hawkmoth Manduca sexta. Proc. Natl. Acad. Sci. USA 2019, 116, 15677–15685. [Google Scholar] [CrossRef]
- Trible, W.; Olivos-Cisneros, L.; McKenzie, S.K.; Saragosti, J.; Chang, N.C.; Matthews, B.J.; Oxley, P.R.; Kronauer, D.J.C. orco Mutagenesis Causes Loss of Antennal Lobe Glomeruli and Impaired Social Behavior in Ants. Cell 2017, 170, 727–735.e710. [Google Scholar] [CrossRef]
- Yan, H.; Opachaloemphan, C.; Mancini, G.; Yang, H.; Gallitto, M.; Mlejnek, J.; Leibholz, A.; Haight, K.; Ghaninia, M.; Huo, L.; et al. An Engineered orco Mutation Produces Aberrant Social Behavior and Defective Neural Development in Ants. Cell 2017, 170, 736–747.e739. [Google Scholar] [CrossRef]
- Chen, Z.; Traniello, I.M.; Rana, S.; Cash-Ahmed, A.C.; Sankey, A.L.; Yang, C.; Robinson, G.E. Neurodevelopmental and transcriptomic effects of CRISPR/Cas9-induced somatic orco mutation in honey bees. J. Neurogenet. 2021, 35, 320–332. [Google Scholar] [CrossRef]
- Chiang, A.; Priya, R.; Ramaswami, M.; Vijayraghavan, K.; Rodrigues, V. Neuronal activity and Wnt signaling act through Gsk3-beta to regulate axonal integrity in mature Drosophila olfactory sensory neurons. Development 2009, 136, 1273–1282. [Google Scholar] [CrossRef]
- Sakurai, T.; Mitsuno, H.; Haupt, S.S.; Uchino, K.; Yokohari, F.; Nishioka, T.; Kobayashi, I.; Sezutsu, H.; Tamura, T.; Kanzaki, R. A single sex pheromone receptor determines chemical response specificity of sexual behavior in the silkmoth Bombyx mori. PLoS Genet. 2011, 7, e1002115. [Google Scholar] [CrossRef]
- Chang, H.; Liu, Y.; Ai, D.; Jiang, X.; Dong, S.; Wang, G. A Pheromone Antagonist Regulates Optimal Mating Time in the Moth Helicoverpa armigera. Curr. Biol. 2017, 27, 1610–1615.e1613. [Google Scholar] [CrossRef]
- Bastin-Heline, L.; de Fouchier, A.; Cao, S.; Koutroumpa, F.; Caballero-Vidal, G.; Robakiewicz, S.; Monsempes, C.; Francois, M.C.; Ribeyre, T.; Maria, A.; et al. A novel lineage of candidate pheromone receptors for sex communication in moths. Elife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Poitout, S.; Buès, R. Elevage de chenilles de vingt-huit espèces de Lépidoptères Noctuidae et de deux espèces d’Arctiidae sur milieu artificiel simple. Particularités de l’élevage selon les espèces. Ann. Zool. Ecol. Anim. 1974, 6, 431–441. [Google Scholar]
- Ljungberg, H.; Anderson, P.; Hansson, B.S. Physiology and morphology of pheromone-specific sensilla on the antennae of male and female Spodoptera littoralis (Lepidoptera: Noctuidae). J. Insect Physiol. 1993, 39, 253–260. [Google Scholar] [CrossRef]
- Quero, C.; Lucas, P.; Renou, M.; Guerrero, A. Behavioral responses of Spodoptera littoralis males to sex pheromone components and virgin females in wind tunnel. J. Chem. Ecol. 1996, 22, 1087–1102. [Google Scholar] [CrossRef] [PubMed]
- de Fouchier, A.; Sun, X.; Monsempes, C.; Mirabeau, O.; Jacquin-Joly, E.; Montagné, M. Evolution of two receptors detecting the same pheromone compound in crop pest moths of the genus Spodoptera. Front. Ecol. Evol. 2015, 3, 95. [Google Scholar] [CrossRef]
- Anton, S.; Chabaud, M.A.; Schmidt-Busser, D.; Gadenne, B.; Iqbal, J.; Juchaux, M.; List, O.; Gaertner, C.; Devaud, J.M. Brief sensory experience differentially affects the volume of olfactory brain centres in a moth. Cell Tissue Res. 2016, 364, 59–65. [Google Scholar] [CrossRef]
- Couton, L.; Minoli, S.; Kieu, K.; Anton, S.; Rospars, J.P. Constancy and variability of identified glomeruli in antennal lobes: Computational approach in Spodoptera littoralis. Cell Tissue Res. 2009, 337, 491–511. [Google Scholar] [CrossRef]
- Ochieng, S.A.; Anderson, P.; Hansson, B.S. Antennal lobe projection patterns of olfactory receptor neurons involved in sex pheromone detection in Spodoptera littoralis (Lepidoptera: Noctuidae). Tissue Cell 1995, 27, 221–232. [Google Scholar] [CrossRef]
- Carlsson, M.A.; Galizia, C.G.; Hansson, B.S. Spatial representation of odours in the antennal lobe of the moth Spodoptera littoralis (Lepidoptera: Noctuidae). Chem. Senses 2002, 27, 231–244. [Google Scholar] [CrossRef][Green Version]
- Krieger, J.; Grosse-Wilde, E.; Gohl, T.; Dewer, Y.M.; Raming, K.; Breer, H. Genes encoding candidate pheromone receptors in a moth (Heliothis virescens). Proc. Natl. Acad. Sci. USA 2004, 101, 11845–11850. [Google Scholar] [CrossRef]
