Posttranscriptional Regulation of Insulin Resistance: Implications for Metabolic Diseases
Abstract
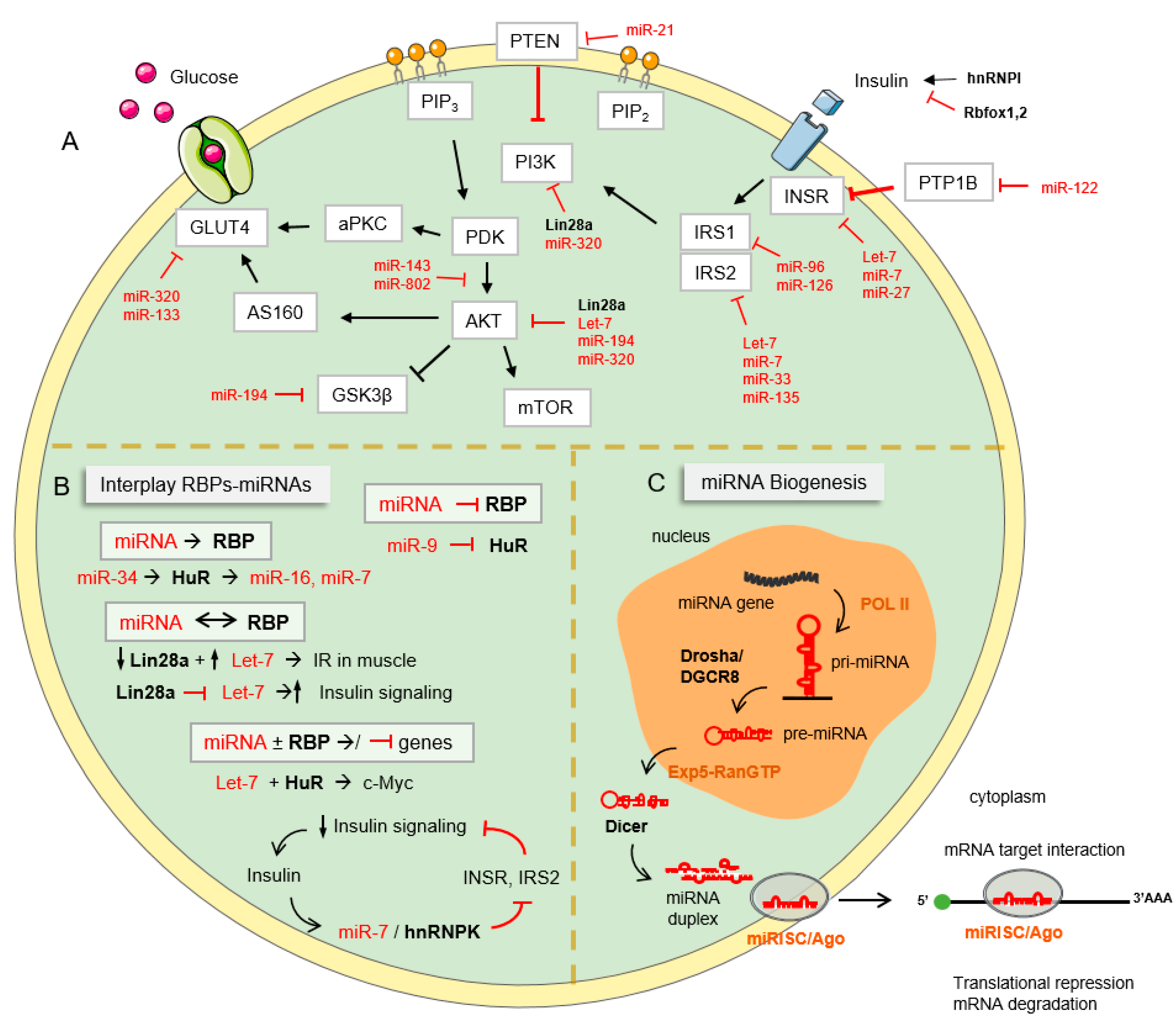
1. The Insulin Signaling Pathway
2. Insulin Resistance
2.1. Definition and Causes of Insulin Resistance (IR)
2.2. Negative Regulation of Insulin Signaling
3. MiRNAs and RBPs: Novel Regulators of Insulin Signaling and Metabolism
3.1. miRNAs
3.2. RBPs
3.2.1. hnRNP Family
3.2.2. Hu Family
3.2.3. NOVA Family
3.2.4. Rbfox Family
3.2.5. CUG-BP Elav-like Family (CELF)
3.2.6. FTO Protein
3.2.7. LIN28 Protein
3.2.8. Dead Box Helicase Superfamily
3.3. Cooperation between RBPs and miRNAs
4. Posttranscriptional Regulation in Metabolic Diseases
4.1. Diabetes
4.2. Obesity
4.3. Cardiovascular Disease
4.4. Alzheimer’s Disease
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Lizcano, J.M.; Alessi, D.R. The insulin signalling pathway. Curr. Biol. 2002, 12, 236–238. [Google Scholar] [CrossRef]
- Rowland, A.F.; Fazakerley, D.J.; James, D.E. Mapping Insulin/GLUT4 Circuitry. Traffic 2011, 12, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Accili, D.; Arden, K.C. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 2004, 117, 421–426. [Google Scholar] [CrossRef]
- Courtney, C.H.; Olefsky, J.M. Insulin resistance. In Mechanisms of Insulin Action: Medical Intelligence Unit; Springer: New York, NY, USA, 2007; pp. 185–209. [Google Scholar]
- Mclaughlin, T.; Ackerman, S.E.; Shen, L.; Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Investig. 2017, 127, 5–13. [Google Scholar] [CrossRef]
- Shimobayashi, M.; Albert, V.; Woelnerhanssen, B.; Frei, I.C.; Weissenberger, D.; Meyer-Gerspach, A.C.; Clement, N.; Moes, S.; Colombi, M.; Meier, J.A.; et al. Insulin resistance causes inflammation in adipose tissue. J. Clin. Investig. 2018, 128, 1538. [Google Scholar] [CrossRef]
- Jiménez-Osorio, A.S.; Monroy, A.; Alavez, S. Curcumin and insulin resistance-Molecular targets and clinical evidences. Biofactors 2016, 42, 561–580. [Google Scholar] [CrossRef]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Den Hartogh, D.J.; Giacca, A.; Tsiani, E. Amelioration of High-Insulin-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol Is Linked to Activation of AMPK and Restoration of GLUT4 Translocation. Nutrients 2020, 12, 914. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin Receptor Signaling in Normal and Insulin-Resistant States. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Gavin, J.R.; Roth, J.; Neville, D.M.; Meyts, P.D.E.; Buellt, D.N. Insulin-Dependent Regulation of Insulin Receptor Concentrations: A Direct Demonstrationin in Cell Culture. Proc. Natl. Acad. Sci. USA 1974, 71, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, J.L. Insulin receptor internalization: Molecular mechanisms and physiopathological implications. Diabetologia 1994, 37 (Suppl. 2), 117–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zick, Y. Ser/Thr phosphorylation of IRS proteins: A molecular basis for insulin resistance. Sci. STKE 2005, 2005, pe4. [Google Scholar] [CrossRef] [PubMed]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central, role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Protein Tyrosine Phosphatases: Structure and Function, Substrate Specificity, and Inhibitor Development. Pharmacol. Toxicol. 2002, 42, 209–234. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Alessi, D.R. The PI3K-PBK1 connection: More than just a road to PKB. Biochem. J. 2000, 346, 561–576. [Google Scholar] [CrossRef]
- Kim, W.; Kyung Lee, E. Post-transcriptional regulation in metabolic diseases. RNA Biol. 2012, 9, 772–780. [Google Scholar] [CrossRef]
- Arif, W.; Datar, G.; Kalsotra, A. Intersections of post-transcriptional gene regulatory mechanisms with intermediary metabolism. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 349–362. [Google Scholar] [CrossRef]
- Cappellani, D.; Macchia, E.; Falorni, A.; Marchetti, P. Insulin autoimmune syndrome (Hirata disease): A comprehensive review fifty years after its first description. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 963–978. [Google Scholar] [CrossRef]
- Støy, J.; Steiner, D.F.; Park, S.Y.; Ye, H.; Philipson, L.H.; Bell, G.I. Clinical and molecular genetics of neonatal diabetes due to mutations in the insulin gene. Rev. Endocr. Metab. Disord. 2010, 11, 205–215. [Google Scholar] [CrossRef]
- Sun, F.; Du, W.; Ma, J.; Gu, M.; Wang, J.; Zhu, H.; Song, H.; Gao, G. A Novel c125 T>G (p.Val42Gly) Mutation in the Human INS Gene Leads to Neonatal Diabetes Mellitus via a Decrease in Insulin Synthesis. Exp. Clin. Endocrinol. Diabetes 2020, 128, 182–189. [Google Scholar] [PubMed]
- Nagarajan, A.; Petersen, M.C.; Nasiri, A.R.; Butrico, G.; Fung, A.; Ruan, H.B.; Kursawe, R.; Caprio, S.; Thibodeau, J.; Bourgeois-Daigneault, M.C.; et al. MARCH1 regulates insulin sensitivity by controlling cell surface insulin receptor levels. Nat. Commun. 2016, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.I.; Kadowaki, T.; Kadowaki, H.; Accili, D.; Cama, A.; McKeon, C. Mutations in insulin-receptor gene in insulin-resistant patients. Diabetes Care 1990, 13, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Maiza, J.C.; Caron-Debarle, M.; Vigouroux, C.; Schneebeli, S. Anti-insulin receptor antibodies related to hypoglycemia in a previously diabetic patient. Diabetes Care 2013, 36, e77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kadowaki, T.; Bevins, C.L.; Cama, A.; Ojamaa, K.; Marcus-Samuels, B.; Kadowaki, H.; Beitz, L.; Mckeon, C.; Taylor, S.I. Two mutant alleles of the insulin receptor gene in a patient with extreme insulin resistance. Science 1988, 240, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Takata, Y.; Sasaoka, T.; Takada, Y.; Morioka, H.; Haruta, T.; Sawa, T.; Iwanishi, M.; Hu, Y.G.; Suzukis, Y.; et al. Two Naturally Occurring Mutations in the Kinase Domain of Insulin Receptor Accelerate Degradation of the Insulin Receptor and Impair the Kinase Activity. J. Biol. Chem. 1994, 269, 31019–31027. [Google Scholar] [CrossRef]
- Grønskov, K.; Vissing, H.; Shymko, R.M.; Tornqvist, H.; De Meyts, P. Mutation of arginine 86 to proline in the insulin receptor alpha-subunit causes lack of transport of the receptor to the plasma membrane, loss of binding affinity and a constitutively activated tyrosine kinase in transfected cells. Biochem. Biophys. Res. Commun. 1993, 192, 905–911. [Google Scholar] [CrossRef]
- George, S.; Johansen, A.; Soos, M.A.; Mortensen, H.; Gammeltoft, S.; Saudek, V.; Siddle, K.; Hansen, L.; O’Rahilly, S. Deletion of V335 from the l2 domain of the insulin receptor results in a conformationally abnormal receptor that is unable to bind insulin and causes Donohue’s syndrome in a human subject. Endocrinology 2003, 144, 631–637. [Google Scholar] [CrossRef]
