The Complex Biological Effects of Pectin: Galectin-3 Targeting as Potential Human Health Improvement?
Abstract
1. Introduction
2. Basic Pectin Molecular Aspects
2.1. Pectin Molecular Weight
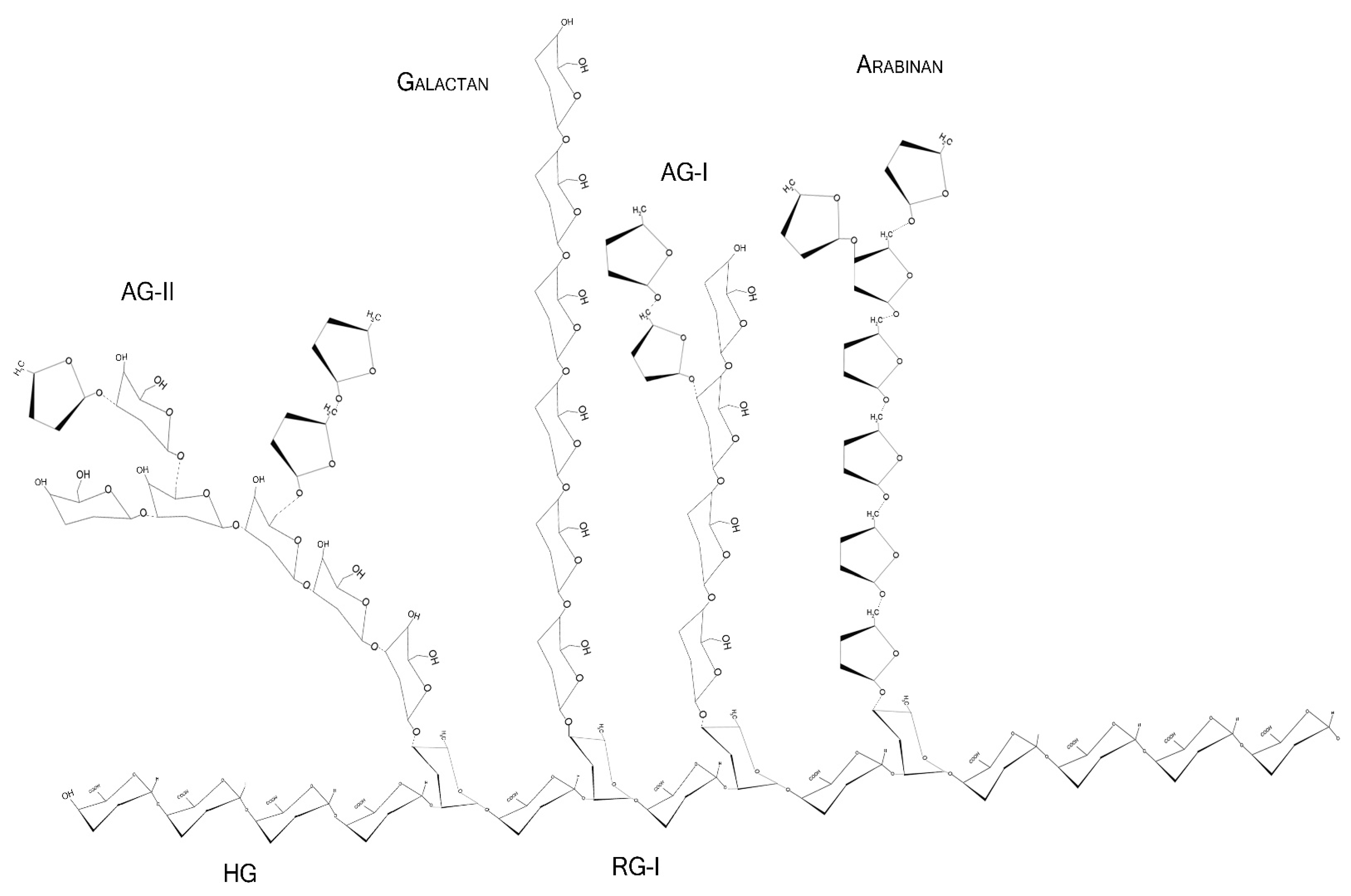
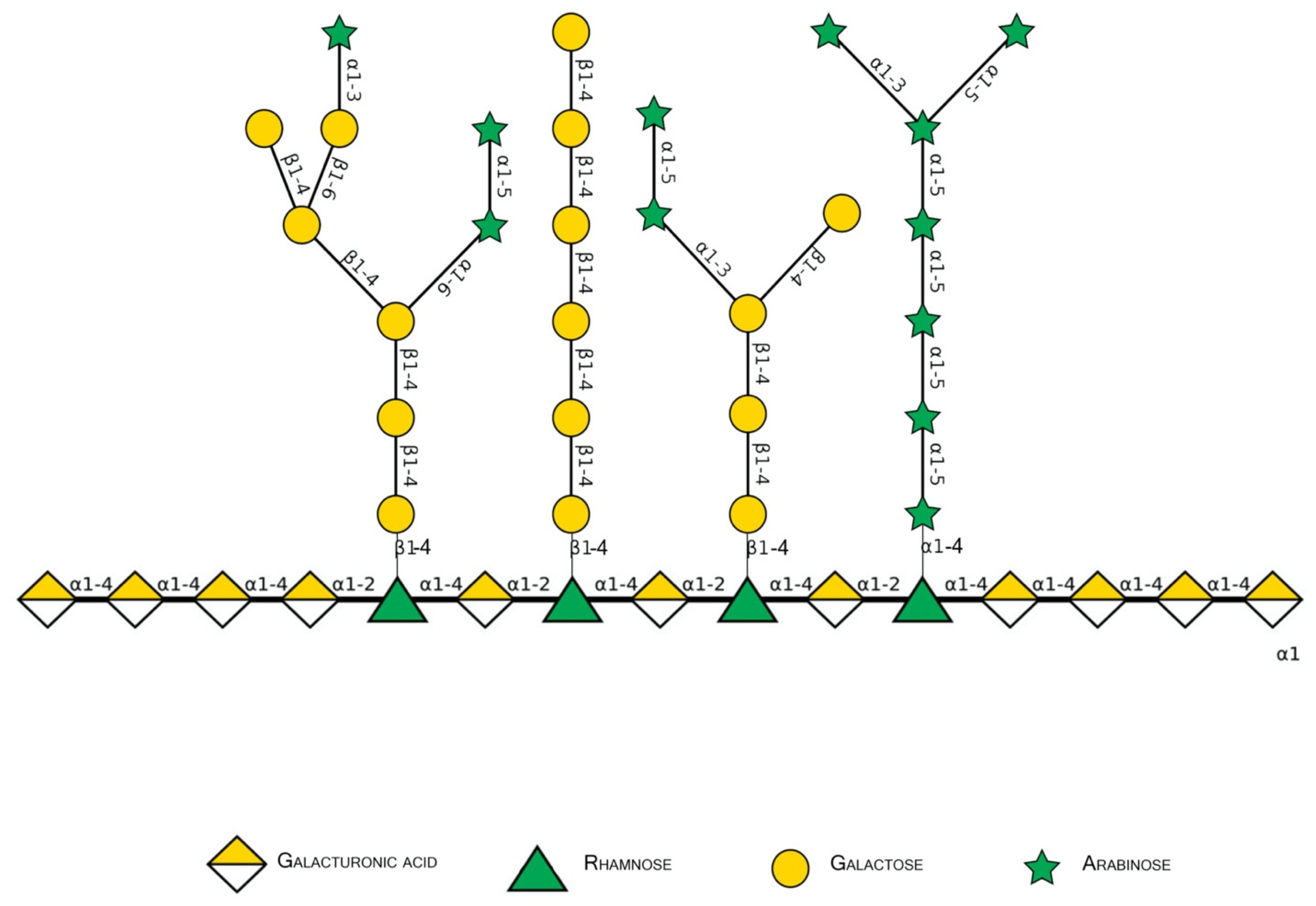
2.2. Monosaccharides, Backbone, and Side Chains
2.3. Esterification Degree
2.4. Rheological Properties
2.5. Food Source
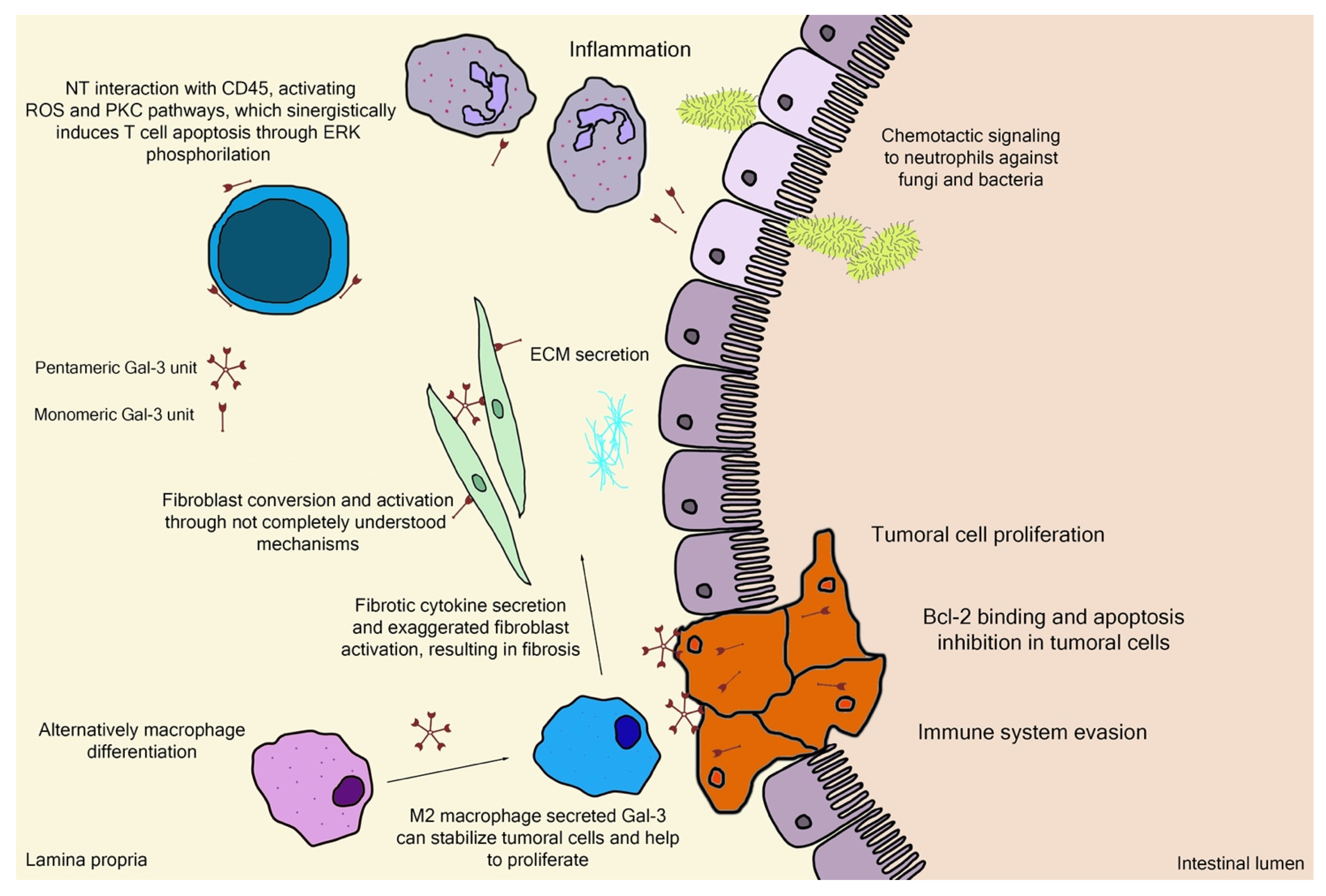
3. Gal-3 Binding Sites and Pectin Interactions
4. Pectin and Gal-3 Controversies
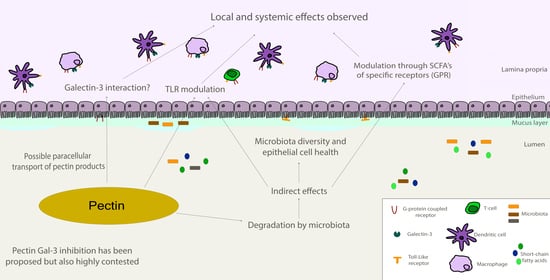
5. Pectin as Dietary Fiber: Some of the Gal-3 Independent Beneficial Effects to Human Health
6. Should Gal-3 Inhibition Be the Main Biological Effect Expected from Pectin?
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef]
- Maxwell, E.G.; Belshaw, N.J.; Waldron, K.W.; Morris, V.J. Pectin—An emerging new bioactive food polysaccharide. Trends Food Sci. Technol. 2012, 24, 64–73. [Google Scholar] [CrossRef]
- Cui, L.; Wang, J.; Huang, R.; Tan, Y.; Zhang, F.; Zhou, Y.; Sun, L. Analysis of pectin from Panax ginseng flower buds and their binding activities to galectin-3. Int. J. Biol. Macromol. 2019, 128, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Fabi, J.P.; Seymour, G.B.; Graham, N.S.; Broadley, M.R.; May, S.T.; Lajolo, F.M.; Cordenunsi, B.R.; Oliveira do Nascimento, J.R. Analysis of ripening-related gene expression in papaya using an Arabidopsis-based microarray. BMC Plant Biol. 2012, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Fabi, J.P.; Broetto, S.G.; da Silva, S.L.G.L.; Zhong, S.; Lajolo, F.M.; do Nascimento, J.R.O. Analysis of papaya cell wall-related genes during fruit ripening indicates a central role of polygalacturonases during pulp softening. PLoS ONE 2014, 9, e105685. [Google Scholar] [CrossRef] [PubMed]
- De Freitas Pedrosa, L.; Lopes, R.G.; Fabi, J.P. The acid and neutral fractions of pectins isolated from ripe and overripe papayas differentially affect galectin-3 inhibition and colon cancer cell growth. Int. J. Biol. Macromol. 2020, 164, 2681–2690. [Google Scholar] [CrossRef] [PubMed]
- Do Prado, S.B.R.; Ferreira, G.F.; Harazono, Y.; Shiga, T.M.; Raz, A.; Carpita, N.C.; Fabi, J.P. Ripening-induced chemical modifications of papaya pectin inhibit cancer cell proliferation. Sci. Rep. 2017, 7, 16564. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol nomenclature for graphical representations of glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C.; Huang, R.; Song, J.; Li, D.; Xia, M. Butyrate from pectin fermentation inhibits intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E-deficient mice. J. Nutr. Biochem. 2018, 56, 175–182. [Google Scholar] [CrossRef]
- Li, W.; Zhang, K.; Yang, H. Pectin Alleviates High Fat (Lard) Diet-Induced Nonalcoholic Fatty Liver Disease in Mice: Possible Role of Short-Chain Fatty Acids and Gut Microbiota Regulated by Pectin. J. Agric. Food Chem. 2018, 66, 8015–8025. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.; Berger, A.; Mensink, R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012, 66, 591–599. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, M.; Yang, Z.; Pan, S. Anti-diabetic effect of citrus pectin in diabetic rats and potential mechanism via PI3K/Akt signaling pathway. Int. J. Biol. Macromol. 2016, 89, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Fotschki, B.; Jurgoński, A.; Juśkiewicz, J.; Kołodziejczyk, K.; Sójka, M. Effects of dietary addition of a low-pectin apple fibre preparation on rats. Pol. J. Food Nutr. Sci. 2014, 64, 193–199. [Google Scholar] [CrossRef]
- Kunzmann, A.T.; Coleman, H.G.; Huang, W.-Y.; Kitahara, C.M.; Cantwell, M.M.; Berndt, S.I. Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am. J. Clin. Nutr. 2015, 102, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Tang, J.-W.; Owusu, L.; Sun, M.-Z.; Wu, J.; Zhang, J. Galectin-3 in cancer. Clin. Chim. Acta 2014, 431, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One molecule for an alphabet of diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef]
- Fortuna-Costa, A.; Gomes, A.M.; Kozlowski, E.O.; Stelling, M.P.; Pavão, M.S.G. Extracellular galectin-3 in tumor progression and metastasis. Front. Oncol. 2014, 4. [Google Scholar] [CrossRef]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.W.; Ho, J.E.; Liu, F.-T.; de Boer, R.A. Galectin-3 activation and inhibition in heart failure and cardiovascular disease: An update. Theranostics 2018, 8, 593–609. [Google Scholar] [CrossRef]
- Filipová, M.; Bojarová, P.; Rodrigues Tavares, M.; Bumba, L.; Elling, L.; Chytil, P.; Gunár, K.; Křen, V.; Etrych, T.; Janoušková, O. Glycopolymers for Efficient Inhibition of Galectin-3: In Vitro Proof of Efficacy Using Suppression of T Lymphocyte Apoptosis and Tumor Cell Migration. Biomacromolecules 2020, 21, 3122–3133. [Google Scholar] [CrossRef]
- Jia, J.; Claude-Taupin, A.; Gu, Y.; Choi, S.W.; Peters, R.; Bissa, B.; Mudd, M.H.; Allers, L.; Pallikkuth, S.; Lidke, K.A.; et al. Galectin-3 Coordinates a Cellular System for Lysosomal Repair and Removal. Dev. Cell 2020, 52, 69–87.e8. [Google Scholar] [CrossRef]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Rhamnogalacturonan i containing homogalacturonan inhibits colon cancer cell proliferation by decreasing ICAM1 expression. Carbohydr. Polym. 2015, 132, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-L.; Huang, E.-Y.; Jhu, E.-W.; Huang, Y.-H.; Su, W.-H.; Chuang, P.-C.; Yang, K.D. Overexpression of galectin-3 enhances migration of colon cancer cells related to activation of the K-Ras-Raf-Erk1/2 pathway. J. Gastroenterol. 2013, 48, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ji, B.; Ramachandran, V.; Wang, H.; Hafley, M.; Logsdon, C.; Bresalier, R.S. Overexpressed galectin-3 in pancreatic cancer induces cell proliferation and invasion by binding ras and activating ras signaling. PLoS ONE 2012, 7, e42699. [Google Scholar] [CrossRef]
- Margadant, C.; Van Den Bout, I.; Van Boxtel, A.L.; Thijssen, V.L.; Sonnenberg, A. Epigenetic regulation of galectin-3 expression by β1 integrins promotes cell adhesion and migration. J. Biol. Chem. 2012, 287, 44684–44693. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, H.; Geng, J.; Zheng, X.; Wei, H.; Sun, R.; Tian, Z. Tumor-released galectin-3, a soluble inhibitory ligand of human NKp30, plays an important role in tumor escape from NK cell attack. J. Biol. Chem. 2014, 289, 33311–33319. [Google Scholar] [CrossRef] [PubMed]
- Voss, J.J.L.P.; Ford, C.A.; Petrova, S.; Melville, L.; Paterson, M.; Pound, J.D.; Holland, P.; Giotti, B.; Freeman, T.C.; Gregory, C.D. Modulation of macrophage antitumor potential by apoptotic lymphoma cells. Cell Death Differ. 2017, 24, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Liu, L.; Zhao, Z.; Zhang, Z.; Guan, Y.; Cheng, H.; Zhou, Y.; Tai, G. The N-terminal tail coordinates with carbohydrate recognition domain to mediate galectin-3 induced apoptosis in T cells. Oncotarget 2017, 8, 49824–49838. [Google Scholar] [CrossRef]
