Marine Origin Ligands of Nicotinic Receptors: Low Molecular Compounds, Peptides and Proteins for Fundamental Research and Practical Applications
Abstract
1. Introduction
2. Marine Low Molecular Weight Compounds Targeting nAChRs
2.1. Biosafety Threat of Shellfish Poisoning with Nicotinergic Ligands
2.2. Cembranoids from Marine Coelentera
2.3. Nereistoxin from Marine Worm and Its Derivatives Used as Insecticides
2.4. Anabaseine, Its Derivatives and Other Marine Alkaloids with Clinical Perspective
3. Marine Origin Peptides Targeting nAChRs
3.1. A Start of the “Conotoxin Era”
3.2. Discovery of New Conotoxins Targeting nAChRs
3.3. Studies on Molecular Bases of nAChR Subtypes Selectivity with Conotoxins
3.4. Medical Perspectives for Conotpeptides Targeting nAChRs
4. Marine Protein Ligands of nAChR—Still an Open Field for Research
4.1. Three-Finger α-Neurotoxins
4.2. Proteomic and Transcriptomic Analyses of Sea Snake Venoms
4.3. Non-Snake Proteins of Marine Origin Acting on nAChRs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishikawa, Y.; Shimada, Y. Fluorescent staining of neuromuscular junctions by using the antibody against acetylcholine receptors of Narke japonica, and double staining with the antibody and erabutoxin b. Brain Res. 1981, 224, 45–54. [Google Scholar] [CrossRef]
- Grady, S.R.; Salminen, O.; Laverty, D.C.; Whiteaker, P.; McIntosh, J.M.; Collins, A.C.; Marks, M.J. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem. Pharmacol. 2007, 74, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Shelukhina, I.V.; Kryukova, E.V.; Lips, K.S.; Tsetlin, V.I.; Kummer, W. Presence of alpha7 nicotinic acetylcholine receptors on dorsal root ganglion neurons proved using knockout mice and selective alpha-neurotoxins in histochemistry. J. Neurochem. 2009, 109, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Yu, T.; Chen, B.; Xu, J.; Chen, W.; Qi, Y.; Zhang, P.; Li, Y.; Kou, Y.; Ma, Y.; et al. Spatial distribution of motor endplates and its adaptive change in skeletal muscle. Theranostics 2019, 9, 734–746. [Google Scholar] [CrossRef]
- Molgó, J.; Aráoz, R.; Benoit, E.; Iorga, B.I. Physical and virtual screening methods for marine toxins and drug discovery targeting nicotinic acetylcholine receptors. Expert Opin. Drug Discov. 2013, 8, 1203–1223. [Google Scholar] [CrossRef] [PubMed]
- Lebbe, E.K.M.M.; Peigneur, S.; Wijesekara, I.; Tytgat, J. Conotoxins targeting nicotinic acetylcholine receptors: An overview. Mar. Drugs 2014, 12, 2970–3004. [Google Scholar] [CrossRef]
- Mir, R.; Karim, S.; Kamal, M.A.; Wilson, C.M.; Mirza, Z. Conotoxins: Structure, therapeutic potential and pharmacological applications. Curr. Pharm. Des. 2016, 22, 582–589. [Google Scholar] [CrossRef]
- Unwin, N. Refined structure of the nicotinic acetylcholine receptor at 4Å resolution. J. Mol. Biol. 2005, 346, 967–989. [Google Scholar] [CrossRef]
- Rahman, M.M.; Teng, J.; Worrell, B.T.; Noviello, C.M.; Lee, M.; Karlin, A.; Stowell, M.H.B.B.; Hibbs, R.E. Structure of the native muscle-type nicotinic receptor and inhibition by snake venom toxins. Neuron 2020, 106, 952–962.e5. [Google Scholar] [CrossRef]
- Noviello, C.M.; Gharpure, A.; Mukhtasimova, N.; Cabuco, R.; Baxter, L.; Borek, D.; Sine, S.M.; Hibbs, R.E. Structure and gating mechanism of the α7 nicotinic acetylcholine receptor. Cell 2021, 184, 2121–2134.e13. [Google Scholar] [CrossRef]
- Celie, P.H.N.; Kasheverov, I.E.; Mordvintsev, D.Y.; Hogg, R.C.; van Nierop, P.; Van Elk, R.; van Rossum-Fikkert, S.E.; Zhmak, M.N.; Bertrand, D.; Tsetlin, V.; et al. Crystal structure of nicotinic acetylcholine receptor homolog AChBP in complex with an alpha-conotoxin PnIA variant. Nat. Struct. Mol. Biol. 2005, 12, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Pan, S.; Xu, M.; Zhang, L.; Yu, J.; Yu, J.; Wu, Y.; Fan, Y.; Li, H.; Kasheverov, I.E.; et al. High Selectivity of an α-Conotoxin LvIA Analogue for α3β2 Nicotinic Acetylcholine Receptors Is Mediated by β2 Functionally Important Residues. J. Med. Chem. 2020, 63, 13656–13668. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtsev, D.; Makarieva, T.; Utkina, N.; Santalova, E.; Kryukova, E.; Methfessel, C.; Tsetlin, V.; Stonik, V.; Kasheverov, I. Marine natural products acting on the acetylcholine-binding protein and nicotinic receptors: From computer modeling to binding studies and electrophysiology. Mar. Drugs 2014, 12, 1859–1875. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Fensin, E.; Gobler, C.J.; Hoeglund, A.E.; Hubbard, K.A.; Kulis, D.M.; Landsberg, J.H.; Lefebvre, K.A.; Provoost, P.; Richlen, M.L.; et al. Marine harmful algal blooms (HABs) in the United States: History, current status and future trends. Harmful Algae 2021, 102, 101975. [Google Scholar] [CrossRef] [PubMed]
- Zingone, A.; Escalera, L.; Aligizaki, K.; Fernández-Tejedor, M.; Ismael, A.; Montresor, M.; Mozetič, P.; Taş, S.; Totti, C. Toxic marine microalgae and noxious blooms in the Mediterranean Sea: A contribution to the Global HAB Status Report. Harmful Algae 2021, 102, 101843. [Google Scholar] [CrossRef]
- Molgó, J.; Marchot, P.; Aráoz, R.; Benoit, E.; Iorga, B.I.; Zakarian, A.; Taylor, P.; Bourne, Y.; Servent, D. Cyclic imine toxins from dinoflagellates: A growing family of potent antagonists of the nicotinic acetylcholine receptors. J. Neurochem. 2017, 142, 41–51. [Google Scholar] [CrossRef]
- Rubio, F.; Kamp, L.; Carpino, J.; Faltin, E.; Loftin, K.; Molgó, J.; Aráoz, R. Colorimetric microtiter plate receptor-binding assay for the detection of freshwater and marine neurotoxins targeting the nicotinic acetylcholine receptors. Toxicon 2014, 91, 45–56. [Google Scholar] [CrossRef]
- Rodríguez, L.P.; Vilariño, N.; Molgó, J.; Aráoz, R.; Botana, L.M. High-throughput receptor-based assay for the detection of spirolides by chemiluminescence. Toxicon 2013, 75, 35–43. [Google Scholar] [CrossRef]
- Rodríguez, L.P.; Vilariño, N.; Molgó, J.; Aráoz, R.; Louzao, M.C.; Taylor, P.; Talley, T.; Botana, L.M. Development of a Solid-Phase Receptor-Based Assay for the Detection of Cyclic Imines Using a Microsphere-Flow Cytometry System. Anal. Chem. 2013, 85, 2340–2347. [Google Scholar] [CrossRef]
- Couesnon, A.; Aráoz, R.; Iorga, B.I.; Benoit, E.; Reynaud, M.; Servent, D.; Molgó, J. The Dinoflagellate Toxin 20-Methyl Spirolide-G Potently Blocks Skeletal Muscle and Neuronal Nicotinic Acetylcholine Receptors. Toxins 2016, 8, 249. [Google Scholar] [CrossRef]
- Kharrat, R.; Servent, D.; Girard, E.; Ouanounou, G.; Amar, M.; Marrouchi, R.; Benoit, E.; Molgó, J. The marine phycotoxin gymnodimine targets muscular and neuronal nicotinic acetylcholine receptor subtypes with high affinity. J. Neurochem. 2008, 107, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Bourne, Y.; Sulzenbacher, G.; Radić, Z.; Aráoz, R.; Reynaud, M.; Benoit, E.; Zakarian, A.; Servent, D.; Molgó, J.; Taylor, P.; et al. Marine Macrocyclic Imines, Pinnatoxins A and G: Structural Determinants and Functional Properties to Distinguish Neuronal α7 from Muscle α12βγδ nAChRs. Structure 2015, 23, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Nieva, J.A.; Krock, B.; Tillmann, U.; Tebben, J.; Zurhelle, C.; Bickmeyer, U. Gymnodimine A and 13-desMethyl Spirolide C Alter Intracellular Calcium Levels via Acetylcholine Receptors. Toxins 2020, 12, 751. [Google Scholar] [CrossRef] [PubMed]
- Duroure, L.; Jousseaume, T.; Aráoz, R.; Barré, E.; Retailleau, P.; Chabaud, L.; Molgó, J.; Guillou, C. 6,6-Spiroimine analogs of (−)-gymnodimine A: Synthesis and biological evaluation on nicotinic acetylcholine receptors. Org. Biomol. Chem. 2011, 9, 8112–8118. [Google Scholar] [CrossRef] [PubMed]
- Kosuge, T.; Zenda, H.; Ochiai, A.; Masaki, N.; Noguchi, M.; Kimura, S.; Narita, H. Isolation and structure determination of a new marine toxin, surugatoxin from the Japanese ivory shell, babylonia japonica. Tetrahedron Lett. 1972, 13, 2545–2548. [Google Scholar] [CrossRef]
- Brown, D.A.; Garthwaite, J.; HayashI, E.; Yamada, S. Action of surugatoxin on nicotinic receptors in the superior cervical ganglion of the rat. Br. J. Pharmacol. 1976, 58, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Hinze, M.E.; Daughtry, J.L.; Lewis, C.A. Access to the Surugatoxin Alkaloids: Chemo-, Regio-, and Stereoselective Oxindole Annulation. J. Org. Chem. 2015, 80, 11258–11265. [Google Scholar] [CrossRef]
- Tsuneki, H.; You, Y.; Toyooka, N.; Sasaoka, T.; Nemoto, H.; Dani, J.A.; Kimura, I. Marine Alkaloids (−)-Pictamine and (−)-Lepadin B Block Neuronal Nicotinic Acetylcholine Receptors. Biol. Pharm. Bull. 2005, 28, 611–614. [Google Scholar] [CrossRef]
- Ma, F.; He, C.; Wang, E.; Tong, R. Collective Asymmetric Total Syntheses of Marine Decahydroquinoline Alkaloid Lepadins A-E, H, and ent-I. Org. Lett. 2021, 23, 6583–6588. [Google Scholar] [CrossRef]
