Effect of the Biopolymer Carrier on Staphylococcus aureus Bacteriophage Lytic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Preparation of the Bacteriophage Solutions
- Culturing of the host bacterial strain. A solid agar plate was inoculated with the host strain (reference strain S. aureus (ATCC 25923)) and incubated overnight at 37 °C. Furthermore, the 3–5 bacterial colonies grown on the plate were inoculated in a liquid medium (trypticase soy broth (TSB, Oxoid, Basingstoke, Hampshire, UK)) and incubated overnight at 37 ± 1 °C.
- Performing the plaque assay to determine the concentration of the bacteriophage cocktails. In test tubes, the overnight-grown host strain was mixed with serially diluted Staphylococcal and Pyo Bacteriophage cocktails. Then, 5 mL of molten 0.7% trypticase soy agar (TSA, Oxoid, Basingstoke, Hampshire, UK) was transferred to each test tube, and the obtained mixture was poured onto a solid TSA plate. After the top agar was solidified, the plates were inverted and placed in the incubator overnight at 37 ± 1 °C. The number of plaque-forming units per mL (PFU/mL) was counted.
- Propagating commercial bacteriophage stocks to obtain higher titre bacteriophage lysates. After plaque assay testing, the webbed plates were selected and flooded with 5–7 mL of TSB. The supernatant and soft-overlay agar was selected. Afterwards, a 2% chloroform (CHCl3) treatment for 2 h at 4 °C, the removal of bacterial debris using centrifugation at 6000× g for 15 min at 4 °C, and filtration using a 0.20 μm filter were performed. The supernatant was then collected, and the final concentration (titre in terms of plaque-forming units per millilitre (PFU/mL)) was determined using plaque assay according to Equation (1):