- Freichel, T.; Heine, V.; Laaf, D.; Mackintosh, E.E.; Sarafova, S.; Elling, L.; Snyder, N.L.; Hartmann, L. Sequence-Defined Heteromultivalent Precision Glycomacromolecules Bearing Sulfonated/Sulfated Nonglycosidic Moieties Preferentially Bind Galectin-3 and Delay Wound Healing of a Galectin-3 Positive Tumor Cell Line in an In Vitro Wound Scratch Assay. Macromol. Biosci. 2020, 20, 2000163. [Google Scholar] [CrossRef]
- Laaf, D.; Bojarová, P.; Elling, L.; Křen, V. Galectin—Carbohydrate Interactions in Biomedicine and Biotechnology. Trends Biotechnol. 2019, 37, 402–415. [Google Scholar] [CrossRef]
- Rajput, V.K.; MacKinnon, A.; Mandal, S.; Collins, P.; Blanchard, H.; Leffler, H.; Sethi, T.; Schambye, H.; Mukhopadhyay, B.; Nilsson, U.J. A Selective Galactose-Coumarin-Derived Galectin-3 Inhibitor Demonstrates Involvement of Galectin-3-glycan Interactions in a Pulmonary Fibrosis Model. J. Med. Chem. 2016, 59, 8141–8147. [Google Scholar] [CrossRef] [PubMed]
- Do Prado, S.B.R.; Santos, G.R.C.; Mourão, P.A.S.; Fabi, J.P. Chelate-soluble pectin fraction from papaya pulp interacts with galectin-3 and inhibits colon cancer cell proliferation. Int. J. Biol. Macromol. 2019, 126, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zheng, J.; Hu, W.; Zheng, X.; He, Q.; Linhardt, R.J.; Ye, X.; Chen, S. Structure-activity relationship of Citrus segment membrane RG-I pectin against Galectin-3: The galactan is not the only important factor. Carbohydr. Polym. 2020, 245, 116526. [Google Scholar] [CrossRef]
- Zhang, T.; Lan, Y.; Zheng, Y.; Liu, F.; Zhao, D.; Mayo, K.H.; Zhou, Y.; Tai, G. Identification of the bioactive components from pH-modified citrus pectin and their inhibitory effects on galectin-3 function. Food Hydrocoll. 2016, 58, 113–119. [Google Scholar] [CrossRef]
- Do Nascimento Oliveira, A.; de Almeida Paula, D.; de Oliveira, E.B.; Saraiva, S.H.; Stringheta, P.C.; Ramos, A.M. Optimization of pectin extraction from Ubá mango peel through surface response methodology. Int. J. Biol. Macromol. 2018, 113, 395–402. [Google Scholar] [CrossRef]
- Chan, S.-Y.; Choo, W.-S. Effect of extraction conditions on the yield and chemical properties of pectin from cocoa husks. Food Chem. 2013, 141, 3752–3758. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Moates, G.K.; Wellner, N.; Waldron, K.W.; Azeredo, H.M.C. Pectin extraction from pomegranate peels with citric acid. Int. J. Biol. Macromol. 2016, 88, 373–379. [Google Scholar] [CrossRef]
- Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Pereira, P.H.F.; Moates, G.K.; Wellner, N.; Mazzetto, S.E.; Waldron, K.W.; Azeredo, H.M.C. Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chem. 2016, 198, 113–118. [Google Scholar] [CrossRef]
- Srivastava, P.; Malviya, R. Sources of pectin, extraction and its applications in pharmaceutical industry—An overview. Indian J. Nat. Prod. Resour. 2011, 2, 10–18. [Google Scholar]
- Gao, X.; Zhi, Y.; Sun, L.; Peng, X.; Zhang, T.; Xue, H.; Tai, G.; Zhou, Y. The inhibitory effects of a rhamnogalacturonan I (RG-I) domain from ginseng Pectin on galectin-3 and its structure-activity relationship. J. Biol. Chem. 2013, 288, 33953–33965. [Google Scholar] [CrossRef]
- Leclere, L.; Van Cutsem, P.; Michiels, C. Anti-cancer activities of pH- or heat-modified pectin. Front. Pharmacol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Leclere, L.; Fransolet, M.; Cote, F.; Cambier, P.; Arnould, T.; Van Cutsem, P.; Michiels, C. Heat-modified citrus pectin induces apoptosis-like cell death and autophagy in HepG2 and A549 cancer cells. PLoS ONE 2015, 10, e0115831. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Bailina, Y.; Ge, Z.; Ding, T.; Ye, X.; Liu, D. Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. J. Food Eng. 2014, 126, 72–81. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Xu, Y.; Cao, Y.; Jiang, Z.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food Chem. 2015, 178, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, W.; Zou, M.; Lv, R.; Wang, D.; Hou, F.; Feng, H.; Ma, X.; Zhong, J.; Ding, T.; et al. Applications of power ultrasound in oriented modification and degradation of pectin: A review. J. Food Eng. 2018, 234, 98–107. [Google Scholar] [CrossRef]
- Zouambia, Y.; Ettoumi, K.Y.; Krea, M.; Moulai-Mostefa, N. A new approach for pectin extraction: Electromagnetic induction heating. Arab. J. Chem. 2017, 10, 480–487. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, H.; Zhi, Z.; Zhang, H.; Linhardt, R.J.; Zhang, F.; Chen, S.; Ye, X. Extraction temperature is a decisive factor for the properties of pectin. Food Hydrocoll. 2021, 112, 106160. [Google Scholar] [CrossRef]
- Blanchard, H.; Bum-Erdene, K.; Bohari, M.H.; Yu, X. Galectin-1 inhibitors and their potential therapeutic applications: A patent review. Expert Opin. Ther. Pat. 2016, 26, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Laaf, D.; Bojarová, P.; Pelantová, H.; Křen, V.; Elling, L. Tailored Multivalent Neo-Glycoproteins: Synthesis, Evaluation, and Application of a Library of Galectin-3-Binding Glycan Ligands. Bioconjugate Chem. 2017, 28, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Stegmayr, J.; Lepur, A.; Kahl-Knutson, B.; Aguilar-Moncayo, M.; Klyosov, A.A.; Field, R.A.; Oredsson, S.; Nilsson, U.J.; Leffler, H. Low or No Inhibitory Potency of the Canonical Galectin Carbohydrate-binding Site by Pectins and Galactomannans. J. Biol. Chem. 2016, 291, 13318–13334. [Google Scholar] [CrossRef]
- Gunning, A.P.; Bongaerts, R.J.M.; Morris, V.J. Recognition of galactan components of pectin by galectin-3. FASEB J. 2009, 23, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yu, L.; Shi, Y.; Lu, J.; Teng, H.; Zhou, Y.; Sun, L. Structural characterization of a rhamnogalacturonan I domain from ginseng and its inhibitory effect on galectin-3. Molecules 2017, 22, 1016. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ma, P.; Shuai, M.; Huang, J.; Sun, C.; Yao, X.; Chen, Z.; Min, X.; Zhang, T. Analysis of the water-soluble polysaccharides from Camellia japonica pollen and their inhibitory effects on galectin-3 function. Int. J. Biol. Macromol. 2020, 159, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Wang, P.; Niu, B.; Kang, J. Environmental stress stability of pectin-stabilized resveratrol liposomes with different degree of esterification. Int. J. Biol. Macromol. 2018, 119, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, G.E.; Simas-Tosin, F.F.; Iacomini, M.; Gorin, P.A.J.; Cordeiro, L.M.C. Rheological behavior of high methoxyl pectin from the pulp of tamarillo fruit (Solanum betaceum). Carbohydr. Polym. 2016, 139, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, U.S.; Pietsch, V.L.; Rentschler, C.; Kurz, T.; Endreß, H.U.; Schuchmann, H.P. Influence of the degree of esterification on the emulsifying performance of conjugates formed between whey protein isolate and citrus pectin. Food Hydrocoll. 2016, 56, 1–8. [Google Scholar] [CrossRef]
- Schmidt, U.S.; Schütz, L.; Schuchmann, H.P. Interfacial and emulsifying properties of citrus pectin: Interaction of pH, ionic strength and degree of esterification. Food Hydrocoll. 2017, 62, 288–298. [Google Scholar] [CrossRef]