- Hauser, T.A.; Hepler, C.D.; Kombo, D.C.; Grinevich, V.P.; Kiser, M.N.; Hooker, D.N.; Zhang, J.; Mountfort, D.; Selwood, A.; Akireddy, S.R.; et al. Comparison of acetylcholine receptor interactions of the marine toxins, 13-desmethylspirolide C and gymnodimine. Neuropharmacology 2012, 62, 2239–2250. [Google Scholar] [CrossRef]
- Hann, R.M.; Pagán, O.R.; Gregory, L.; Jácome, T.; Rodríguez, A.D.; Ferchmin, P.A.; Lu, R.; Eterovic, V.A. Characterization of Cembranoid Interaction with the Nicotinic Acetylcholine Receptor. J. Pharmacol. Exp. Ther. 1998, 287. [Google Scholar]
- Delpech, V.R.; Ihara, M.; Coddou, C.; Matsuda, K.; Sattelle, D.B. Action of nereistoxin on recombinant neuronal nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. Invertebr. Neurosci. 2003 51 2003, 5, 29–35. [Google Scholar] [CrossRef]
- Ferchmin, P.A.; Pagán, O.R.; Ulrich, H.; Szeto, A.C.; Hann, R.M.; Eterović, V.A. Actions of octocoral and tobacco cembranoids on nicotinic receptors. Toxicon 2009, 54, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Iwamaru, A.; Iwado, E.; Kondo, S.; Newman, R.A.; Vera, B.; Rodríguez, A.D.; Kondo, Y. Eupalmerin acetate, a novel anticancer agent from Caribbean gorgonian octocorals, induces apoptosis in malignant glioma cells via the c-Jun NH2-terminal kinase pathway. Mol. Cancer Ther. 2007, 6, 184–192. [Google Scholar] [CrossRef]
- Ulrich, H.; Akk, G.; Nery, A.A.; Trujillo, C.A.; Rodriguez, A.D.; Eterović, V.A. Mode of cembranoid action on embryonic muscle acetylcholine receptor. J. Neurosci. Res. 2008, 86, 93–107. [Google Scholar] [CrossRef]
- Kudryavtsev, D.S.; Shelukhina, I.V.; Son, L.V.; Ojomoko, L.O.; Kryukova, E.V.; Lyukmanova, E.N.; Zhmak, M.N.; Dolgikh, D.A.; Ivanov, I.A.; Kasheverov, I.E.; et al. Neurotoxins from snake venoms and α-Conotoxin ImI inhibit functionally active Ionotropic γ-aminobutyric acid (GABA) receptors. J. Biol. Chem. 2015, 290, 22747–22758. [Google Scholar] [CrossRef]
- Li, P.; Reichert, D.E.; Rodríguez, A.D.; Manion, B.D.; Evers, A.S.; Eterović, V.A.; Steinbach, J.H.; Akk, G. Mechanisms of potentiation of the mammalian GABAA receptor by the marine cembranoid eupalmerin acetate. Br. J. Pharmacol. 2008, 153, 598–608. [Google Scholar] [CrossRef]
- Gergalova, G.; Lykhmus, O.; Kalashnyk, O.; Koval, L.; Chernyshov, V.; Kryukova, E.; Tsetlin, V.; Komisarenko, S.; Skok, M. Mitochondria Express α7 Nicotinic Acetylcholine Receptors to Regulate Ca2+ Accumulation and Cytochrome c Release: Study on Isolated Mitochondria. PLoS One 2012, 7, e31361. [Google Scholar] [CrossRef]
- Rojas-Colón, L.A.; Dash, P.K.; Morales-Vías, F.A.; Lebrón-Dávila, M.; Ferchmin, P.A.; Redell, J.B.; Maldonado-Martínez, G.; Vélez-Torres, W.I. 4R-cembranoid confers neuroprotection against LPS-induced hippocampal inflammation in mice. J. Neuroinflammation 2021, 18, 1–13. [Google Scholar] [CrossRef]
- Lee, S.J.; Tomizawa, M.; Casida, J.E. Nereistoxin and Cartap Neurotoxicity Attributable to Direct Block of the Insect Nicotinic Receptor/Channel. J. Agric. Food Chem. 2003, 51, 2646–2652. [Google Scholar] [CrossRef]
- Crossthwaite, A.J.; Bigot, A.; Camblin, P.; Goodchild, J.; Lind, R.J.; Slater, R.; Maienfisch, P. The invertebrate pharmacology of insecticides acting at nicotinic acetylcholine receptors. J. Pestic. Sci. 2017, 42, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Dong, Q.; Li, S.; Guo, J.; Wang, X.; Zhu, G. Developmental toxicity of cartap on zebrafish embryos. Aquat. Toxicol. 2009, 95, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Boorugu, H.K.; Chrispal, A. Cartap hydrochloride poisoning: A clinical experience. Indian J. Crit. Care Med. 2012, 16, 58. [Google Scholar] [CrossRef] [PubMed]
- Suganthan, N.; Manmathan, R.; Kumanan, T. Rhabdomyolysis and acute kidney injury associated with thiocyclam hydrogen oxalate (Evisect) poisoning. SAGE Open Med. Case Rep. 2020, 8, 2050313X20954942. [Google Scholar] [CrossRef]
- Dai, J.; Jiang, C.; Gao, G.; Zhu, L.; Chai, Y.; Chen, H.; Liu, X. Dissipation pattern and safety evaluation of cartap and its metabolites during tea planting, tea manufacturing and brewing. Food Chem. 2020, 314, 126165. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Wang, Z.; Lee, S.M.Y.; Wang, L.H.; Wang, R. Constraining the Teratogenicity of Pesticide Pollution by a Synthetic Nanoreceptor. Chem.—Asian J. 2018, 13, 41–45. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, L.; Jing, J.; Zhuang, M.; Xin, J.; Zhou, Y.; Feng, X.; Zhang, H. Development, optimization, and validation of a method for detection of cartap, thiocyclam, thiosultap-monosodium, and thiosultap-disodium residues in plant foods by GC-ECD. Food Chem. 2022, 371, 131198. [Google Scholar] [CrossRef]
- Dai, J.; Chen, H.; Gao, G.; Zhu, L.; Chai, Y.; Liu, X. Simultaneous determination of cartap and its metabolite in tea using hydrophilic interaction chromatography tandem mass spectrometry and the combination of dispersive solid phase extraction and solid phase extraction. J. Chromatogr. A 2019, 1600, 148–157. [Google Scholar] [CrossRef]
- Park, Y.Y.; Choe, S.; Lee, H.; Jo, J.; Park, Y.Y.; Kim, E.; Pyo, J.; Jung, J.H. Advanced analytical method of nereistoxin using mixed-mode cationic exchange solid-phase extraction and GC/MS. Forensic Sci. Int. 2015, 252, 143–149. [Google Scholar] [CrossRef]
- Yang, Y.; Hou, J.; Huo, D.; Wang, X.; Li, J.; Xu, G.; Bian, M.; He, Q.; Hou, C.; Yang, M. Green emitting carbon dots for sensitive fluorometric determination of cartap based on its aggregation effect on gold nanoparticles. Microchim. Acta 2019, 186, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, X.; Jiang, W.; Liu, H. Determination of nereistoxin-related insecticide via quantum-dots-doped covalent organic frameworks in a molecularly imprinted network. Microchim. Acta 2020, 187, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Noguchi, S.; Yamamoto, M.; Nishiyama, K.; Kitamura, Y.; Ihara, T. Electrochemical Sensing of Neurotoxic Agents Based on Their Electron Transfer Promotion Effect on an Au Electrode. Anal. Chem. 2017, 89, 5742–5747. [Google Scholar] [CrossRef] [PubMed]
- Khoshroo, A.; Fattahi, A. Electrochemical analysis of anionic analytes in weakly supported media using electron transfer promotion effect: A case study on nitrite. Sci. Rep. 2020 101 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Yamamoto, N.; Todoriki, M.; Jin, J. Sonochemical preparation of gold nanoparticles for sensitive colorimetric determination of nereistoxin insecticides in environmental samples. Talanta 2018, 188, 651–657. [Google Scholar] [CrossRef]
- Xie, S.; Tang, C.; Liu, H.; Zhang, T.E.; Tang, Y.; Teng, L.; Zhang, J. An electroanalytical platform for nereistoxin-related insecticide detection based on DNA conformational switching and exonuclease III assisted target recycling. Analyst 2020, 145, 946–952. [Google Scholar] [CrossRef]
- Kem, W.R.; Abbott, B.C.; Coates, R.M. Isolation and structure of a hoplonemertine toxin. Toxicon 1971, 9, 15–22. [Google Scholar] [CrossRef]
- Kem, W.; Soti, F.; Wildeboer, K.; LeFrancois, S.; MacDougall, K.; Wei, D.Q.; Chou, K.C.; Arias, H.R. The Nemertine Toxin Anabaseine and Its Derivative DMXBA (GTS-21): Chemical and Pharmacological Properties. Mar. Drugs 2006, 4, 255–273. [Google Scholar] [CrossRef]
- Andrud, K.; Xing, H.; Gabrielsen, B.; Bloom, L.; Mahnir, V.; Lee, S.; Green, B.T.; Lindstrom, J.; Kem, W. Investigation of the Possible Pharmacologically Active Forms of the Nicotinic Acetylcholine Receptor Agonist Anabaseine. Mar. Drugs 2019, 17, 614. [Google Scholar] [CrossRef]
- Kem, W.R. The brain α7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimer’s disease: Studies with DMXBA (GTS-21). Behav. Brain Res. 2000, 113, 169–181. [Google Scholar] [CrossRef]
- Hibbs, R.E.; Sulzenbacher, G.; Shi, J.; Talley, T.T.; Conrod, S.; Kem, W.R.; Taylor, P.; Marchot, P.; Bourne, Y. Structural determinants for interaction of partial agonists with acetylcholine binding protein and neuronal α7 nicotinic acetylcholine receptor. EMBO J. 2009, 28, 3040–3051. [Google Scholar] [CrossRef]
- Martin, L.F.; Kem, W.R.; Freedman, R. Alpha-7 nicotinic receptor agonists: Potential new candidates for the treatment of schizophrenia. Psychopharmacol. 2004, 174, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Olincy, A.; Harris, J.G.; Johnson, L.L.; Pender, V.; Kongs, S.; Allensworth, D.; Ellis, J.; Zerbe, G.O.; Leonard, S.; Stevens, K.E.; et al. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch. Gen. Psychiatry 2006, 63, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R.; Olincy, A.; Buchanan, R.W.; Harris, J.G.; Gold, J.M.; Johnson, L.; Allensworth, D.; Guzman-Bonilla, A.; Clement, B.; Ball, M.P.; et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am. J. Psychiatry 2008, 165, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Celanire, S.; Poli, S. (Eds.) Small Molecule Therapeutics for Schizophrenia; Topics in Medicinal Chemistry; Springer International Publishing: Cham, Switzerland, 2015; Volume 13, ISBN 978-3-319-11501-6. [Google Scholar]