- Cocozza, S.; Porcellini, A.; Riccardi, G.; Monticelli, A.; Condorelli, G.; Ferrara, A.; Pianese, L.; Miele, C.; Capaldo, B.; Beguinot, F.; et al. NIDDM associated with mutation in tyrosine kinase domain of insulin receptor gene. Diabetes 1992, 41, 521–526. [Google Scholar] [CrossRef]
- Haruta, T.; Imamura, T.; Iwanishi, M.; Egawa, K.; Goji, K.; Kobayashi, M. Amplification and analysis of promoter region of insulin receptor gene in a patient with leprechaunism associated with severe insulin resistance. Metabolism 1995, 44, 430–437. [Google Scholar] [CrossRef]
- Hribal, M.L.; Tornei, F.; Pujol, A.; Menghini, R.; Barcaroli, D.; Lauro, D.; Amoruso, R.; Lauro, R.; Bosch, F.; Sesti, G.; et al. Transgenic mice overexpressing human G972R IRS-1 show impaired insulin action and insulin secretion. J. Cell Mol. Med. 2008, 12, 2096–2106. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, L.E.; Cruz, M.; Martínez-Nava, G.A.; Madrid-Marina, V.; Parra, E.; García-Mena, J.; Espinoza-Rojo, M.; Estrada-Velasco, B.I.; Piza-Roman, L.F.; Aguilera, P.; et al. A Replication Study of the IRS1, CAPN10, TCF7L2, and PPARG Gene Polymorphisms Associated with Type 2 Diabetes in Two Different Populations of Mexico. Ann. Hum. Genet. 2011, 75, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.L.; Li, Y.; Vanni, C.; Mammarella, S.; Veschi, S.; Della Loggia, F.; Mariani-Costantini, R.; Battista, P.; Quon, M.J.; Cama, A. Clinical case seminar: A novel T608R missense mutation in insulin receptor substrate-1 identified in a subject with type 2 diabetes impairs metabolic insulin signaling. J. Clin. Endocrinol. Metab. 2003, 88, 1468–1475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rung, J.; Cauchi, S.; Albrechtsen, A.; Shen, L.; Rocheleau, G.; Cavalcanti-Proença, C.; Bacot, F.; Balkau, B.; Belisle, A.; Borch-Johnsen, K.; et al. Genetic variant near IRS1 is associated with type 2 diabetes, insulin resistance and hyperinsulinemia. Nat. Genet. 2009, 41, 1110–1115. [Google Scholar] [CrossRef]
- Ishihara, H.; Sasaoka, T.; Kagawa, S.; Murakami, S.; Fukui, K.; Kawagishi, Y.; Yamazaki, K.; Sato, A.; Iwata, M.; Urakaze, M.; et al. Association of the polymorphisms in the 5′-untranslated region of PTEN gene with type 2 diabetes in a Japanese population. FEBS Lett. 2003, 554, 450–454. [Google Scholar] [CrossRef]
- Pal, A.; Barber, T.M.; Van de Bunt, M.; Rudge, S.A.; Zhang, Q.; Lachlan, K.L.; Cooper, N.S.; Linden, H.; Levy, J.C.; Wakelam, M.J.O.; et al. PTEN Mutations as a Cause of Constitutive Insulin Sensitivity and Obesity. N. Engl. J. Med. 2012, 367, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Wandel, S.; Schürmann, A.; Becker, W.; Summers, S.A.; Shanahan, M.F.; Joost, H.G. Mutation of two conserved arginine residues in the glucose transporter GLUT4 supresses transport activity, but not glucose-inhibitable binding of inhibitory ligands. Naunyn Schmiedebergs Arch. Pharmacol. 1995, 353, 36–41. [Google Scholar] [CrossRef]
- George, S.; Rochford, J.J.; Wolfrum, C.; Gray, S.L.; Schinner, S.; Wilson, J.C.; Soos, M.A.; Murgatroyd, P.R.; Williams, R.M.; Acerini, C.L.; et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science 2004, 304, 1325–1328. [Google Scholar] [CrossRef]
- Prudente, S.; Scarpelli, D.; Chandalia, M.; Zhang, Y.Y.; Morini, E.; Del Guerra, S.; Perticone, F.; Li, R.; Powers, C.; Andreozzi, F.; et al. The TRIB3 Q84R polymorphism and risk of early-onset type 2 diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 190–196. [Google Scholar] [CrossRef]
- Garofalo, R.S.; Orena, S.J.; Rafidi, K.; Torchia, A.J.; Stock, J.L.; Hildebrandt, A.L.; Coskran, T.; Black, S.C.; Brees, D.J.; Wicks, J.R.; et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKBβ. J. Clin. Investig. 2003, 112, 197–208. [Google Scholar] [CrossRef]
- Lu, M.; Wan, M.; Leavens, K.F.; Chu, Q.; Monks, B.R.; Fernandez, S.; Ahima, R.S.; Ueki, K.; Kahn, C.R.; Birnbaum, M.J. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat. Med. 2012, 18, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.D.M.C.B.; Pulawa, L.K.; Jensen, D.R.; Eckel, R.H. Overexpressing human lipoprotein lipase in mouse skeletal muscle is associated with insulin resistance. Diabetes 2001, 50, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Özcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Özdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Holland, W.L.; Brozinick, J.T.; Wang, L.P.; Hawkins, E.D.; Sargent, K.M.; Liu, Y.; Narra, K.; Hoehn, K.L.; Knotts, T.A.; Siesky, A.; et al. Inhibition of Ceramide Synthesis Ameliorates Glucocorticoid-, Saturated-Fat-, and Obesity-Induced Insulin Resistance. Cell Metab. 2007, 5, 167–179. [Google Scholar] [CrossRef]
- Yu, C.; Chen, Y.; Cline, G.W.; Zhang, D.; Zong, H.; Wang, Y.; Bergeron, R.; Kim, J.K.; Cushman, S.W.; Cooney, G.J.; et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 2002, 277, 50230–50236. [Google Scholar] [CrossRef]
- Schinner, S.; Scherbaum, W.A.; Bornstein, S.R.; Barthel, A. Molecular mechanisms of insulin resistance. Diabet. Med. 2005, 22, 674–682. [Google Scholar] [CrossRef]
- Kamei, N.; Tobe, K.; Suzuki, R.; Ohsugi, M.; Watanabe, T.; Kubota, N.; Ohtsuka-Kowatari, N.; Kumagai, K.; Sakamoto, K.; Kobayashi, M.; et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 2006, 281, 26602–26614. [Google Scholar] [CrossRef]
- Ozes, O.N.; Akca, H.; Mayo, L.D.; Gustin, J.A.; Maehama, T.; Dixon, J.E.; Donner, D.B. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc. Natl. Acad. Sci. USA 2001, 98, 4640–4645. [Google Scholar] [CrossRef]
- Rotter, V.; Nagaev, I.; Smith, U. Interleukin-6 (IL-6) Induces Insulin Resistance in 3T3-L1 Adipocytes and Is, Like IL-8 and Tumor Necrosis Factor-α, Overexpressed in Human Fat Cells from Insulin-resistant Subjects. J. Biol. Chem. 2003, 278, 45777–45784. [Google Scholar] [CrossRef]
- Jager, J.; Grémeaux, T.; Cormont, M.; Le Marchand-Brustel, Y.; Tanti, J.F. Interleukin-1β-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 2007, 148, 241–251. [Google Scholar] [CrossRef]
- Morino, K.; Petersen, K.F.; Dufour, S.; Befroy, D.; Frattini, J.; Shatzkes, N.; Neschen, S.; White, M.F.; Bilz, S.; Sono, S.; et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J. Clin. Investig. 2005, 115, 3587–3593. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; Seng, K.G.; Turner, N.; Campbell, L.V.; Chisholm, D.J. Markers of mitochondrial biogenesis and metabolism are lower in overweight and obese insulin-resistant subjects. J. Clin. Endocrinol. Metab. 2007, 92, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Szendroedi, J.; Roden, M. Mitochondrial fitness and insulin sensitivity in humans. Diabetologia 2008, 51, 2155–2167. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.D.; Banks, L.; Caterini, J.E.; Thompson, S.; Noseworthy, M.D.; Rayner, T.; Syme, C.; McCrindle, B.W.; Hamilton, J. The association among skeletal muscle phosphocreatine recovery, adiposity, and insulin resistance in children. Pediatr. Obes. 2017, 12, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Kacerovsky-Bielesz, G.; Chmelik, M.; Ling, C.; Pokan, R.; Szendroedi, J.; Farukuoye, M.; Kacerovsky, M.; Schmid, A.I.; Gruber, S.; Wolzt, M.; et al. Short-term exercise training does not stimulate skeletal muscle ATP synthesis in relatives of humans with type 2 diabetes. Diabetes 2009, 58, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Ripley, E.M.; Clarke, G.D.; Hamidi, V.; Martinez, R.A.; Settles, F.D.; Solis, C.; Deng, S.; Abdul-Ghani, M.; Tripathy, D.; Defronzo, R.A. Reduced skeletal muscle phosphocreatine concentration in type 2 diabetic patients: A quantitative image-based phosphorus-31 MR spectroscopy study. Am. J. Physiol.-Endocrinol. Metab. 2018, 315, E229–E239. [Google Scholar] [CrossRef]