- Sakurai, T.; Nakagawa, T.; Mitsuno, H.; Mori, H.; Endo, Y.; Tanoue, S.; Yasukochi, Y.; Touhara, K.; Nishioka, T. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. Proc. Natl. Acad. Sci. USA 2004, 101, 16653–16658. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.; Grosse-Wilde, E.; Gohl, T.; Breer, H. Candidate pheromone receptors of the silkmoth Bombyx mori. Eur. J. Neurosci. 2005, 21, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Cao, S.; Zhang, Z.; Kong, X.; Liu, F.; Wang, G.; Zhang, S. Evolution of sex pheromone receptors in Dendrolimus punctatus Walker (lepidoptera: Lasiocampidae) is divergent from other moth species. Insect Biochem. Mol. Biol. 2020, 122, 103375. [Google Scholar] [CrossRef]
- Yuvaraj, J.K.; Jordan, M.D.; Zhang, D.D.; Andersson, M.N.; Lofstedt, C.; Newcomb, R.D.; Corcoran, J.A. Sex pheromone receptors of the light brown apple moth, Epiphyas postvittana, support a second major pheromone receptor clade within the Lepidoptera. Insect Biochem. Mol. Biol. 2022, 141, 103708. [Google Scholar] [CrossRef] [PubMed]
- Trebels, B.; Dippel, S.; Goetz, B.; Graebner, M.; Hofmann, C.; Hofmann, F.; Schmid, F.R.; Uhl, M.; Vuong, M.P.; Weber, V.; et al. Metamorphic development of the olfactory system in the red flour beetle (Tribolium castaneum, HERBST). BMC Biol. 2021, 19, 155. [Google Scholar] [CrossRef] [PubMed]
- Devaud, J.M.; Acebes, A.; Ferrus, A. Odor exposure causes central adaptation and morphological changes in selected olfactory glomeruli in Drosophila. J. Neurosci. 2001, 21, 6274–6282. [Google Scholar] [CrossRef]
- Karpati, Z.; Dekker, T.; Hansson, B.S. Reversed functional topology in the antennal lobe of the male European corn borer. J. Exp. Biol. 2008, 211, 2841–2848. [Google Scholar] [CrossRef]
- Sakurai, T.; Mitsuno, H.; Mikami, A.; Uchino, K.; Tabuchi, M.; Zhang, F.; Sezutsu, H.; Kanzaki, R. Targeted disruption of a single sex pheromone receptor gene completely abolishes in vivo pheromone response in the silkmoth. Sci. Rep. 2015, 5, 11001. [Google Scholar] [CrossRef]
- Maguire, S.E.; Afify, A.; Goff, L.A.; Potter, C.J. A Feedback Mechanism Regulates Odorant Receptor Expression in the Malaria Mosquito, Anopheles gambiae. bioRxiv 2020. [Google Scholar] [CrossRef]
- Dobritsa, A.A.; van der Goes van Naters, W.; Warr, C.G.; Steinbrecht, R.A.; Carlson, J.R. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron 2003, 37, 827–841. [Google Scholar] [CrossRef]
- Kurtovic, A.; Widmer, A.; Dickson, B.J. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 2007, 446, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Jefferis, G.S.; Vyas, R.M.; Berdnik, D.; Ramaekers, A.; Stocker, R.F.; Tanaka, N.K.; Ito, K.; Luo, L. Developmental origin of wiring specificity in the olfactory system of Drosophila. Development 2004, 131, 117–130. [Google Scholar] [CrossRef]
- Li, H.; Horns, F.; Wu, B.; Xie, Q.; Li, J.; Li, T.; Luginbuhl, D.J.; Quake, S.R.; Luo, L. Classifying Drosophila Olfactory Projection Neuron Subtypes by Single-Cell RNA Sequencing. Cell 2017, 171, 1206–1220.e1222. [Google Scholar] [CrossRef] [PubMed]
- Task, D.; Lin, C.-C.; Vulpe, A.; Afify, A.; Ballou, S.; Brbić, M.; Schlegel, P.; Jefferis, G.S.X.E.; Li, H.; Menuz, K.; et al. Chemoreceptor Co-Expression in Drosophila Olfactory Neurons. bioRxiv 2021. [Google Scholar] [CrossRef]
- Younger, M.A.; Herre, M.; Goldman, O.V.; Lu, T.-C.; Caballero-Vidal, G.; Qi, Y.; Gilbert, Z.N.; Gong, Z.; Morita, T.; Rahiel, S.; et al. Non-Canonical Odor Coding in the Mosquito. bioRxiv 2022. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koutroumpa, F.; Monsempès, C.; Anton, S.; François, M.-C.; Montagné, N.; Jacquin-Joly, E. Pheromone Receptor Knock-Out Affects Pheromone Detection and Brain Structure in a Moth. Biomolecules 2022, 12, 341. https://doi.org/10.3390/biom12030341
Koutroumpa F, Monsempès C, Anton S, François M-C, Montagné N, Jacquin-Joly E. Pheromone Receptor Knock-Out Affects Pheromone Detection and Brain Structure in a Moth. Biomolecules. 2022; 12(3):341. https://doi.org/10.3390/biom12030341
Chicago/Turabian StyleKoutroumpa, Fotini, Christelle Monsempès, Sylvia Anton, Marie-Christine François, Nicolas Montagné, and Emmanuelle Jacquin-Joly. 2022. "Pheromone Receptor Knock-Out Affects Pheromone Detection and Brain Structure in a Moth" Biomolecules 12, no. 3: 341. https://doi.org/10.3390/biom12030341
APA StyleKoutroumpa, F., Monsempès, C., Anton, S., François, M.-C., Montagné, N., & Jacquin-Joly, E. (2022). Pheromone Receptor Knock-Out Affects Pheromone Detection and Brain Structure in a Moth. Biomolecules, 12(3), 341. https://doi.org/10.3390/biom12030341