- Beinat, C.; Banister, S.D.; Herrera, M.; Law, V.; Kassiou, M. The Therapeutic Potential of α7 Nicotinic Acetylcholine Receptor (α7 nAChR) Agonists for the Treatment of the Cognitive Deficits Associated with Schizophrenia. CNS Drugs 2015, 29, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R. α7-Nicotinic Acetylcholine Receptor Agonists for Cognitive Enhancement in Schizophrenia. Annu. Rev. Med. 2014, 65, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.T.; Uddin, M.S.; Jeandet, P.; Emran, T.B.; Mitra, S.; Albadrani, G.M.; Sayed, A.A.; Abdel-Daim, M.M.; Simal-Gandara, J. Anti-Alzheimer’s Molecules Derived from Marine Life: Understanding Molecular Mechanisms and Therapeutic Potential. Mar. Drugs 2021, 19, 251. [Google Scholar] [CrossRef]
- Kem, W.R.; Olincy, A.; Johnson, L.; Harris, J.; Wagner, B.D.; Buchanan, R.W.; Christians, U.; Freedman, R. Pharmacokinetic Limitations on Effects of an Alpha7-Nicotinic Receptor Agonist in Schizophrenia: Randomized Trial with an Extended-Release Formulation. Neuropsychopharmacology 2018, 43, 583–589. [Google Scholar] [CrossRef]
- Lewis, A.S.; Olincy, A.; Buchanan, R.W.; Kem, W.R.; Picciotto, M.R.; Freedman, R. Effects of a nicotinic agonist on the Brief Psychiatric Rating Scale five-factor subscale model in schizophrenia. Schizophr. Res. 2018, 195, 568–569. [Google Scholar] [CrossRef]
- Gaidhani, N.; Tucci, F.C.; Kem, W.R.; Beaton, G.; Uteshev, V.V. Therapeutic efficacy of α7 ligands after acute ischaemic stroke is linked to conductive states of α7 nicotinic ACh receptors. Br. J. Pharmacol. 2021, 178, 1684–1704. [Google Scholar] [CrossRef]
- Pruekprasert, N.; Meng, Q.; Gu, R.; Xie, H.; Liu, Y.; Liu, C.; Cooney, R.N. A7 nicotinic acetylcholine receptor agonists regulate inflammation and growth hormone resistance in sepsis. Shock 2021, 56, 1057–1065. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Ochani, M.; Yang, L.H.; Gallowitsch-Puerta, M.; Ochani, K.; Lin, X.; Levi, J.; Parrish, W.R.; Rosas-Ballina, M.; Czura, C.J.; et al. Selective α7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit. Care Med. 2007, 35, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, S.; Khan, M.A.S.; Yasuhara, S.; Goto, T.; Kem, W.R.; Tompkins, R.G.; Kaneki, M.; Jeevendra Martyn, J.A. Prevention of burn-induced inflammatory responses and muscle wasting by GTS-21, a specific agonist for α7 nicotinic acetylcholine receptors. Shock 2017, 47, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U. The cholinergic anti-inflammatory pathway alleviates acute lung injury. Mol. Med. 2020, 26, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Douaoui, S.; Djidjik, R.; Boubakeur, M.; Ghernaout, M.; Touil-boukoffa, C.; Oumouna, M.; Derrar, F.; Amrani, Y. GTS-21, an α7nAChR agonist, suppressed the production of key inflammatory mediators by PBMCs that are elevated in COPD patients and associated with impaired lung function. Immunobiology 2020, 225, 151950. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Jiang, L.; Li, Q.; Sokulsky, L.; Wanyan, Y.; Wang, L.; Liu, X.; Zhou, L.; Tay, H.L.; Zhang, G.; et al. A selective α7 nicotinic acetylcholine receptor agonist, PNU-282987, attenuates ILC2s activation and Alternaria-induced airway inflammation. Front. Immunol. 2020, 11, 598165. [Google Scholar] [CrossRef] [PubMed]
- Al-Sharea, A.; Lee, M.K.S.; Whillas, A.; Flynn, M.C.; Chin-Dusting, J.; Murphy, A.J. Nicotinic acetylcholine receptor alpha 7 stimulation dampens splenic myelopoiesis and inhibits atherogenesis in Apoe−/− mice. Atherosclerosis 2017, 265, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kox, M.; Pompe, J.C.; Gordinou De Gouberville, M.C.; Van Der Hoeven, J.G.; Hoedemaekers, C.W.; Pickkers, P. Effects of the α7 nicotinic acetylcholine receptor agonist gts-21 on the innate immune response in humans. Shock 2011, 36, 5–11. [Google Scholar] [CrossRef]
- Xie, H.; Yepuri, N.; Meng, Q.; Dhawan, R.; Leech, C.A.; Chepurny, O.G.; Holz, G.G.; Cooney, R.N. Therapeutic potential of α7 nicotinic acetylcholine receptor agonists to combat obesity, diabetes, and inflammation. Rev. Endocr. Metab. Disord. 2020, 21, 431–447. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Kurokawa, M.; Ozaki, N.; Nara, K.; Atou, K.; Takada, E.; Kamochi, H.; Suzuki, N. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-κB phosphorylation and nuclear factor-κB transcriptional activity through nicotinic acetylcholine receptor α7. Clin. Exp. Immunol. 2006, 146, 116–123. [Google Scholar] [CrossRef]
- Zoheir, N.; Lappin, D.F.; Nile, C.J. Acetylcholine and the alpha 7 nicotinic receptor: A potential therapeutic target for the treatment of periodontal disease? Inflamm. Res. 2012, 61, 915–926. [Google Scholar] [CrossRef][Green Version]
- Gaidhani, N.; Kem, W.R.; Uteshev, V.V. Spleen is not required for therapeutic effects of 4OH-GTS-21, a selective α7 nAChR agonist, in the sub-acute phase of ischemic stroke in rats. Brain Res. 2021, 1751, 147196. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.K.; Loring, R.H. GTS-21 has cell-specific anti-inflammatory effects independent of α7 nicotinic acetylcholine receptors. PLoS One 2019, 14, e0214942. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.S.; Khan, M.F.; Kashiwagi, S.; Kem, W.R.; Yasuhara, S.; Kaneki, M.; Tompkins, R.G.; Martyn, J.A.J. An ALPHA7 Nicotinic Acetylcholine Receptor Agonist (GTS-21) Promotes C 2 C 12 Myonuclear Accretion in Association with Release of Interleukin-6 (IL-6) and Improves Survival in Burned Mice. Shock 2017, 48, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Amamiya, T.; Mizoguchi, H.; Kawanishi, S.; Kuroda, E.; Kitamura, R.; Ito, A.; Saito, Y.; Tawa, M.; Nagasawa, T.; et al. Alpha7 nicotinic acetylcholine receptor-specific agonist DMXBA (GTS-21) attenuates Aβ accumulation through suppression of neuronal γ-secretase activity and promotion of microglial amyloid-β phagocytosis and ameliorates cognitive impairment in. Neurobiol. Aging 2018, 62, 197–209. [Google Scholar] [CrossRef]
- Zhou, Y.; Leung-Pitt, Y.; Deng, H.; Ren, Y.; You, Z.; Kem, W.R.; Shen, S.; Zhang, W.; Mao, J.; Martyn, J.A.J. Nonopioid GTS-21 mitigates burn injury pain in rats by decreasing spinal cord inflammatory responses. Anesth. Analg. 2021, 132, 240–252. [Google Scholar] [CrossRef]
- Kolodziej, M.A.; Gött, H.; Kopischke, B.; Bender, M.K.F.; Weigand, M.A.; Di Fazio, P.; Schwarm, F.P.; Uhle, F. Antiproliferative effect of GTS-21 in glioblastoma cells. Oncol. Lett. 2021, 22, 1–6. [Google Scholar] [CrossRef]
- Mashimo, M.; Fujii, T.; Ono, S.; Moriwaki, Y.; Misawa, H.; Kawashima, K. Minireview: Divergent roles of α7 nicotinic acetylcholine receptors expressed on antigen-presenting cells and CD4+ T cells in the regulation of T cell differentiation. Int. Immunopharmacol. 2020, 82, 106306. [Google Scholar] [CrossRef]
- Matera, C.; Quadri, M.; Sciaccaluga, M.; Pomè, D.Y.; Fasoli, F.; De Amici, M.; Fucile, S.; Gotti, C.; Dallanoce, C.; Grazioso, G. Modification of the anabaseine pyridine nucleus allows achieving binding and functional selectivity for the α3β4 nicotinic acetylcholine receptor subtype. Eur. J. Med. Chem. 2016, 108, 392–405. [Google Scholar] [CrossRef]
- Chojnacka, K.; Papke, R.L.; Horenstein, N.A. Synthesis and evaluation of a conditionally-silent agonist for the α7 nicotinic acetylcholine receptor. Bioorg. Med. Chem. Lett. 2013, 23, 4145–4149. [Google Scholar] [CrossRef]
- Kudryavtsev, D.S.; Spirova, E.N.; Shelukhina, I.V.; Son, L.V.; Makarova, Y.V.; Utkina, N.K.; Kasheverov, I.E.; Tsetlin, V.I. Makaluvamine G from the Marine Sponge Zyzzia fuliginosa Inhibits Muscle nAChR by Binding at the Orthosteric and Allosteric Sites. Mar. Drugs 2018, 16, 109. [Google Scholar] [CrossRef]
- Kudryavtsev, D.; Isaeva, A.; Barkova, D.; Spirova, E.; Mukhutdinova, R.; Kasheverov, I.; Tsetlin, V. Point Mutations of Nicotinic Receptor α1 Subunit Reveal New Molecular Features of G153S Slow-Channel Myasthenia. Molecules 2021, 26, 1278. [Google Scholar] [CrossRef] [PubMed]