- Simoneau, J.; Veerkamp, J.H.; Turcotte, L.P.; Kelley, D.E. Markers of capacity to utilize fatty acids in human skeletal muscle: Relation to insulin resistance and obesity and effects of weight loss. FASEB J. 1999, 13, 2051–2060. [Google Scholar] [CrossRef]
- Mogensen, M.; Sahlin, K.; Fernström, M.; Glintborg, D.; Vind, B.F.; Beck-Nielsen, H.; Højlund, K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 2007, 56, 1592–1599. [Google Scholar] [CrossRef]
- Lefort, N.; Glancy, B.; Bowen, B.; Willis, W.T.; Bailowitz, Z.; De Filippis, E.A.; Brophy, C.; Meyer, C.; Højlund, K.; Yi, Z.; et al. Increased reactive oxygen species production and lower abundance of complex I subunits and carnitine palmitoyltransferase 1B protein despite normal mitochondrial respiration in insulin-resistant human skeletal muscle. Diabetes 2010, 59, 2444–2452. [Google Scholar] [CrossRef]
- Nishikawa, T.; Araki, E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 2007, 9, 343–353. [Google Scholar] [CrossRef]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W.; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, O.P.; Lehtovirta, M.; Hastoy, B.; Chandra, V.; Krentz, N.A.J.; Kleiner, S.; Jain, D.; Richard, A.M.; Abaitua, F.; Beer, N.L.; et al. Loss of ZnT8 function protects against diabetes by enhanced insulin secretion. Nat. Genet. 2019, 51, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Rutter, G.A.; Chimienti, F. SLC30A8 mutations in type 2 diabetes. Diabetologia 2015, 58, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Rosenkranz, E.; Overbeck, S.; Warmuth, S.; Mocchegiani, E.; Giacconi, R.; Weiskirchen, R.; Karges, W.; Rink, L. Disturbed zinc homeostasis in diabetic patients by in vitro and in vivo analysis of insulinomimetic activity of zinc. J. Nutr. Biochem. 2012, 23, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Tepaamorndech, S.; Kirschke, C.P.; Pedersen, T.L.; Keyes, W.R.; Newman, J.W.; Huang, L. Zinc transporter 7 deficiency affects lipid synthesis in adipocytes by inhibiting insulin-dependent Akt activation and glucose uptake. FEBS J. 2016, 283, 378–394. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kirschke, C.P.; Lay, Y.A.E.; Levy, L.B.; Lamirande, D.E.; Zhang, P.H. Znt7-null mice are more susceptible to diet-induced glucose intolerance and insulin resistance. J. Biol. Chem. 2012, 287, 33883–33896. [Google Scholar] [CrossRef] [PubMed]
- Hug, N.; Longman, D.; Cáceres, J. Mechanism and regulation of the nonsense-mediated decay pathway. Nucleic. Acids Res. 2016, 44, 1483–1495. [Google Scholar] [CrossRef]
- Re, A.; Joshi, T.; Kulberkyte, E.; Morris, Q.; Workman, C.T. RNA—Protein Interactions: An Overview. RNA Seq. Struct. Funct. Comput. Bioinform. Methods 2014, 1097, 491–521. [Google Scholar]
- Lukong, K.E.; Fatimy, R.E. Implications of RNA-binding Proteins for Human Diseases. eLS 2012, 10, a0023866. [Google Scholar]
- Picchiarelli, G.; Dupuis, L. Role of RNA Binding Proteins with prion-like domains in muscle and neuromuscular diseases. Cell Stress 2020, 4, 76–91. [Google Scholar] [CrossRef]
- Thelen, M.P.; Kye, M.J. The Role of RNA Binding Proteins for Local mRNA Translation: Implications in Neurological Disorders. Front. Mol. Biosci. 2020, 6, 161. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hernando, C.; Ramírez, C.M.; Goedeke, L.; Suárez, Y. MicroRNAs in metabolic disease. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Baugh, L.R.; Sternberg, P.W. DAF-16/FOXO Regulates Transcription of cki-1/Cip/Kip and Repression of lin-4 during C. elegans L1 Arrest. Curr. Biol. 2006, 16, 780–785. [Google Scholar] [CrossRef]
- Boehm, M.; Slack, F. Physiology: A developmental timing microRNA and its target regulate life span in C. elegans. Science 2005, 310, 1954–1957. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Corley, M.; Burns, M.C.; Yeo, G.W. How RNA-Binding Proteins Interact with RNA: Molecules and Mechanisms. Mol. Cell 2020, 78, 9–29. [Google Scholar] [CrossRef]
- Kilchert, C.; Sträßer, K.; Kunetsky, V.; Änkö, M.L. From parts lists to functional significance—RNA–protein interactions in gene regulation. Wiley Interdiscip. Rev. RNA 2020, 11, e1582. [Google Scholar] [CrossRef]
- Geuens, T.; Bouhy, D.; Timmerman, V. The hnRNP family: Insights into their role in health and disease. Hum. Genet. 2016, 135, 851–867. [Google Scholar] [CrossRef]
- Han, S.P.; Tang, Y.H.; Smith, R. Functional diversity of the hnRNPs: Past, present and perspectives. Biochem. J. 2010, 430, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Dzwonek, A.; Mikula, M.; Ostrowski, J. The diverse involvement of heterogeneous nuclear ribonucleoprotein K in mitochondrial response to insulin. FEBS Lett. 2006, 580, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Gorospe, M.; Multitasking, P. HuR in the Mammalian Genotoxic Response: Post-Transcriptional Multitasking HuR in the Mammalian Genotoxic Response. Cell Cycle 2003, 2, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Hinman, M.N.; Lou, H. Diverse molecular functions of Hu proteins. Cell Mol. Life Sci. 2008, 65, 3168–3181. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.W.; Preet, R.; Dhir, T.; Dixon, D.A.; Brody, J.R. Understanding and targeting the disease-related RNA binding protein human antigen R (HuR). Wiley Interdiscip. Rev. RNA 2020, 11, e1581. [Google Scholar] [CrossRef]
- Ramírez, C.M.; Lin, C.S.; Abdelmohsen, K.; Goedeke, L.; Yoon, J.H.; Madrigal-Matute, J.; Martin-Ventura, J.L.; Vo, D.T.; Uren, P.J.; Penalva, L.O.; et al. RNA binding protein HuR regulates the expression of ABCA1. J. Lipid Res. 2014, 55, 1066–1076. [Google Scholar] [CrossRef]
- Buckanovich, J. The Onconeural Antigen Nova-l Is a Neuron-Specific Protein, the Activity of which Is Inhibited Paraneoplastic Antibodies by. J. Neurosci. 1996, 16, 1114–1122. [Google Scholar] [CrossRef]
- Lewis, H.A.; Chen, H.; Edo, C.; Buckanovich, R.J.; Yang, Y.Y.L.; Musunuru, K.; Zhong, R.; Darnell, R.B.; Burley, S.K. Crystal structures of Nova-1 and Nova-2 K-homology RNA-binding domains. Structure 1999, 7, 191–203. [Google Scholar] [CrossRef]