- Jacob, E.M.; Borah, A.; Jindal, A.; Pillai, S.C.; Yamamoto, Y.; Maekawa, T.; Kumar, D.N.S. Synthesis and characterization of citrus-derived pectin nanoparticles based on their degree of esterification. J. Mater. Res. 2020, 35, 1514–1522. [Google Scholar] [CrossRef]
- Wan, L.; Chen, Q.; Huang, M.; Liu, F.; Pan, S. Physiochemical, rheological and emulsifying properties of low methoxyl pectin prepared by high hydrostatic pressure-assisted enzymatic, conventional enzymatic, and alkaline de-esterification: A comparison study. Food Hydrocoll. 2019, 93, 146–155. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Extraction, purification and characterization of pectin from alternative sources with potential technological applications. Food Res. Int. 2018, 113, 327–350. [Google Scholar] [CrossRef]
- Begum, R.; Yusof, Y.A.; Aziz, M.G.; Uddin, M.B. Structural and functional properties of pectin extracted from jackfruit (Artocarpus heterophyllus) waste: Effects of drying. Int. J. Food Prop. 2017, 20, S190–S201. [Google Scholar] [CrossRef]
- Karnik, D.; Wicker, L. Emulsion stability of sugar beet pectin fractions obtained by isopropanol fractionation. Food Hydrocoll. 2018, 74, 249–254. [Google Scholar] [CrossRef]
- Juttulapa, M.; Piriyaprasarth, S.; Takeuchi, H.; Sriamornsak, P. Effect of high-pressure homogenization on stability of emulsions containing zein and pectin. Asian J. Pharm. Sci. 2017, 12, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Petkowicz, C.L.O.; Vriesmann, L.C.; Williams, P.A. Pectins from food waste: Extraction, characterization and properties of watermelon rind pectin. Food Hydrocoll. 2017, 65, 57–67. [Google Scholar] [CrossRef]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, Characterisation, and Application of Pectin from Tropical and Sub-Tropical Fruits: A Review. Food Rev. Int. 2020, 38, 282–312. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, X.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason. Sonochemistry 2013, 20, 222–231. [Google Scholar] [CrossRef]
- Basanta, M.F.; Ponce, N.M.A.; Rojas, A.M.; Stortz, C.A. Effect of extraction time and temperature on the characteristics of loosely bound pectins from Japanese plum. Carbohydr. Polym. 2012, 89, 230–235. [Google Scholar] [CrossRef]
- Kosmala, M.; Milala, J.; Kołodziejczyk, K.; Markowski, J.; Zbrzeźniak, M.; Renard, C.M.G.C. Dietary fiber and cell wall polysaccharides from plum (Prunus domestica L.) fruit, juice and pomace: Comparison of composition and functional properties for three plum varieties. Food Res. Int. 2013, 54, 1787–1794. [Google Scholar] [CrossRef]
- Moreno, L.; Nascimento, R.F.; Zielinski, A.A.F.; Wosiacki, G.; Canteri, M.H.G. Extraction and characterization of pectic substances in Myrciaria cauliflora (Jaboticaba sabará) fruit. Rev. Strict. Sensu 2016, 1, 1–11. [Google Scholar] [CrossRef][Green Version]
- Sayah, M.Y.; Chabir, R.; Benyahia, H.; Kandri, Y.R.; Chahdi, F.O.; Touzani, H.; Errachidi, F. Yield, esterification degree and molecular weight evaluation of pectins isolated from orange and grapefruit peels under different conditions. PLoS ONE 2016, 11, e0161751. [Google Scholar] [CrossRef]
- Hao, M.; Yuan, X.; Cheng, H.; Xue, H.; Zhang, T.; Zhou, Y.; Tai, G. Comparative studies on the anti-tumor activities of high temperature- and pH-modified citrus pectins. Food Funct. 2013, 4, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Do Prado, S.B.R.; Melfi, P.R.; Castro-Alves, V.C.; Broetto, S.G.; Araújo, E.S.; Do Nascimento, J.R.O.; Fabi, J.P. Physiological degradation of pectin in papaya cell walls: Release of long chains galacturonans derived from insoluble fractions during postharvest fruit ripening. Front. Plant Sci. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Prado, S.B.R.; Beukema, M.; Jermendi, E.; Schols, H.A.; de Vos, P.; Fabi, J.P. Pectin Interaction with Immune Receptors is Modulated by Ripening Process in Papayas. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Flores-Ibarra, A.; Vértesy, S.; Medrano, F.J.; Gabius, H.J.; Romero, A. Crystallization of a human galectin-3 variant with two ordered segments in the shortened N-terminal tail. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Su, J.; Zhang, T.; Wang, P.; Liu, F.; Tai, G.; Zhou, Y. The water network in galectin-3 ligand binding site guides inhibitor design. Acta Biochim. Biophys. Sin. 2015, 47, 192–198. [Google Scholar] [CrossRef]
- Ippel, H.; Miller, M.C.; Vértesy, S.; Zheng, Y.; Cañada, F.J.; Suylen, D.; Umemoto, K.; Romanò, C.; Hackeng, T.; Tai, G.; et al. Intra- and intermolecular interactions of human galectin-3: Assessment by full-assignment-based NMR. Glycobiology 2016, 26, 888–903. [Google Scholar] [CrossRef]
- Kim, S.-J.; Chun, K.-H. Non-classical role of Galectin-3 in cancer progression: Translocation to nucleus by carbohydrate-recognition independent manner. BMB Rep. 2020, 53, 173–180. [Google Scholar] [CrossRef]
- Ruvolo, P.P. Galectin 3 as a guardian of the tumor microenvironment. Biochim. Biophys. Acta—Mol. Cell Res. 2016, 1863, 427–437. [Google Scholar] [CrossRef]
- Chan, Y.-C.; Lin, H.-Y.; Tu, Z.; Kuo, Y.-H.; Hsu, S.-T.D.; Lin, C.-H. Dissecting the structure–Activity relationship of galectin—Ligand interactions. Int. J. Mol. Sci. 2018, 19, 392. [Google Scholar] [CrossRef]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Miller, M.C.; Zheng, Y.; Zhang, Z.; Xue, H.; Zhao, D.; Su, J.; Mayo, K.H.; Zhou, Y.; Tai, G. Macromolecular assemblies of complex polysaccharides with galectin-3 and their synergistic effects on function. Biochem. J. 2017, 474, 3849–3868. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Ippel, H.; Suylen, D.; Klyosov, A.A.; Traber, P.G.; Hackeng, T.; Mayo, K.H. Binding of polysaccharides to human galectin-3 at a noncanonical site in its carbohydrate recognition domain. Glycobiology 2015, 26, 88–99. [Google Scholar] [CrossRef]
- Hu, S.; Kuwabara, R.; Beukema, M.; Ferrari, M.; de Haan, B.J.; Walvoort, M.T.C.; de Vos, P.; Smink, A.M. Low methyl-esterified pectin protects pancreatic β-cells against diabetes-induced oxidative and inflammatory stress via galectin-3. Carbohydr. Polym. 2020, 249, 116863. [Google Scholar] [CrossRef]
- Xu, G.-R.; Zhang, C.; Yang, H.-X.; Sun, J.-H.; Zhang, Y.; Yao, T.-T.; Li, Y.; Ruan, L.; An, R.; Li, A.-Y. Modified citrus pectin ameliorates myocardial fibrosis and inflammation via suppressing galectin-3 and TLR4/MyD88/NF-κB signaling pathway. Biomed. Pharmacother. 2020, 126, 110071. [Google Scholar] [CrossRef]
- Ilmer, M.; Mazurek, N.; Byrd, J.C.; Ramirez, K.; Hafley, M.; Alt, E.; Vykoukal, J.; Bresalier, R.S. Cell surface galectin-3 defines a subset of chemoresistant gastrointestinal tumor-initiating cancer cells with heightened stem cell characteristics. Cell Death Dis. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Y.; Zhao, D.; Yan, J.; Sun, C.; Zhou, Y.; Tai, G. Multiple approaches to assess pectin binding to galectin-3. Int. J. Biol. Macromol. 2016, 91, 994–1001. [Google Scholar] [CrossRef]
