Abstract
Progress in developing disease-modifying therapies in Parkinson’s disease (PD) can only be achieved through reliable objective markers that help to identify subjects at risk. This includes an early and accurate diagnosis as well as continuous monitoring of disease progression and therapy response. Although PD diagnosis still relies mainly on clinical features, encouragingly, advances in biomarker discovery have been made. The cerebrospinal fluid (CSF) is a biofluid of particular interest to study biomarkers since it is closest to the brain structures and therefore could serve as an ideal source to reflect ongoing pathologic processes. According to the key pathophysiological mechanisms, the CSF status of α-synuclein species, markers of amyloid and tau pathology, neurofilament light chain, lysosomal enzymes and markers of neuroinflammation provide promising preliminary results as candidate biomarkers. Untargeted approaches in the field of metabolomics provide insights into novel and interconnected biological pathways. Markers based on genetic forms of PD can contribute to identifying subgroups suitable for gene-targeted treatment strategies that might also be transferable to sporadic PD. Further validation analyses in large PD cohort studies will identify the CSF biomarker or biomarker combinations with the best value for clinical and research purposes.
1. Introduction
As one of the most common neurodegenerative disorders, Parkinson’s disease (PD) is also the fastest growing neurological disorder with regard to age-standardized rates of prevalence, disability and deaths [1]. Despite the relentless efforts and progress in unraveling the pathophysiological mechanisms of PD, a breakthrough in disease-modifying therapies is still lacking. The current PD diagnostic criteria mainly rely on the core motor symptoms—bradykinesia, rigidity and rest tremor. Even though these criteria are correctly applied, the rate of misdiagnosis is still up to 20% due to clinical overlap with parkinsonism of other etiologies [2]. Major differential diagnoses of PD include atypical parkinsonian syndromes (APSs) that share similar motor features of bradykinesia and rigor but are neuropathologically distinct disease entities. As with PD, multiple system atrophy (MSA) and dementia with Lewy bodies (DLB) belong to the spectrum of synucleinopathies, whereas progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS) represent tauopathies. By the time of the first motor signs, at least 50% of nigral dopaminergic neurons are already lost [3], confounding the prospect to substantially alter the disease course. The asymptomatic preclinical and prodromal phases arising with the first non-motor and subtle motor signs provide an optimal window of therapeutic opportunity [4]. The possibility that early onset and progression of neurodegeneration could be accompanied by molecular changes measurable even before clinical onset constitutes the driving force of the biomarker search. Objective and reliable biomarkers are urgently needed, firstly, that identify PD in pre-motor stages and indicate susceptibility to the disease. Secondly, biomarkers should support the clinical diagnosis and define disease subtype and severity. Thirdly, biomarkers should reliably track disease progression and serve as meaningful endpoints for clinical trials to testify the impact on disease modification of an intervention.
Cerebrospinal fluid (CSF) represents the preferred source for biomarker discovery because of its direct contact with the extracellular space in the brain where an unrestricted bidirectional flow of molecules takes place between these compartments, secluded from the systemic circulation by the blood–brain barrier. However, only 20% of CSF proteins are brain-derived, while 80% are derived from filtration of the peripheral blood [5]. Nevertheless, compared to other peripheral fluids, these 20% brain-derived components have the greatest potential to truly reflect the state of the brain under pathological conditions. CSF is obtained by lumbar puncture, a procedure which is feasible in PD research participants, with a manageable rate of headache and lower back pain as adverse events [6]. Monitoring of disease progression and treatment response could be more challenging if repeated lumbar punctures would be required given the relative invasiveness of the procedure. Recent developments in CSF biomarker research in PD will be summarized in the following. Here, focus is directed to markers that reflect the pathophysiological mechanisms involved in PD including genetic aspects and examples of untargeted biomarker discovery approaches, regarding their usefulness and limitations (Figure 1).
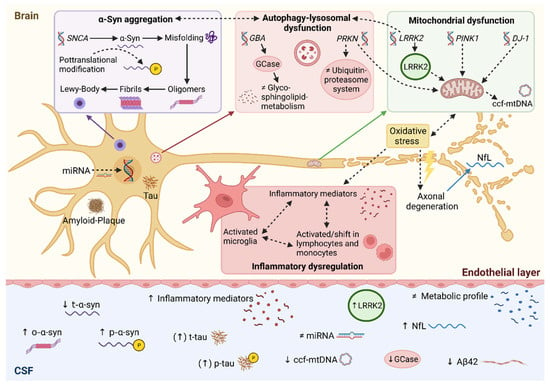
Figure 1.
Synopsis of CSF biomarkers under investigation in Parkinson’s disease. Pathophysiological links between autophagy-lysosomal disruption, mitochondrial dysfunction and neuroinflammation leading to α-synuclein accumulation. Accordingly, molecular changes can be detected in CSF serving as candidate biomarkers in Parkinson’s disease. Solid lines represent direct relations, whereas dotted lines represent multi-step processes. ↑ increased; ↓ decreased; ≠ unaltered; Aβ42, amyloid beta peptide 1-42; α-syn, α-synuclein; ccf-mtDNA, circulating cell-free mitochondrial DNA; miRNA, microRNA; NfL, neurofilament light chain; o-α-syn, oligomeric α-synuclein; p-α-syn, phosphorylated α-synuclein; t-α-syn, total α-synuclein; p-tau, phosphorylated tau; t-tau, total tau protein. Created with BioRender.com (accessed on 8 January 2022).
2. Alpha-Synuclein
Abnormal deposition of α-synuclein (α-syn) in the form of Lewy bodies represents the major neuropathological feature in PD associated with dopaminergic cell death. Numerous factors including genetic predisposition and post-translational modifications are considered to promote misfolding and aggregation of α-syn, leading to the subsequent formation of oligomers, amyloid-like fibrils and Lewy bodies [7].
Based on the central role of α-syn in PD pathogenesis, great attention has been paid to α-syn levels in CSF as a promising biomarker. Notably, the majority of studies consistently report lower CSF levels of total α-syn (t-α-syn) as compared to healthy controls [8,9,10], in contrast to the inconclusive findings in peripheral blood [11,12,13]. However, CSF t-α-syn levels vary greatly among studies, likely due to clinical heterogeneity and methodological differences that could compromise diagnostic accuracy. Indeed, a pooled sensitivity of 78–88% and a specificity of 40–57% for t-α-syn in CSF are still unsatisfactory to sufficiently discriminate PD from controls [14]. Differential diagnosis cannot be supported by t-α-syn in CSF alone, since MSA and DLB, and even tauopathies such as PSP and CBS, also show reduced levels [15,16]. The significance of CSF α-syn in Alzheimer’s disease (AD) is still unclear despite Lewy body co-pathology in AD. Correlations of the CSF α-syn level with AD markers have been reported [17,18]. Since Lewy bodies have also been found in neurologically asymptomatic elderly individuals [19], it would be important to target disease-specific forms of α-syn. Longitudinal observations of t-α-syn dynamics in CSF revealed discrepant findings showing an increase in t-α-syn throughout the disease course [20,21], but also a decrease without correlation with disease progression [22]. Divergence of α-syn concentrations in CSF could additionally result from the fact that α-syn is deposited in Lewy bodies but also released from degenerating synapses [23].
Apart from the total level of α-syn, other species of α-syn can be measured in CSF. Post-translationally modified phosphorylated α-syn (p-α-syn) levels in CSF were increased in PD patients compared to controls [24] and decreased during the disease course [20], indicating disease progression. Interestingly, it has been shown that p-α-syn could be detected by ultrasensitive immunoassays only in plasma, but not in CSF [25]. This finding underlines the instability of post-translationally modified species and the possible influence of matrix effects leading to interference between proteins or other constituents present in the CSF and the assay used for detection. For improving the diagnostic utility, CSF levels of oligomeric α-syn (o-α-syn) and the ratio of o-α-syn/t-α-syn have been investigated and found to be elevated in PD patients [20,26,27]. CSF levels of o-α-syn showed a longitudinal increase, and the change in o-α-syn/t-α-syn showed an association with motor deterioration, particularly in the postural-instability and gait-difficulty dominant PD subgroup [20].
In recent years, two ultrasensitive protein amplification assays, Protein Misfolding Cyclic Amplification (PMCA) and the Real-Time Quaking-Induced Conversion (RT-QuIC), have been introduced to detect aggregated and misfolded α-syn in CSF, yielding high diagnostic accuracy (AUC 0.93 for PMCA and 0.89 for RT-QuIC) in distinguishing PD from controls [28]. A high sensitivity (95.3%) and specificity (98%) of α-syn seeding activity were demonstrated by applying RT-QuIC to accurately discriminate synucleinopathies [29]. Excellent separation of MSA from PD/DLB subjects could be achieved by PMCA-based o-α-syn analysis [29,30]. Interestingly, the PMCA method could also detect specific α-syn strains forming different conformational aggregates that could reliably distinguish between MSA and PD [31]. Further efforts are needed to confirm the ability of different α-syn species or conformational states to validate the clinical value in terms of precise PD diagnostics. The robustness of immunoassays or aggregation assays relies on the quality of standardized antigens for quantification. In addition, blood contamination constitutes a considerable challenge for accurate quantification of the α-syn level in CSF [32].