- Kasheverov, I.E.I.; Shelukhina, I.I.V.; Kudryavtsev, D.S.D.; Makarieva, T.N.T.; Spirova, E.N.E.; Guzii, A.A.G.; Stonik, V.A.V.; Tsetlin, V.V.I. 6-Bromohypaphorine from Marine Nudibranch Mollusk Hermissenda crassicornis is an Agonist of Human α7 Nicotinic Acetylcholine Receptor. Mar. Drugs 2015, 13, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Paguigan, N.D.; Tun, J.O.; Leavitt, L.S.; Lin, Z.; Chase, K.; Dowell, C.; Deering-Rice, C.E.; Lim, A.L.; Karthikeyan, M.; Hughen, R.W.; et al. Nicotinic Acetylcholine Receptor Partial Antagonist Polyamides from Tunicates and Their Predatory Sea Slugs. ACS Chem. Neurosci. 2021, 12, 2693–2704. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.R.; Luque, A.; Olivera, B.M.; Barrett, J.; Cruz, L.J. Peptide toxins from Conus geographus venom. J. Biol. Chem. 1981, 256, 4734–4740. [Google Scholar] [CrossRef]
- McManus, O.B.; Musick, J.R.; Gonzalez, C. Peptides isolated from the venom of Conus geographus block neuromuscular transmission. Neurosci. Lett. 1981, 25, 57–62. [Google Scholar] [CrossRef]
- Lewis, R.J.; Dutertre, S.; Vetter, I.; Christie, M.J. Conus Venom Peptide Pharmacology. Pharmacol. Rev. 2012, 64, 259–298. [Google Scholar] [CrossRef]
- Jin, A.H.; Muttenthaler, M.; Dutertre, S.; Himaya, S.W.A.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Conotoxins: Chemistry and Biology. Chem. Rev. 2019, 119, 11510–11549. [Google Scholar] [CrossRef]
- Luo, S.; Christensen, S.; Zhangsun, D.; Wu, Y.; Hu, Y.; Zhu, X.; Chhabra, S.; Norton, R.S.; McIntosh, J.M. A novel inhibitor of alpha9alpha10 nicotinic acetylcholine receptors from Conus vexillum delineates a new conotoxin superfamily. PLoS One 2013, 8, e54648. [Google Scholar] [CrossRef]
- Luo, S.; Zhangsun, D.; Harvey, P.J.; Kaas, Q.; Wu, Y.; Zhu, X.; Hu, Y.; Li, X.; Tsetlin, V.I.; Christensen, S.; et al. Cloning, synthesis, and characterization of αO-conotoxin GeXIVA, a potent α9α10 nicotinic acetylcholine receptor antagonist. Proc. Natl. Acad. Sci. USA 2015, 112, E4026–E4035. [Google Scholar] [CrossRef]
- Christensen, S.B.; Bandyopadhyay, P.K.; Olivera, B.M.; McIntosh, J.M. αS-conotoxin GVIIIB potently and selectively blocks α9α10 nicotinic acetylcholine receptors. Biochem. Pharmacol. 2015, 96, 349–356. [Google Scholar] [CrossRef]
- Rybin, M.J.; O’Brien, H.; Ramiro, I.B.L.; Azam, L.; McIntosh, J.M.; Olivera, B.M.; Safavi-Hemami, H.; Yoshikami, D. αM-conotoxin MIIIJ blocks nicotinic acetylcholine receptors at neuromuscular junctions of frog and fish. Toxins 2020, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, T.; Kompella, S.N.; Yan, M.; Lu, A.; Wang, Y.; Shao, X.; Chi, C.; Adams, D.J.; Ding, J.; et al. Conotoxin αD-GeXXA utilizes a novel strategy to antagonize nicotinic acetylcholine receptors. Sci. Rep. 2015, 5, 14261. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Sámano, A.C.; Falcón, A.; Zamudio, F.; Batista, C.V.F.; Michel-Morfín, J.E.; Landa-Jaime, V.; López-Vera, E.; Jeziorski, M.C.; Aguilar, M.B. αD-Conotoxins in Species of the Eastern Pacific: The Case of Conus princeps from Mexico. Toxins 2019, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, K.; Wang, S.; Sun, T.; Yu, S.; Dai, Q.; Liu, Z. Cloning, expression and functional characterization of a D-superfamily conotoxin Lt28.1 with previously undescribed cysteine pattern. Peptides 2017, 94, 64–70. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, X.; Harvey, P.J.; Kaas, Q.; Zhangsun, D.; Craik, D.J.; Luo, S. Single Amino Acid Substitution in α-Conotoxin TxID Reveals a Specific α3β4 Nicotinic Acetylcholine Receptor Antagonist. J. Med. Chem. 2018, 61, 9256–9265. [Google Scholar] [CrossRef]
- Lebbe, E.K.M.M.; Peigneur, S.; Maiti, M.; Mille, B.G.; Devi, P.; Ravichandran, S.; Lescrinier, E.; Waelkens, E.; D’Souza, L.; Herdewijn, P.; et al. Discovery of a new subclass of α-conotoxins in the venom of Conus australis. Toxicon 2014, 91, 145–154. [Google Scholar] [CrossRef]
- Wilson, D.T.; Bansal, P.S.; Carter, D.A.; Vetter, I.; Nicke, A.; Dutertre, S.; Daly, N.L. Characterisation of a novel A-superfamily conotoxin. Biomedicines 2020, 8, 128. [Google Scholar] [CrossRef]
- Favreau, P.; Benoit, E.; Hocking, H.G.; Carlier, L.; D’Hoedt, D.; Leipold, E.; Markgraf, R.; Schlumberger, S.; Córdova, M.A.; Gaertner, H.; et al. A novel µ-conopeptide, CnIIIC, exerts potent and preferential inhibition of NaV1.2/1.4 channels and blocks neuronal nicotinic acetylcholine receptors. Br. J. Pharmacol. 2012, 166, 1654–1668. [Google Scholar] [CrossRef]
- Jin, A.H.; Cristofori-Armstrong, B.; Rash, L.D.; Román-González, S.A.; Espinosa, R.A.; Lewis, R.J.; Alewood, P.F.; Vetter, I. Novel conorfamides from Conus austini venom modulate both nicotinic acetylcholine receptors and acid-sensing ion channels. Biochem. Pharmacol. 2019, 164, 342–348. [Google Scholar] [CrossRef]
- Wang, S.; Du, T.; Liu, Z.; Wang, S.; Wu, Y.; Ding, J.; Jiang, L.; Dai, Q. Characterization of a T-superfamily conotoxin TxVC from Conus textile that selectively targets neuronal nAChR subtypes. Biochem. Biophys. Res. Commun. 2014, 454, 151–156. [Google Scholar] [CrossRef]
- Azam, L.; McIntosh, J.M. Alpha-conotoxins as pharmacological probes of nicotinic acetylcholine receptors. Acta Pharmacol. Sin. 2009, 30, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Muttenthaler, M.; Akondi, K.B.; Alewood, P.F. Structure-activity studies on alpha-conotoxins. Curr. Pharm. Des. 2011, 17, 4226–4241. [Google Scholar] [CrossRef] [PubMed]
- Kauferstein, S.; Porth, C.; Kendel, Y.; Wunder, C.; Nicke, A.; Kordis, D.; Favreau, P.; Koua, D.; Stöcklin, R.; Mebs, D. Venomic study on cone snails (Conus spp.) from South Africa. Toxicon 2011, 57, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Kompella, S.N.; Akondi, K.B.; Melaun, C.; Daly, N.L.; Luetje, C.W.; Alewood, P.F.; Craik, D.J.; Adams, D.J.; Marí, F. RegIIA: An α4/7-conotoxin from the venom of Conus regius that potently blocks α3β4 nAChRs. Biochem. Pharmacol. 2012, 83, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Inserra, M.C.; Kompella, S.N.; Vetter, I.; Brust, A.; Daly, N.L.; Cuny, H.; Craik, D.J.; Alewood, P.F.; Adams, D.J.; Lewis, R.J. Isolation and characterization of α-conotoxin LsIA with potent activity at nicotinic acetylcholine receptors. Biochem. Pharmacol. 2013, 86, 791–799. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, C.; Liu, Z.; Wang, X.; Liu, N.; Du, W.; Dai, Q. Structural and functional characterization of a novel α-conotoxin Mr1.7 from Conus marmoreus targeting neuronal nAChR α3β2, α9α10 and α6/α3β2β3 subtypes. Mar. Drugs 2015, 13, 3259–3275. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Liu, N.; Wu, C.; Jiang, J.; Yue, J.; Jing, Y.; Dai, Q. Diversity and evolution of conotoxins in Conus virgo, Conus eburneus, Conus imperialis and Conus marmoreus from the South China Sea. Toxicon 2012, 60, 982–989. [Google Scholar] [CrossRef]
- Luo, S.; Zhangsun, D.; Wu, Y.; Zhu, X.; Hu, Y.; McIntyre, M.; Christensen, S.; Akcan, M.; Craik, D.J.; McIntosh, J.M. Characterization of a novel α-conotoxin from Conus textile that selectively targets α6/α3β2β3 nicotinic acetylcholine receptors. J. Biol. Chem. 2013, 288, 894–902. [Google Scholar] [CrossRef]
- Luo, S.; Zhangsun, D.; Schroeder, C.I.; Zhu, X.; Hu, Y.; Wu, Y.; Weltzin, M.M.; Eberhard, S.; Kaas, Q.; Craik, D.J.; et al. A novel α4/7-conotoxin LvIA from Conus lividus that selectively blocks α3β2 vs. α6/α3β2β3 nicotinic acetylcholine receptors. FASEB J. 2014, 28, 1842–1853. [Google Scholar] [CrossRef]
- Chen, J.; Liang, L.; Ning, H.; Cai, F.; Liu, Z.; Zhang, L.; Zhou, L.; Dai, Q. Cloning, Synthesis and Functional Characterization of a Novel α-Conotoxin Lt1.3. Mar. Drugs 2018, 16, 112. [Google Scholar] [CrossRef]
- Guo, M.; Yu, J.; Zhu, X.; Zhangsun, D.; Luo, S. Characterization of an α 4/7-Conotoxin LvIF from Conus lividus That Selectively Blocks α3β2 Nicotinic Acetylcholine Receptor. Mar. Drugs 2021, 19, 398. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Huang, B.; Tae, H.-S.; Liu, Z.; Yu, S.; Li, L.; Zhang, L.; Adams, D.J.; Guo, C.; Dai, Q. α-Conotoxin Bt1.8 from Conus betulinus selectively inhibits α6/α3β2β3 and ɑ3β2 nicotinic acetylcholine receptor subtypes. J. Neurochem. 2021, 159, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Lebbe, E.K.M.; Peigneur, S.; Maiti, M.; Devi, P.; Ravichandran, S.; Lescrinier, E.; Ulens, C.; Waelkens, E.; D’Souza, L.; Herdewijn, P.; et al. Structure-Function Elucidation of a New α-Conotoxin, Lo1a, from Conus longurionis. J. Biol. Chem. 2014, 289, 9573–9583. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Le Caer, J.-P.; Aráoz, R.; Thai, R.; Lamthanh, H.; Benoit, E.; Molgó, J. Isolation, purification and functional characterization of alpha-BnIA from Conus bandanus venom. Toxicon 2014, 91, 155–163. [Google Scholar] [CrossRef]
- Peng, C.; Chen, W.; Sanders, T.; Chew, G.; Liu, J.; Hawrot, E.; Chi, C. Chemical synthesis and characterization of two α4/7-conotoxins. Acta Biochim. Biophys. Sin. 2010, 42, 745–753. [Google Scholar] [CrossRef]