- Zheng, Y.; Su, J.; Miller, M.C.; Geng, J.; Xu, X.; Zhang, T.; Mayzel, M.; Zhou, Y.; Mayo, K.H.; Tai, G. Topsy-turvy binding of negatively-charged homogalacturonan oligosaccharides to galectin-3. Glycobiology 2020, 31, 341–350. [Google Scholar] [CrossRef]
- Miller, M.C.; Zheng, Y.; Zhou, Y.; Tai, G.; Mayo, K.H. Galectin-3 binds selectively to the terminal, non-reducing end of β(1→4)-galactans, with overall affinity increasing with chain length. Glycobiology 2019, 29, 74–84. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, F.; Liu, X.; St. Ange, K.; Zhang, A.; Li, Q.; Linhardt, R.J. Isolation of a lectin binding rhamnogalacturonan-I containing pectic polysaccharide from pumpkin. Carbohydr. Polym. 2017, 163, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Zheng, Y.; Yan, J.; Zhou, Y.; Tai, G.; Mayo, K.H. Novel polysaccharide binding to the N-terminal tail of galectin-3 is likely modulated by proline isomerization. Glycobiology 2017, 27, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, E.M.; Bovin, N.V. Specificity of human galectins on cell surfaces. Biochemistry 2015, 80, 846–856. [Google Scholar] [CrossRef] [PubMed]
- García Caballero, G.; Beckwith, D.; Shilova, N.V.; Gabba, A.; Kutzner, T.J.; Ludwig, A.-K.; Manning, J.C.; Kaltner, H.; Sinowatz, F.; Cudic, M.; et al. Influence of protein (human galectin-3) design on aspects of lectin activity. Histochem. Cell Biol. 2020, 154, 135–153. [Google Scholar] [CrossRef]
- Gao, X.; Zhi, Y.; Zhang, T.; Xue, H.; Wang, X.; Foday, A.D.; Tai, G.; Zhou, Y. Analysis of the neutral polysaccharide fraction of MCP and its inhibitory activity on galectin-3. Glycoconj. J. 2012, 29, 159–165. [Google Scholar] [CrossRef]
- Farhadi, S.A.; Liu, R.; Becker, M.W.; Phelps, E.A.; Hudalla, G.A. Physical tuning of galectin-3 signaling. Proc. Natl. Acad. Sci. USA 2021, 118, 1–10. [Google Scholar] [CrossRef]
- Cecioni, S.; Imberty, A.; Vidal, S. Glycomimetics versus multivalent glycoconjugates for the design of high affinity lectin ligands. Chem. Rev. 2015, 115, 525–561. [Google Scholar] [CrossRef] [PubMed]
- Hevey, R. Strategies for the development of glycomimetic drug candidates. Pharmaceuticals 2019, 12, 55. [Google Scholar] [CrossRef]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Modified sugar beet pectin induces apoptosis of colon cancer cells via an interaction with the neutral sugar side-chains. Carbohydr. Polym. 2016, 136, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Li, S.; Fan, Y.; Gao, X.; Hao, M.; Wang, J.; Zhang, X.; Tai, G.; Zhou, Y. Comparative studies of the antiproliferative effects of ginseng polysaccharides on HT-29 human colon cancer cells. Med. Oncol. 2011, 28, 175–181. [Google Scholar] [CrossRef]
- Pynam, H.; Dharmesh, S.M. A xylorhamnoarabinogalactan I from Bael (Aegle marmelos L.) modulates UV/DMBA induced skin cancer via galectin-3 & gut microbiota. J. Funct. Foods 2019, 60, 103425. [Google Scholar] [CrossRef]
- Popov, S.V.; Ovodov, Y.S. Polypotency of the immunomodulatory effect of pectins. Biochemistry 2013, 78, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Liu, D.-D.; Ning, H.-M.; Liu, D.; Sun, J.-Y.; Huang, X.-J.; Dong, Y.; Geng, M.-Y.; Yun, S.-F.; Yan, J.; et al. Modified citrus pectin inhibited bladder tumor growth through downregulation of galectin-3. Acta Pharmacol. Sin. 2018, 39, 1885–1893. [Google Scholar] [CrossRef]
- Hossein, G.; Halvaei, S.; Heidarian, Y.; Dehghani-Ghobadi, Z.; Hassani, M.; Hosseini, H.; Naderi, N.; Sheikh Hassani, S. Pectasol-C Modified Citrus Pectin targets Galectin-3-induced STAT3 activation and synergize paclitaxel cytotoxic effect on ovarian cancer spheroids. Cancer Med. 2019, 8, 4315–4329. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elsaad, N.M.; Elkashef, W.F. Modified citrus pectin stops progression of liver fibrosis by inhibiting galectin-3 and inducing apoptosis of stellate cells. Can. J. Physiol. Pharmacol. 2016, 94, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, E.; Ibarrola, J.; Calvier, L.; Fernandez-Celis, A.; Leroy, C.; Cachofeiro, V.; Rossignol, P.; Lopez-Andres, N. Galectin-3 blockade reduces renal fibrosis in two normotensive experimental models of renal damage. PLoS ONE 2016, 11, e0166272. [Google Scholar] [CrossRef]
- Calvier, L.; Miana, M.; Reboul, P.; Cachofeiro, V.; Martinez-Martinez, E.; De Boer, R.A.; Poirier, F.; Lacolley, P.; Zannad, F.; Rossignol, P.; et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 67–75. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chou, C.-H.; Wu, X.-M.; Chang, Y.-Y.; Hung, C.-S.; Chen, Y.-H.; Tzeng, Y.-L.; Wu, V.-C.; Ho, Y.-L.; Hsieh, F.-J.; et al. Aldosterone induced galectin-3 secretion in vitro and in vivo: From cells to humans. PLoS ONE 2014, 9, e95254. [Google Scholar] [CrossRef] [PubMed]
- Calvier, L.; Martinez-Martinez, E.; Miana, M.; Cachofeiro, V.; Rousseau, E.; Sádaba, J.R.; Zannad, F.; Rossignol, P.; López-Andrés, N. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015, 3, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Yang, S.; Li, J.-C.; Feng, J.-X. Galectin 3 inhibition attenuates renal injury progression in cisplatin-induced nephrotoxicity. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, M.; Coutrot, M.; Michel, T.; Boutin, L.; Genest, M.; Poirier, F.; Launay, J.M.; Kane, B.; Kinugasa, S.; Prakoura, N.; et al. Acute Kidney Injury Induces Remote Cardiac Damage and Dysfunction through the Galectin-3 Pathway. JACC Basic Transl. Sci. 2019, 4, 717–732. [Google Scholar] [CrossRef]
- Ibarrola, J.; Matilla, L.; Martínez-Martínez, E.; Gueret, A.; Fernández-Celis, A.; Henry, J.P.; Nicol, L.; Jaisser, F.; Mulder, P.; Ouvrard-Pascaud, A.; et al. Myocardial Injury after Ischemia/Reperfusion Is Attenuated by Pharmacological Galectin-3 Inhibition. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, S.; Hao, X.; Zhang, Y.; Deng, W. Perindopril and a Galectin-3 inhibitor improve ischemic heart failure in rabbits by reducing gal-3 expression and myocardial fibrosis. Front. Physiol. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vergaro, G.; Prud’Homme, M.; Fazal, L.; Merval, R.; Passino, C.; Emdin, M.; Samuel, J.L.; Cohen Solal, A.; Delcayre, C. Inhibition of Galectin-3 Pathway Prevents Isoproterenol-Induced Left Ventricular Dysfunction and Fibrosis in Mice. Hypertension 2016, 67, 606–612. [Google Scholar] [CrossRef]
- Ibarrola, J.; Martínez-Martínez, E.; Sádaba, J.R.; Arrieta, V.; García-Peña, A.; Álvarez, V.; Fernández-Celis, A.; Gainza, A.; Rossignol, P.; Ramos, V.C.; et al. Beneficial effects of galectin-3 blockade in vascular and aortic valve alterations in an experimental pressure overload model. Int. J. Mol. Sci. 2017, 18, 1664. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Zhao, Z.; Lin, Z.; Geng, J.; Guan, Y.; Song, C.; Zhou, Y.; Tai, G. Selective effects of ginseng pectins on galectin-3-mediated T cell activation and apoptosis. Carbohydr. Polym. 2019, 219, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.S.; Liu, E.; Paniagua, S.M.; Sarma, A.A.; Zampierollo, G.; López, B.; Díez, J.; Wang, T.J.; Ho, J.E. Galectin-3 Inhibition With Modified Citrus Pectin in Hypertension. JACC Basic Transl. Sci. 2021, 6, 12–21. [Google Scholar] [CrossRef]