- Ule, J.; Stefani, G.; Mele, A.; Ruggiu, M.; Wang, X.; Taneri, B.; Gaasterland, T.; Blencowe, B.J.; Darnell, R.B. An RNA map predicting Nova-dependent splicing regulation. Nature 2006, 444, 580–586. [Google Scholar] [CrossRef]
- Lewis, H.A.; Musunuru, K.; Jensen, K.B.; Edo, C.; Chen, H.; Darnell, R.B.; Burley, S.K. Sequence-specific RNA binding by a Nova KH domain: Implications for paraneoplastic disease and the fragile X syndrome. Cell 2000, 100, 323–332. [Google Scholar] [CrossRef]
- Buckanovich, R.; Posner, J.B.; Darneli, R.B. Nova, the Paraneoplastic Ri Antigen, Is Homologous to an RNA-Binding Protein and Is Specifically Expressed in the Developing Motor System. Neuron 1993, 11, 657–672. [Google Scholar] [CrossRef]
- Arnell, R.O.B.D. The neuronal RNA-binding protein Nova-2 is implicated as the autoantigen targeted in POMA patients with dementia. Proc. Natl. Acad. Sci. USA 1998, 95, 13254–13259. [Google Scholar]
- Juan-Mateu, J.; Rech, T.H.; Villate, O.; Lizarraga-Mollinedo, E.; Wendt, A.; Turatsinze, J.V.; Brondani, L.A.; Nardelli, T.R.; Nogueira, T.C.; Esguerra, J.L.S.S.; et al. Neuron-enriched RNA-binding proteins regulate pancreatic beta cell function and survival. J. Biol. Chem. 2017, 292, 3466–3480. [Google Scholar] [CrossRef] [PubMed]
- Villate, O.; Turatsinze, J.; Mascali, L.G.; Grieco, F.A.; Nogueira, C.; Cunha, D.A.; Nardelli, T.R.; Sammeth, M.; Salunkhe, V.A.; Esguerra, J.L.S.; et al. Nova1 is a master regulator of alternative splicing in pancreatic beta cells. Nucleic Acids Res. 2014, 42, 11818–11830. [Google Scholar] [CrossRef] [PubMed]
- Begg, B.E.; Jens, M.; Wang, P.Y.; Minor, C.M.; Christopher, B. Concentration-dependent splincing is enabled by Rbfox motifs of intermediate affinity. Nat. Struct. Mol. Biol. 2021, 27, 901–912. [Google Scholar] [CrossRef]
- Ladd, A.N.; Charlet-B, N.; Cooper, T.A. The CELF Family of RNA Binding Proteins Is Implicated in Cell-Specific and Developmentally Regulated Alternative Splicing. Mol. Cell Biol. 2001, 21, 1285–1296. [Google Scholar] [CrossRef]
- Dasgupta, T.; Ladd, A.N. The importance of CELF control: Molecular and biological roles of the CUG-BP, Elav-like family of RNA-binding proteins. Wiley Interdiscip. Rev. RNA 2012, 3, 104–121. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; Sun, B.-F.; Zhao, Y.-L.; Yang, Y.-G. FTO and Obesity: Mechanisms of Association. Curr. Diabetes Rep. 2014, 14, 486. [Google Scholar] [CrossRef]
- Scuteri, A.; Sanna, S.; Chen, W.M.; Uda, M.; Albai, G.; Strait, J.; Najjar, S.; Nagaraja, R.; Orrú, M.; Usala, G.; et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007, 3, 1200–1210. [Google Scholar] [CrossRef]
- Bravard, A.; Lefai, E.; Meugnier, E.; Pesenti, S.; Disse, E.; Vouillarmet, J.; Peretti, N.; Rabasa-Lhoret, R.; Laville, M.; Vidal, H.; et al. FTO is increased in muscle during type 2 diabetes, and its overexpression in myotubes alters insulin signaling, enhances lipogenesis and ROS production, and induces mitochondrial dysfunction. Diabetes 2011, 60, 258–268. [Google Scholar] [CrossRef]
- Li, H.; Ren, Y.; Mao, K.; Hua, F.; Yang, Y.; Wei, N.; Yue, C.; Li, D.; Zhang, H. FTO is involved in Alzheimer’s disease by targeting TSC1-mTOR-Tau signaling. Biochem. Biophys. Res. Commun. 2018, 498, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Baltimore, D. RNA-binding protein Lin28 in cancer and immunity. Cancer Lett. 2016, 375, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Wei, J.; Bishopric, N.H. A cardiac myocyte-restricted Lin28/let-7 regulatory axis promotes hypoxia-mediated apoptosis by inducing the AKT signaling suppressor PIK3IP1. Biochim. Biophys. Acta-Mol. Basis Dis. 2016, 1862, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, M.; Lin, J.; Hu, J.; Zhang, R.; Li, C.; Wei, T.; Sun, D.; Wei, J.; Wang, H. Lin28a protects against diabetic cardiomyopathy via the PKA/ROCK2 pathway. Biochem. Biophys. Res. Commun. 2016, 469, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Ng, S.C.; Segr, A.V.; Shinoda, G.; Shah, S.P.; Einhorn, W.S.; Takeuchi, A.; Engreitz, J.M.; Hagan, J.P.; Kharas, M.G.; et al. The Lin28/let-7 axis regulates glucose metabolism. Cell 2011, 147, 81–94. [Google Scholar] [CrossRef]
- Thornton, J.E.; Gregory, R.I. How does Lin28 let-7 control development and disease? Trends Cell Biol. 2012, 22, 474–482. [Google Scholar] [CrossRef]
- Gustafson, E.A.; Wessel, G.M. DEAD-box helicases: Posttranslational regulation and function. Biochem. Biophys. Res. Commun. 2010, 395, 1–6. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, M.; Cai, Z.; Liu, H.; Zhong, W.; Hao, Q.; Cheng, D.; Hu, X.; Hou, J.; Xu, P.; et al. RNA-binding protein DDX1 is responsible for fatty acid-mediated repression of insulin translation. Nucleic Acids Res. 2018, 46, 12052–12066. [Google Scholar] [CrossRef]
- Iadevaia, V.; Gerber, A.P. Combinatorial control of mRNA fates by RNA-binding proteins and non-coding RNAs. Biomolecules 2015, 5, 2207–2222. [Google Scholar] [CrossRef]
- Treiber, T.; Treiber, N.; Plessmann, U.; Harlander, S.; Daiß, J.L.; Eichner, N.; Lehmann, G.; Schall, K.; Urlaub, H.; Meister, G. A Compendium of RNA-Binding Proteins that Regulate MicroRNA Biogenesis. Mol. Cell 2017, 66, 270–284.e13. [Google Scholar] [CrossRef]
- Connerty, P.; Ahadi, A.; Hutvagner, G. RNA Binding Proteins in the miRNA pathway. Int. J. Mol. Sci. 2015, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Coller, H. Functional Interactions Between microRNAs and RNA Binding Proteins. MicroRNA 2012, 1, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Janas, M.M.; Wang, B.; Harris, A.S.; Aguiar, M.; Shaffer, J.M.; Subrahmanyam, Y.V.B.K.; Behlke, M.A.; Wucherpfennig, K.W.; Gygi, S.P.; Gagnon, E.; et al. Alternative RISC assembly: Binding and repression of microRNA-mRNA duplexes by human Ago proteins. RNA 2012, 18, 2041–2055. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.J.D.; Marsden, P.A. Competition and collaboration between RNA-binding proteins and microRNAs. Wiley Interdiscip. Rev. RNA 2014, 5, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Hyeon, H.K.; Kuwano, Y.; Srikantan, S.; Eun, K.L.; Martindale, J.L.; Gorospe, M. HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev. 2009, 23, 1743–1748. [Google Scholar]
- Jeyabal, P.; Thandavarayan, R.A.; Joladarashi, D.; Suresh Babu, S.; Krishnamurthy, S.; Bhimaraj, A.; Youker, K.A.; Kishore, R.; Krishnamurthy, P. MicroRNA-9 inhibits hyperglycemia-induced pyroptosis in human ventricular cardiomyocytes by targeting ELAVL1. Biochem. Biophys. Res. Commun. 2016, 471, 423–429. [Google Scholar] [CrossRef]
- Srikantan, S.; Tominaga, K.; Gorospe, M. Functional Interplay between RNA-Binding Protein HuR and microRNAs. Curr. Protein Pept. Sci. 2012, 13, 372–379. [Google Scholar] [CrossRef]