- Jin, A.H.; Vetter, I.; Dutertre, S.; Abraham, N.; Emidio, N.B.; Inserra, M.; Murali, S.S.; Christie, M.J.; Alewood, P.F.; Lewis, R.J. MrIC, a novel α-conotoxin agonist in the presence of PNU at endogenous α7 nicotinic acetylcholine receptors. Biochemistry 2014, 53, 1–3. [Google Scholar] [CrossRef]
- Mueller, A.; Starobova, H.; Inserra, M.C.; Jin, A.-H.; Deuis, J.R.; Dutertre, S.; Lewis, R.J.; Alewood, P.F.; Daly, N.L.; Vetter, I. α-Conotoxin MrIC is a biased agonist at α7 nicotinic acetylcholine receptors. Biochem. Pharmacol. 2015, 94, 155–163. [Google Scholar] [CrossRef]
- Safavi-Hemami, H.; Siero, W.A.; Kuang, Z.; Williamson, N.A.; Karas, J.A.; Page, L.R.; MacMillan, D.; Callaghan, B.; Kompella, S.N.; Adams, D.J.; et al. Embryonic Toxin Expression in the Cone Snail Conus victoriae. J. Biol. Chem. 2011, 286, 22546–22557. [Google Scholar] [CrossRef]
- Kompella, S.N.; Hung, A.; Clark, R.J.; Marí, F.; Adams, D.J. Alanine Scan of α-Conotoxin RegIIA Reveals a Selective α3β4 Nicotinic Acetylcholine Receptor Antagonist. J. Biol. Chem. 2015, 290, 1039–1048. [Google Scholar] [CrossRef]
- Abraham, N.; Healy, M.; Ragnarsson, L.; Brust, A.; Alewood, P.F.; Lewis, R.J. Structural mechanisms for α-conotoxin activity at the human α3β4 nicotinic acetylcholine receptor. Sci. Rep. 2017, 7, 45466. [Google Scholar] [CrossRef]
- Quinton, L.; Servent, D.; Girard, E.; Molgó, J.; Le Caer, J.-P.; Malosse, C.; Haidar, E.A.; Lecoq, A.; Gilles, N.; Chamot-Rooke, J. Identification and functional characterization of a novel α-conotoxin (EIIA) from Conus ermineus. Anal. Bioanal. Chem. 2013, 405, 5341–5351. [Google Scholar] [CrossRef] [PubMed]
- Echterbille, J.; Gilles, N.; Araóz, R.; Mourier, G.; Amar, M.; Servent, D.; De Pauw, E.; Quinton, L. Discovery and characterization of EIIB, a new α-conotoxin from Conus ermineus venom by nAChRs affinity capture monitored by MALDI-TOF/TOF mass spectrometry. Toxicon 2017, 130, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhangsun, D.; Zhu, X.; Kaas, Q.; Zhangsun, M.; Harvey, P.J.; Craik, D.J.; McIntosh, J.M.; Luo, S. α-conotoxin [S9A]TxID potently discriminates between α3β4 and α6/α3β4 nicotinic acetylcholine receptors. J. Med. Chem. 2017, 60, 5826–5833. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhangsun, D.; Zhu, X.; Wu, Y.; Hu, Y.; Christensen, S.; Harvey, P.J.; Akcan, M.; Craik, D.J.; McIntosh, J.M. Characterization of a novel α-conotoxin TxID from Conus textile that potently blocks rat α3β4 nicotinic acetylcholine receptors. J. Med. Chem. 2013, 56, 9655–9663. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.N.T.; Abraham, N.; Lewis, R.J. Rigidity of loop 1 contributes to equipotency of globular and ribbon isomers of α-conotoxin AusIA. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Li, L.; Liu, N.; Ding, R.; Wang, S.; Liu, Z.; Li, H.; Zheng, X.; Dai, Q. A novel 4/6-type alpha-conotoxin ViIA selectively inhibits nAchR α3β2 subtype. Acta Biochim. Biophys. Sin. (Shanghai) 2015, 47, 1023–1028. [Google Scholar] [CrossRef]
- Hoggard, M.F.; Rodriguez, A.M.; Cano, H.; Clark, E.; Tae, H.S.; Adams, D.J.; Godenschwege, T.A.; Marí, F. In vivo and in vitro testing of native α-conotoxins from the injected venom of Conus purpurascens. Neuropharmacology 2017, 127, 253–259. [Google Scholar] [CrossRef]
- Giribaldi, J.; Wilson, D.; Nicke, A.; El Hamdaoui, Y.; Laconde, G.; Faucherre, A.; Maati, H.M.O.; Daly, N.L.; Enjalbal, C.; Dutertre, S. Synthesis, Structure and Biological Activity of CIA and CIB, Two α-Conotoxins from the Predation-Evoked Venom of Conus catus. Toxins 2018, 10, 222. [Google Scholar] [CrossRef]
- van Hout, M.; Valdes, A.; Christensen, S.B.; Tran, P.T.; Watkins, M.; Gajewiak, J.; Jensen, A.A.; Olivera, B.M.; McIntosh, J.M. α-Conotoxin VnIB from Conus ventricosus is a potent and selective antagonist of α6β4* nicotinic acetylcholine receptors. Neuropharmacology 2019, 157, 107691. [Google Scholar] [CrossRef]
- Peigneur, S.; Devi, P.; Seldeslachts, A.; Ravichandran, S.; Quinton, L.; Tytgat, J. Structure-Function Elucidation of a New α-Conotoxin, MilIA, from Conus milneedwardsi. Mar. Drugs 2019, 17, 535. [Google Scholar] [CrossRef]
- Tae, H.-S.; Gao, B.; Jin, A.-H.; Alewood, P.F.; Adams, D.J. Globular and ribbon isomers of Conus geographus α-conotoxins antagonize human nicotinic acetylcholine receptors. Biochem. Pharmacol. 2021, 190, 114638. [Google Scholar] [CrossRef] [PubMed]
- Giribaldi, J.; Haufe, Y.; Evans, E.R.J.; Wilson, D.T.; Daly, N.L.; Enjalbal, C.; Nicke, A.; Dutertre, S. Synthesis, Structural and Pharmacological Characterizations of CIC, a Novel α-Conotoxin with an Extended N-Terminal Tail. Mar. Drugs 2021, 19, 141. [Google Scholar] [CrossRef]
- Peng, C.; Tang, S.; Pi, C.; Liu, J.; Wang, F.; Wang, L.; Zhou, W.; Xu, A. Discovery of a novel class of conotoxin from Conus litteratus, lt14a, with a unique cysteine pattern. Peptides 2006, 27, 2174–2181. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Ren, Z.; Zeng, X.; You, Y.; Pan, W.; Zhou, M.; Wang, L.; Xu, A. Structure-function relationship of conotoxin lt14a, a potential analgesic with low cytotoxicity. Peptides 2011, 32, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Ye, M.; Wang, Y.; Shao, X.; Yuan, D.; Liu, J.; Hawrot, E.; Wang, C.; Chi, C. A new subfamily of conotoxins belonging to the A-superfamily. Peptides 2010, 31, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Tae, H.S.; Xu, S.; Shao, X.; Adams, D.J.; Wang, C. Identification of a Novel O-Conotoxin Reveals an Unusual and Potent Inhibitor of the Human α9α10 Nicotinic Acetylcholine Receptor. Mar. Drugs 2017, 15, 170. [Google Scholar] [CrossRef]
- Bergeron, Z.L.; Chun, J.B.; Baker, M.R.; Sandall, D.W.; Peigneur, S.; Yu, P.Y.C.; Thapa, P.; Milisen, J.W.; Tytgat, J.; Livett, B.G.; et al. A ‘conovenomic’ analysis of the milked venom from the mollusk-hunting cone snail Conus textile—The pharmacological importance of post-translational modifications. Peptides 2013, 49, 145–158. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, F.; Du, W. Solution structure of a novel α-conotoxin with a distinctive loop spacing pattern. Amino Acids 2012, 43, 389–396. [Google Scholar] [CrossRef]
- Kryukova, E.V.; Ivanov, I.A.; Lebedev, D.S.; Spirova, E.N.; Egorova, N.S.; Zouridakis, M.; Kasheverov, I.E.; Tzartos, S.J.; Tsetlin, V.I. Orthosteric and/or allosteric binding of α-conotoxins to nicotinic acetylcholine receptors and their models. Mar. Drugs 2018, 16, 460. [Google Scholar] [CrossRef]
- Ellison, M.; Feng, Z.P.; Park, A.J.; Zhang, X.; Olivera, B.M.; McIntosh, J.M.; Norton, R.S. α-RgIA, a Novel Conotoxin That Blocks the α9α10 nAChR: Structure and Identification of Key Receptor-Binding Residues. J. Mol. Biol. 2008, 377, 1216–1227. [Google Scholar] [CrossRef]
- Huynh, P.N.; Harvey, P.J.; Gajewiak, J.; Craik, D.J.; Michael McIntosh, J. Critical residue properties for potency and selectivity of α-Conotoxin RgIA towards α9α10 nicotinic acetylcholine receptors. Biochem. Pharmacol. 2020, 181, 114124. [Google Scholar] [CrossRef] [PubMed]
- Azam, L.; McIntosh, J.M. Molecular basis for the differential sensitivity of rat and human α9α10 nAChRs to α-conotoxin RgIA. J. Neurochem. 2012, 122, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Azam, L.; Papakyriakou, A.; Zouridakis, M.; Giastas, P.; Tzartos, S.J.; McIntosh, J.M. Molecular Interaction of α-Conotoxin RgIA with the Rat α9α10 Nicotinic Acetylcholine Receptor. Mol. Pharmacol. 2015, 87, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Kompella, S.N.; Adams, D.J.; Craik, D.J.; Kaas, Q. Determination of the α-conotoxin Vc1.1 binding site on the α9α10 nicotinic acetylcholine receptor. J. Med. Chem. 2013, 56, 3557–3567. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Tae, H.S.; Xu, Q.; Jiang, T.; Adams, D.J.; Yu, R. α-Conotoxin Vc1.1 Structure-Activity Relationship at the Human α9α10 Nicotinic Acetylcholine Receptor Investigated by Minimal Side Chain Replacement. ACS Chem. Neurosci. 2019, 10, 4328–4336. [Google Scholar] [CrossRef]
- Leffler, A.E.; Kuryatov, A.; Zebroski, H.A.; Powell, S.R.; Filipenko, P.; Hussein, A.K.; Gorson, J.; Heizmann, A.; Lyskov, S.; Tsien, R.W.; et al. Discovery of peptide ligands through docking and virtual screening at nicotinic acetylcholine receptor homology models. Proc. Natl. Acad. Sci. USA 2017, 114, E8100–E8109. [Google Scholar] [CrossRef]
- Katz, D.; Dimattia, M.A.; Sindhikara, D.; Li, H.; Abraham, N.; Leffler, A.E. Potency- and Selectivity-Enhancing Mutations of Conotoxins for Nicotinic Acetylcholine Receptors Can Be Predicted Using Accurate Free-Energy Calculations. Mar. Drugs 2021, 19, 367. [Google Scholar] [CrossRef]