- Portacci, A.; Diaferia, F.; Santomasi, C.; Dragonieri, S.; Boniello, E.; Di Serio, F.; Carpagnano, G.E. Galectin-3 as prognostic biomarker in patients with COVID-19 acute respiratory failure. Respir. Med. 2021, 187, 106556. [Google Scholar] [CrossRef]
- Kuśnierz-Cabala, B.; Maziarz, B.; Dumnicka, P.; Dembiński, M.; Kapusta, M.; Bociąga-Jasik, M.; Winiarski, M.; Garlicki, A.; Grodzicki, T.; Kukla, M. Diagnostic significance of serum galectin-3 in hospitalized patients with COVID-19—A preliminary study. Biomolecules 2021, 11, 1136. [Google Scholar] [CrossRef] [PubMed]
- Gaughan, A.E.; Sethi, T.; Quinn, T.; Hirani, N.; Mills, A.; Annya, M.; Mackinnon, A.; Aslanis, V.; Li, F.; Connor, R.O.; et al. GB0139, an inhaled small molecule inhibitor of galectin-3, in COVID-19 pneumonitis: A randomised, controlled, open-label, phase 2a experimental medicine trial of safety, pharmacokinetics, and potential therapeutic value. medRxiv 2022. [Google Scholar] [CrossRef]
- Sakurai, M.H.; Matsumoto, T.; Kiyohara, H.; Yamada, H. Detection and tissue distribution of anti-ulcer peptic polysaccharides from Bepleurum falcatum by polyclonal antibody. Planta Med. 1996, 62, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Busato, B.; de Almeida Abreu, E.C.; de Oliveira Petkowicz, C.L.; Martinez, G.R.; Noleto, G.R. Pectin from Brassica oleracea var. italica triggers immunomodulating effects in vivo. Int. J. Biol. Macromol. 2020, 161, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, P.; Zhang, H. Pectin in cancer therapy: A review. Trends Food Sci. Technol. 2015, 44, 258–271. [Google Scholar] [CrossRef]
- Majee, S.B.; Avlani, D.; Ghosh, P.; Biswas, G.R. Therapeutic and pharmaceutical benefits of native and modified plant pectin. J. Med. Plants Res. 2018, 12, 1–6. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Huang, Z.-L.; Yang, G.-H.; Lu, W.-Q.; Yu, N.-R. Inhibitory effect of modified citrus pectin on liver metastases in a mouse colon cancer model. World J. Gastroenterol. 2008, 14, 7386–7391. [Google Scholar] [CrossRef]
- Courts, F.L. Profiling of modified citrus pectin oligosaccharide transport across Caco-2 cell monolayers. PharmaNutrition 2013, 1, 22–31. [Google Scholar] [CrossRef]
- Huang, P.-H.; Fu, L.-C.; Huang, C.-S.; Wang, Y.-T.; Wu, M.-C. The uptake of oligogalacturonide and its effect on growth inhibition, lactate dehydrogenase activity and galactin-3 release of human cancer cells. Food Chem. 2012, 132, 1987–1995. [Google Scholar] [CrossRef]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef]
- Fan, Y.; Sun, L.; Yang, S.; He, C.; Tai, G.; Zhou, Y. The roles and mechanisms of homogalacturonan and rhamnogalacturonan I pectins on the inhibition of cell migration. Int. J. Biol. Macromol. 2018, 106, 207–217. [Google Scholar] [CrossRef]
- Shao, A.; Wu, H.; Zhang, J. Letter by Shao et al Regarding Article, “Modified Citrus Pectin Prevents Blood-Brain Barrier Disruption in Mouse Subarachnoid Hemorrhage by Inhibiting Galectin-3”. J. Biol. Chem. 2019, 50, e22. [Google Scholar] [CrossRef]
- Leffler, H. Letter by Leffler Regarding Article, “Modified Citrus Pectin Prevents Blood-Brain Barrier Disruption in Mouse Subarachnoid Hemorrhage by Inhibiting Galectin-3”. J. Biol. Chem. 2019, 50, e136. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Liu, L.; Nakano, F.; Kawakita, F.; Kanamaru, H.; Nakatsuka, Y.; Okada, T.; Suzuki, H. Modified citrus pectin prevents blood-brain barrier disruption in mouse Subarachnoid hemorrhage by inhibiting Galectin-3. Stroke 2018, 49, 2743–2751. [Google Scholar] [CrossRef]
- Hirani, N.; MacKinnon, A.C.; Nicol, L.; Ford, P.; Schambye, H.; Pedersen, A.; Nilsson, U.J.; Leffler, H.; Sethi, T.; Tantawi, S.; et al. Target inhibition of galectin-3 by inhaled TD139 in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2021, 57, 1–13. [Google Scholar] [CrossRef]
- Bumba, L.; Laaf, D.; Spiwok, V.; Elling, L.; Křen, V.; Bojarová, P. Poly-N-acetyllactosamine Neo-glycoproteins as nanomolar ligands of human galectin-3: Binding kinetics and modeling. Int. J. Mol. Sci. 2018, 19, 372. [Google Scholar] [CrossRef] [PubMed]
- Laaf, D.; Steffens, H.; Pelantová, H.; Bojarová, P.; Křen, V.; Elling, L. Chemo-Enzymatic Synthesis of Branched N-Acetyllactosamine Glycan Oligomers for Galectin-3 Inhibition. Adv. Synth. Catal. 2017, 359, 4015–4024. [Google Scholar] [CrossRef]
- Fischöder, T.; Laaf, D.; Dey, C.; Elling, L. Enzymatic synthesis of N-acetyllactosamine (LacNAc) type 1 oligomers and characterization as multivalent galectin ligands. Molecules 2017, 22, 1320. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Havenaar, R. Intestinal health functions of colonic microbial metabolites: A review. Benef. Microbes 2011, 2, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Gurav, A.; Paschall, A.V.; Coe, G.L.; Chaudhary, K.; Cai, Y.; Kolhe, R.; Martin, P.; Browning, D.; Huang, L.; et al. An essential role of Ffar2 (Gpr43) in dietary fibre-mediated promotion of healthy composition of gut microbiota and suppression of intestinal carcinogenesis. Oncogenesis 2016, 5, e238. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396–406. [Google Scholar] [CrossRef]
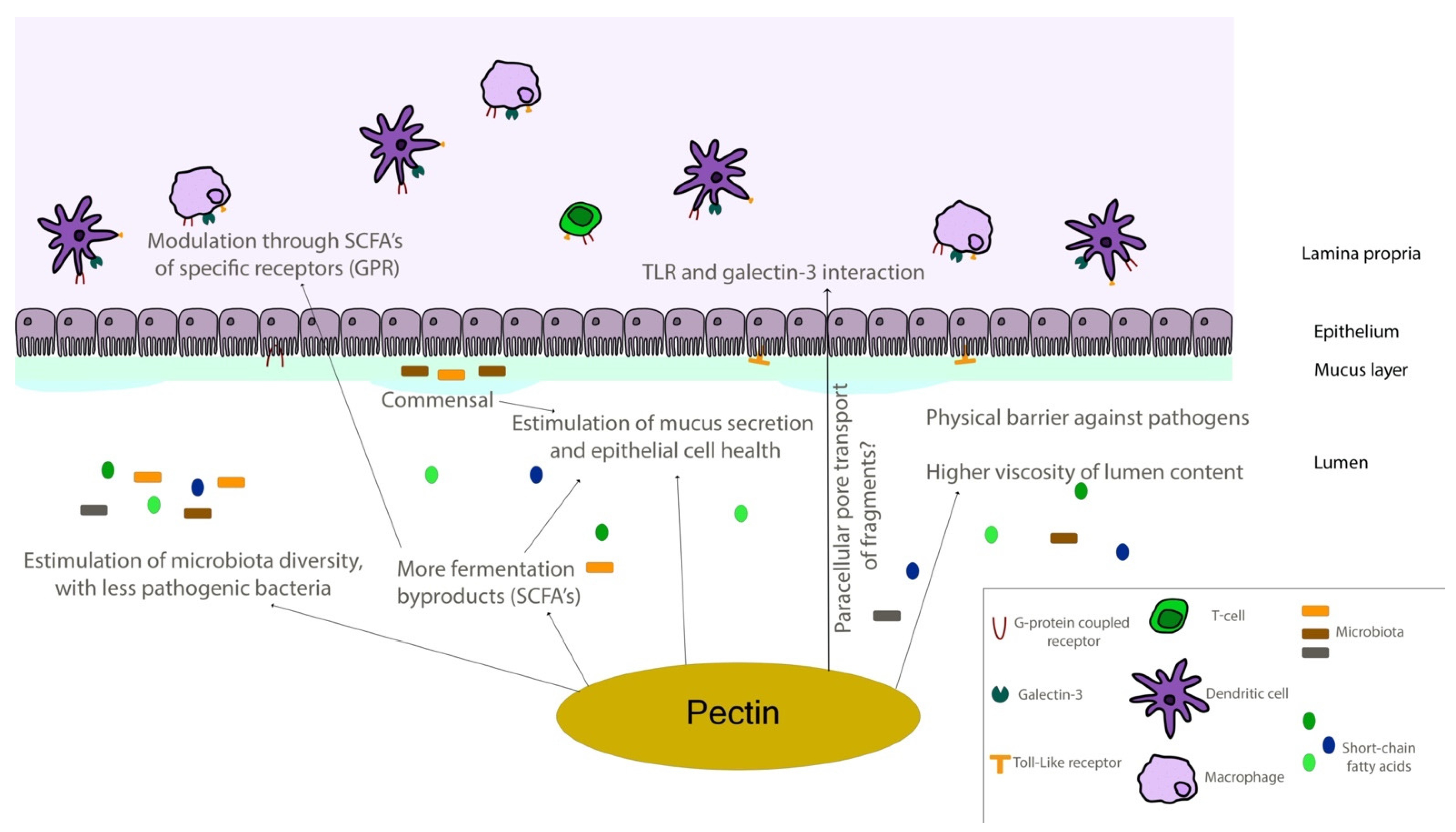
- Beukema, M.; Faas, M.M.; de Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef]
- Tian, L.; Scholte, J.; Borewicz, K.; van den Bogert, B.; Smidt, H.; Scheurink, A.J.W.; Gruppen, H.; Schols, H.A. Effects of pectin supplementation on the fermentation patterns of different structural carbohydrates in rats. Mol. Nutr. Food Res. 2016, 60, 2256–2266. [Google Scholar] [CrossRef]
- Tian, L.; Bruggeman, G.; van den Berg, M.; Borewicz, K.; Scheurink, A.J.W.; Bruininx, E.; de Vos, P.; Smidt, H.; Schols, H.A.; Gruppen, H. Effects of pectin on fermentation characteristics, carbohydrate utilization, and microbial community composition in the gastrointestinal tract of weaning pigs. Mol. Nutr. Food Res. 2017, 61, 1–10. [Google Scholar] [CrossRef]
- Ferreira-Lazarte, A.; Moreno, F.J.; Cueva, C.; Gil-Sánchez, I.; Villamiel, M. Behaviour of citrus pectin during its gastrointestinal digestion and fermentation in a dynamic simulator (simgi®). Carbohydr. Polym. 2019, 207, 382–390. [Google Scholar] [CrossRef]
- Chen, J.; Liang, R.-H.; Liu, W.; Li, T.; Liu, C.-M.; Wu, S.-S.; Wang, Z.-J. Pectic-oligosaccharides prepared by dynamic high-pressure microfluidization and their in vitro fermentation properties. Carbohydr. Polym. 2013, 91, 175–182. [Google Scholar] [CrossRef]
- Onumpai, C.; Kolida, S.; Bonnin, E.; Rastall, R.A. Microbial utilization and selectivity of pectin fractions with various structures. Appl. Environ. Microbiol. 2011, 77, 5747–5754. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Khan, T.M.; Duncan, S.H.; Harmsen, H.J.M.; Garcia-Gil, L.J.; Flint, H.J. Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host-derived substrates for growth. Appl. Environ. Microbiol. 2012, 78, 420–428. [Google Scholar] [CrossRef]