- Fernández-de Frutos, M.; Galán-Chilet, I.; Goedeke, L.; Kim, B.; Pardo-Marqués, V.; Pérez-García, A.; Herrero, J.I.; Fernández-Hernando, C.; Kim, J.; Ramírez, C.M. MicroRNA 7 Impairs Insulin Signaling and Regulates Aβ Levels through Posttranscriptional Regulation of the Insulin Receptor Substrate 2, Insulin Receptor, Insulin-Degrading Enzyme, and Liver X Receptor Pathway. Mol. Cell Biol. 2019, 39, e00170-19. [Google Scholar] [CrossRef]
- Chakraborty, C.; Doss, C.G.P.; Bandyopadhyay, S.; Agoramoorthy, G. Influence of miRNA in insulin signaling pathway and insulin resistance: Micro-molecules with a major role in type-2 diabetes. Wiley Interdiscip. Rev. RNA 2014, 5, 697–712. [Google Scholar] [CrossRef]
- Chen, H.; Lan, H.Y.; Roukos, D.H.; Cho, W.C. Application of microRNAs in diabetes mellitus. J. Endocrinol. 2014, 222, R1–R10. [Google Scholar] [CrossRef]
- Nutter, C.A.; Kuyumcu-Martinez, M.N. Emerging roles of RNA-binding proteins in diabetes and their therapeutic potential in diabetic complications. Wiley Interdiscip. Rev. RNA 2018, 9, e1459. [Google Scholar] [CrossRef] [PubMed]
- Knoch, K.P.; Nath-Sain, S.; Petzold, A.; Schneider, H.; Beck, M.; Wegbrod, C.; Sönmez, A.; Münster, C.; Friedrich, A.; Roivainen, M.; et al. PTBP1 is required for glucose-stimulated cap-independent translation of insulin granule proteins and Coxsackieviruses in beta cells. Mol. Metab. 2014, 3, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Knoch, K.P.; Meisterfeld, R.; Kersting, S.; Bergert, H.; Altkrüger, A.; Wegbrod, C.; Jäger, M.; Saeger, H.D.; Solimena, M. cAMP-dependent phosphorylation of PTB1 promotes the expression of insulin secretory granule proteins in β cells. Cell Metab. 2006, 3, 123–134. [Google Scholar] [CrossRef]
- Knoch, K.P.; Bergetr, H.; Borgonovo, B.; Saeger, H.D.; Altkrüger, A.; Verkade, P.; Solimena, M. Polypyrimidine tract-binding protein promotes insulin secretory granule biogenesis. Nat. Cell Biol. 2004, 6, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Poy, M.N.; Hausser, J.; Trajkovski, M.; Braun, M.; Collins, S.; Rorsman, P.; Zavolan, M.; Stoffel, M. miR-375 maintains normal pancreatic α- and β-cell mass. Proc. Natl. Acad. Sci. USA 2009, 106, 5813–5818. [Google Scholar] [CrossRef] [PubMed]
- Baroukh, N.; Ravier, M.A.; Loder, M.K.; Hill, E.V.; Bounacer, A.; Scharfmann, R.; Rutter, G.A.; Van Obberghen, E. MicroRNA-124a regulates foxa2 expression and intracellular signaling in pancreatic β-cell lines. J. Biol. Chem. 2007, 282, 19575–19588. [Google Scholar] [CrossRef]
- Puigserver, P.; Rodgers, J.T. Foxa2, a novel transcriptional regulator of insulin sensitivity. Nat. Med. 2006, 12, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, D.; Roy, U.; Garg, S.; Ghosh, S.; Pathak, S.; Kolthur-Seetharam, U. Sirt1 and mir-9 expression is regulated during glucose-stimulated insulin secretion in pancreatic β-islets. FEBS J. 2011, 278, 1167–1174. [Google Scholar] [CrossRef]
- Sun, L.L.; Jiang, B.G.; Li, W.T.; Zou, J.J.; Shi, Y.Q.; Liu, Z.M. MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression. Diabetes Res. Clin. Pract. 2011, 91, 94–100. [Google Scholar] [CrossRef]
- Jeong, H.J.; Park, S.Y.; Yang, W.M.; Lee, W. The induction of miR-96 by mitochondrial dysfunction causes impaired glycogen synthesis through translational repression of IRS-1 in SK-Hep1 cells. Biochem. Biophys. Res. Commun. 2013, 434, 503–508. [Google Scholar] [CrossRef]
- Ryu, H.S.; Park, S.-Y.; Ma, D.; Zhang, J.; Lee, W. Correction: The Induction of MicroRNA Targeting IRS-1 Is Involved in the Development of Insulin Resistance under Conditions of Mitochondrial Dysfunction in Hepatocytes. PLoS ONE 2011, 6, e17343. [Google Scholar] [CrossRef]
- Benito-Vicente, A.; Uribe, K.B.; Rotllan, N.; Ramírez, C.M.; Jebari-Benslaiman, S.; Goedeke, L.; Canfrán-Duque, A.; Galicia-García, U.; De Urturi, D.S.; Aspichueta, P.; et al. MiR-27b modulates insulin signaling in hepatocytes by regulating insulin receptor expression. Int.J. Mol. Sci. 2020, 21, 8675. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Goedeke, L.; Smibert, P.; Ramírez, C.M.; Warrier, N.P.; Andreo, U.; Cirera-Salinas, D.; Rayner, K.; Suresh, U.; Pastor-Pareja, J.C.; et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 9232–9237. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Srivastava, R.; Srivastava, A.K.; Ali, S.; Datta, M. MiR-135a targets IRS2 and regulates insulin signaling and glucose uptake in the diabetic gastrocnemius skeletal muscle. Biochim. Biophys. Acta-Mol. Basis Dis. 2013, 1832, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Seo, S.Y.; Kim, T.H.; Kim, S.G. Decrease of microRNA-122 causes hepatic insulin resistance by inducing protein tyrosine phosphatase 1B, which is reversed by licorice flavonoid. Hepatology 2012, 56, 2209–2220. [Google Scholar] [CrossRef]
- Trajkovski, M.; Hausser, J.; Soutschek, J.; Bhat, B.; Akin, A.; Zavolan, M.; Heim, M.H.; Stoffel, M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 2011, 474, 649–653. [Google Scholar] [CrossRef]
- Latouche, C.; Natoli, A.; Reddy-Luthmoodoo, M.; Heywood, S.E.; Armitage, J.A.; Kingwell, B.A. MicroRNA-194 modulates glucose metabolism and its skeletal muscle expression is reduced in diabetes. PLoS ONE 2016, 11, e0155108. [Google Scholar] [CrossRef]
- Li, J.; Gong, L.; Liu, S.; Zhang, Y.; Zhang, C.; Tian, M.; Lu, H.; Bu, P.; Yang, J.; Ouyang, C.; et al. Adipose HuR protects against diet-induced obesity and insulin resistance. Nat. Commun. 2019, 10, 2375. [Google Scholar] [CrossRef]
- Jordan, S.D.; Krüger, M.; Willmes, D.M.; Redemann, N.; Wunderlich, F.T.; Brönneke, H.S.; Merkwirth, C.; Kashkar, H.; Olkkonen, V.M.; Böttger, T.; et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat. Cell Biol. 2011, 13, 434–448. [Google Scholar] [CrossRef]
- Kornfeld, J.-W.; Baitzel, C.; Könner, A.C.; Nicholls, H.T.; Vogt, M.C.; Herrmanns, K.; Scheja, L.; Haumaitre, C.; Wolf, A.M.; Knippschild, U.; et al. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature 2013, 494, 111–115. [Google Scholar] [CrossRef]
- Ling, H.-Y.; Ou, H.-S.; Feng, S.-D.; Zhang, X.-Y.; Tuo, Q.-H.; Chen, L.-X.; Zhu, B.-Y.; Gao, Z.-P.; Tang, C.-K.; Yin, W.-D.; et al. CHANGES IN microRNA (miR) PROFILE AND EFFECTS OF miR-320 IN INSULIN-RESISTANT 3T3-L1 ADIPOCYTES. Clin. Exp. Pharmacol. Physiol. 2009, 36, e32–e39. [Google Scholar] [CrossRef]
- Ling, H.-Y.; Hu, B.; Hu, X.-B.; Zhong, J.; Feng, S.-D.; Qin, L.; Liu, G.; Wen, G.-B.; Liao, D.-F. MiRNA-21 Reverses High Glucose and High Insulin Induced Insulin Resistance in 3T3-L1 Adipocytes through Targeting Phosphatase and Tensin Homologue. Exp. Clin. Endocrinol. Diabetes 2012, 120, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, M.; Sgroi, A.; Veyrat-Durebex, C.; Rubbia-Brandt, L.; Buhler, L.H.; Foti, M. Unsaturated fatty acids inhibit the expression of tumor suppressor phosphatase and tensin homolog(PTEN) via microRNA-21 up-regulation in hepatocytes. Hepatology 2009, 49, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Buchan, R.J.; Cook, S.A. MicroRNA-223 regulates Glut4 expression and cardiomyocyte glucose metabolism. Cardiovasc. Res. 2010, 86, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Ono, K.; Nishi, H.; Iwanaga, Y.; Nagao, K.; Kinoshita, M.; Kuwabara, Y.; Takanabe, R.; Hasegawa, K.; Kita, T.; et al. MicroRNA-133 regulates the expression of GLUT4 by targeting KLF15 and is involved in metabolic control in cardiac myocytes. Biochem. Biophys. Res. Commun. 2009, 389, 315–320. [Google Scholar] [CrossRef]