2.1.2. Preparation of the Mixtures of Biopolymers/Bacteriophages
2.2. Characterization
3. Results and Discussion
3.1. Bacteriophages Propagation
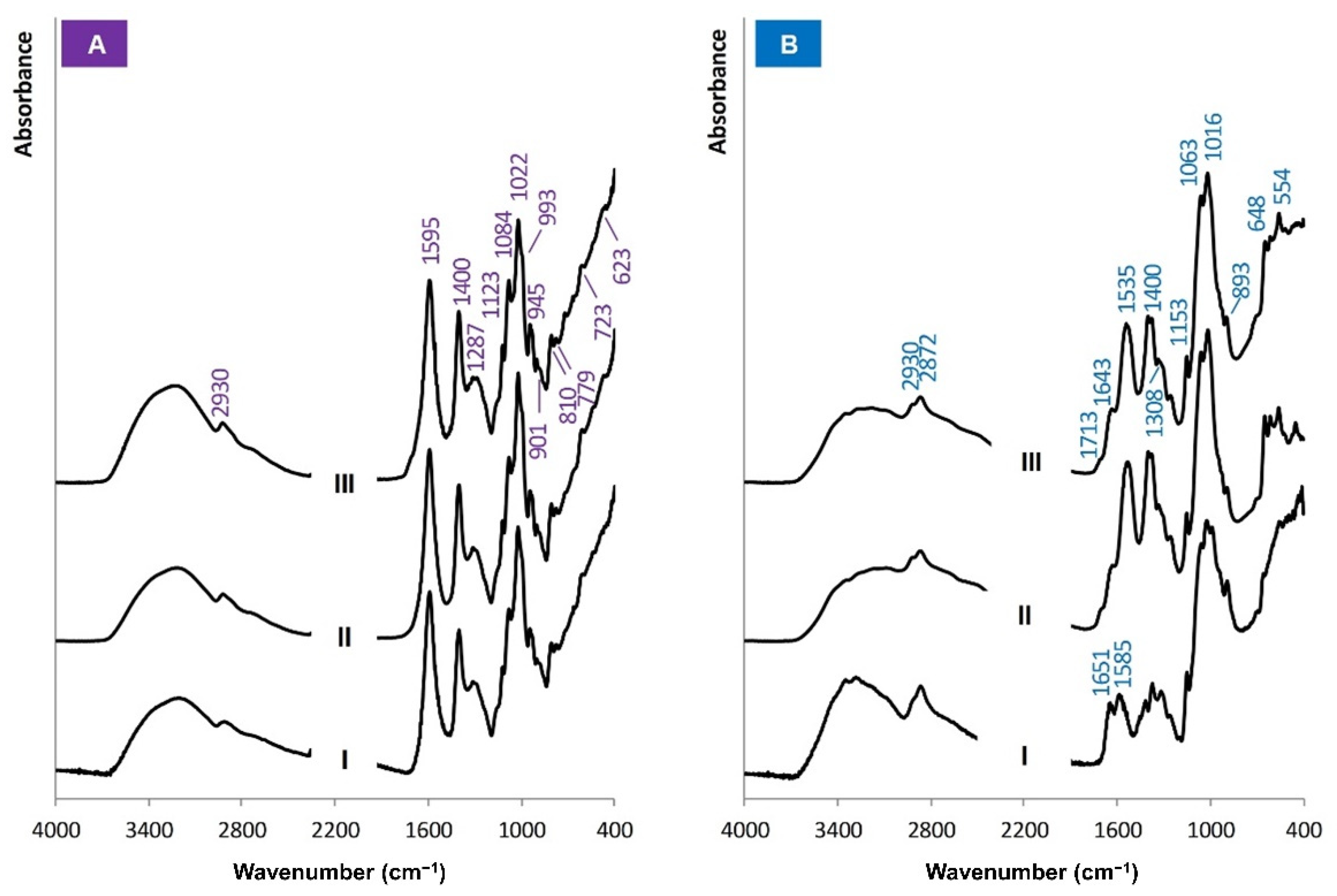
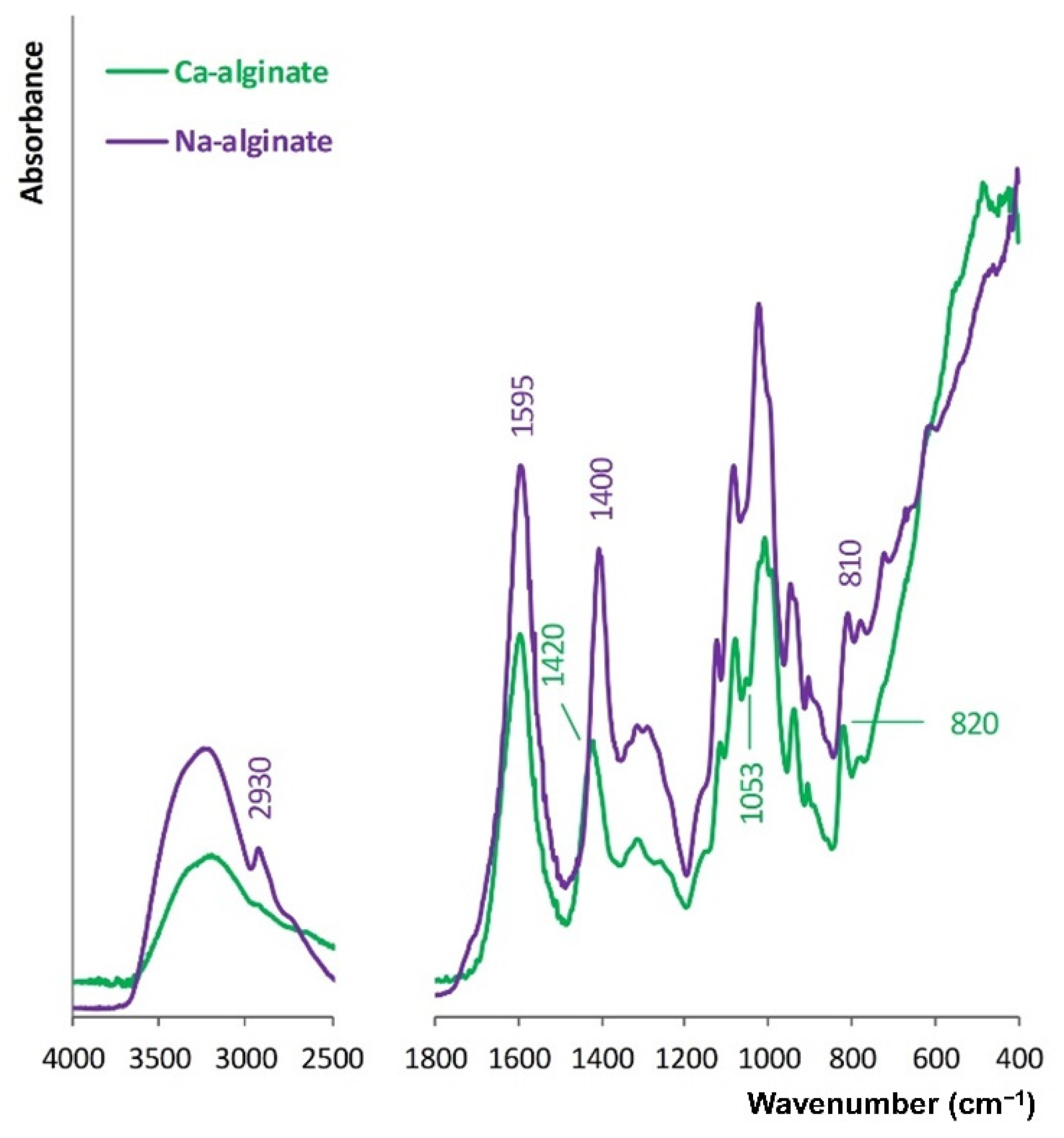
3.2. Molecular Structure of the Biopolymers
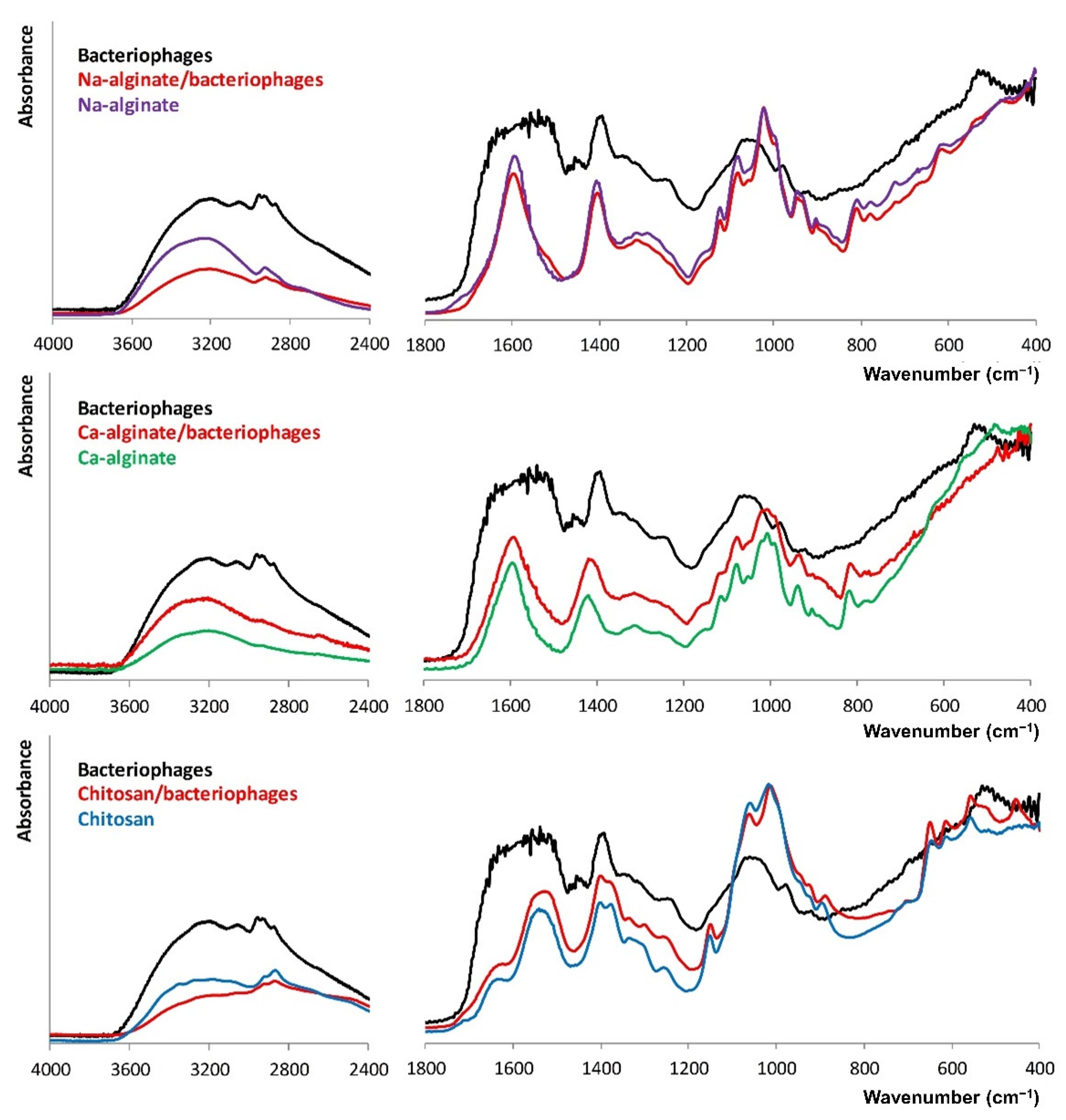
3.3. Bacteriophages Stability
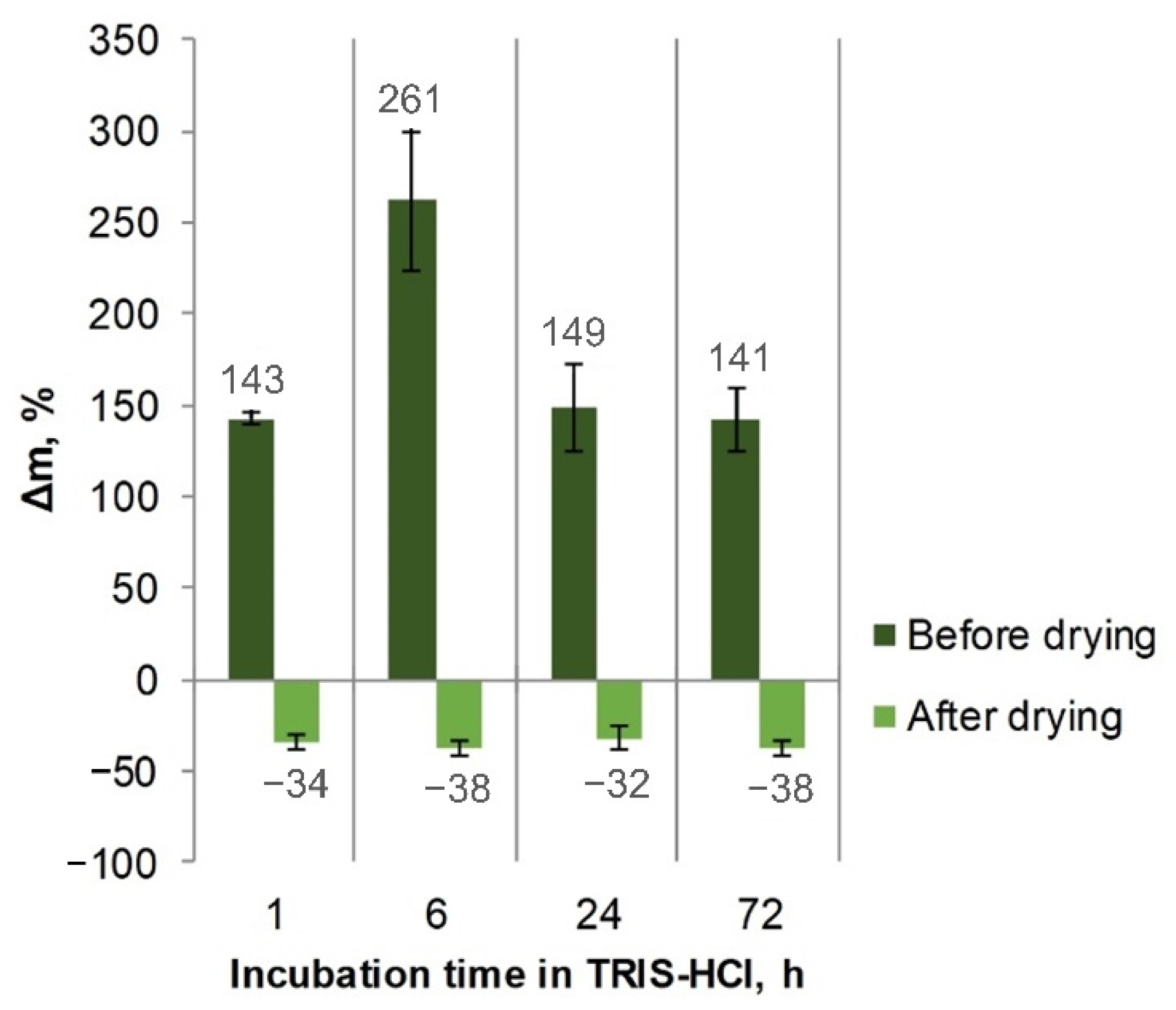
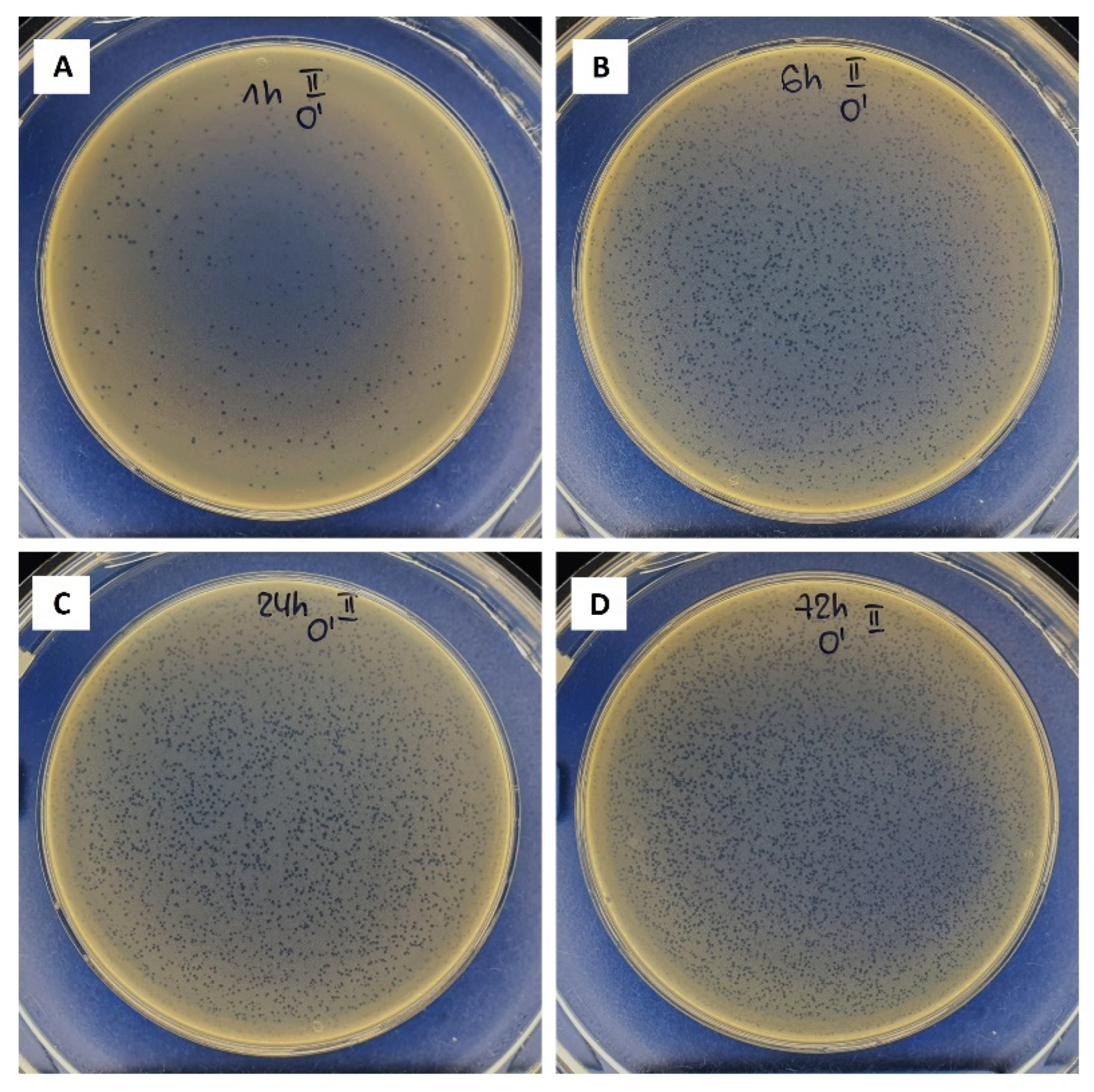
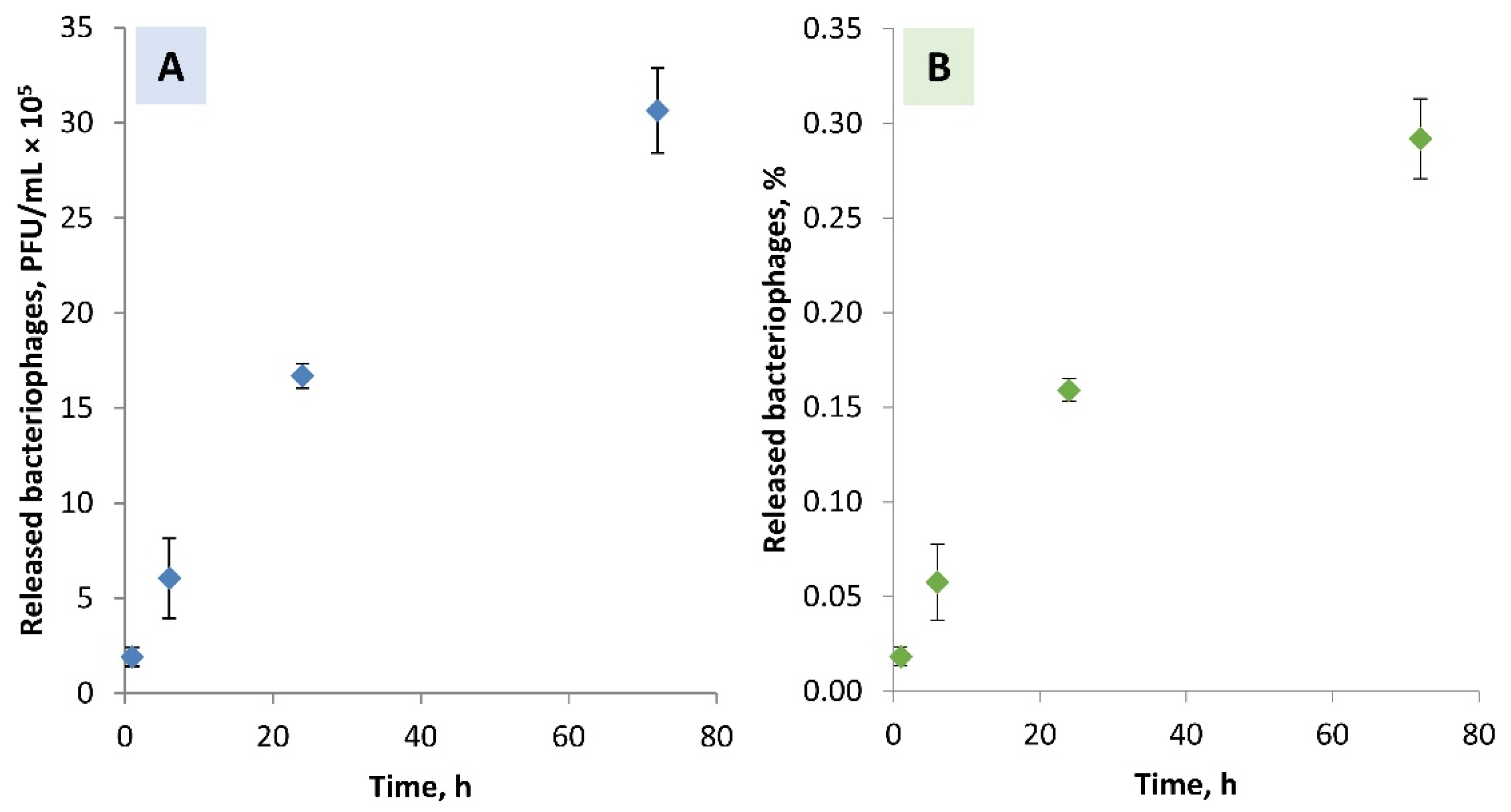
3.4. Ca-Alginate Stability and Bacteriophages Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Cai, W.J.; Ren, Z.; Han, P. The Role of Staphylococcal Biofilm on the Surface of Implants in Orthopedic Infection. Microorganisms 2022, 10, 1909. [Google Scholar] [CrossRef] [PubMed]