3. Amyloid-Beta and Tau Protein
Besides the PD-defining synuclein pathology, other age-related neurodegenerative pathologies can coexist in PD brains including amyloid plaques and tau-containing neurofibrillary tangles that are classical features of AD [33,34,35]. In AD, the diagnostic usefulness of CSF biomarkers has already been proven, which provides a great impetus to implement CSF biomarkers in other neurodegenerative disorders such as PD. Amyloid-β (Aβ) and tau protein can interact with α-syn, thus promoting their mutual accumulation that contributes to the accelerated cognitive decline in PD [36]. Analogous to AD, several studies reported that a lower CSF level of amyloid-beta1-42 (Aβ42) at baseline compared to controls could predict cognitive impairment possibly reflecting amyloid pathology [27,37,38,39]. The reduction in the CSF Aβ42 level in LBD, AD and PD with dementia (PDD) tends to be more pronounced compared to PD, but differential diagnosis remains difficult solely based on Aβ42 levels and requires the combination of other markers [40,41]. In the Parkinson’s Progression Markers Initiative (PPMI) cohort, early PD subjects were followed for up to three years [42]. Aβ42 in CSF showed a greater decrease during the disease course, and a lower Aβ42 level at baseline predicted a modest decline not only in cognitive but also in autonomic and motor functions in early PD. Interestingly, a low baseline CSF Aβ42 level was also able to predict the progression of dopa-resistant gait impairments in PD [43]. By normalizing the Aβ42 concentration to amyloid-beta1-40 (Aβ40), the most abundant form of Aβ peptides, the Aβ42/Ab40 ratio can correct for interindividual differences and should hence be included in future PD investigations.
Although total tau (t-tau) and phosphorylated tau (p-tau) are implicated in tau pathology and cognitive dysfunction in AD, the role of CSF tau species in PD has not been clarified yet. There are mixed results showing reduced [44], similar [45] and increased CSF levels of t-tau and p-tau, particularly in PD with manifest dementia [46]. With regard to differential diagnosis of MSA, higher t-tau levels have been observed in MSA compared to PD [47,48]. The increase in t-tau and p-tau in CSF could mirror unspecific events of neuronal damage caused by stroke or viral encephalitis and is even higher in patients with Creutzfeld–Jakob disease [49]. However, even for tauopathies other than AD (frontotemporal dementia (FTD) and PSP), CSF tau concentrations are not significantly different from healthy controls [50]. Lower secretion of tau proteins in the extracellular space for these tauopathies and alternative disease-specific tau processing that could escape detection from available assays have been discussed. Therefore, the question whether an increase in tau levels in APS could reflect a more rapid progression compared to PD needs to be further elucidated. At least in combination with Aβ42, increased t-tau and p-tau levels in CSF allowed the prediction of subsequent decline in cognitive tasks involving both memory and executive functions [51].
4. Neurofilament Light Chain
Neurofilament light chain (NfL) is a subunit of neurofilaments that is exclusively expressed in neurons. It is the main structural component of large, myelinated axons. Following axonal damage and membrane disruption, neuronal signals are interrupted and NfL is released in the interstitial space [52]. As a general marker of axonal injury, NfL has been extensively studied in the field of neurodegeneration. Higher NfL levels have been reported in the CSF of PSP and MSA patients in comparison to PD patients, consistent with the more aggressive neurodegeneration in these disease entities [53,54]. A comprehensive meta-analysis exhibited no differences in the mean CSF NfL values of PD and PDD/DLB patients, but increased levels in MSA, PSP and CBS patients [55]. NfL levels in CSF were investigated in a longitudinal cohort of de novo PD patients (DeNoPa), demonstrating the highest levels in other neurodegenerative diseases including MSA and DLB compared to PD and the lowest levels in controls [56]. Higher NfL levels in CSF could perfectly separate MSA patients from controls, and higher cut-off values enabled excellent discrimination of MSA from PD and DLB (97% sensitivity, 90% specificity) since NfL was not elevated in the CSF of most PD and DLB cases [30]. A recent study demonstrated the highest CSF NfL level in PD subjects with cognitive impairment and a moderately elevated level in PD subjects with normal cognitive function compared to the control group [57]. Although CSF NfL correlated with motor and cognitive impairment, the conversion to cognitive impairment could not be predicted by the baseline NfL level. Moreover, it has been shown that NfL correlates with age, and lacking age-specific reference values could hamper the accurate distinction of PD from elderly controls [57,58]. These results underline the potential of NfL as a candidate for a biomarker panel in combination with other markers, but there is also great overlap across the different studies and disease groups and a critical correlation with the aging of the patients.
5. Lysosomal Biomarkers
The autophagy–lysosomal pathway is a key route for the intracellular degradation of proteins. A disturbed autophagy–lysosomal system can cause a reduced degradation of α-syn, thus enhancing α-syn accumulation in PD [59]. This hypothesis is strengthened by the identification of mutations in the GBA gene, encoding the lysosomal enzyme glucocerebrosidase (GCase), as the most common genetic risk factor for PD [60,61]. It can be assumed that reduced GCase activity elevates the α-syn level by stabilizing toxic soluble oligomeric α-syn which, in turn, leads to decreased lysosomal activity in a bidirectional pathogenic loop [62]. Not only GBA but also an excessive burden of 54 other lysosomal storage disorder gene variants has been linked to PD [63]. Following this pathophysiological pathway, markers of lysosomal metabolism have been investigated in CSF as candidate biomarkers for PD.
GCase is an enzyme that hydrolyzes glucosylceramide (GlcCer) and glucosylsphingosine (GlcSph) into glucose and ceramide or sphingosine, respectively [64]. CSF GCase activity has been shown to be significantly reduced in PD as well as in DLB compared to controls [65,66,67,68,69,70]. In PD patients carrying a GBA mutation (GBA-PD), CSF GCase activity was significantly lower compared to sporadic PD (sPD), whereas GBA-PD with severe mutations showed the lowest enzyme activity, indicating an accelerated pathological condition in GBA mutations [71]. However, other studies reported a reduced GCase activity in the CSF of PD patients irrespective of GBA mutations [67,68]. This could be partially explained by the fact that aging also leads to a progressive decline in CGase activity [72]. Activities of other lysosomal hydrolases, such as β-hexosaminidase and β-galactosidase, were also found reduced in the CSF of GBA-PD and sPD compared to controls [67,70]. Regarding downstream metabolites, a significant elevation in ceramide species has been reported in PD and other Lewy body spectrum disorders independent of GBA mutation [64,71]. Kurzawa-Akanbi and colleagues postulated an upregulation of ceramides in response to cell stress [64]. Ceramides were found heavily loaded in extracellular vesicles together with neurodegeneration-linked proteins including α-syn and tau, possibly mediating α-syn aggregation by interaction of these molecules. A moderate diagnostic accuracy of lysosomal enzymes could only be yielded in combination with other markers. A combination of GCase activtity, the o-/t-α-syn ratio and age showed the best performance in discriminating PD from controls independent of GBA mutation status (sensitivity of 82% and specificity of 71%) [68]. The diagnostic accuracy could be further improved by using a combined lysosomal enzyme profile (β-glucocerebrosidase, cathepsin D and β-hexosaminidase) [67].
Some studies even suggest a prognostic value of lysosomal markers. In the longitudinal observation of the PPMI cohort including GBA-PD and sPD, the glucosylceramide fraction was increased, whereas the sphingomyelin fraction was reduced in the CSF of GBA-PD patients compared to controls [73]. However, a higher ratio of glucosylceramide to sphingomyelin significantly associated with an accelerated cognitive decline in sPD compared to the sPD subjects with a lower ratio. Importantly, this finding indicates that genetically derived findings could be transferred to sPD and used for stratification in clinical trials. Another study reported that reduced lysosomal enzymes GCase and cathepsin D significantly associated with more advanced motor stages in Hoehn and Yahr (H&Y), while lower cathepsin D and β-hexosaminidase activities significantly associated with worse cognitive performance [67].
In conclusion, lysosomal biomarkers are promising since lysosomal metabolism appears to be crucially involved in the pathophysiology of PD and significant alterations could be measured. While lysosomal progression markers need further investigation, the diagnostic accuracy can be enhanced by considering PD-related pathologies in combination, such as CSF levels of α-syn or AD core biomarkers [67,68]. Therefore, the potential lysosomal biomarkers for diagnosis should be included in a robust biomarker panel covering the different pathological pathways of PD.
6. Inflammatory Biomarkers
Neuroinflammation plays a major role in the pathology of PD, since α-syn triggers the activation of the innate and adaptive immune systems [74]. Humoral and cellular components of the immune system have been investigated in CSF as candidate biomarkers for PD. Phenotyping of CSF immune cells by multiparameter flow cytometry in PD patients revealed a shift in cell proportions from classical monocytes (CD14+/CD16−) to non-classical monocytes (CD14+/CD16+) in PD patients compared to controls, which was not the case in the peripheral blood [75]. In PD patients, the fraction of activated T lymphocytes was found to be increased, whereas the absolute numbers of these cell populations were not significantly altered [75]. Studies on the humoral inflammatory profile highlight two promising inflammatory markers, namely, monocyte chemoattractant protein-1 (MCP-1) and YKL-40 (chitinase-3-like protein 1). MCP-1 is a chemokine that is involved in the recruitment of monocytes and spreading of inflammation. Elevated MCP-1 levels were detected in the CSF of PD [75] as well as MSA patients in comparison to controls [76]. On the contrary, other studies reported comparable MCP-1 levels in the CSF among PD, MSA and controls [77,78,79,80,81,82]. These discrepancies might be explained by clinical heterogeneity, diagnostic uncertainties and different assays used. Despite the diagnostic insufficiency, a positive correlation was found with motor progression (H&Y) [77,78] and even the severity of depression as a non-motor symptom [82]. YKL-40 is a glycoprotein primarily expressed in microglia and astrocytes. There are inconsistent results on the trend of CSF levels and differentiating biomarker potential among PD, APS and controls [76,81,83,84,85,86]. However, an increase in YKL-40 in CSF over time in PD correlated significantly with faster cognitive decline [21]. Further studies support its potential to predict cognition biomarkers in AD [81] and PD [83].