- Hone, A.J.; Ruiz, M.; Scadden, M.; Christensen, S.; Gajewiak, J.; Azam, L.; McIntosh, J.M. Positional scanning mutagenesis of α-conotoxin PeIA identifies critical residues that confer potency and selectivity for α6/ α3β2β3 and α3β2 nicotinic acetylcholine receptors. J. Biol. Chem. 2013, 288, 25428–25439. [Google Scholar] [CrossRef]
- Kim, H.W.; McIntosh, J.M. α6 nAChR subunit residues that confer α-conotoxin BuIA selectivity. FASEB J. 2012, 26, 4102–4110. [Google Scholar] [CrossRef]
- Wei, N.; Chu, Y.; Liu, H.; Xu, Q.; Jiang, T.; Yu, R. Antagonistic mechanism of α-conotoxin BuIA toward the human α3β2 nicotinic acetylcholine receptor. ACS Chem. Neurosci. 2021, 12, 4535–4545. [Google Scholar] [CrossRef] [PubMed]
- Grishin, A.A.; Cuny, H.; Hung, A.; Clark, R.J.; Brust, A.; Akondi, K.; Alewood, P.F.; Craik, D.J.; Adams, D.J. Identifying Key Amino Acid Residues That Affect α-Conotoxin AuIB Inhibition of α3β4 Nicotinic Acetylcholine Receptors. J. Biol. Chem. 2013, 288, 34428–34442. [Google Scholar] [CrossRef]
- Zhangsun, D.; Zhu, X.; Wu, Y.; Hu, Y.; Kaas, Q.; Craik, D.J.; McIntosh, J.M.; Luo, S. Key residues in the nicotinic acetylcholine receptor β2 subunit contribute to α-conotoxin LvIA binding. J. Biol. Chem. 2015, 290, 9855–9862. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Li, R.; Ren, J.; Zhangsun, D.; Zhu, X.; Wu, Y.; Luo, S. Alanine-scanning Mutagenesis of α-conotoxin GI reveals the residues crucial for activity at the muscle acetylcholine receptor. Mar. Drugs 2018, 16, 507. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Ren, J.; Xiong, Y.; Wu, Y.; Zhangsun, M.; Zhangsun, D.; Zhu, X.; Luo, S. Identification of crucial residues in α-conotoxin EI inhibiting muscle nicotinic acetylcholine receptor. Toxins 2019, 11, 603. [Google Scholar] [CrossRef]
- Hone, A.J.; Scadden, M.; Gajewiak, J.; Christensen, S.; Lindstrom, J.; McIntosh, J.M. α-Conotoxin PeIA[S9H,V10A,E14N] potently and selectively blocks α6β2β3 versus α6β4 nicotinic acetylcholine receptors. Mol. Pharmacol. 2012, 82, 972–982. [Google Scholar] [CrossRef]
- Xu, Q.; Tae, H.-S.; Wang, Z.; Jiang, T.; Adams, D.J.; Yu, R. Rational design of α-conotoxin RegIIA analogues selectively inhibiting the human α3β2 nicotinic acetylcholine receptor through computational scanning. ACS Chem. Neurosci. 2020, 11, 2804–2811. [Google Scholar] [CrossRef]
- Hone, A.J.; Kaas, Q.; Kearns, I.; Hararah, F.; Gajewiak, J.; Christensen, S.; Craik, D.J.; McIntosh, J.M. Computational and Functional Mapping of Human and Rat α6β4 Nicotinic Acetylcholine Receptors Reveals Species-Specific Ligand-Binding Motifs. J. Med. Chem. 2021, 64, 1685–1700. [Google Scholar] [CrossRef]
- Kompella, S.N.; Cuny, H.; Hung, A.; Adams, D.J. Molecular Basis for Differential Sensitivity of α-Conotoxin RegIIA at Rat and Human Neuronal Nicotinic Acetylcholine Receptors. Mol. Pharmacol. 2015, 88, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, X.; Zhang, L.; Kudryavtsev, D.; Kasheverov, I.; Lei, Y.; Zhangsun, D.; Tsetlin, V.; Luo, S. Species specificity of rat and human α7 nicotinic acetylcholine receptors towards different classes of peptide and protein antagonists. Neuropharmacology 2018, 139, 226–237. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, X.; Zhangsun, M.; Wu, Y.; Yu, J.; Harvey, P.J.; Kaas, Q.; Zhangsun, D.; Craik, D.J.; Luo, S. Engineered conotoxin differentially blocks and discriminates rat and human α7 nicotinic acetylcholine receptors. J. Med. Chem. 2021, 64, 5620–5631. [Google Scholar] [CrossRef]
- Lin, B.; Xu, M.; Zhu, X.; Wu, Y.; Liu, X.; Zhangsun, D.; Hu, Y.; Xiang, S.H.; Kasheverov, I.E.; Tsetlin, V.I.; et al. From crystal structure of α-conotoxin GIC in complex with Ac-AChBP to molecular determinants of its high selectivity for α3β2 nAChR. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, X.; Yu, J.; Yu, J.; Luo, S.; Wang, X. The crystal structure of Ac-AChBP in complex with α-conotoxin LvIA reveals the mechanism of its selectivity towards different nAChR subtypes. Protein Cell 2017, 8, 675–685. [Google Scholar] [CrossRef]
- Hone, A.J.; Talley, T.T.; Bobango, J.; Melo, C.H.; Hararah, F.; Gajewiak, J.; Christensen, S.; Harvey, P.J.; Craik, D.J.; Michael McIntosh, J. Molecular determinants of α-conotoxin potency for inhibition of human and rat α6β4 nicotinic acetylcholine receptors. J. Biol. Chem. 2018, 293, 17838–17852. [Google Scholar] [CrossRef]
- Zouridakis, M.; Papakyriakou, A.; Ivanov, I.A.; Kasheverov, I.E.; Tsetlin, V.; Tzartos, S.; Giastas, P. Crystal structure of the monomeric extracellular domain of α9 nicotinic receptor subunit in complex with α-conotoxin RgIA: Molecular dynamics insights into RgIA binding to α9α10 nicotinic receptors. Front. Pharmacol. 2019, 10, 474. [Google Scholar] [CrossRef]
- Lin, B.; Xiang, S.; Li, M. Residues Responsible for the Selectivity of α-Conotoxins for Ac-AChBP or nAChRs. Mar. Drugs 2016, 14, 173. [Google Scholar] [CrossRef]
- Cuny, H.; Yu, R.; Tae, H.S.; Kompella, S.N.; Adams, D.J. α-Conotoxins active at α3-containing nicotinic acetylcholine receptors and their molecular determinants for selective inhibition. Br. J. Pharmacol. 2018, 175, 1855–1868. [Google Scholar] [CrossRef]
- Gulsevin, A.; Papke, R.L.; Stokes, C.; Tran, H.N.T.; Jin, A.H.; Vetter, I.; Meiler, J. The Allosteric Activation of α7 nAChR by α-Conotoxin MrIC Is Modified by Mutations at the Vestibular Site. Toxins 2021, 13, 555. [Google Scholar] [CrossRef]
- Walsh Jr, R.M.; Roh, S.-H.; Gharpure, A.; Morales-Perez, C.L.; Teng, J.; Hibbs, R.E. Structural principles of distinct assemblies of the human α4β2 nicotinic receptor. Nature 2018, 557, 261–265. [Google Scholar] [CrossRef]
- Gharpure, A.; Teng, J.; Zhuang, Y.; Noviello, C.M.; Walsh, R.M.; Cabuco, R.; Howard, R.J.; Zaveri, N.T.; Lindahl, E.; Hibbs, R.E. Agonist Selectivity and Ion Permeation in the α3β4 Ganglionic Nicotinic Receptor. Neuron 2019, 104, 501–511.e6. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Zhou, Y.; Zhang, M.; Chen, H.; Eric Xu, H.; Sun, D.; Liu, L.; Tian, C. Structural basis of human α7 nicotinic acetylcholine receptor activation. Cell Res. 2021, 31, 713–716. [Google Scholar] [CrossRef]
- Delgado-Vélez, M.; Quesada, O.; Villalobos-Santos, J.C.; Maldonado-Hernández, R.; Asmar-Rovira, G.; Stevens, R.C.; Lasalde-Dominicci, J.A. Pursuing High-Resolution Structures of Nicotinic Acetylcholine Receptors: Lessons Learned from Five Decades. Molecules 2021, 26, 5753. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.E.; Deer, T.R. Ziconotide: A clinical update and pharmacologic review. Expert Opin. Pharmacother. 2013, 14, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Safavi-Hemami, H.; Brogan, S.E.; Olivera, B.M. Pain therapeutics from cone snail venoms: From Ziconotide to novel non-opioid pathways. J. Proteom. 2019, 190, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Pope, J.E.; Hanes, M.C.; McDowell, G.C. Intrathecal Therapy for Chronic Pain: A Review of Morphine and Ziconotide as Firstline Options. Pain Med. 2019, 20, 784–798. [Google Scholar] [CrossRef]
- Durek, T.; Craik, D.J. Therapeutic conotoxins: A US patent literature survey. Expert Opin. Ther. Pat. 2015, 25, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Peng, C.; Yang, J.; Yi, Y.; Zhang, J.; Shi, Q. Cone Snails: A Big Store of Conotoxins for Novel Drug Discovery. Toxins 2017, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tae, H.S.; Chu, Y.; Jiang, T.; Adams, D.J.; Yu, R. Medicinal chemistry, pharmacology, and therapeutic potential of α-conotoxins antagonizing the α9α10 nicotinic acetylcholine receptor. Pharmacol. Ther. 2021, 222, 107792. [Google Scholar] [CrossRef]
- Romero, H.K.; Christensen, S.B.; Di Cesare Mannelli, L.; Gajewiak, J.; Ramachandra, R.; Elmslie, K.S.; Vetter, D.E.; Ghelardini, C.; Iadonato, S.P.; Mercado, J.L.; et al. Inhibition of α9α10 nicotinic acetylcholine receptors prevents chemotherapy-induced neuropathic pain. Proc. Natl. Acad. Sci. USA 2017, 114, E1825–E1832. [Google Scholar] [CrossRef]
- Zheng, N.; Christensen, S.B.; Dowell, C.; Purushottam, L.; Skalicky, J.J.; McIntosh, J.M.; Chou, D.H.-C. Discovery of methylene thioacetal-incorporated α-RgIA analogues as potent and stable antagonists of the human α9α10 nicotinic acetylcholine receptor for the treatment of neuropathic pain. J. Med. Chem. 2021, 64, 9513–9524. [Google Scholar] [CrossRef]
- Gajewiak, J.; Christensen, S.B.; Dowell, C.; Hararah, F.; Fisher, F.; Huynh, P.N.; Olivera, B.M.; McIntosh, J.M. Selective Penicillamine Substitution Enables Development of a Potent Analgesic Peptide that Acts through a Non-Opioid-Based Mechanism. J. Med. Chem. 2021, 64, 9271–9278. [Google Scholar] [CrossRef]