- Rosas, S.; Ong, A.C.; Buller, L.T.; Sabeh, K.G.; Law, T.Y.; Roche, M.W.; Hernandez, V.H. Season of the year influences infection rates following total hip arthroplasty. World J. Orthop. 2017, 8, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.T.; Garcia, A.J. Scaffold-based anti-infection strategies in bone repair. Ann. Biomed. Eng. 2015, 43, 515–528. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.-N.; Kim, E.S.; Oh, M.-D. Oral antibiotic treatment of staphylococcal bone and joint infections in adults. J. Antimicrob. Chemother. 2014, 69, 309–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wassif, R.K.; Elkayal, M.; Shamma, R.N.; Elkheshen, S.A. Recent advances in the local antibiotics delivery systems for management of osteomyelitis. Drug Deliv. 2021, 28, 2392–2414. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Missirlis, Y.F. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cells Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Renata, C. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef] [Green Version]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Racenis, K.; Rezevska, D.; Madelane, M.; Lavrinovics, E.; Djebara, S.; Petersons, A.; Kroica, J. Use of phage cocktail BFC 1.10 in combination with ceftazidime-avibactam in the treatment of multidrug-resistant Pseudomonas aeruginosa femur osteomyelitis—A case report. Front. Med. 2022, 9, 851310. [Google Scholar] [CrossRef]
- Qadir, M.I.; Mobeen, T.; Masood, A. Phage therapy: Progress in pharmacokinetics. Braz. J. Pharm. Sci. 2018, 54, e17093. [Google Scholar] [CrossRef]