Elevated CSF levels of C-reactive protein (CRP), an acute phase protein and a commonly applied inflammatory marker, showed a significant correlation with the severity of motor (H&Y, UPDRS III) and non-motor (cognition, depression and fatigue) symptoms in PD and APS [82,84]. Further studies detected significantly higher CRP levels in PDD and MSA compared with non-demented PD and controls [82,84], supporting its predictive value for cognitive decline [87]. Several other potential inflammatory markers have been suggested including TNF-α [75,88,89,90], fractalkine [91,92], MIP1α (CCL3) [92], IL-1β [89], IL-2 and -6 [75,84,89], IL-8 [84], TGF-β1 and IFN-γ [89,93,94,95]. Each marker on its own can only cover a restricted biological domain and fails to reach statistical significance. Accordingly, combining different markers into robust large panels can lead to higher diagnostic sensitivity [78,88,89,90,93]. For instance, CRP and a cytokine set (TNF-α, IL-1β, IFN-γ) were able to distinguish PD from MSA [89]. The p-tau/α-syn ratio combined with TNF-α could separate PD patients from controls (AUC > 0.9) [90]. Therefore, inflammatory biomarkers should be included in comprehensive panels as they may particularly reflect motor and non-motor PD progression, particularly in more aggressive PD forms [94].
7. Metabolomics
A non-hypothesis-driven approach for the discovery of new biomarkers can be achieved by untargeted metabolomics [96]. Metabolomics has attracted attention in recent years, revealing multiple novel metabolic pathways linked to the pathogenesis of PD. Several studies demonstrated altered metabolic profiles in PD, PD subgroups, APS and other neurological conditions [97,98,99,100,101,102,103]. By utilizing machine learning algorithms, 14 CSF metabolites were identified that enabled distinguishing early PD from controls with high accuracy [104]. These metabolites indicated alterations of the amino acid metabolism in PD, although the exact mechanisms associated with PD need to be clarified. Another cohort with early PD patients (DeNoPa) reported a significant decrease in dehydroascorbic acid CSF levels and a significant increase in fructose, mannose and threonic acid CSF levels—molecules that are involved in the antioxidative stress response, glycation and inflammation—compared to controls [105]. Moreover, a link between altered metabolic profiles and the tricarboxylic acid cycle has been observed, which are implicated in mitochondrial dysfunction and increased oxidative stress [106]. By using liquid chromatography–tandem mass spectrometry, a recently published study detected increased CSF levels of intermediates of the proline metabolic pathway in PD and APS subjects that could discriminate them from controls but not among parkinsonian syndromes [97].
Concerning PD medication-related sequelae, dysregulations in bile acid biosynthesis and glycosphingolipid/glycerophospholipid metabolism were able to distinguish PD patients suffering levodopa-induced dyskinesia (LID) from PD without LID and controls, pointing towards a further association between dysregulated lipid metabolism and neuroinflammation [98]. Intriguingly, an altered glycosphingolipid metabolic pathway was strongly associated with the severity of dyskinetic movements. In terms of PD progression markers, CSF concentrations of the main dopamine metabolite and end product of dopamine catabolism, homovanillate, showed only a slight change over time and a weak correlation with worsening of disease severity, lacking sufficiency to reflect disease progression [107]. To sum up, these findings illustrate the complexity of the multiple metabolic pathways involved in the pathogenesis of PD. Further studies are needed to better understand the pathophysiological context and validate these promising metabolites.
8. Genetic Perspective
Even though only a minority of PD cases (5–10%) are caused by monogenic mutations, understanding the genetic basis has provided fundamental insights into PD pathogenesis and led to the development of gene-targeted treatment strategies [108,109]. Genetic PD includes autosomal-dominant (SNCA, LRRK2) and autosomal-recessive forms (Parkin, PINK1, DJ-1) and the most common genetic risk factor GBA. A genetic trait is considered stable and indicates the predisposition to develop a disease, albeit with incomplete and variable penetrance [110]. In addition to the gene mutation itself, gene products at the transcriptional and post-transcriptional levels in CSF could serve as markers that possibly reflect the pathophysiological processes underlying PD.
The biomarker potential of α-syn (encoded by SNCA) and GCase (encoded by GBA) has been extensively discussed in the previous sections. Although SNCA mutations are very rare, the finding of α-syn containing Lewy bodies establishes a pivotal link between genetic and sporadic forms of PD [111]. GBA mutations represent a risk factor for PD with a reduced penetrance, and about 9.1% of GBA mutation carriers will develop PD [112]. Ambroxol is a chaperone for GCase and discussed as a novel neuroprotective agent. In acellular CSF, the inhibitory effect of ambroxol can be measured by decreased GCase activity, whereas in tissue, CGase activity is supposed to increase, and target engagement can be determined by upregulation of CSF GCase protein levels [113]. Among LRRK2 mutations, G2019S is the most frequent variant, causing monogenic PD with age- and population-dependent incomplete penetrance [114,115]. LRRK2 encodes a multifunctional protein including a kinase domain and exerts its physiological role in cytoskeletal maintenance, mitochondrial function and autophagy [116]. Since most pathogenic variants lead to increased kinase activity, pharmacological LRRK2 inhibition has been proposed as a counteractive therapeutic strategy. LRRK2 was first detected in exosomes purified from CSF [117]. A more reliable parameter to quantify LRRK2 kinase activity is the measurement of the level of autophosphorylation at pS1292 [118]. Compared to pS1292-LRRK2 levels in urinary exosomes, CSF levels were much higher (about 5-fold) but failed to discriminate LRRK2 mutation carriers/PD patients from non-carriers/controls, possibly due to saturation effects. By using an improved LRRK2 monoclonal antibody technique, absolute quantification of the LRRK2 protein has revealed elevated CSF LRRK2 levels in G2019S-PD compared to sPD and non-manifesting G2019 carriers [119]. Autosomal-recessive forms of PD with typical early onset are most frequently linked to mutations in Parkin, followed by PINK1 and very rarely DJ-1 [108]. These genes participate in mitochondrial quality control, disruption of which is thought to significantly contribute to PD pathogenesis [120,121]. PINK1 is a mitochondrial kinase that phosphorylates Parkin, an E3 ubiquitin ligase, to eliminate damaged mitochondria. DJ-1 is also involved in mitochondrial regulation and antioxidative stress mechanisms. In response to oxidative stress, circulating cell-free mitochondrial DNA (ccf-mtDNA) is released from cells. Paradoxically, a lower ccf-mtDNA level was reported in the CSF of PD patients, which could be explained by shutting down energy production prior to cell death [122]. The CSF ccf-mtDNA level could be influenced by medication as demonstrated by the inverse correlation with treatment [123]. Results regarding DJ-1 are inconclusive since increased and decreased CSF levels have been reported in PD [124]. Therefore, it remains disputable whether DJ-1 in CSF is able to differentiate PD from APS or controls [47,125].
MicroRNAs (miRNAs) are small noncoding RNAs that are involved in the post-transcriptional regulation of gene expression through inhibition of translation and degradation of target mRNAs [126]. They can be detected cell-free or within extracellular vesicles, particularly exosomes, and remain stable in various body fluids such as CSF [127]. Distinct miRNA signatures have been found in the CSF of early [128] as well as more advanced PD stages [129,130,131], differentiating them from controls and APS [130,132]. However, since standardized methodological and normalization approaches are still missing, inconsistencies between miRNA studies and lack of reproducibility hamper miRNAs from becoming more widely used as biomarkers [133].
9. Conclusions
The research of CSF biomarkers has deepened our understanding of the biological and molecular processes occurring in the brain. Table 1 summarizes candidate CSF biomarkers for PD under current investigation. In view of the pathophysiological evidence, α-syn species have the strongest rationale of use and should constitute the basis of composite biomarker panels. Fortunately, novel techniques such as PMCA and RT-QuIC have improved the detection of α-syn aggregates. CSF Aβ42 has proved its prognostic use for cognitive impairment in PD. Other biomarkers related to axonal damage (tau proteins and NfL) are not specific for PD diagnosis but can help assess PD progression. Moreover, multiple novel candidate biomarkers have been identified within known (lysosomal, inflammatory, mitochondrial dysfunction, LRRK2) and novel biological pathways associated with PD (metabolomics). Given the complexity and intricate interplay of different pathophysiological mechanisms, applying a panel of markers reflecting different aspects of disease-related pathways simultaneously would be most promising. In order to optimize the utility of CSF biomarkers, analytical validation is needed by establishing standardization of techniques (including assays for measurement, sample collection and handling procedures) across different laboratories that can ensure the reproducibility of results and the generation of relatable cut-off and calibration values. Further validation analyses in large PD cohort studies will identify the CSF biomarker or biomarker combinations with the best value for clinical and research purposes. Importantly, confounding factors such as age should be excluded by adjusting models accordingly. Even though laborious and invasive, the number of longitudinal CSF studies needs to be expanded for prognostic assessment. Large multicentric longitudinal biomarker studies including PD at the very early stages (i.e., pre-motor PD) will allow identifying relevant molecular changes for early diagnostic accuracy. Furthermore, the prognostic value of certain markers needs to be ascertained for their use as indicators of disease progression and treatment-associated changes that are imperative for proving the effectiveness of novel disease-modifying therapeutics.