- Carstens, B.B.; Berecki, G.; Daniel, J.T.; Lee, H.S.; Jackson, K.A.V.; Tae, H.; Sadeghi, M.; Castro, J.; O’Donnell, T.; Deiteren, A.; et al. Structure–Activity Studies of Cysteine-Rich α-Conotoxins that Inhibit High-Voltage-Activated Calcium Channels via GABA B Receptor Activation Reveal a Minimal Functional Motif. Angew. Chemie 2016, 128, 4770–4774. [Google Scholar] [CrossRef]
- Mohammadi, S.A.; Christie, M.J. Conotoxin interactions with α9α10-nAChRs: Is the α9α10-nicotinic acetylcholine receptor an important therapeutic target for pain management? Toxins 2015, 7, 3916–3932. [Google Scholar] [CrossRef] [PubMed]
- Hone, A.J.; Servent, D.; McIntosh, J.M. α9-containing nicotinic acetylcholine receptors and the modulation of pain. Br. J. Pharmacol. 2018, 175, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Bony, A.R.; McArthur, J.R.; Finol-Urdaneta, R.K.; Adams, D.J. Analgesic α-conotoxins modulate native and recombinant GIRK1/2 channels via activation of GABAB receptors and reduce neuroexcitability. Br. J. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Li, X.; Hu, Y.; Wu, Y.; Huang, Y.; Yu, S.; Ding, Q.; Zhangsun, D.; Luo, S. Anti-hypersensitive effect of intramuscular administration of αO-conotoxin GeXIVA[1,2] and GeXIVA[1,4] in rats of neuropathic pain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 66, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Zhangsun, D.; Yu, G.; Su, R.; Luo, S. The α9α10 nicotinic acetylcholine receptor antagonist αo-conotoxin GeXIVA[1,2] alleviates and reverses chemotherapy-induced neuropathic pain. Mar. Drugs 2019, 17, 265. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, L.; Qin, M.; You, Y.; Pan, W.; Zhou, L.; Sun, D.; Xu, A. Pharmacological characterization of conotoxin lt14a as a potent non-addictive analgesic. Toxicon 2015, 96, 57–67. [Google Scholar] [CrossRef]
- Wu, Y.; Qiang, Y.; Zhang, G.; Zhou, M. Acute toxicity and micronucleus test of conotoxin lt14a in mice. Basic Clin. Pharmacol. Toxicol. 2021, 129, 52–60. [Google Scholar] [CrossRef]
- Napier, I.A.; Klimis, H.; Rycroft, B.K.; Jin, A.H.; Alewood, P.F.; Motin, L.; Adams, D.J.; Christie, M.J. Intrathecal α-conotoxins Vc1.1, AuIB and MII acting on distinct nicotinic receptor subtypes reverse signs of neuropathic pain. Neuropharmacology 2012, 62, 2202–2207. [Google Scholar] [CrossRef]
- Liu, C.; Wu, P.; Zhu, H.; Grieco, P.; Yu, R.; Gao, X.; Wu, G.; Wang, D.; Xu, H.; Qi, W. Rationally Designed α-Conotoxin Analogues Maintained Analgesia Activity and Weakened Side Effects. Molecules 2019, 24, 337. [Google Scholar] [CrossRef]
- Zhu, X.; Bi, J.; Yu, J.; Li, X.; Zhang, Y.; Zhangsun, D.; Luo, S. Recombinant Expression and Characterization of α-Conotoxin LvIA in Escherichia coli. Mar. Drugs 2016, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Bao, J.; Zhangsun, M.; Dong, S.; Zhangsun, D.; Luo, S. αO-Conotoxin GeXIVA inhibits the growth of breast cancer cells via interaction with α9 nicotine acetylcholine receptors. Mar. Drugs 2020, 18, 195. [Google Scholar] [CrossRef] [PubMed]
- Terpinskaya, T.I.; Osipov, A.V.; Kryukova, E.V.; Kudryavtsev, D.S.; Kopylova, N.V.; Yanchanka, T.L.; Palukoshka, A.F.; Gondarenko, E.A.; Zhmak, M.N.; Tsetlin, V.I.; et al. α-Conotoxins and α-Cobratoxin Promote, while Lipoxygenase and Cyclooxygenase Inhibitors Suppress the Proliferation of Glioma C6 Cells. Mar. Drugs 2021, 19, 118. [Google Scholar] [CrossRef] [PubMed]
- Reina Improgo, M.; Soll, L.G.; Tapper, A.R.; Gardner, P.D. Nicotinic acetylcholine receptors mediate lung cancer growth. Front. Physiol. 2013, 4, 251. [Google Scholar] [CrossRef]
- Qian, J.; Liu, Y.-Q.; Sun, Z.-H.; Zhangsun, D.-T.; Luo, S.-L. Identification of nicotinic acetylcholine receptor subunits in different lung cancer cell lines and the inhibitory effect of alpha-conotoxin TxID on lung cancer cell growth. Eur. J. Pharmacol. 2019, 865, 172674. [Google Scholar] [CrossRef]
- Sato, S.; Tamiya, N. The amino acid sequences of erabutoxins, neurotoxic proteins of sea-snake (Laticauda semifasciata) venom. Biochem. J. 1971, 122, 453–461. [Google Scholar] [CrossRef][Green Version]
- Sato, S.; Abe, T.; Tamiya, N. Binding of iodinated erabutoxin b, a sea snake toxin, to the endplates of the mouse diaphragm. Toxicon 1970, 8, 313–314. [Google Scholar] [CrossRef]
- Low, B.W.; Preston, H.S.; Sato, A.; Rosen, L.S.; Searl, J.E.; Rudko, A.D.; Richardson, J.S. Three dimensional structure of erabutoxin b neurotoxic protein: Inhibitor of acetylcholine receptor. Proc. Natl. Acad. Sci. USA 1976, 73, 2991–2994. [Google Scholar] [CrossRef]
- Tsernoglou, D.; Petsko, G.A. The crystal structure of a post-synaptic neurotoxin from sea snake at 2.2 Å resolution. FEBS Lett. 1976, 68, 1–4. [Google Scholar] [CrossRef]
- Tamiya, N.; Yagi, T. Studies on sea snake venom. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 41–52. [Google Scholar] [CrossRef]
- Gulsevin, A.; Meiler, J. An Investigation of Three-Finger Toxin—nAChR Interactions through Rosetta Protein Docking. Toxins 2020, 12, 598. [Google Scholar] [CrossRef] [PubMed]
- Pillet, L.; Tremeau, O.; Ducancel, F.; Drevet, P.; Zinn-Justin, S.; Pinkasfeld, S.; Boulain, J.C.; Menez, A.; Trémeau, O.; Ducancel, F.; et al. Genetic engineering of snake toxins. Role of invariant residues in the structural and functional properties of a curaremimetic toxin, as probed by site-directed mutagenesis. J. Biol. Chem. 1993, 268, 909–916. [Google Scholar] [CrossRef]
- Barber, C.M.; Isbister, G.K.; Hodgson, W.C. Alpha neurotoxins. Toxicon 2013, 66, 47–58. [Google Scholar] [CrossRef]
- Durban, J.; Sasa, M.; Calvete, J.J. Venom gland transcriptomics and microRNA profiling of juvenile and adult yellow-bellied sea snake, Hydrophis platurus, from Playa del Coco (Guanacaste, Costa Rica). Toxicon 2018, 153, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Neale, V.; Sotillo, J.; Seymour, J.; Wilson, D. The venom of the spine-bellied sea snake (Hydrophis curtus): Proteome, toxin diversity and intraspecific variation. Int. J. Mol. Sci. 2017, 18, 2695. [Google Scholar] [CrossRef]
- Tan, C.H.; Tan, K.Y.; Lim, S.E.; Tan, N.H. Venomics of the beaked sea snake, Hydrophis schistosus: A minimalist toxin arsenal and its cross-neutralization by heterologous antivenoms. J. Proteomics 2015, 126, 121–130. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Q.; Wang, C.; Wang, B.; Qiu, L.; Zou, S.; Zhang, F.; Liu, G.; Zhang, L. A comparative analysis of the proteomes and biological activities of the venoms from two sea snakes, Hydrophis curtus and Hydrophis cyanocinctus, from Hainan, China. Toxicon 2020, 187, 35–46. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Wen, L.; Miao, Y.-F.; Du, Y.; Sun, Y.; Yin, Y.; Lin, C.-X.; Lin, L.-H.; Ji, X.; Gao, J.-F. Venom-gland transcriptomic, venomic, and antivenomic profiles of the spine-bellied sea snake (Hydrophis curtus) from the South China Sea. BMC Genomics 2021, 22, 520. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Sun, Y.; Du, Y.; Li, J.-Q.; Lv, J.-G.; Qu, Y.-F.; Lin, L.-H.; Lin, C.-X.; Ji, X.; Gao, J.-F. Venom of the annulated sea snake Hydrophis cyanocinctus: A biochemically simple but genetically complex weapon. Toxins 2021, 13, 548. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Tan, K.Y. De Novo venom-gland transcriptomics of spine-bellied sea snake (Hydrophis curtus) from Penang, Malaysia-next-generation sequencing, functional annotation and toxinological correlation. Toxins 2021, 13, 127. [Google Scholar] [CrossRef]
- Lomonte, B.; Pla, D.; Sasa, M.; Tsai, W.C.; Solórzano, A.; Ureña-Díaz, J.M.; Fernández-Montes, M.L.; Mora-Obando, D.; Sanz, L.; Gutiérrez, J.M.; et al. Two color morphs of the pelagic yellow-bellied sea snake, Pelamis platura, from different locations of Costa Rica: Snake venomics, toxicity, and neutralization by antivenom. J. Proteom. 2014, 103, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Gutiérrez, J.M.; Rasmussen, A.R.; Engmark, M.; Gravlund, P.; Sanders, K.L.; Lohse, B.; Lomonte, B. Danger in the reef: Proteome, toxicity, and neutralization of the venom of the olive sea snake, Aipysurus laevis. Toxicon 2015, 107, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Wong, K.Y.; Tan, K.Y.; Tan, N.H. Venom proteome of the yellow-lipped sea krait, Laticauda colubrina from Bali: Insights into subvenomic diversity, venom antigenicity and cross-neutralization by antivenom. J. Proteomics 2017, 166, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Tan, K.Y.; Ng, T.S.; Sim, S.M.; Tan, N.H. Venom proteome of spine-bellied sea snake (Hydrophis curtus) from Penang, Malaysia: Toxicity correlation, immunoprofiling and cross-neutralization by Sea Snake Antivenom. Toxins 2018, 11, 3. [Google Scholar] [CrossRef]