- Scott, K.P.; Martin, J.C.; Duncan, S.H.; Flint, H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 2014, 87, 30–40. [Google Scholar] [CrossRef]
- Merheb, R.; Abdel-Massih, R.M.; Karam, M.C. Immunomodulatory effect of natural and modified Citrus pectin on cytokine levels in the spleen of BALB/c mice. Int. J. Biol. Macromol. 2019, 121, 1–5. [Google Scholar] [CrossRef]
- Amorim, J.C.; Vriesmann, L.C.; Petkowicz, C.L.O.; Martinez, G.R.; Noleto, G.R. Modified pectin from Theobroma cacao induces potent pro-inflammatory activity in murine peritoneal macrophage. Int. J. Biol. Macromol. 2016, 92, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, G.E.; Winnischofer, S.M.B.; Ramirez, M.I.; Iacomini, M.; Cordeiro, L.M.C. The influence of sweet pepper pectin structural characteristics on cytokine secretion by THP-1 macrophages. Food Res. Int. 2017, 102, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.V.; Ovodova, R.G.; Golovchenko, V.V.; Popova, G.Y.; Viatyasev, F.V.; Shashkov, A.S.; Ovodov, Y.S. Chemical composition and anti-inflammatory activity of a pectic polysaccharide isolated from sweet pepper using a simulated gastric medium. Food Chem. 2011, 124, 309–315. [Google Scholar] [CrossRef]
- Ishisono, K.; Yabe, T.; Kitaguchi, K. Citrus pectin attenuates endotoxin shock via suppression of Toll-like receptor signaling in Peyer’s patch myeloid cells. J. Nutr. Biochem. 2017, 50, 38–45. [Google Scholar] [CrossRef]
- Vogt, L.M.; Sahasrabudhe, N.M.; Ramasamy, U.; Meyer, D.; Pullens, G.; Faas, M.M.; Venema, K.; Schols, H.A.; de Vos, P. The impact of lemon pectin characteristics on TLR activation and T84 intestinal epithelial cell barrier function. J. Funct. Foods 2016, 22, 398–407. [Google Scholar] [CrossRef]
- Wang, H.; Bi, H.; Gao, T.; Zhao, B.; Ni, W.; Liu, J. A homogalacturonan from Hippophae rhamnoides L. Berries enhance immunomodulatory activity through TLR4/MyD88 pathway mediated activation of macrophages. Int. J. Biol. Macromol. 2018, 107, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N.; Noh, K.T.; Jeong, Y.-I.; Jung, I.D.; Kang, H.K.; Cha, G.S.; Lee, S.J.; Seo, J.K.; Kang, D.H.; Hwang, T.-H.; et al. Rhamnogalacturonan II is a Toll-like receptor 4 agonist that inhibits tumor growth by activating dendritic cell-mediated CD8+ T cells. Exp. Mol. Med. 2013, 45, e8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sahasrabudhe, N.M.; Beukema, M.; Tian, L.; Troost, B.; Scholte, J.; Bruininx, E.; Bruggeman, G.; van den Berg, M.; Scheurink, A.; Schols, H.A.; et al. Dietary fiber pectin directly blocks toll-like receptor 2—1 and prevents doxorubicin-induced ileitis. Front. Immunol. 2018, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Kuwabara, R.; Chica, C.E.N.; Smink, A.M.; Koster, T.; Medina, J.D.; de Haan, B.J.; Beukema, M.; Lakey, J.R.T.; García, A.J.; et al. Toll-like receptor 2-modulating pectin-polymers in alginate-based microcapsules attenuate immune responses and support islet-xenograft survival. Biomaterials 2021, 266, 120460. [Google Scholar] [CrossRef] [PubMed]
- Kolatsi-Joannou, M.; Price, K.L.; Winyard, P.J.; Long, D.A. Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS ONE 2011, 6, e18683. [Google Scholar] [CrossRef]
- Mai, C.W.; Kang, Y.B.; Pichika, M.R. Should a Toll-like receptor 4 (TLR-4) agonist or antagonist be designed to treat cancer? TLR-4: Its expression and effects in the ten most common cancers. OncoTargets Ther. 2013, 6, 1573–1587. [Google Scholar] [CrossRef]
- Kaczanowska, S.; Joseph, A.M.; Davila, E. TLR agonists: Our best frenemy in cancer immunotherapy. J. Leukoc. Biol. 2013, 93, 847–863. [Google Scholar] [CrossRef]
- Pradere, J.P.; Dapito, D.H.; Schwabe, R.F. The Yin and Yang of Toll-like receptors in cancer. Oncogene 2014, 33, 3485–3495. [Google Scholar] [CrossRef]
- Wang, H.; Gao, T.; Du, Y.; Yang, H.; Wei, L.; Bi, H.; Ni, W. Anticancer and immunostimulating activities of a novel homogalacturonan from Hippophae rhamnoides L. berry. Carbohydr. Polym. 2015, 131, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Sato-Kaneko, F.; Yao, S.; Ahmadi, A.; Zhang, S.S.; Hosoya, T.; Kaneda, M.M.; Varner, J.A.; Pu, M.; Messer, K.S.; Guiducci, C.; et al. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight 2017, 2, 1–18. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Karim, N.; Gowd, V.; Xie, J.; Zheng, X.; Chen, W. Pectin-chitosan conjugated nanoliposome as a promising delivery system for neohesperidin: Characterization, release behavior, cellular uptake, and antioxidant property. Food Hydrocoll. 2019, 95, 432–444. [Google Scholar] [CrossRef]
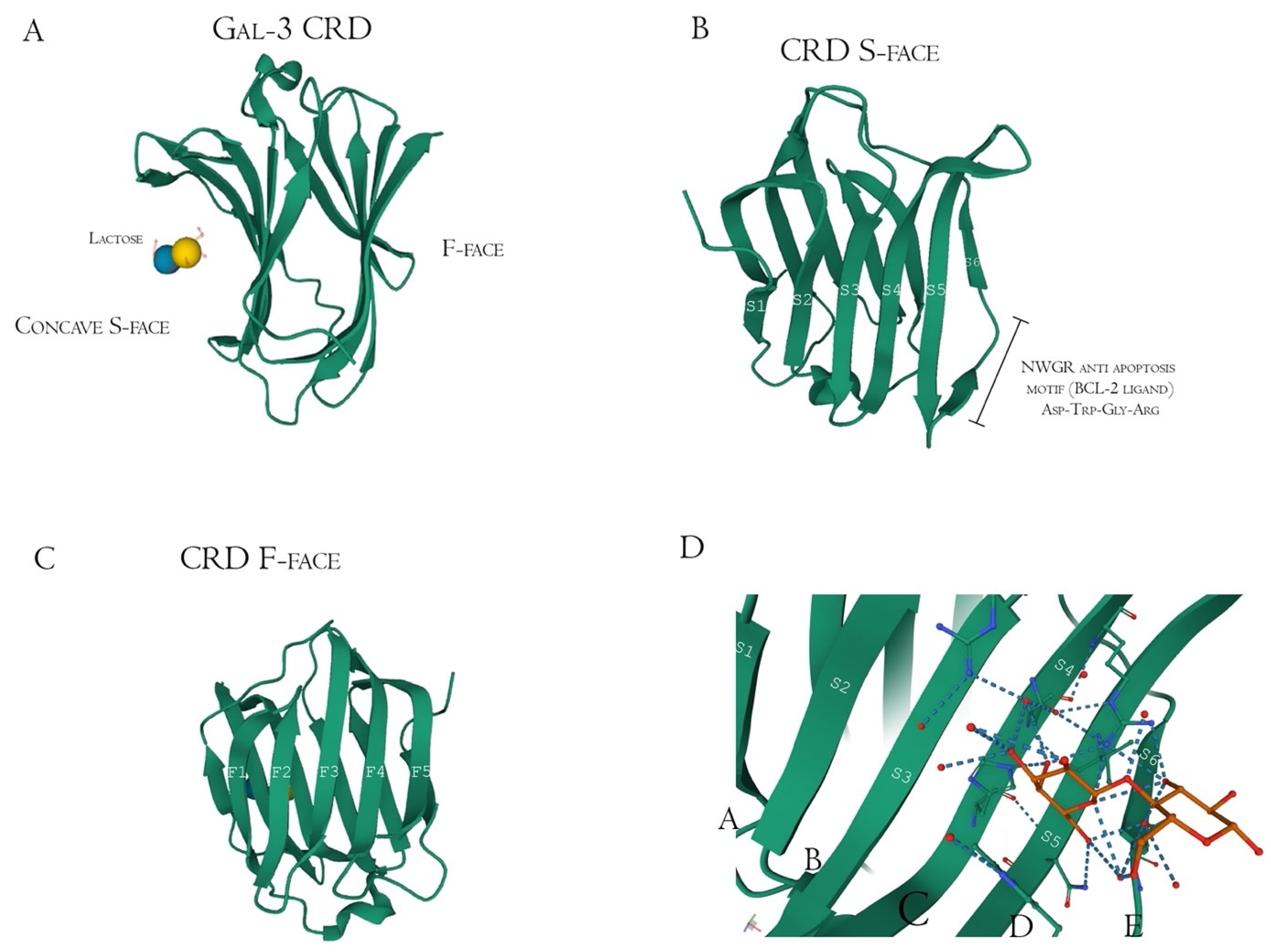
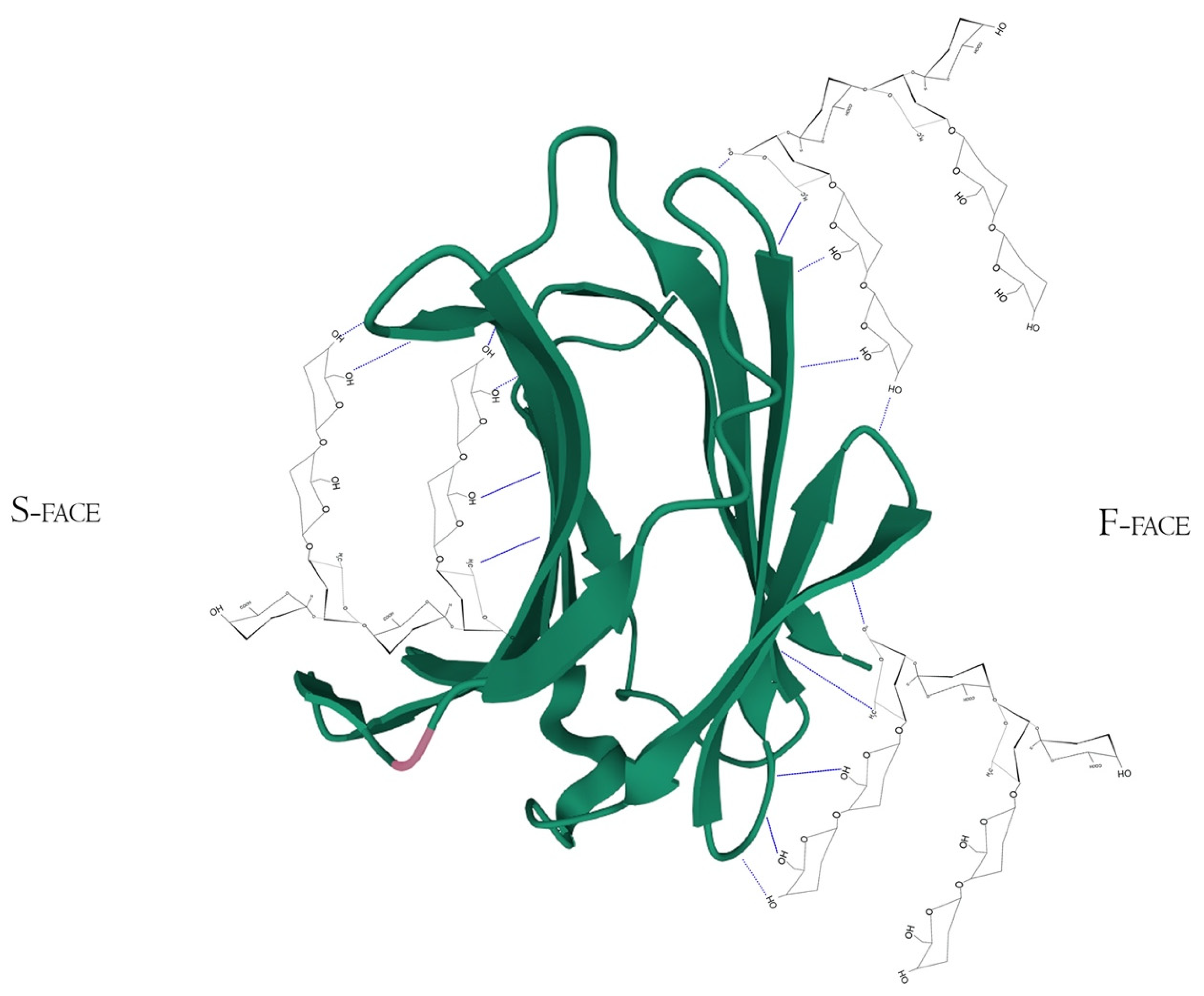
| Authors | Polysaccharide Residue | Analysis Method | Binding Evaluation |
|---|---|---|---|
| Wu et al., 2020 [33] | RG-I from citrus canning process water | Surface plasmon resonance | Smooth binding curve through SPR with decreased affinity with galactan side-chain removal |
| Zhang et al., 2016 [34] | MCP, RG-I-4, and p-galactan | Gal-3 hemagglutination, bio-layer interferometry, and surface plasmon resonance | RG-I-4 demonstrated higher Gal-3 avidity in comparison to the other two polysaccharides, with a KD at sub-micromolar range (RG-I-4 and p-galactan), but no significant result when testing competitive assays with known S-face inhibitors such as lactose |
| Gao et al., 2013 [40] | Ginseng RG-I-4 domain | Gal-3 hemagglutination and surface plasmon resonance | RG-I-4 inhibited G3H and was bound specifically to CRD with high affinity with Ara residue location in the RG-I, changing the activity detected at the G3H assay |
| Gunning, Bongaerts, Morris et al., 2009 [51] | RG-I, PG, and galactans | Atomic force microscopy, fluorescence microscopy, nuclear magnetic resonance, and flow cytometry | Galactan binding to Gal-3 is lectin-saccharide highly specific, while RG-I has low specificity, and PG was not specific. The data suggest that the lesser “sterical crowding” of the galactans alongside its beta-1,4 linear chain could be the reason for the better performance observed |
| Shi et al., 2017 [52] | Ginseng RG-I-3A domain | Bio-layer interferometry, Gal-3 hemagglutination | Binding kinetics of RG-I-3A showed a high binding affinity with a KD of 28 nM through and also presented notable G3H inhibition |
| Zhang et al., 2017 [83] | MCP-derived RG-I and HG portions | Gal-3 hemagglutination, bio-layer interferometry, ELISA, and nuclear magnetic resonance | Gal-3 bound to both portions separately but with a much more notable avidity when a combination of them (RG + HG) is performed, suggesting that this interaction exposes more binding sites at the lectin |
| Miller et al., 2015 [84] | Galactomannans (GM) and polymannan | Nuclear magnetic resonance | The primary binding surface of the GM’s located mainly at F-face beta-sheets (7,8 and 9) |