- Higaki, S.; Muramatsu, M.; Matsuda, A.; Matsumoto, K.; Satoh, J.-i.; Michikawa, M.; Niida, S. Defensive effect of microRNA-200b/c against amyloid-beta peptide-induced toxicity in Alzheimer’s disease models. PLoS ONE 2018, 13, e0196929. [Google Scholar] [CrossRef]
- Hu, Y.K.; Wang, X.; Li, L.; Du, Y.H.; Ye, H.T.; Li, C.Y. MicroRNA-98 induces an Alzheimer’s disease-like disturbance by targeting insulin-like growth factor 1. Neurosci. Bull. 2013, 29, 745–751. [Google Scholar] [CrossRef]
- Liu, H.; Chu, W.; Gong, L.; Gao, X.; Wang, W. MicroRNA-26b is upregulated in a double transgenic mouse model of Alzheimer’s disease and promotes the expression of amyloid-β by targeting insulin-like growth factor 1. Mol. Med. Rep. 2016, 13, 2809–2814. [Google Scholar] [CrossRef]
- Fenn, A.M.; Smith, K.M.; Lovett-Racke, A.E.; Guerau-de-Arellano, M.; Whitacre, C.C.; Godbout, J.P. Increased micro-RNA 29b in the aged brain correlates with the reduction of insulin-like growth factor-1 and fractalkine ligand. Neurobiol. Aging 2013, 34, 2748–2758. [Google Scholar] [CrossRef]
- Petersmann, A.; Müller-Wieland, D.; Müller, U.A.; Landgraf, R.; Nauck, M.; Freckmann, G.; Heinemann, L.; Schleicher, E. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127, S1–S7. [Google Scholar] [CrossRef]
- James, W.P.T. The fundamental drivers of the obesity epidemic. Obes. Rev. 2008, 9 (Suppl. 1), 6–13. [Google Scholar] [CrossRef] [PubMed]
- Guay, C.; Regazzi, R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 2013, 9, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Gorospe, M. Minireview: Posttranscriptional regulation of the insulin and insulin-like growth factor systems. Endocrinology 2010, 151, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Tak, H.; Kim, C.; Kang, H.; Ji, E.; Ahn, S.; Jung, M.; Kim, H.L.; Lee, J.H.; Kim, W.; et al. RNA binding protein HuD contributes to β-cell dysfunction by impairing mitochondria dynamics. Cell Death Differ. 2020, 27, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, H.; Kang, H.; Shin, J.J.; Tak, H.; Kim, W.; Gorospe, M.; Lee, E.K. RNA-binding protein HuD reduces triglyceride production in pancreatic β cells by enhancing the expression of insulin-induced gene 1. Biochim. Biophys. Acta-Gene Regul. Mech. 2016, 1859, 675–685. [Google Scholar] [CrossRef]
- Zhai, K.; Gu, L.; Yang, Z.; Mao, Y.; Jin, M.; Chang, Y.; Yuan, Q.; Leblais, V.; Wang, H.; Fischmeister, R.; et al. RNA-binding protein CUGBP1 regulates insulin secretion via activation of phosphodiesterase 3B in mice. Diabetologia 2016, 59, 1959–1967. [Google Scholar] [CrossRef]
- Ouaamari, A.E.; Baroukh, N.; Martens, G.A.; Lebrun, P.; Pipeleers, D.; Van Obberghen, E. MiR-375 targets 3′l-Phosphoinositide-Dependent protein Kinase-1 and regulates Glucose-Induced biological responses in pancreatic β-Cells. Diabetes 2008, 57, 2708–2717. [Google Scholar] [CrossRef]
- Nieto, M.; Hevia, P.; Garcia, E.; Klein, D.; Alvarez-Cubela, S.; Bravo-Egana, V.; Rosero, S.; Damaris Molano, R.; Vargas, N.; Ricordi, C.; et al. Antisense miR-7 impairs insulin expression in developing pancreas and in cultured pancreatic buds. Cell Transplant. 2012, 21, 1761–1774. [Google Scholar] [CrossRef]
- Guay, C.; Roggli, E.; Nesca, V.; Jacovetti, C.; Regazzi, R. Diabetes mellitus, a microRNA-related disease? Transl. Res. 2011, 157, 253–264. [Google Scholar] [CrossRef]
- Kumar, M.; Nath, S.; Prasad, H.K.; Sharma, G.D.; Li, Y. MicroRNAs: A new ray of hope for diabetes mellitus. Protein Cell 2012, 3, 726–738. [Google Scholar] [CrossRef]
- Zampetaki, A.; Kiechl, S.; Drozdov, I.; Willeit, P.; Mayr, U.; Prokopi, M.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Bonora, E.; et al. Plasma MicroRNA profiling reveals loss of endothelial MiR-126 and other MicroRNAs in type 2 diabetes. Circ. Res. 2010, 107, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhu, J.; Han, W.; Jiang, X.; Xu, M.; Zhao, Y.; Dong, Q.; Pang, Z.; Guan, Q.; Gao, L.; et al. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: A clinical study. Acta Diabetol. 2011, 48, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xing, W.; Xie, L. Regulatory Roles of MicroRNAs in Diabetes. Int. J. Mol. Sci. 2016, 17, 1729. [Google Scholar] [CrossRef]
- Wood, A.R.; Tyrrell, J.; Beaumont, R.; Jones, S.E.; Tuke, M.A.; Ruth, K.S.; Yaghootkar, H.; Freathy, R.M.; Murray, A.; Frayling, T.M.; et al. Variants in the FTO and CDKAL1 loci have recessive effects on risk of obesity and type 2 diabetes, respectively. Diabetologia 2016, 59, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Hess, M.; Hess, S.; Meyer, K.; Verhagen, L.; Koch, L.; Brönneke, H.; Dietrich, M.; Jordan, S.; Saletore, Y.; Elemento, O.; et al. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat. Neurosci. 2013, 16, 1042–1048. [Google Scholar] [CrossRef]
- Fawcett, K.; Barroso, I. The genetics of obesity: FTO leads the way. Trends Genet. 2010, 26, 266–274. [Google Scholar] [CrossRef]
- Esau, C.; Kang, X.; Peralta, E.; Hanson, E.; Marcusson, E.G.; Ravichandran, L.V.; Sun, Y.; Koo, S.; Perera, R.J.; Jain, R.; et al. MicroRNA-143 regulates adipocyte differentiation. J. Biol. Chem. 2004, 279, 52361–52365. [Google Scholar] [CrossRef]
- Xie, H.; Sun, L.; Lodish, H.F. Targeting microRNAs in obesity. Expert Opin. Ther. Targets 2009, 13, 1227–1238. [Google Scholar] [CrossRef]
- Kang, T.; Lu, W.; Xu, W.; Anderson, L.; Bacanamwo, M.; Thompson, W.; Chen, Y.E.; Liu, D. MicroRNA-27 (miR-27) Targets Prohibitin and Impairs Adipocyte Differentiation and Mitochondrial Function in Human Adipose-derived Stem Cells. J. Biol. Chem. 2013, 288, 34394. [Google Scholar] [CrossRef]
- Lin, Q.; Gao, Z.; Alarcon, R.; Ye, J.; Yun, Z. A role of miR-27 in the regulation of adipogenesis. FEBS J. 2009, 276, 2348–2358. [Google Scholar] [CrossRef]
- Price, N.L.; Holtrup, B.; Kwei, S.L.; Wabitsch, M.; Rodeheffer, M.; Bianchini, L.; Suárez, Y.; Fernández-Hernando, C. SREBP-1c/MicroRNA 33b Genomic Loci Control Adipocyte Differentiation. Mol. Cell Biol. 2016, 36, 1180–1193. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Guo, X. The clinical potential of circulating microRNAs in obesity. Nat. Rev. Endocrinol. 2019, 15, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Danielson, K.M.; Benton, M.C.; Ziegler, O.; Shah, R.; Stubbs, R.S.; Das, S.; Macartney-Coxson, D. miRNA Signatures of Insulin Resistance in Obesity. Obesity 2017, 25, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, H.M.; Miller, N.; McAnena, O.J.; O’Brien, T.; Kerin, M.J. Differential miRNA expression in omental adipose tissue and in the circulation of obese patients identifies novel metabolic biomarkers. J. Clin. Endocrinol. Metab. 2011, 96, E846–E850. [Google Scholar] [CrossRef]