- Ponce, D.; López-Vera, E.; Aguilar, M.B.; Sánchez-Rodr\’\iguez, J. Preliminary results of the in vivo and in vitro characterization of a tentacle venom fraction from the jellyfish Aurelia aurita. Toxins 2013, 5, 2420–2433. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.S.; Olek, A.J. An extract of lionfish (Pterois volitans) spine tissue contains acetylcholine and a toxin that affects neuromuscular transmission. Toxicon 1989, 27, 1367–1376. [Google Scholar] [CrossRef]
- Becerra-Amezcua, M.P.; Hernández-Sámano, A.C.; Puch-Hau, C.; Aguilar, M.B.; Collí-Dulá, R.C. Effect of pterois volitans (lionfish) venom on cholinergic and dopaminergic systems. Environ. Toxicol. Pharmacol. 2020, 77, 103359. [Google Scholar] [CrossRef] [PubMed]
- Kendel, Y.; Melaun, C.; Kurz, A.; Nicke, A.; Peigneur, S.; Tytgat, J.; Wunder, C.; Mebs, D.; Kauferstein, S. Venomous Secretions from Marine Snails of the Terebridae Family Target Acetylcholine Receptors. Toxins 2013, 5, 1043–1050. [Google Scholar] [CrossRef]
- Omaga, C.A.; Carpio, L.D.; Imperial, J.S.; Daly, N.L.; Gajewiak, J.; Flores, M.S.; Espino, S.S.; Christensen, S.; Filchakova, O.M.; López-Vera, E.; et al. Structure and Biological Activity of a Turripeptide from Unedogemmula bisaya Venom. Biochemistry 2017, 56, 6051–6060. [Google Scholar] [CrossRef]
- Hernández-Sámano, A.C.; Falcón, A.; Zamudio, F.; Ortíz-Arellano, M.A.; López-Vera, E.; Aguilar, M.B. A turripeptide from Polystira nobilis venom inhibits human α3β2 and α7 nicotinic acetylcholine receptors. Insect Biochem. Mol. Biol. 2020, 124, 103416. [Google Scholar] [CrossRef]
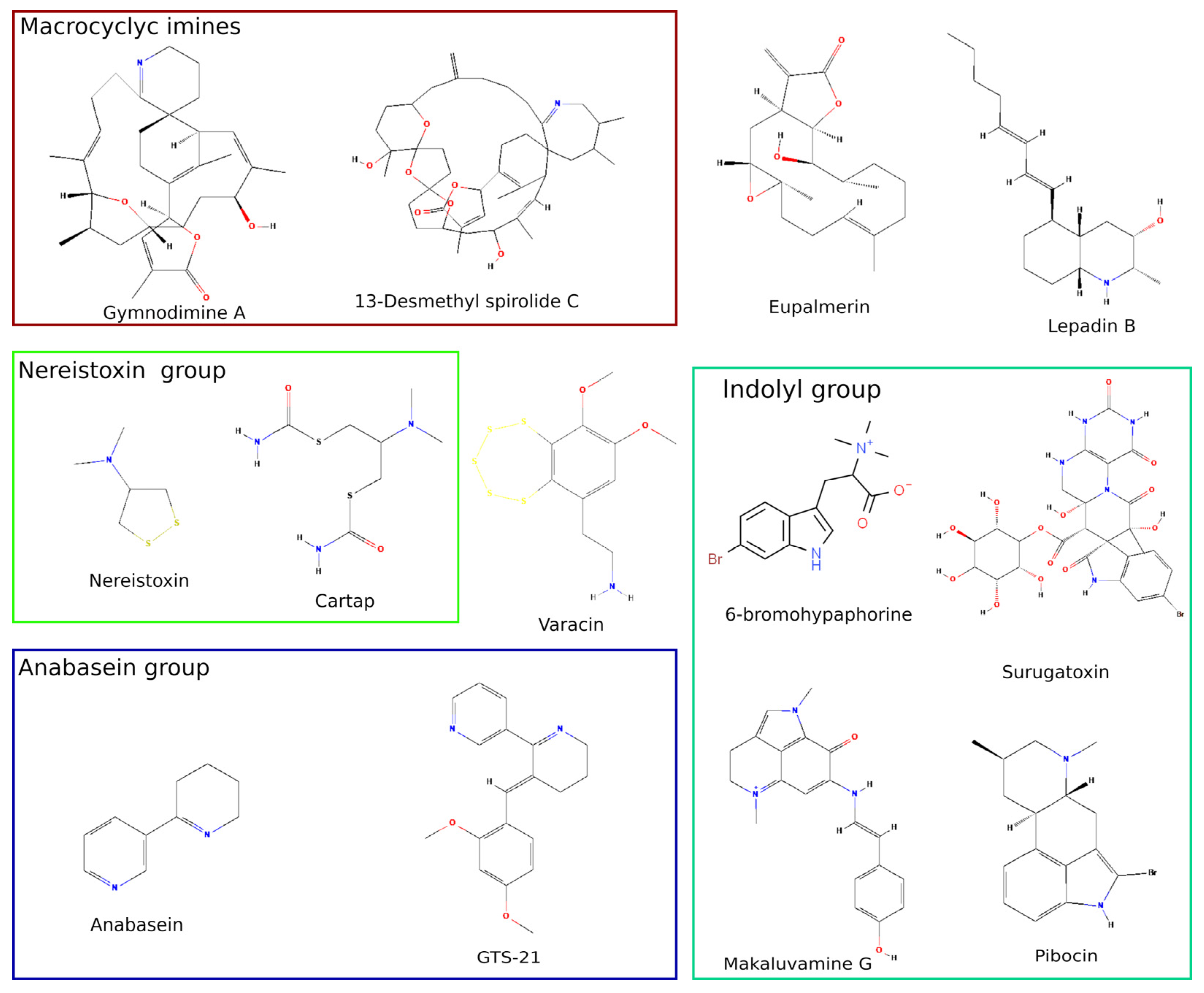

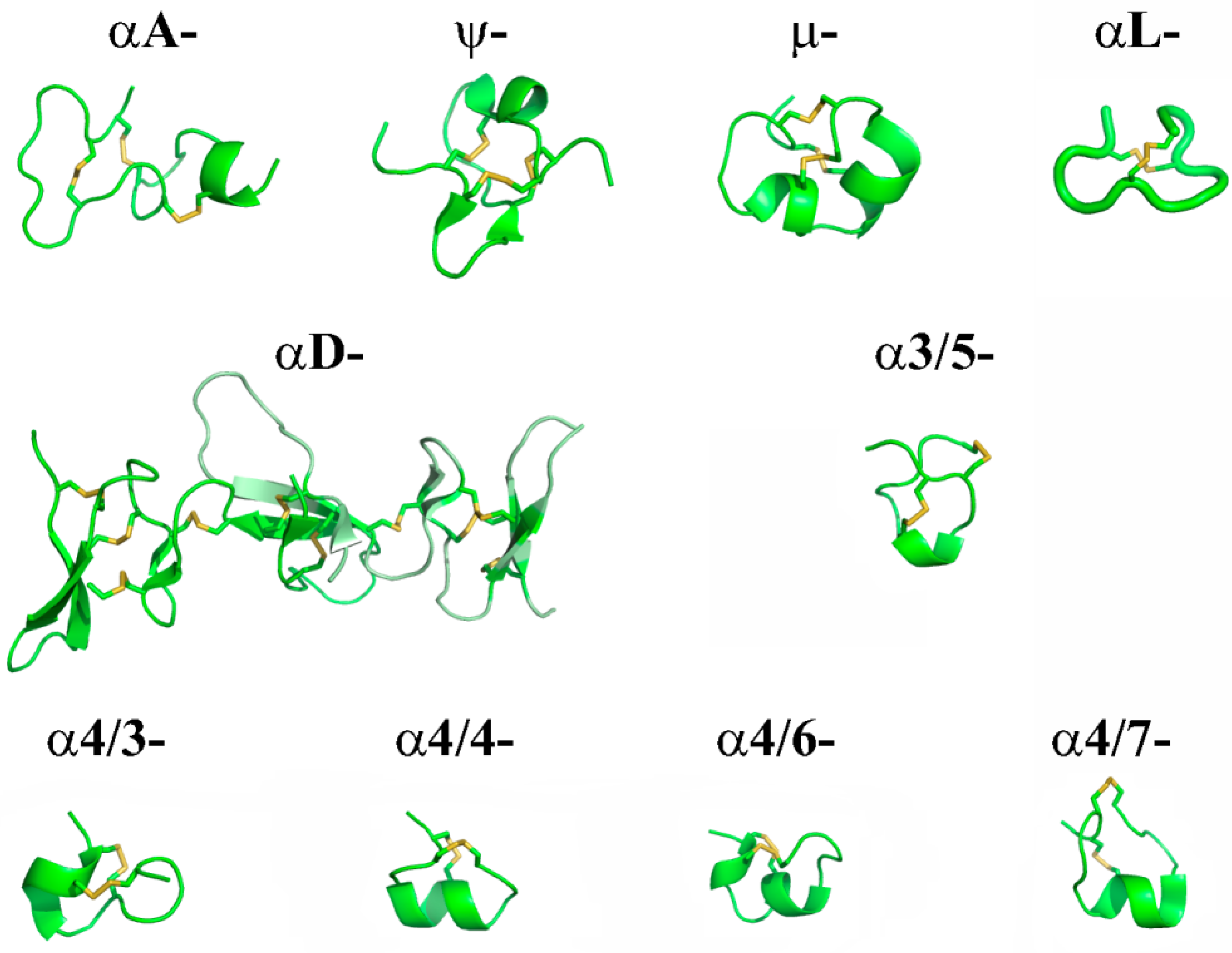
| Low-Molecular Weight Compound | Target (nAChRs) | IC50 ± SEM, or (CI95% 1) (nM) Functional Studies | IC50 ± SEM (nM) Binding Studies | Ki ± SEM (nM) Binding Studies | Ref. |
|---|---|---|---|---|---|
| gymnodimine A | α4β2 (human) | 0.5 ± 0.1 b | - | 70 ± 19 | [30] |
| α4β2 (rat) | - | - | 68 ± 18 | ||
| α4β4 (human) | 8 ± 5 b | - | 36 ± 8 | ||
| α6β3β4α5 (human) | - | - | 1.0 ± 0.3 | ||
| α7 (human) | 2.0 ± 0.1 b | - | 1.0 ± 0.1 | ||
| α3*(human) | 1.0 ± 0.1 b | - | 30 ± 17 | ||
| α3*(rat) | 63 ± 2 b | - | 8 ± 3 | ||
| (α1)2β1γδ (human) | 357 ± 121 b | - | 440 ± 215 | ||
| (α1)2β1γδ (mouse) | - | 1.38 ± 0.19 | - | [21] | |
| α7-5HT3 (chick) | - | 0.33 ± 0.08 | - | ||
| α3β2 | - | 6.25 ± 1.10 | - | ||
| α4β2 | - | 15.50 ± 0.19 | - | ||
| surugatoxin | α3*(rat superior cervical ganglia) | 58 | - | - | [26] |
| 13-desmethyl-spirolide C | α4β2 (human) | 0.7 ± 0.1 b | - | 96 ± 34 | [30] |
| α4β2 (rat) | - | - | 120 ± 15 | ||
| α4β4 (human) | 22 ± 4 b | - | 43 ± 5 | ||
| α6β3β4α5 (human) | - | - | 2.0 ± 0.2 | ||
| α7(human) | 0.4 ± 0.1 b | - | 0.7 ± 0.2 | ||
| α3*(human) | 3.0 ± 0.5 b | - | 47 ± 16 | ||
| α3*(rat) | 40 ± 1 b | - | 24 ± 11 | ||
| (α1)2β1γδ (human) | 11 ± 3 b | - | 31 ± 6 | ||
| 20-methyl spirolide G | (α1)2β1γδ (Torpedo) | 0.36 (0.29−0.45) a | - | 0.028 ± 0.005 | [20] |
| α7(human) | 0.48 (0.09–2.50) a | - | - | ||
| α7-5HT3 (chick) | 2.1 (1.4−3.1) a | - | 0.11 ± 0.08 | ||
| α4β2 (human) | - | - | 3.60 ± 0.07 | ||
| α3β2 (human) | - | - | 0.040 ± 0.001 | ||
| lepadin B | α4β2 (mouse) | 900 (700−1200) a | - | - | [28] |
| α7 (mouse) | 700 (500−900) a | - | - | ||
| cembranoids | (α1)2β1γδ (Torpedo) | - | - | 435 ± 157 | [31] |
| nereistoxin | α4β2 (chiken) | 40,000 | - | - | [32] |
| α7 (chiken) | 33,000 | - | - | ||
| ALS/β2 (Drosophila/chiken) | 15,000 | - | - | ||
| SAD/β2 (Drosophila/chiken) | 13,000 | - | - | ||
| varacin | (α1)2β1εδ (mouse) | 8700 ± 400 a | - | - | [13] |
| (α1)2β1γδ (Torpedo) | - | 10,000 ± 1000 | - | ||
| α7 (human) | - | 19,000 ± 1000 | - | ||
| L. stagnalis AChBP | - | - | 790 ± 100 | ||
| makaluvamine G | (α1)2β1εδ (mouse) | 3300 ± 300 a | - | - | [13] |
| (α1)2β1γδ (Torpedo) | - | 2800 ± 300 | - |
| Name | Species | Year | Sequence | Targets | Refs. | ||
|---|---|---|---|---|---|---|---|
| nAChR Subtype | Affinity (nM) | Key Ligand Determinants * | |||||
| α-Conotoxins | |||||||
| α-peptide | C. tinianus | 2011 | GGCCSHPACQNNPDYC * | α3β2; α4β2; α7 | nd | nd | [114] |
| Vc1.2 | C.victoriae (embryos) | 2011 | GCCSNPACMVNNPQIC * | α3β2; | 75; | N5, A7, N11, N12; | [129] |
| α7; | 637; | nd; | |||||
| α9α10 | ~1000 | nd | |||||
| RegIIA | C. regius | 2012 | GCCSHPACNVNNPHIC * | α3β2; | 33; | H14; | [115,130] |
| α3β4; | 97; | N9, H14; | |||||
| α7 | 103 | N9, N11, N12, H14 | |||||
| Mr1.7 | C. marmoreus | 2012 | PECCTHPACHVSHPELC * | α3β2; | 53.1; | H13; | [117,118] |
| α9α10; | 185.7; | E2, S12, H13; | |||||
| α6/α3β2β3 | 284.2 | nd | |||||
| TxIB | C. textile | 2013 | GCCSDPPCRNKHPDLC * | α6/α3β2β3 | 28.4 | nd | [119] |
| LsIA | C. limpusi | 2013 | SGCCSNPACRVNNPNIC * | α3β2; | 10; | S1; | [116,131] |
| α7; | 10; | R10, N12; | |||||
| α3α5β2 | 31 | nd | |||||
| EIIA | C. ermineus | 2013 | ZTOGCCWNPACVKNRC * | α1β1γδ | 0.46 and 105 | nd | [132] |
| EIIB | C. ermineus | 2017 | ZTOGCCWHPACGKNRC * | α1β1γδ | 2.2 | nd | [133] |
| MrIC | C. marmoreus | 2014 | PECCTHPACHVSNPELC * | α7 | 1900 | nd | [127,128] |
| TxID | C. textile | 2013 | GCCSHPVCSAMSPIC * | α3β4; | 3.6–12.5; | G1, H5, P6, V7, M11, P13; | [134,135] |
| α6/α3β4 | 33.9–94.1 | G1, H5, P6, V7, S9, M11, P13 | |||||
| Lo1a | C. longurionis | 2014 | EGCCSNPACRTNHPEVCD * | α7 | 3240 | nd | [124] |
| BnIA | C. bandanus | 2014 | CCSHPACSVNNPDIC * | α7 | ~1000 | nd | [125] |
| AusIA | C. australis | 2014 | SCCARNPACRHNHPCV * | α7 | 10,000–47,000 | R5, P7, R10 | [107,136] |
| LvIA | C. lividus | 2014 | GCCSHPACNVDHPEIC * | α3β2; | 8.7–15.6; | G1, H5, P6, N9, H12, P13, I15; | [12,120] |
| α3β4; | 148–283; | H5, P6, N9, D11, H12; | |||||
| α6/α3β2β3; | 108; | nd; | |||||
| α6/α3β4 | 121 | nd | |||||
| ViIA | C. virgo | 2015 | RDCCSNPPCAHNNPDC * | α3β2 | 845.5 | H11 | [137] |
| PIC | C. purpurascens | 2017 | SGCCKHPACGKNRC | α1β1ε/γδ; α3β2 | nd | nd | [138] |
| CIA | C. catus | 2018 | NGRCCHPACGKHFSC | α1β1γδ; α3β2 | 5.7; 2060 | nd | [139] |
| CIB | C. catus | 2018 | GCCSNPVCHLEHSNLC | α3β2; α7 | 128.9; 1510 | nd | [139] |
| Lt1.3 | C. litteratus | 2018 | GCCSHPACSGNNPYFC * | α3β2 | 44.8 | N11, N12, P13, F15 | [121] |
| VnIB | C.ventricosus | 2019 | GGCCSHPVCYTKNPNCG * | α6β4; α3β4; α6/α3β4 | 12; 320; 5.3–18 | nd | [140] |
| MilIA | C.milneed-wardsi | 2019 | DMCCHPACMNHFNC | α1β1ε/γδ | 11,000–13,000 | M9, N10, H11 | [141] |
| GIB | C. geographus | 2021 | ECCNPACGRHYSCKG * | α1β1εδ; α9α10 | 116; 1113 | nd | [142] |
| G1.5 | C. geographus | 2021 | GCCSHPACSGNNPEYCRQ* | α3β2; α3β4; α7; α9α10 | 35.7; 1928; 1935; 569 | nd | [142] |
| CIC | C. catus | 2021 | ASGADTCCSNPACQVQHSDLC | α3β2; α6/α3β2β3 | 3510; 1030 | nd | [143] |
| LvIF | C. lividus | 2021 | GCCSHPACAGNNQDIC * | α3β2; α6/α3β2β3 | 9.0; 14.4 | nd | [122] |
| Bt1.8 | C. betulinus | 2021 | GCCSNPACILNNPNQC * | α6/α3β2β3; α3β2 | 2.1; 9.4 | I9, N11, N12; I9, L10, N11, N12, N14, Q15 | [123] |
| αL-Conotoxins | |||||||
| lt14a | C. litteratus | 2006 | MCPPLCKPSCTNC * | neuronal nAChRs | nd | nd | [144,145] |
| Pu14a | C. pulicarius | 2010 | DCPPHPVPGMHKCVCLKTC | α1β1γδ; α6α3β2 | <1000; ~1000 | nd | [146] |
| αO-Conotoxins | |||||||
| GeXIVA | C. generalis | 2015 | TCRSSGRYCRSPYDRRRRYCRRITDACV * | α9α10 | 4.61 | nd | [100] |
| GeXXVIIA | C. generalis | 2017 | ALMSTGTNYRLLKTCRGSGRYCRSPYDCRRRYCRRISDACV | α9α10; α1β1εδ | 16.2; 774 | C-terminal part (27–41); N-terminal part (1–26) | [147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasheverov, I.; Kudryavtsev, D.; Shelukhina, I.; Nikolaev, G.; Utkin, Y.; Tsetlin, V. Marine Origin Ligands of Nicotinic Receptors: Low Molecular Compounds, Peptides and Proteins for Fundamental Research and Practical Applications. Biomolecules 2022, 12, 189. https://doi.org/10.3390/biom12020189
Kasheverov I, Kudryavtsev D, Shelukhina I, Nikolaev G, Utkin Y, Tsetlin V. Marine Origin Ligands of Nicotinic Receptors: Low Molecular Compounds, Peptides and Proteins for Fundamental Research and Practical Applications. Biomolecules. 2022; 12(2):189. https://doi.org/10.3390/biom12020189
Chicago/Turabian StyleKasheverov, Igor, Denis Kudryavtsev, Irina Shelukhina, Georgy Nikolaev, Yuri Utkin, and Victor Tsetlin. 2022. "Marine Origin Ligands of Nicotinic Receptors: Low Molecular Compounds, Peptides and Proteins for Fundamental Research and Practical Applications" Biomolecules 12, no. 2: 189. https://doi.org/10.3390/biom12020189
APA StyleKasheverov, I., Kudryavtsev, D., Shelukhina, I., Nikolaev, G., Utkin, Y., & Tsetlin, V. (2022). Marine Origin Ligands of Nicotinic Receptors: Low Molecular Compounds, Peptides and Proteins for Fundamental Research and Practical Applications. Biomolecules, 12(2), 189. https://doi.org/10.3390/biom12020189