Table 1.
Overview of CSF biomarkers according to key pathologic mechanisms involved in PD with diagnostic and prognostic relevance indicating disease severity and progression.
Author Contributions
Conceptualization, E.H.K. and L.T.; methodology, E.H.K. and L.T.; software, S.T.; investigation, E.H.K., L.B. and S.T.; writing—original draft preparation, L.B., S.T. and E.H.K.; writing—E.H.K., L.B. and S.T.; review and editing, E.H.K., L.B. and S.T.; visualization, E.H.K. and S.T.; supervision, L.T., R.G. and K.G.; project administration, E.H.K. and L.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Feigin, V.L.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Abyu, G.Y.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; et al. GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef]
- Rizzo, G.; Copetti, M.; Arcuti, S.; Martino, D.; Fontana, A.; Logroscino, G. Accuracy of clinical diagnosis of Parkinson disease. Neurology 2016, 86, 566–576. [Google Scholar] [CrossRef]
- Cheng, H.-C.; Ulane, C.M.; Burke, R. Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 2010, 67, 715–725. [Google Scholar] [CrossRef]
- Mahlknecht, P.; Seppi, K.; Poewe, W. The Concept of Prodromal Parkinson’s Disease. J. Park. Dis. 2015, 5, 681–697. [Google Scholar] [CrossRef]
- Reiber, H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin. Chim. Acta 2001, 310, 173–186. [Google Scholar] [CrossRef]
- Prakash, N.; Caspell-Garcia, C.; Coffey, C.; Siderowf, A.; Tanner, C.M.; Kieburtz, K.; Mollenhauer, B.; Galasko, D.; Merchant, K.; Foroud, T.; et al. Feasibility and safety of lumbar puncture in the Parkinson’s disease research participants: Parkinson’s Progression Marker Initiative (PPMI). Park. Relat. Disord. 2019, 62, 201–209. [Google Scholar] [CrossRef]
- Meade, R.M.; Fairlie, D.P.; Mason, J.M. Alpha-synuclein structure and Parkinson’s disease—Lessons and emerging principles. Mol. Neurodegener. 2019, 14, 29. [Google Scholar] [CrossRef]
- Førland, M.G.; Tysnes, O.; Aarsland, D.; Maple-Grødem, J.; Pedersen, K.F.; Alves, G.; Lange, J. The value of cerebrospinal fluid α-synuclein and the tau/α-synuclein ratio for diagnosis of neurodegenerative disorders with Lewy pathology. Eur. J. Neurol. 2019, 27, 43–50. [Google Scholar] [CrossRef]
- Shim, K.H.; Kang, M.J.; Suh, J.W.; Pyun, J.-M.; Ryoo, N.; Park, Y.H.; Youn, Y.C.; Jang, J.-W.; Jeong, J.H.; Park, K.W.; et al. CSF total tau/α-synuclein ratio improved the diagnostic performance for Alzheimer’s disease as an indicator of tau phosphorylation. Alzheimer Res. Ther. 2020, 12, 83. [Google Scholar] [CrossRef]
- Chahine, L.M.; Beach, T.G.; Brumm, M.C.; Adler, C.H.; Coffey, C.S.; Mosovsky, S.; Caspell-Garcia, C.; Serrano, G.E.; Munoz, D.G.; White, C.L.; et al. In vivo distribution of α-synuclein in multiple tissues and biofluids in Parkinson disease. Neurology 2020, 95, e1267–e1284. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Wang, X.; Zhang, L.; Jiang, S.; Yuan, Y.; Li, J.; Zhu, L.; Zhang, K. Relationship between the plasma levels of neurodegenerative proteins and motor subtypes of Parkinson’s disease. J. Neural Transm. 2016, 124, 353–360. [Google Scholar] [CrossRef]
- Chang, C.-W.; Yang, S.-Y.; Yang, C.-C.; Chang, C.-W.; Wu, Y.-R. Plasma and Serum Alpha-Synuclein as a Biomarker of Diagnosis in Patients with Parkinson’s Disease. Front. Neurol. 2020, 10, 1388. [Google Scholar] [CrossRef]
- Fayyad, M.; Salim, S.; Majbour, N.; Erskine, D.; Stoops, E.; Mollenhauer, B.; El-Agnaf, O.M.A. Parkinson’s disease biomarkers based on α-synuclein. J. Neurochem. 2019, 150, 626–636. [Google Scholar] [CrossRef]
- Parnetti, L.; Gaetani, L.; Eusebi, P.; Paciotti, S.; Hansson, O.; El-Agnaf, O.; Mollenhauer, B.; Blennow, K.; Calabresi, P. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol. 2019, 18, 573–586. [Google Scholar] [CrossRef]
- Schulz, I.; Kruse, N.; Gera, R.G.; Kremer, T.; Cedarbaum, J.; Barbour, R.; Zago, W.; Schade, S.; Otte, B.; Bartl, M.; et al. Systematic Assessment of 10 Biomarker Candidates Focusing on α-Synuclein-Related Disorders. Mov. Disord. 2021, 36, 2874–2887. [Google Scholar] [CrossRef]
- Foulds, P.G.; Yokota, O.; Thurston, A.; Davidson, Y.; Ahmed, Z.; Holton, J.; Thompson, J.C.; Akiyama, H.; Arai, T.; Hasegawa, M.; et al. Post mortem cerebrospinal fluid α-synuclein levels are raised in multiple system atrophy and distinguish this from the other α-synucleinopathies, Parkinson’s disease and Dementia with Lewy bodies. Neurobiol. Dis. 2012, 45, 188–195. [Google Scholar] [CrossRef]
- Twohig, D.; Rodriguez-Vieitez, E.; Sando, S.B.; Berge, G.; Lauridsen, C.; Møller, I.; Grøntvedt, G.R.; Bråthen, G.; Patra, K.; Bu, G.; et al. The relevance of cerebrospinal fluid α-synuclein levels to sporadic and familial Alzheimer’s disease. Acta Neuropathol. Commun. 2018, 6, 130. [Google Scholar] [CrossRef]
- Vergallo, A.; Bun, R.; Toschi, N.; Baldacci, F.; Zetterberg, H.; Blennow, K.; Cavedo, E.; Lamari, F.; Habert, M.O.; Dubois, B.; et al. Association of cerebrospinal fluid α-synuclein with total and phospho-tau 181 protein concentrations and brain amyloid load in cognitively normal subjective memory complainers stratified by Alzheimer’s disease biomarkers. Alzheimer’s Dement. 2018, 14, 1623–1631. [Google Scholar] [CrossRef]
- Parkkinen, L.; Pirttilä, T.; Tervahauta, M.; Alafuzoff, I. Widespread and abundant alpha-synuclein pathology in a neurologically unimpaired subject. Neuropathology 2005, 25, 304–314. [Google Scholar] [CrossRef]
- Majbour, N.; Msc, N.N.V.; Eusebi, P.; Chiasserini, D.; Ardah, M.; Varghese, S.; Haque, M.E.; Tokuda, T.; Auinger, P.; Calabresi, P.; et al. Longitudinal changes in CSF alpha-synuclein species reflect Parkinson’s disease progression. Mov. Disord. 2016, 31, 1535–1542. [Google Scholar] [CrossRef]
- Hall, S.; Surova, Y.; Öhrfelt, A.; Blennow, K.; Zetterberg, H.; Hansson, O.; the Swedish BioFINDER Study. Longitudinal Measurements of Cerebrospinal Fluid Biomarkers in Parkinson’s Disease. Mov. Disord. 2016, 31, 898–905. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Ms, C.J.C.; Coffey, C.S.; Taylor, P.; Singleton, A.; Shaw, L.M.; Trojanowski, J.Q.; Frasier, M.; Simuni, T.; Iranzo, A.; et al. Longitudinal analyses of cerebrospinal fluid α-Synuclein in prodromal and early Parkinson’s disease. Mov. Disord. 2019, 34, 1354–1364. [Google Scholar] [CrossRef]
- Paoletti, F.P.; Gaetani, L.; Parnetti, L. The Challenge of Disease-Modifying Therapies in Parkinson’s Disease: Role of CSF Biomarkers. Biomolecules 2020, 10, 335. [Google Scholar] [CrossRef]
- Eusebi, P.; Giannandrea, D.; Biscetti, L.; Abraha, I.; Chiasserini, D.; Orso, M.; Calabresi, P.; Parnetti, L. Diagnostic utility of cerebrospinal fluid α-synuclein in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2017, 32, 1389–1400. [Google Scholar] [CrossRef]
- Cariulo, C.; Martufi, P.; Verani, M.; Azzollini, L.; Bruni, G.; Weiss, A.; Deguire, S.M.; Lashuel, H.A.; Scaricamazza, E.; Sancesario, G.M.; et al. Phospho-S129 Alpha-Synuclein Is Present in Human Plasma but Not in Cerebrospinal Fluid as Determined by an Ultrasensitive Immunoassay. Front. Neurosci. 2019, 13, 889. [Google Scholar] [CrossRef]