- Oses, M.; Margareto Sanchez, J.; Portillo, M.; Aguilera, C.; Labayen, I. Circulating miRNAs as Biomarkers of Obesity and Obesity-Associated Comorbidities in Children and Adolescents: A Systematic Review. Nutrients 2019, 11, 2890. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: The missing links. The Claude Bernard Lecture 2009. Diabetologia 2010, 53, 1270–1287. [Google Scholar] [CrossRef]
- Kashyap, S.R.; DeFronzo, R.A. The insulin resistance syndrome: Physiological considerations. Diabetes Vasc. Dis. Res. 2007, 4, 13–19. [Google Scholar] [CrossRef]
- Janus, A.; Szahidewicz-Krupska, E.; Mazur, G.; Doroszko, A. Insulin resistance and endothelial dysfunction constitute a common therapeutic target in cardiometabolic disorders. Mediat. Inflamm. 2016, 2016, 3634948. [Google Scholar] [CrossRef]
- Fernández-Hernando, C.; József, L.; Jenkins, D.; Di Lorenzo, A.; Sessa, W. Absence of Akt1 reduces vascular smooth muscle cell migration and survival and induces features of plaque vulnerability and cardiac dysfunction during atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Rotllan, N.; Chamorro-Jorganes, A.; Araldi, E.; Wanschel, A.C.; Aryal, B.; Aranda, J.F.; Goedeke, L.; Salerno, A.G.; Ramírez, C.M.; Sessa, W.C.; et al. Hematopoietic Akt2 deficiency attenuates the progression of atherosclerosis. FASEB J. 2015, 29, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Paukku, K.; Backlund, M.; De Boer, R.A.; Kalkkinen, N.; Kontula, K.K.; Lehtonen, J.Y.A. Regulation of AT1R expression through HuR by insulin. Nucleic Acids Res. 2012, 40, 5250–5261. [Google Scholar] [CrossRef] [PubMed]
- Nutter, C.A.; Jaworski, E.A.; Verma, S.K.; Deshmukh, V.; Wang, Q.; Botvinnik, O.B.; Lozano, M.J.; Abass, I.J.; Ijaz, T.; Brasier, A.R.; et al. Dysregulation of RBFOX2 Is an Early Event in Cardiac Pathogenesis of Diabetes. Cell Rep. 2016, 15, 2200–2213. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Song, Y.H.; Geng, Y.J.; Lin, Q.X.; Shan, Z.X.; Lin, S.G.; Li, Y. Glucose induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1. Biochem. Biophys. Res. Commun. 2008, 376, 548–552. [Google Scholar] [CrossRef]
- Shan, Z.X.; Lin, Q.X.; Fu, Y.H.; Deng, C.Y.; Zhou, Z.L.; Zhu, J.N.; Liu, X.Y.; Zhang, Y.Y.; Li, Y.; Lin, S.G.; et al. Upregulated expression of miR-1/miR-206 in a rat model of myocardial infarction. Biochem. Biophys. Res. Commun. 2009, 381, 597–601. [Google Scholar] [CrossRef]
- Wang, X.H.; Qian, R.Z.; Zhang, W.; Chen, S.F.; Jin, H.M.; Hu, R.M. MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clin. Exp. Pharmacol. Physiol. 2009, 36, 181–188. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, X.; Shan, P.F. MicroRNAs and Cardiovascular Disease in Diabetes Mellitus. BioMed Res. Int. 2017, 2017, 4080364. [Google Scholar] [CrossRef]
- Arunachalam, G.; Lakshmanan, A.P.; Samuel, S.M.; Triggle, C.R.; Ding, H. Molecular interplay between microRNA-34a and sirtuin1 in hyperglycemia-mediated impaired angiogenesis in endothelial cells: Effects of metformins. J. Pharmacol. Exp. Ther. 2016, 356, 314–323. [Google Scholar] [CrossRef]
- Reddy, M.A.; Das, S.; Zhuo, C.; Jin, W.; Wang, M.; Lanting, L.; Natarajan, R. Regulation of vascular smooth muscle cell dysfunction under diabetic conditions by MIR-504. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 864–873. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.M.; Xi, Y.; Wu, G.; Shelat, H.; Gao, S.; Cheng, J.; Geng, Y.J. MicroRNA-1/133 targeted dysfunction of potassium channels KCNE1 and KCNQ1 in human cardiac progenitor cells with simulated hyperglycemia. Int. J. Cardiol. 2013, 167, 1076–1078. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Frisardi, V.; Solfrizzi, V.; Seripa, D.; Capurso, C.; Santamato, A.; Sancarlo, D.; Vendemiale, G.; Pilotto, A.; Panza, F. Metabolic-cognitive syndrome: A cross-talk between metabolic syndrome and Alzheimer’s disease. Ageing Res. Rev. 2010, 9, 399–417. [Google Scholar] [CrossRef]
- Kim, B.; Feldman, E.L. Insulin resistance in the nervous system. Trends Endocrinol. Metab. 2012, 23, 133–141. [Google Scholar] [CrossRef]
- Talbot, K.; Wang, H.-Y.; Kazi, H.; Han, L.-Y.; Bakshi, K.; Stucky, A.; Fuino, R.; Kawaguchi, K.; Samoyedny, A.; Wilson, R.; et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Investig. 2012, 122, 1316–1338. [Google Scholar] [CrossRef]
- De la Monte, S.M. Type 3 diabetes is sporadic Alzheimer-s disease: Mini-review. Eur. Neuropsychopharmacol. 2014, 24, 1954–1960. [Google Scholar] [CrossRef]
- Bedse, G.; Di Domenico, F.; Serviddio, G.; Cassano, T. Aberrant insulin signaling in Alzheimer’s disease: Current knowledge. Front. Neurosci. 2015, 9, 204. [Google Scholar] [CrossRef]
- Craft, S.; Watson, G.S. Insulin and neurodegenerative disease: Shared and specific mechanisms. Lancet Neurol. 2004, 3, 169–178. [Google Scholar] [CrossRef]
- Zhao, W.Q.; Chen, H.; Quon, M.J.; Alkon, D.L. Insulin and the insulin receptor in experimental models of learning and memory. Eur. J. Pharmacol. 2004, 490, 71–81. [Google Scholar] [CrossRef]
- Kurochkin, I.V.; Guarnera, E.; Berezovsky, I.N. Insulin-Degrading Enzyme in the Fight against Alzheimer’s Disease. Trends Pharmacol. Sci. 2018, 39, 49–58. [Google Scholar] [CrossRef]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, V.; Thingore, C.; Juvekar, A. Insulin resistance: A connecting link between Alzheimer’s disease and metabolic disorder. Metab. Brain Dis. 2021, 36, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.S.; Craft, S. The Role of Insulin Resistance in the Pathogenesis of Alzheimer’s Disease. CNS Drugs 2003, 17, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Jash, K.; Gondaliya, P.; Kirave, P.; Kulkarni, B.; Sunkaria, A.; Kalia, K. Cognitive dysfunction: A growing link between diabetes and Alzheimer’s disease. Drug Dev. Res. 2020, 81, 144–164. [Google Scholar] [CrossRef]
- Adlakha, Y.K.; Saini, N. Brain microRNAs and insights into biological functions and therapeutic potential of brain enriched miRNA-128. Mol. Cancer 2014, 13, 33. [Google Scholar] [CrossRef]
- Motti, D.; Bixby, J.L.; Lemmon, V.P. MicroRNAs and neuronal development. Semin. Fetal Neonatal. Med. 2012, 17, 347–352. [Google Scholar] [CrossRef]
- O’Carroll, D.; Schaefer, A. General principals of miRNA biogenesis and regulation in the brain. Neuropsychopharmacology 2013, 38, 39–54. [Google Scholar] [CrossRef]
- Abe, M.; Bonini, N.M. MicroRNAs and neurodegeneration: Role and impact. Trends Cell Biol. 2013, 23, 30–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Ao, X.; Yu, W.; Zhang, L.; Wang, Y.; Chang, W. The Role of Non-coding RNAs in Alzheimer’s Disease: From Regulated Mechanism to Therapeutic Targets and Diagnostic Biomarkers. Front. Aging Neurosci. 2021, 13, 654978. [Google Scholar] [CrossRef]