| Zheng et al., 2020 [89] | MCP-derived HGs of varying molecular weights | Nuclear magnetic resonance heteronuclear single quantum coherence spectroscopy and crystallography | Higher molecular weight HGs demonstrated more perturbances at F-face resonances and involved more S-face beta-sheets at the binding footprint. A possible binding of Gal-3 to the non-terminal HG sites is suggested, and it is shown a different S-face binding pattern of HG’s compared to lactose |
| Miller et al., 2019 [90] | Galactan oligosaccharides of varying chain lengths | Nuclear magnetic resonance heteronuclear single quantum coherence spectroscopy | Binding affinity at the terminal non-reducing end of the galactans in the CRD S-face (beta-sheets 4, 5, and 6 chemical shifts mostly) increases with the increase in chain length |
| Zhao et al., 2017 [91] | Pumpkin RG-I-containing pectin | Surface plasmon resonance | Moderate binding affinity towards Gal-3 through SPR, with a fast association between protein and polysaccharide (KA) and slow dissociation (KD) |
| Miller et al., 2017 [92] | Ginseng RG-I-4 domain | Nuclear magnetic resonance heteronuclear single quantum coherence spectroscopy | Epitopes from RG-I-4 bind to three different labeled Gal-3 sites, two at the CRD and another one at NT. At lower concentrations, the F-face site is more activated, turning to S-face at higher ones |
| Authors | Treatment | Study Type | Treatment Target | Observed Experimental Effects |
|---|---|---|---|---|
| Pedrosa, Lopes and Fabi, 2020 [7] | Papaya pectin acid and neutral fractions | In vitro | HCT 116, HT-29, and HCT-116 Gal-3−/− | Gal-3-mediated agglutination inhibition, cell viability decrease in both WT and knockout cells (suggesting Gal-3 independent pathways) |
| Chen et al., 2018 [10] | SCFAs | In vivo | Male apoE−/− mice | Stimulation of Lxrα mediated genes expression related to intestinal cholesterol uptake and excretion; improved blood lipid profiles and anti-atherosclerotic property |
| Li, Zhang, and Yang 2018 [11] | CP | In vivo | Healthy male C57BL/6J mice | Pectin-supplemented high-fat diet mice had reduced lower liver damage, lipid accumulation, and total serum triglyceride |
| Brouns et al., 2012 [12] | Different DM and MW apple and citrus pectin (CP) | Human intervention | Mildly hyper-cholesterolemic men and women | Higher DM apple and citrus pectin lowered between 7 and 10% low-density lipoprotein cholesterol (LDL-C) compared to control |
| Liu et al., 2016 [13] | CP | In vivo | Male Sprague-Dawley rats with induced type 2 diabetes | Enhanced glucose tolerance, blood lipid levels, reduced insulin resistance, pAKT expression upregulation, and glycogen synthase kinase 3 β (GSK3β) downregulation |
| Fotschki et al., 2014 [14] | Apple fiber (low pectin) | In vivo | Male Wistar rats | Disaccharidase activity reduction, higher SCFA production, reduced serum glucose concentration |
| Prado et al., 2019 [32] | Chelate-soluble fraction of papaya pectin | In vitro | HCT 116 and HT-29 human colon cancer cells | Gal-3-mediated agglutination inhibition, similar to lactose control; pre-treatment with lactose suggests cell Gal-3 independent proliferation reduction for one of the fractions (3CSF) |
| Wu et al., 2020 [33] | CP fragments | In vitro | MCF-7 human breast cancer and A549 human lung carcinoma | Significant binding affinities to Gal-3; dose-responsive cell proliferation inhibition in vitro, not necessarily related to Gal-3 |
| Gao et al., 2013 [40] | MCP, ginseng pectin fractions, potato galactans, and RG-I | In vitro | HT-29 human colon cancer cell line | RG I-4 from ginseng strongly inhibited Gal-3 mediated hemagglutination; better inhibition of cell adhesion and homotypic cell aggregation than lactose |
| Stegmayr et al., 2016 [50] | MCP | In vitro | JIMT-1 breast cancer cells | No Gal-3 inhibition was detected; however, MCP pre-incubation resulted in the accumulation of Gal-3 molecules around intracellular vesicles |
| Prado et al., 2020 [73] | Papaya pectins from different ripening periods | In vitro | THP-1 human monocytic cell | Different TLR’s activation and inhibition depend on the ripening period |
| Hu et al., 2020 [85] | Lemon pectin | In vitro | Human pancreatic beta-cell | Unspecific and unspecified reduction of deleterious effects of inflammatory cytokines with very low (5%) degree of esterification pectin at cell culture |
| Xu et al., 2020 [86] | MCP | In vivo | Male Wistar rats | Down-regulation of Gal-3, TLR, and MyD88, decreased expression of IL-1β, IL-18, and TNF-α |
| Maxwell et al., 2016 [99] | Sugar beet and CP | In vitro | HT-29 human colon cancer cell line | Cell proliferation control and induction of apoptosis |
| Pynam and Dharmesh, 2019 [101] | Bael fruit pectin fragments | In vitro and in vivo | Healthy Swiss albino mice and B16F10 cell line | Microbiota protection, tyrosinase down-regulation, Gal-3 binding, downregulation of Gal-3 gene, IL10 and IL17 cytokines |
| Fang et al., 2018 [103] | MCP | In vitro | Human urinary bladder cancer (UBC) cells | Gal-3 down-regulation and inactivation of Akt signaling pathway, a decrease in Cyclin B1, G2/M phase arrest, Caspase-3 activation |
| Hossein et al., 2019 [104] | MCP | In vitro | SKOV-3 and SOC (serous ovarian cancer) cells | Synergistic effect of PTX and MCP increasing caspase-3 activity and decreasing cyclin D1 expression level |
| Abu-Elsaad and Elkashef, 2016 [105] | MCP | In vivo | Adult male Sprague-Dawley rats | Decreased liver fibrosis and necroinflammation, a decrease in MDA, TIMP-1, Col1A1, and Gal-3, increase in Caspase-3, gluthatione, and superoxide dismutase expression |
| Martinez-Martinez et al., 2016 [106] | MCP | In vivo | Adult male Wistar rats | Attenuation of renal fibrosis-related biomarkers, osteopontin, cytokine A2, albuminuria and TGF-β1 |
| Calvier et al., 2015 [109] | MCP | In vivo | Adult male Wistar rats, C57BJ6 WT and Gal-3−/− mice | Reverted fibrosing markers and Gal-3 augmentation levels, similarly to spironolactone |
| Li et al., 2018 [110] | MCP | In vitro and in vivo | HEK293 cells and C57BL/6 male mice | Amelioration of renal interstitial fibrosis, lower collagen I and fibronectin in the kidney, reduced IL-1β mRNA levels, lower Gal-3 expression |
| Prud’homme et al., 2019 [111] | MCP | Cohort and in vivo | C57BL6/J and C57BL6/J Gal-3 KO male mice | Cardiac fibrosis induced by model prevented by MCP treatment, IL-1β level maintained, protected, treated mice against renal inflammation |
| Ibarrola et al., 2019 [112] | MCP | In vivo | Male Wistar rats | BNP serum level normalization, lower Gal-3 cardiac expression, reticulocalbin-3 and fumarase in the myocardium, IL-1β and CRP in serum |
| Li et al., 2019 [113] | MCP and perindopril | In vivo | New Zealand male rabbits | Gal-3, collagen I, and III downregulation |
| Vergaro et al., 2016 [114] | MCP | In vivo | Transgenic mice with aldosterone synthase gene overexpression | Reduced cardiac hypertrophy, fibrosis, Coll-1, and Coll-3 genes expression and also enhanced anti-inflammatory and anti-fibrotic effects when synergistically acting with Canrenoate |