- Tokuda, T.; Qureshi, M.M.; Ardah, M.T.; Varghese, S.; Shehab, S.A.S.; Kasai, T.; Ishigami, N.; Tamaoka, A.; Nakagawa, M.; El-Agnaf, O.M.A. Detection of elevated levels of -synuclein oligomers in CSF from patients with Parkinson disease. Neurology 2010, 75, 1766–1770. [Google Scholar] [CrossRef]
- Eparnetti, L.; Efarotti, L.; Eeusebi, P.; Echiasserini, D.; Carlo, C.E.; Egiannandrea, D.; Esalvadori, N.; Elisetti, V.; Etambasco, N.; Erossi, A.; et al. Differential role of CSF alpha-synuclein species, tau, and Aβ42 in Parkinson’s Disease. Front. Aging Neurosci. 2014, 6, 53. [Google Scholar] [CrossRef]
- Kang, U.J.; Boehme, A.K.; Bs, G.F.; Shahnawaz, M.; Ma, T.; Hutten, S.J.; Green, A.; Soto, C. Comparative study of cerebrospinal fluid α-synuclein seeding aggregation assays for diagnosis of Parkinson’s disease. Mov. Disord. 2019, 34, 536–544. [Google Scholar] [CrossRef]
- Rossi, M.; Candelise, N.; Baiardi, S.; Capellari, S.; Giannini, G.; Orrù, C.D.; Antelmi, E.; Mammana, A.; Hughson, A.G.; Calandra-Buonaura, G.; et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 2020, 140, 49–62. [Google Scholar] [CrossRef]
- Singer, W.; Schmeichel, A.M.; Shahnawaz, M.; Schmelzer, J.D.; Boeve, B.F.; Sletten, D.M.; Gehrking, T.L.; Gehrking, J.A.; Olson, A.D.; Savica, R.; et al. Alpha-Synuclein Oligomers and Neurofilament Light Chain in Spinal Fluid Differentiate Multiple System Atrophy from Lewy Body Synucleinopathies. Ann. Neurol. 2020, 88, 503–512. [Google Scholar] [CrossRef]
- Shahnawaz, M.; Mukherjee, A.; Pritzkow, S.; Mendez, N.; Rabadia, P.; Liu, X.; Hu, B.; Schmeichel, A.; Singer, W.; Wu, G.; et al. Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature 2020, 578, 273–277. [Google Scholar] [CrossRef]
- Barkovits, K.; Kruse, N.; Linden, A.; Tönges, L.; Pfeiffer, K.; Mollenhauer, B.; Marcus, K. Blood Contamination in CSF and Its Impact on Quantitative Analysis of Alpha-Synuclein. Cells 2020, 9, 370. [Google Scholar] [CrossRef]
- Compta, Y.; Parkkinen, L.; O’Sullivan, S.S.; Vandrovcova, J.; Holton, J.L.; Collins, C.; Lashley, T.; Kallis, C.; Williams, D.R.; de Silva, R.; et al. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: Which is more important? Brain 2011, 134, 1493–1505. [Google Scholar] [CrossRef]
- Irwin, D.J.; Lee, V.M.-Y.; Trojanowski, J.Q. Parkinson’s disease dementia: Convergence of α-synuclein, tau and amyloid-β pathologies. Nat. Rev. Neurosci. 2013, 14, 626–636. [Google Scholar] [CrossRef]
- Jellinger, K.A.; Seppi, K.; Wenning, G.K.; Poewe, W. Impact of coexistent Alzheimer pathology on the natural history of Parkinson’s disease. J. Neural Transm. 2002, 109, 329–339. [Google Scholar] [CrossRef]
- Clinton, L.K.; Blurton-Jones, M.; Myczek, K.; Trojanowski, J.Q.; LaFerla, F.M. Synergistic Interactions between Aβ, Tau, and -Synuclein: Acceleration of Neuropathology and Cognitive Decline. J. Neurosci. 2010, 30, 7281–7289. [Google Scholar] [CrossRef]
- Alves, G.; Lange, J.; Blennow, K.; Zetterberg, H.; Andreasson, U.; Førland, M.G.; Tysnes, O.-B.; Larsen, J.P.; Pedersen, K.F. CSF A 42 predicts early-onset dementia in Parkinson disease. Neurology 2014, 82, 1784–1790. [Google Scholar] [CrossRef]
- Stav, A.L.; Aarsland, D.; Johansen, K.K.; Hessen, E.; Auning, E.; Fladby, T. Amyloid-β and α-synuclein cerebrospinal fluid biomarkers and cognition in early Parkinson’s disease. Park. Relat. Disord. 2015, 21, 758–764. [Google Scholar] [CrossRef]
- Hall, S.; Surova, Y.; Öhrfelt, A.; Zetterberg, H.; Lindqvist, D.; Hansson, O. CSF biomarkers and clinical progression of Parkinson disease. Neurology 2014, 84, 57–63. [Google Scholar] [CrossRef]
- Vranová, H.P.; Hényková, E.; Kaiserová, M.; Menšíková, K.; Vaštík, M.; Mareš, J.; Hluštík, P.; Zapletalová, J.; Strnad, M.; Stejskal, D.; et al. Tau protein, beta-amyloid1–42 and clusterin CSF levels in the differential diagnosis of Parkinsonian syndrome with dementia. J. Neurol. Sci. 2014, 343, 120–124. [Google Scholar] [CrossRef]
- Kaerst, L.; Kuhlmann, A.; Wedekind, D.; Stoeck, K.; Lange, P.; Zerr, I. Using Cerebrospinal Fluid Marker Profiles in Clinical Diagnosis of Dementia with Lewy Bodies, Parkinson’s Disease, and Alzheimer’s Disease. J. Alzheimer Dis. 2013, 38, 63–73. [Google Scholar] [CrossRef]
- Irwin, D.J.; Fedler, J.; Coffey, C.S.; Ms, C.C.; Kang, J.H.; Simuni, T.; Foroud, T.; Toga, A.W.; Tanner, C.M.; Kieburtz, K.; et al. Evolution of Alzheimer’s Disease Cerebrospinal Fluid Biomarkers in Early Parkinson’s Disease. Ann. Neurol. 2020, 88, 574–587. [Google Scholar] [CrossRef]
- Rochester, L.; Galna, B.; Lord, S.; Yarnall, A.; Morris, R.; Duncan, G.; Khoo, T.K.; Mollenhauer, B.; Burn, D. Decrease in Aβ42 predicts dopa-resistant gait progression in early Parkinson disease. Neurology 2017, 88, 1501–1511. [Google Scholar] [CrossRef]
- Hall, S.; Öhrfelt, A.; Constantinescu, R.; Andreasson, U.; Surova, Y.; Bostrom, F.; Nilsson, C.; Widner, H.; Decraemer, H.; Nägga, K.; et al. Accuracy of a Panel of 5 Cerebrospinal Fluid Biomarkers in the Differential Diagnosis of Patients with Dementia and/or Parkinsonian Disorders. Arch. Neurol. 2012, 69, 1445–1452. [Google Scholar] [CrossRef]
- Alves, G.; Brønnick, K.; Aarsland, D.; Blennow, K.; Zetterberg, H.; Ballard, C.; Kurz, M.W.; Andreasson, U.; Tysnes, O.-B.; Larsen, J.P.; et al. CSF amyloid- and tau proteins, and cognitive performance, in early and untreated Parkinson’s Disease: The Norwegian ParkWest study. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1080–1086. [Google Scholar] [CrossRef]
- Hu, X.; Yang, Y.; Gong, D. Changes of cerebrospinal fluid Aβ42, t-tau, and p-tau in Parkinson’s disease patients with cognitive impairment relative to those with normal cognition: A meta-analysis. Neurol. Sci. 2017, 38, 1953–1961. [Google Scholar] [CrossRef]
- Herbert, M.K.; Eeftens, J.M.; Aerts, M.B.; Esselink, R.A.J.; Bloem, B.R.; Kuiperij, H.B.; Verbeek, M.M. CSF levels of DJ-1 and tau distinguish MSA patients from PD patients and controls. Parkinsonism Relat. Disord. 2014, 20, 112–115. [Google Scholar] [CrossRef]
- Abdo, W.F.; Bloem, B.R.; Van Geel, W.J.; Esselink, R.A.J.; Verbeek, M.M. CSF neurofilament light chain and tau differentiate multiple system atrophy from Parkinson’s disease. Neurobiol. Aging 2007, 28, 742–747. [Google Scholar] [CrossRef]
- Palmio, J.; Suhonen, J.; Keränen, T.; Hulkkonen, J.; Peltola, J.; Pirttilä, T. Cerebrospinal fluid tau as a marker of neuronal damage after epileptic seizure. Seizure 2009, 18, 474–477. [Google Scholar] [CrossRef]
- Brinkmalm, A.; Portelius, E.; Brinkmalm, G.; Pannee, J.; Dahlén, R.; Gobom, J.; Blennow, K.; Zetterberg, H. Fluid-based proteomics targeted on pathophysiological processes and pathologies in neurodegenerative diseases. J. Neurochem. 2018, 151, 417–434. [Google Scholar] [CrossRef]
- Liu, C.; Cholerton, B.; Shi, M.; Ginghina, C.; Cain, K.C.; Auinger, P.; Zhang, J. CSF tau and tau/Aβ42 predict cognitive decline in Parkinson’s disease. Park. Relat. Disord. 2015, 21, 271–276. [Google Scholar] [CrossRef]
- Zetterberg, H. Neurofilament Light: A Dynamic Cross-Disease Fluid Biomarker for Neurodegeneration. Neuron 2016, 91, 1–3. [Google Scholar] [CrossRef]