- Wang, B.; Sun, F.; Dong, N.; Sun, Z.; Diao, Y.; Zheng, C.; Sun, J.; Yang, Y.; Jiang, D. MicroRNA-7 directly targets insulin-like growth factor 1 receptor to inhibit cellular growth and glucose metabolism in gliomas. Diagn. Pathol. 2014, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.M.; Maes, O.C.; Chertkow, H.M.; Wang, E. MicroRNA Expression in Alzheimer Blood Mononuclear Cells. Gene Regul. Syst. Biol. 2007, 1, GRSB.S361. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, J.P.; Ward, J.; Taylor, I.A.; Waters, M.; Shi, Y.; Cannon, B.; Kelnar, K.; Kemppainen, J.; Brown, D.; Chen, C.; et al. Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J. Alzheimer’s Dis. 2008, 14, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, P.N.; Dua, P.; Hill, J.M.; Bhattacharjee, S.; Zhao, Y.; Lukiw, W.J. MicroRNA (miRNA) speciation in Alzheimer’s disease (AD) cerebrospinal fluid (CSF) and extracellular fluid (ECF). Int. J. Biochem. Mol. Biol. 2012, 3, 365–373. [Google Scholar]
- Lukiw, W.J.; Alexandrov, P.N. Regulation of complement factor H (CFH) by multiple miRNAs in Alzheimer’s disease (AD) brain. Mol. Neurobiol. 2012, 46, 11–19. [Google Scholar] [CrossRef]
- Lehmann, S.M.; Krüger, C.; Park, B.; Derkow, K.; Rosenberger, K.; Baumgart, J.; Trimbuch, T.; Eom, G.; Hinz, M.; Kaul, D.; et al. An unconventional role for miRNA: Let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 2012, 15, 827–835. [Google Scholar] [CrossRef]
- Geekiyanage, H.; Jicha, G.A.; Nelson, P.T.; Chan, C. Blood serum miRNA: Non-invasive biomarkers for Alzheimer’s disease. Exp. Neurol. 2012, 235, 491–496. [Google Scholar] [CrossRef]
- Ramírez, C.M.; Goedeke, L.; Fernández-Hernando, C. “Micromanaging” metabolic syndrome. Cell Cycle 2011, 10, 3249–3252. [Google Scholar] [CrossRef]
- Goedeke, L.; Fernández-Hernando, C. MicroRNAs: A connection between cholesterol metabolism and neurodegeneration. Neurobiol. Dis. 2014, 72 Pt A, 48–53. [Google Scholar] [CrossRef]
| Cause | Gene/Protein | Mechanism | Reference |
|---|---|---|---|
| Reduced insulin quantity or function | - | Autoimmune antibodies | [20] |
| INS | Mutations in the insulin gene | [21,22] | |
| Reduced INSR availability | - | Reduced exposure in the membrane | [23,24] |
| - | Autoimmune antibodies | [25] | |
| INSR mutations | INSR | Accelerated degradation | [26,27] |
| In the ligand-binding domain | [28,29] | ||
| In the tyrosine kinase domain | [27,30] | ||
| Reduced mRNA expression | [31] | ||
| Mutations in other elements of the pathway | IRS-1 | Impaired insulin signaling | [32,33,34,35] |
| PTEN | Impaired end of signaling | [36,37] | |
| GLUT-4 | Reduced glucose internalization in target tissues | [38] | |
| AKT and its targets | Impaired insulin signaling | [39,40,41,42] | |
| Lipotoxicity | Lipoprotein lipase | Overexpression in muscle | [43] |
| IKK and JNK | Endoplasmic reticulum stress by circulating FFA or ceramides | [44,45] | |
| Serine/threonine (Ser/Thr) kinases (PKC-θ, PKCβII and PKCδ) | DAG-induced activation | [46] | |
| IKKβ and NFκB | DAG-induced inhibition | [47] | |
| Inflammation | Proinflammatory cytokines (MCP-1, TNF-α, IL1β or IL-6) | Adipocytes and macrophages triggered in obesity Inhibition of several steps of the insulin pathway | [15,48,49,50,51] |
| Mitochondrial Dysfunction | - | Reduced content and/or biogenesis | [52,53] |
| - | Decreased ATP production and phosphocreatine recovery | [54,55,56,57] | |
| Citrate synthase | Decreased activity | [58] | |
| - | Lower OxPhos capacity | [59] | |
| - | Increased ROS | [60,61,62] | |
| Alterations in the zinc metabolism | Zinc transporters (ZnT8, SLC30A8, ZnT7) | Regulation of multiple zinc-dependent effectors | [63,64,65,66,67] |
| Regulator | Tissue/Cell Type | Target Genes | Function | Disease | Reference |
|---|---|---|---|---|---|
| hnRNPI | β-cells | INS | ↑ Insulin secretion | T2DM | [123,124,125] |
| Rbfox1,2 | β-cells | INS | ↓ Insulin secretion | T2DM | [94] |
| miR-375 | β-cells | CAV-1 | β-cell development | T2DM | [126] |
| miR-124 | β-cells | FOXA2 | ↓ Insulin secretion | T2DM | [127,128] |
| miR-9 | β-cells | SIRT1 | ↓ Insulin secretion | T2DM | [129] |
| miR-15a | β-cells | UCP2 | ↑ Insulin biosynthesis and secretion | T2DM | [130] |
| Lin28/Let-7 | Muscle | IGF1R, INSR, IRS2, AKT | Insulin signaling | T2DM | [104,106] |
| miR-96, miR-126 | Hepatocytes | IRS1 | ↓ Insulin signaling | T2DM | [131,132] |
| miR-27, miR-33 | Hepatocytes | INSR, IRS2 | ↓ Insulin signaling | T2DM | [133,134] |
| miR-135 | Skeletal muscle | IRS2, PI3K/AKT | ↓ Insulin signaling | T2DM | [135] |
| miR-122 | Liver | PTP1B | ↑ Insulin signaling | T2DM | [136] |
| miR-103, miR-107 | Adipocytes | CAV-1 | ↓Insulin sensitivity | T2DM | [137] |
| miR-194 | Skeletal muscle | AKT, GSK3β | ↓Glucose metabolism | T2DM | [138] |
| HuR | Adipocytes | INSIG1 | ↑ Insulin sensitivity | Obesity | [139] |
| miR-143, miR-802 | Liver | PKD/AKT | ↓ Insulin signaling | Obesity | [140,141] |
| miR-320 | Adipocytes | PI3K, GLUT4, AKT | ↓Insulin sensitivity | Obesity | [142] |
| miR-21 | Liver, adipose tissue | PTEN | ↑ Insulin signaling | Obesity | [143,144] |
| Lin28a | Heart | PI3K, AKT | ↑ Insulin sensitivity | CVD | [105,106] |
| miR-223 miR-133 | Cardiomyocytes | GLUT4 GLUT4 | ↑ Glucose uptake ↓ Glucose uptake | CVD CVD | [145] [146] |
| miR-200b/c | Murine primary neurons | S6K1 | ↑ Insulin signaling | AD | [147] |
| miR-7 | Neuronal cells | INSR, IRS2, IDE | ↓ Insulin signaling | AD | [119] |
| miR-26, miR-29, miR-98 | Neuronal cells | IGF1 | ↑ Aβ production and Tau phosphorylation | AD | [148,149,150] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-García, A.; Torrecilla-Parra, M.; Fernández-de Frutos, M.; Martín-Martín, Y.; Pardo-Marqués, V.; Ramírez, C.M. Posttranscriptional Regulation of Insulin Resistance: Implications for Metabolic Diseases. Biomolecules 2022, 12, 208. https://doi.org/10.3390/biom12020208
Pérez-García A, Torrecilla-Parra M, Fernández-de Frutos M, Martín-Martín Y, Pardo-Marqués V, Ramírez CM. Posttranscriptional Regulation of Insulin Resistance: Implications for Metabolic Diseases. Biomolecules. 2022; 12(2):208. https://doi.org/10.3390/biom12020208
Chicago/Turabian StylePérez-García, Ana, Marta Torrecilla-Parra, Mario Fernández-de Frutos, Yolanda Martín-Martín, Virginia Pardo-Marqués, and Cristina M. Ramírez. 2022. "Posttranscriptional Regulation of Insulin Resistance: Implications for Metabolic Diseases" Biomolecules 12, no. 2: 208. https://doi.org/10.3390/biom12020208
APA StylePérez-García, A., Torrecilla-Parra, M., Fernández-de Frutos, M., Martín-Martín, Y., Pardo-Marqués, V., & Ramírez, C. M. (2022). Posttranscriptional Regulation of Insulin Resistance: Implications for Metabolic Diseases. Biomolecules, 12(2), 208. https://doi.org/10.3390/biom12020208