| Ibarrola et al., 2017 [115] | MCP | In vivo | Male Wistar rats | Gal-3, mRNA expression normalized, collagen I, fibronectin, α-SMA, TGF-β1, and CTGF mRNA expression reduced compared to pressure overload group, vascular inflammatory markers expression was also controlled |
| Xue et al., 2019 [116] | Ginseng pectin fractions | In vitro and In vivo | Jurkat (human leukemia cells) and male IRC mice | MCP inhibited IL-2 expression, and the three pectin fractions utilized reversed cleaved caspase-3 formation alongside lactose. MCP and ginseng pectins inhibited ROS production in vitro. Reduced tumor weight and increased IL-2 secretion in vivo |
| Lau et al., 2021 [117] | MCP | Interventional trial | Participants with high Gal-3 levels and hypertension | MCP had no impact regarding attenuating of cardiac-related risk factors |
| Busato et al., 2020 [122] | Broccoli stalks pectin | In vitro and in vivo | Female albino swiss mice and peritoneal exsudate cells | Macrophage activation and higher phagocytic activity; IL-10 presence was higher at peritoneal fluid in vivo, but not at in vitro model |
| Liu et al., 2008 [125] | MCP | In vitro and in vivo | CT-26 cells and Balb/c female mice | MCP did not alter Gal-3 expression at metastatic liver cells, although it did inhibit tumor growth and metastatic rate |
| Courts, 2013 [126] | MCP | In vitro | Caco-2 monolayer | MCP fragments were absorbed through paracellular and to a lower degree by transcellular transports at in vitro culture |
| Huang et al., 2012 [127] | Enzyme-treated CP | In vitro and In vivo | HepG2, A549, Colo 205, and HEK293 cells, BALB/c mice | Altered membrane permeability (LDH release) in the cancer cell lines; low weight oligogalacturonide was absorbed by the mice and the tumor cells, enhancing Gal-3 release to the medium |
| Fan et al., 2018 [129] | Ginseng RG-I enriched pectins | In vitro | L-929 fibroblast cells | Modulation of cell migration and adhesion, independent of Gal-3 |
| Nishikawa et al., 2018 [130] | Modified citrus pectin (MCP) | In vivo | Male C57BL/6 mice | Attenuated blood-brain barrier disruption Gal-3 upregulation, inactivation of ERK 1/2, STAT and MMP |
| Sivaprakasam et al., 2016 [143] | 2% inulin, 2% pectin, and 1% cellulose | In vivo | Human colon cancer tissue and Ffar-2−/− C57BL/6J mice | Microbiota modulation, promotion of Bifidobacterium growth, and reduction of Prevotellaceae |
| Kim et al., 2013 [144] | SCFAs | In vivo | WT, GPR41−/− and GPR43−/− mice | Activation of intestinal epithelial cells to produce chemokines and cytokines, GPR’s were essential in T effector cell activation and signaling pathways |
| Tian et al., 2016 [146] | Sugar beet, soy, low DM, and high DM citrus pectin | In vivo | Male Wistar rats | More stimulation of Lactobacillus and Lachnospiraceae growth in sugar beet pectin, higher production of SCFA’s for low DM citrus and soy pectin |
| Tian et al., 2017 [147] | Low DM and high DM citrus pectin | In vivo | Piglets | The slower fermentation process, alteration of main fermentation region, and higher Bacteroidetes predominance |
| Ferreira-Lazarte et al., 2019 [148] | CP | In vitro | Dynamic gastric simulator with healthy volunteer fecal slurry donated | Growth stimulation of Bifidobacterium spp., Bacteroides spp., and Faecalobacterium prausnitzii, high increase in acetate and butyrate production |
| Chen et al., 2013 [149] | Apple pectin oligosaccharides | In vitro | Fecal batch culture fermentation | Increased numbers of Lactobacillus and Bifidobacteria, a higher concentration of acetic, lactic, and propionic acid decreased number of Clostridia and Bacteroides |
| Onumpai et al., 2011 [150] | Potato galactan, methylated citrus pectin, beet arabinan, Arabidopsis thaliana RG-I | In vitro | Fecal batch culture fermentation | Higher Bifidobacterium populations and higher SCFA’s yield increased Bacteroides-Prevotella groups |
| Merheb, Abdel-Massih, and Karam, 2019 [153] | CP and MCP | In vivo | Female BALB/c mice | Upregulation of IL-17, IFN-γ, and TNF-α through IL-4 cytokine secretion in the spleen |
| Amorim et al., 2016 [154] | Theobroma cacao pod husk modified pectin | In vivo | Female albino Swiss mice | Promotion of macrophage differentiation, nitric oxide production, and upregulation of IL-12, TNF-α, and IL-10 secretion |
| Do Nascimento et al., 2017 [155] | Sweet pepper pectin | In vitro | THP-1 human monocytic cell | Modulation of TNF-α, IL-1β, and IL-10 production and secretion |
| Popov et al., 2011 [156] | Sweet pepper pectin | In vivo | Male BALB/c mice | Higher IL-10 production with lower TNF-α release |
| Ishisono et al., 2017 [157] | CP | In vivo | Male C57BL/6 mice | Suppression of IL-6 secretion from TLR activated macrophages and CD11c+ cells |
| Vogt et al., 2016 [158] | Different DM lemon pectin | In vitro | T84 intestinal epithelial cells | NF-kB/AP-1 activation through TLR/MyD88 and protective effects in the intestinal barrier |
| Wang et al., 2018 [159] | Hippophae rhamnoides L. berries pectin | In vivo | Cyclophosphamide induced immunosuppressive mice | Macrophage activation, MyD88 increased expression and upregulated expression of TLR4 |
| Park et al., 2013 [160] | RG-II from P. ginseng | In vivo and In vitro | C57BL6 WT, TCR KO, TLR KO mice, and BMDC cells | Facilitation of CD8+ T cells, induced production of TNF-α, IL-12, IFN-γ, and IL-1β during dendritic cell maturation |
| Sahasrabudhe et al., 2018 [161] | Lemon pectins with different DM | In vitro and In vivo | HEK-Blue WT and mutated cell lines, female C57BL/6 mice | Inhibition of TLR2-1 heterodimer, prevention of ileitis in the mice model |
| Hu et al., 2021 [162] | Lemon pectins with different DM | In vivo | Sprague-Dawley male rats and C57BL/6 mice | Reduced peri-capsular fibrosis in vivo and decreased DAMP-induced TLR2 immune activation in vitro |
| Kolatsi-Jannou et al., 2011 [163] | MCP | In vivo | Male C57BL/6J mice | Reduced Gal-3 expression, reduced renal cell proliferation, apoptosis, fibrosis, and proinflammatory cytokine expression |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrosa, L.d.F.; Raz, A.; Fabi, J.P. The Complex Biological Effects of Pectin: Galectin-3 Targeting as Potential Human Health Improvement? Biomolecules 2022, 12, 289. https://doi.org/10.3390/biom12020289
Pedrosa LdF, Raz A, Fabi JP. The Complex Biological Effects of Pectin: Galectin-3 Targeting as Potential Human Health Improvement? Biomolecules. 2022; 12(2):289. https://doi.org/10.3390/biom12020289
Chicago/Turabian StylePedrosa, Lucas de Freitas, Avraham Raz, and João Paulo Fabi. 2022. "The Complex Biological Effects of Pectin: Galectin-3 Targeting as Potential Human Health Improvement?" Biomolecules 12, no. 2: 289. https://doi.org/10.3390/biom12020289
APA StylePedrosa, L. d. F., Raz, A., & Fabi, J. P. (2022). The Complex Biological Effects of Pectin: Galectin-3 Targeting as Potential Human Health Improvement? Biomolecules, 12(2), 289. https://doi.org/10.3390/biom12020289