- Bäckström, D.C.; Domellöf, M.E.; Linder, J.; Olsson, B.; Öhrfelt, A.; Trupp, M.; Zetterberg, H.; Blennow, K.; Forsgren, L. Cerebrospinal Fluid Patterns and the Risk of Future Dementia in Early, Incident Parkinson Disease. JAMA Neurol. 2015, 72, 1175–1182. [Google Scholar] [CrossRef]
- Hu, X.; Yang, Y.; Gong, D. Cerebrospinal fluid levels of neurofilament light chain in multiple system atrophy relative to Parkinson’s disease: A meta-analysis. Neurol. Sci. 2016, 38, 407–414. [Google Scholar] [CrossRef]
- Bridel, C.; van Wieringen, W.N.; Zetterberg, H.; Tijms, B.M.; Teunissen, C.E.; Alvarez-Cermeño, J.C.; Andreasson, U.; Axelsson, M.; Bäckström, D.C.; Bartos, A.; et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology. JAMA Neurol. 2019, 76, 1035–1048. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Dakna, M.; Kruse, N.; Galasko, D.; Foroud, T.; Zetterberg, H.; Schade, S.; Gera, R.G.; Wang, W.; Gao, F.; et al. Validation of Serum Neurofilament Light Chain as a Biomarker of Parkinson’s Disease Progression. Mov. Disord. 2020, 35, 1999–2008. [Google Scholar] [CrossRef]
- Lerche, S.; Wurster, I.; Röben, B.; Zimmermann, M.; Machetanz, G.; Wiethoff, S.; Dehnert, M.; Rietschel, L.; Riebenbauer, B.; Deuschle, C.; et al. CSF NFL in a Longitudinally Assessed PD Cohort: Age Effects and Cognitive Trajectories. Mov. Disord. 2020, 35, 1138–1144. [Google Scholar] [CrossRef]
- Oosterveld, L.P.; Verberk, I.M.V.; Majbour, N.K.; El-Agnaf, O.M.; Weinstein, H.C.; Berendse, H.W.; Teunissen, C.E.; van de Berg, W.D. CSF or Serum Neurofilament Light Added to α-Synuclein Panel Discriminates Parkinson’s from Controls. Mov. Disord. 2019, 35, 288–295. [Google Scholar] [CrossRef]
- Moors, T.; Paciotti, S.; Chiasserini, D.; Calabresi, P.; Parnetti, L.; Beccari, T.; van de Berg, W. Lysosomal Dysfunction and α-Synuclein Aggregation in Parkinson’s Disease: Diagnostic Links. Mov. Disord. 2016, 31, 791–801. [Google Scholar] [CrossRef]
- Nalls, M.A.; Duran, R.; Lopez, G.; Kurzawa-Akanbi, M.; McKeith, I.; Chinnery, P.F.; Morris, C.; Theuns, J.; Crosiers, D.; Cras, P.; et al. A Multicenter Study of Glucocerebrosidase Mutations in Dementia with Lewy Bodies. JAMA Neurol. 2013, 70, 727–735. [Google Scholar] [CrossRef]
- Sidransky, E.; Nalls, M.A.; Aasly, J.O.; Aharon-Peretz, J.; Annesi, G.; Barbosa, E.R.; Bar-Shira, A.; Berg, D.; Bras, J.; Brice, A.; et al. Multicenter Analysis of Glucocerebrosidase Mutations in Parkinson’s Disease. New Engl. J. Med. 2009, 361, 1651–1661. [Google Scholar] [CrossRef]
- Mazzulli, J.R.; Xu, Y.-H.; Sun, Y.; Knight, A.L.; McLean, P.J.; Caldwell, G.A.; Sidransky, E.; Grabowski, G.A.; Krainc, D. Gaucher Disease Glucocerebrosidase and α-Synuclein Form a Bidirectional Pathogenic Loop in Synucleinopathies. Cell 2011, 146, 37–52. [Google Scholar] [CrossRef]
- Robak, L.A.; Jansen, I.E.; Van Rooij, J.; Uitterlinden, A.G.; Kraaij, R.; Jankovic, J.; Heutink, P.; Shulman, J.M.; Nalls, M.; Plagnol, V.; et al. Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain 2017, 140, 3191–3203. [Google Scholar] [CrossRef]
- Kurzawa-Akanbi, M.; Tammireddy, S.; Fabrik, I.; Gliaudelytė, L.; Doherty, M.K.; Heap, R.; Matečko-Burmann, I.; Burmann, B.M.; Trost, M.; Lucocq, J.M.; et al. Altered ceramide metabolism is a feature in the extracellular vesicle-mediated spread of alpha-synuclein in Lewy body disorders. Acta Neuropathol. 2021, 142, 961–984. [Google Scholar] [CrossRef]
- Paciotti, S.; Gatticchi, L.; Beccari, T.; Parnetti, L. Lysosomal enzyme activities as possible CSF biomarkers of synucleinopathies. Clin. Chim. Acta 2019, 495, 13–24. [Google Scholar] [CrossRef]
- Parnetti, L.; Paciotti, S.; Farotti, L.; Bellomo, G.; Sepe, F.N.; Eusebi, P. Parkinson’s and Lewy body dementia CSF biomarkers. Clin. Chim. Acta 2019, 495, 318–325. [Google Scholar] [CrossRef]
- Parnetti, L.; Paciotti, S.; Eusebi, P.; Dardis, A.; Zampieri, S.; Chiasserini, D.; Tasegian, A.; Tambasco, N.; Bembi, B.; Calabresi, P.; et al. Cerebrospinal fluid β-glucocerebrosidase activity is reduced in parkinson’s disease patients. Mov. Disord. 2017, 32, 1423–1431. [Google Scholar] [CrossRef]
- Parnetti, L.; Chiasserini, D.; Persichetti, E.; Eusebi, P.; Varghese, S.; Msc, M.M.Q.; Dardis, A.; Deganuto, M.; De Carlo, C.; Castrioto, A.; et al. Cerebrospinal fluid lysosomal enzymes and alpha-synuclein in Parkinson’s disease. Mov. Disord. 2014, 29, 1019–1027. [Google Scholar] [CrossRef]
- Parnetti, L.; Balducci, C.; Pierguidi, L.; De Carlo, C.; Peducci, M.; D’Amore, C.; Padiglioni, C.; Mastrocola, S.; Persichetti, E.; Paciotti, S.; et al. Cerebrospinal fluid β-glucocerebrosidase activity is reduced in Dementia with Lewy Bodies. Neurobiol. Dis. 2009, 34, 484–486. [Google Scholar] [CrossRef]
- Balducci, C.; Pierguidi, L.; Persichetti, E.; Parnetti, L.; Sbaragli, M.; Tassi, C.; Orlacchio, A.; Calabresi, P.; Beccari, T.; Rossi, A. Lysosomal hydrolases in cerebrospinal fluid from subjects with Parkinson’s disease. Mov. Disord. 2007, 22, 1481–1484. [Google Scholar] [CrossRef]
- Lerche, S.; Schulte, C.; Wurster, I.; Machetanz, G.; Roeben, B.; Zimmermann, M.; Deuschle, C.; Hauser, A.; Böhringer, J.; Krägeloh-Mann, I.; et al. The Mutation Matters: CSF Profiles of GCase, Sphingolipids, α-Synuclein in PD GBA. Mov. Disord. 2021, 36, 1216–1228. [Google Scholar] [CrossRef]
- Rocha, E.M.; Smith, G.A.; Park, E.; Cao, H.; Brown, E.; Hallett, P.; Isacson, O. Progressive decline of glucocerebrosidase in aging and P arkinson’s disease. Ann. Clin. Transl. Neurol. 2015, 2, 433–438. [Google Scholar] [CrossRef]
- Huh, Y.E.; Park, H.; Chiang, M.S.R.; Tuncali, I.; Liu, G.; Locascio, J.J.; Shirvan, J.; Hutten, S.J.; Rotunno, M.S.; Viel, C.; et al. Glucosylceramide in cerebrospinal fluid of patients with GBA-associated and idiopathic Parkinson’s disease enrolled in PPMI. NPJ Park. Dis. 2021, 7, 102. [Google Scholar] [CrossRef]
- Mosley, R.L.; Hutter-Saunders, J.A.; Stone, D.K.; Gendelman, H.E. Inflammation and Adaptive Immunity in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009381. [Google Scholar] [CrossRef]
- Schröder, J.B.; Pawlowski, M.; zu Horste, G.M.; Gross, C.; Wiendl, H.; Meuth, S.G.; Ruck, T.; Warnecke, T. Immune Cell Activation in the Cerebrospinal Fluid of Patients with Parkinson’s Disease. Front. Neurol. 2018, 9, 1081. [Google Scholar] [CrossRef]
- Magdalinou, N.K.; Paterson, R.W.; Schott, J.; Fox, N.; Mummery, C.; Blennow, K.; Bhatia, K.; Morris, H.; Giunti, P.; Warner, T.; et al. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1240–1247. [Google Scholar] [CrossRef]
- Santaella, A.; Kuiperij, H.B.; Van Rumund, A.; Esselink, R.A.J.; Van Gool, A.J.; Bloem, B.R.; Verbeek, M.M. Cerebrospinal fluid monocyte chemoattractant protein 1 correlates with progression of Parkinson’s disease. NPJ Park. Dis. 2020, 6, 21. [Google Scholar] [CrossRef]
- Santaella, A.; Kuiperij, H.B.; van Rumund, A.; Esselink, R.A.J.; van Gool, A.J.; Bloem, B.R.; Verbeek, M.M. Inflammation biomarker discovery in Parkinson’s disease and atypical parkinsonisms. BMC Neurol. 2020, 20, 26. [Google Scholar] [CrossRef]
- Jabbari, E.; Woodside, J.; Guo, T.; Magdalinou, N.K.; Chelban, V.; Athauda, D.; Lees, A.J.; Foltynie, T.; Houlden, H.; Church, A.; et al. Proximity extension assay testing reveals novel diagnostic biomarkers of atypical parkinsonian syndromes. J. Neurol. Neurosurg. Psychiatry 2019, 90, 768–773. [Google Scholar] [CrossRef]
- Rydbirk, R.; Elfving, B.; Andersen, M.D.; Langbøl, M.A.; Folke, J.; Winge, K.; Pakkenberg, B.; Brudek, T.; Aznar, S. Cytokine profiling in the prefrontal cortex of Parkinson’s Disease and Multiple System Atrophy patients. Neurobiol. Dis. 2017, 106, 269–278. [Google Scholar] [CrossRef]
- Wennstrom, M.; Surova, Y.; Hall, S.; Nilsson, C.; Minthon, L.; Hansson, O.; Nielsen, H.M. The Inflammatory Marker YKL-40 Is Elevated in Cerebrospinal Fluid from Patients with Alzheimer’s but Not Parkinson’s Disease or Dementia with Lewy Bodies. PLoS ONE 2015, 10, e0135458. [Google Scholar] [CrossRef]
- Lindqvist, D.; Hall, S.; Surova, Y.; Nielsen, H.M.; Janelidze, S.; Brundin, L.; Hansson, O. Cerebrospinal fluid inflammatory markers in Parkinson’s disease—Associations with depression, fatigue, and cognitive impairment. Brain Behav. Immun. 2013, 33, 183–189. [Google Scholar] [CrossRef]
- Bartl, M.; Dakna, M.; Galasko, D.; Hutten, S.J.; Foroud, T.; Quan, M.; Marek, K.; Siderowf, A.; Franz, J.; Trenkwalder, C.; et al. Biomarkers of neurodegeneration and glial activation validated in Alzheimer’s disease assessed in longitudinal cerebrospinal fluid samples of Parkinson’s disease. PLoS ONE 2021, 16, e0257372. [Google Scholar] [CrossRef]
- Hall, S.; Janelidze, S.; Surova, Y.; Widner, H.; Zetterberg, H.; Hansson, O. Cerebrospinal fluid concentrations of inflammatory markers in Parkinson’s disease and atypical parkinsonian disorders. Sci. Rep. 2018, 8, 13276. [Google Scholar] [CrossRef]
- Magdalinou, N.; Noyce, A.; Pinto, R.; Lindstrom, E.; Holmén-Larsson, J.; Holtta, M.; Blennow, K.; Morris, H.; Skillbäck, T.; Warner, T.; et al. Identification of candidate cerebrospinal fluid biomarkers in parkinsonism using quantitative proteomics. Park. Relat. Disord. 2017, 37, 65–71. [Google Scholar] [CrossRef]
- Olsson, B.; Constantinescu, R.; Holmberg, B.; Andreasen, N.; Blennow, K.; Zetterberg, H. The glial marker YKL-40 is decreased in synucleinopathies. Mov. Disord. 2013, 28, 1882–1885. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Zimmermann, J.; Sixel-Döring, F.; Focke, N.K.; Wicke, T.; Ebentheuer, J.; Ms, M.S.; Bs, E.L.; Friede, T.; Trenkwalder, C.; et al. Baseline predictors for progression 4 years after Parkinson’s disease diagnosis in the De Novo Parkinson Cohort (DeNoPa). Mov. Disord. 2018, 34, 67–77. [Google Scholar] [CrossRef]
- Majbour, N.K.; Aasly, J.O.; Hustad, E.; Thomas, M.A.; Vaikath, N.N.; Elkum, N.; van de Berg, W.; Tokuda, T.; Mollenhauer, B.; Berendse, H.W.; et al. CSF total and oligomeric α-Synuclein along with TNF-α as risk biomarkers for Parkinson’s disease: A study in LRRK2 mutation carriers. Transl. Neurodegener. 2020, 9, 1–10. [Google Scholar] [CrossRef]
- Starhof, C.; Winge, K.; Heegaard, N.H.H.; Skogstrand, K.; Friis, S.; Hejl, A. Cerebrospinal fluid pro-inflammatory cytokines differentiate parkinsonian syndromes. J. Neuroinflamm. 2018, 15, 305. [Google Scholar] [CrossRef]
- Delgado-Alvarado, M.; Gago, B.; Gorostidi, A.; Jiménez-Urbieta, H.; Aguayo, R.D.; Navalpotro, I.; Ruiz-Martínez, J.; Bergareche, A.; Martí-Massó, J.F.; Martínez-Lage, P.; et al. Tau/α-synuclein ratio and inflammatory proteins in Parkinson’s disease: An exploratory study. Mov. Disord. 2017, 32, 1066–1073. [Google Scholar] [CrossRef]
- Hatcher-Martin, J.M.; McKay, J.L.; Pybus, A.F.; Sommerfeld, B.; Howell, J.C.; Goldstein, F.C.; Wood, L.; Hu, W.T.; Factor, S.A. Cerebrospinal fluid biomarkers in Parkinson’s disease with freezing of gait: An exploratory analysis. NPJ Park. Dis. 2021, 7, 105. [Google Scholar] [CrossRef]
- Compta, Y.; Dias, S.P.; Giraldo, D.M.; Pérez-Soriano, A.; Muñoz, E.; Saura, J.; Fernández, M.; Bravo, P.; Cámara, A.; Pulido-Salgado, M.; et al. Cerebrospinal fluid cytokines in multiple system atrophy: A cross-sectional Catalan MSA registry study. Park. Relat. Disord. 2019, 65, 3–12. [Google Scholar] [CrossRef]
- Eidson, L.N.; Kannarkat, G.T.; Barnum, C.J.; Chang, J.; Chung, J.; Caspell-Garcia, C.; Taylor, P.; Mollenhauer, B.; Schlossmacher, M.G.; Ereshefsky, L.; et al. Candidate inflammatory biomarkers display unique relationships with alpha-synuclein and correlate with measures of disease severity in subjects with Parkinson’s disease. J. Neuroinflamm. 2017, 14, 164. [Google Scholar] [CrossRef]
- Harms, A.S.; Ferreira, S.A.; Romero-Ramos, M. Periphery and brain, innate and adaptive immunity in Parkinson’s disease. Acta Neuropathol. 2021, 141, 527–545. [Google Scholar] [CrossRef]
- King, E.; Thomas, A. Systemic Inflammation in Lewy Body Diseases. Alzheimer Dis. Assoc. Disord. 2017, 31, 346–356. [Google Scholar] [CrossRef]
- Botas, A.; Campbell, H.M.; Han, X.; Maletic-Savatic, M. Chapter Two—Metabolomics of Neurodegenerative Diseases. In International Review of Neurobiology: Omic Studies of Neurodegenerative Disease: Part B; Hurley, M.J., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 53–80. ISBN 0074-7742. [Google Scholar]
- Plewa, S.; Poplawska-Domaszewicz, K.; Florczak-Wyspianska, J.; Klupczynska-Gabryszak, A.; Sokol, B.; Miltyk, W.; Jankowski, R.; Kozubski, W.; Kokot, Z.J.; Matysiak, J. The Metabolomic Approach Reveals the Alteration in Human Serum and Cerebrospinal Fluid Composition in Parkinson’s Disease Patients. Pharmaceuticals 2021, 14, 935. [Google Scholar] [CrossRef]
- Santos-Lobato, B.L.; Gardinassi, L.G.; Bortolanza, M.; Peti, A.P.F.; Pimentel, V.; Faccioli, L.H.; Del-Bel, E.A.; Tumas, V. Metabolic Profile in Plasma AND CSF of LEVODOPA-induced Dyskinesia in Parkinson’s Disease: Focus on Neuroinflammation. Mol. Neurobiol. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Iwaoka, K.; Otsuka, C.; Maeda, T.; Yamahara, K.; Kato, K.; Takahashi, K.; Takahashi, K.; Terayama, Y. Impaired metabolism of kynurenine and its metabolites in CSF of parkinson’s disease. Neurosci. Lett. 2019, 714, 134576. [Google Scholar] [CrossRef]
- Havelund, J.F.; Heegaard, N.H.H.; Færgeman, N.J.K.; Gramsbergen, J.B. Biomarker Research in Parkinson’s Disease Using Metabolite Profiling. Metabolites 2017, 7, 42. [Google Scholar] [CrossRef]
- Wuolikainen, A.; Jonsson, P.; Ahnlund, M.; Antti, H.; Marklund, S.L.; Moritz, T.; Forsgren, L.; Andersen, P.M.; Trupp, M. Multi-platform mass spectrometry analysis of the CSF and plasma metabolomes of rigorously matched amyotrophic lateral sclerosis, Parkinson’s disease and control subjects. Mol. BioSyst. 2016, 12, 1287–1298. [Google Scholar] [CrossRef]
- Öhman, A.; Forsgren, L. NMR metabonomics of cerebrospinal fluid distinguishes between Parkinson’s disease and controls. Neurosci. Lett. 2015, 594, 36–39. [Google Scholar] [CrossRef]
- Trupp, M.; Jonsson, P.; Öhrfelt, A.; Zetterberg, H.; Obudulu, O.; Malm, L.; Wuolikainen, A.; Linder, J.; Moritz, T.; Blennow, K.; et al. Metabolite and Peptide Levels in Plasma and CSF Differentiating Healthy Controls from Patients with Newly Diagnosed Parkinson’s Disease. J. Park. Dis. 2014, 4, 549–560. [Google Scholar] [CrossRef]
- Stoessel, D.; Schulte, C.; Dos Santos, M.C.T.; Scheller, D.; Rebollo-Mesa, I.; Deuschle, C.; Walther, D.; Schauer, N.; Berg, D.; Da Costa, A.N.; et al. Promising Metabolite Profiles in the Plasma and CSF of Early Clinical Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 51. [Google Scholar] [CrossRef]
- Trezzi, J.-P.; Galozzi, S.; Jaeger, C.; Barkovits, K.; Brockmann, K.; Maetzler, W.; Berg, D.; Marcus, K.; Betsou, F.; Hiller, K.; et al. Distinct metabolomic signature in cerebrospinal fluid in early parkinson’s disease. Mov. Disord. 2017, 32, 1401–1408. [Google Scholar] [CrossRef]
- Willkommen, D.; Lucio, M.; Moritz, F.; Forcisi, S.; Kanawati, B.; Smirnov, K.S.; Schroeter, M.; Sigaroudi, A.; Schmitt-Kopplin, P.; Michalke, B. Metabolomic investigations in cerebrospinal fluid of Parkinson’s disease. PLoS ONE 2018, 13, e0208752. [Google Scholar] [CrossRef]
- LeWitt, P.A.; Li, J.; Lu, M.; Guo, L.; Auinger, P. Metabolomic biomarkers as strong correlates of Parkinson disease progression. Neurology 2017, 88, 862–869. [Google Scholar] [CrossRef]
- Lill, C.M. Genetics of Parkinson’s disease. Mol. Cell. Probes 2016, 30, 386–396. [Google Scholar] [CrossRef]
- Polissidis, A.; Petropoulou-Vathi, L.; Nakos-Bimpos, M.; Rideout, H.J. The Future of Targeted Gene-Based Treatment Strategies and Biomarkers in Parkinson’s Disease. Biomolecules 2020, 10, 912. [Google Scholar] [CrossRef]
- Wang, C.-D.; Chan, P. Clinicogenetics of Parkinson′s disease: A drawing but not completed picture. Neuroimmunol. Neuroinflamm. 2014, 1, 115–126. [Google Scholar] [CrossRef][Green Version]
- Reed, X.; Bandrés-Ciga, S.; Blauwendraat, C.; Cookson, M.R. The role of monogenic genes in idiopathic Parkinson’s disease. Neurobiol. Dis. 2018, 124, 230–239. [Google Scholar] [CrossRef]
- Riboldi, G.M.; Di Fonzo, A.B. GBA, Gaucher Disease, and Parkinson’s Disease: From Genetic to Clinic to New Therapeutic Approaches. Cells 2019, 8, 364. [Google Scholar] [CrossRef]
- Mullin, S.; Smith, L.; Lee, K.; D’Souza, G.; Woodgate, P.; Elflein, J.; Hällqvist, J.; Toffoli, M.; Streeter, A.; Hosking, J.; et al. Ambroxol for the Treatment of Patients with Parkinson Disease With and Without Glucocerebrosidase Gene Mutations: A Nonrandomized, Noncontrolled Trial. JAMA Neurol. 2020, 77, 427–434. [Google Scholar] [CrossRef]
- Healy, D.G.; Falchi, M.; O’Sullivan, S.S.; Bonifati, V.; Durr, A.; Bressman, S.; Brice, A.; Aasly, J.; Zabetian, C.P.; Goldwurm, S.; et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: A case-control study. Lancet Neurol. 2008, 7, 583–590. [Google Scholar] [CrossRef]
- Lee, A.J.; Wang, Y.; Alcalay, R.N.; Mejia-Santana, H.; Saunders-Pullman, R.; Bressman, S.; Corvol, J.-C.; Brice, A.; Lesage, S.; Mangone, G.; et al. Penetrance estimate of LRRK2 p.G2019S mutation in individuals of non-Ashkenazi Jewish ancestry. Mov. Disord. 2017, 32, 1432–1438. [Google Scholar] [CrossRef]
- Mancini, A.; Mazzocchetti, P.; Sciaccaluga, M.; Megaro, A.; Bellingacci, L.; Beccano-Kelly, D.A.; Di Filippo, M.; Tozzi, A.; Calabresi, P. From Synaptic Dysfunction to Neuroprotective Strategies in Genetic Parkinson’s Disease: Lessons from LRRK2. Front. Cell. Neurosci. 2020, 14, 158. [Google Scholar] [CrossRef]
- Fraser, K.B.; Moehle, M.S.; Daher, J.P.; Webber, P.J.; Williams, J.Y.; Stewart, C.A.; Yacoubian, T.A.; Cowell, R.M.; Dokland, T.; Ye, T.; et al. LRRK2 secretion in exosomes is regulated by 14-3-3. Hum. Mol. Genet. 2013, 22, 4988–5000. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Ye, T.; Mabrouk, O.S.; Maltbie, T.; Aasly, J.; West, A.B. Elevated LRRK2 autophosphorylation in brain-derived and peripheral exosomes in LRRK2 mutation carriers. Acta Neuropathol. Commun. 2017, 5, 86. [Google Scholar] [CrossRef]
- Mabrouk, O.S.; Chen, S.; Edwards, A.L.; Yang, M.; Hirst, W.D.; Graham, D.L. Quantitative Measurements of LRRK2 in Human Cerebrospinal Fluid Demonstrates Increased Levels in G2019S Patients. Front. Neurosci. 2020, 14, 526. [Google Scholar] [CrossRef]
- Trempe, J.-F.; Fon, E.A. Structure and Function of Parkin, PINK1, and DJ-1, the Three Musketeers of Neuroprotection. Front. Neurol. 2013, 4, 38. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, D.; Chen, L.; Choo, Y.S.; Ma, H.; Tang, C.; Xia, K.; Jiang, W.; Ronai, Z.; Zhuang, X.; et al. Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation. J. Clin. Investig. 2009, 119, 650–660. [Google Scholar] [CrossRef]
- Pyle, A.; Brennan, R.; Kurzawa-Akanbi, M.; Yarnall, A.; Thouin, A.; Mollenhauer, B.; Burn, D.; Chinnery, P.F.; Hudson, G. Reduced cerebrospinal fluid mitochondrial DNA is a biomarker for early-stage Parkinson’s disease. Ann. Neurol. 2015, 78, 1000–1004. [Google Scholar] [CrossRef]
- Lowes, H.; Pyle, A.; Santibanez-Koref, M.; Hudson, G. Circulating cell-free mitochondrial DNA levels in Parkinson’s disease are influenced by treatment. Mol. Neurodegener. 2020, 15, 10. [Google Scholar] [CrossRef]
- Farotti, L.; Paoletti, F.P.; Simoni, S.; Parnetti, L. Unraveling Pathophysiological Mechanisms of Parkinson’s Disease: Contribution of CSF Biomarkers. Biomark. Insights 2020, 15. [Google Scholar] [CrossRef]
- Salvesen, L.; Bech, S.; Lokkegaard, A.; Hjermind, L.E.; Nielsen, J.E.; Pakkenberg, B.; Tanassi, J.T.; Heegaard, N.H.; Winge, K. The DJ-1 concentration in cerebrospinal fluid does not differentiate among parkinsonian syndromes. Park. Relat. Disord. 2012, 18, 899–901. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Sohel, M.H. Extracellular/Circulating MicroRNAs: Release Mechanisms, Functions and Challenges. Achiev. Life Sci. 2016, 10, 175–186. [Google Scholar] [CrossRef]
- Dos Santos, M.C.T.; Barreto-Sanz, M.A.; Correia, B.R.S.; Bell, R.; Widnall, C.; Perez, L.T.; Berteau, C.; Schulte, C.; Scheller, D.; Berg, D.; et al. miRNA-based signatures in cerebrospinal fluid as potential diagnostic tools for early stage Parkinson’s disease. Oncotarget 2018, 9, 17455–17465. [Google Scholar] [CrossRef]
- Gui, Y.; Liu, H.; Zhang, L.; Lv, W.; Hu, X. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget 2015, 6, 37043–37053. [Google Scholar] [CrossRef]
- Marques, T.M.; Kuiperij, B.; Bruinsma, I.B.; Van Rumund, A.; Aerts, M.B.; Esselink, R.A.J.; Bloem, B.R.; Verbeek, M.M. MicroRNAs in Cerebrospinal Fluid as Potential Biomarkers for Parkinson’s Disease and Multiple System Atrophy. Mol. Neurobiol. 2016, 54, 7736–7745. [Google Scholar] [CrossRef]
- Gomes, L.C.; Roser, A.; Jain, G.; Centeno, T.P.; Maass, F.; Schilde, L.; May, C.; Schneider, A.; Bähr, M.; Marcus, K.; et al. MicroRNAs from extracellular vesicles as a signature for Parkinson’s disease. Clin. Transl. Med. 2021, 11, e357. [Google Scholar] [CrossRef]
- Starhof, C.; Hejl, A.-M.; Heegaard, N.H.; Carlsen, A.L.; Burton, M.; Lilje, B.; Winge, K. The biomarker potential of cell-free microRNA from cerebrospinal fluid in Parkinsonian Syndromes. Mov. Disord. 2018, 34, 246–254. [Google Scholar] [CrossRef]
- Van den Berg, M.M.J.; Krauskopf, J.; Ramaekers, J.G.; Kleinjans, J.C.S.; Prickaerts, J.; Briedé, J.J. Circulating microRNAs as potential biomarkers for psychiatric and neurodegenerative disorders. Prog. Neurobiol. 2020, 185, 101732. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).