- Monk, A.B.; Rees, C.D.; Barrow, P.; Hagens, S.; Harper, D.R. Bacteriophage applications: Where are we now? Lett. Appl. Microbial. 2010, 51, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Thung, T.Y.; Lee, E.; Premarathne, J.M.K.J.K.; Nurzafirah, M.; Kuan, C.H.; Elexson, N.; Tan, C.W.; Malcolm, T.T.H.; New, C.Y.; Ramzi, O.S.B.; et al. Bacteriophages and their applications. Food Res. 2018, 2, 404–414. [Google Scholar] [CrossRef]
- Davies, E.V.; Winstanley, C.; Fothergill, J.L.; James, C.E. The role of temperate bacteriophages in bacterial infection. FEMS Microbiol. Lett. 2016, 363, fnw015. [Google Scholar] [CrossRef] [Green Version]
- Hyman, P. Phages for phage therapy: Isolation, characterization, and host range breadth. Pharmaceuticals 2019, 12, 35. [Google Scholar] [CrossRef] [Green Version]
- Fulgione, A.; Ianniello, F.; Papaianni, M.; Contaldi, F.; Sgamma, T.; Giannini, C.; Pastore, S.; Velotta, R.; Della Ventura, B.; Roveri, N.; et al. Biomimetic hydroxyapatite nanocrystals are an active carrier for Salmonella bacteriophages. Int. J. Nanomed. 2019, 14, 2219–2232. [Google Scholar] [CrossRef] [Green Version]
- Vasilev, K.; Cook, J.; Griesser, H.J. Antibacterial surfaces for biomedical devices. Expert Rev. Med. Devices 2009, 6, 553–567. [Google Scholar] [CrossRef]
- Zimmerli, W. Clinical presentation and treatment of orthopaedic implant-associated infection. J. Intern. Med. 2014, 276, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Choinska-Pulit, A.; Mitula, P.; Sliwka, P.; Laba, W.; Skaradzinska, A. Bacteriophage encapsulation: Trends and potential applications. Trends Food Sci. Technol. 2015, 45, 212–221. [Google Scholar] [CrossRef]
- Lone, A.; Anany, H.; Hakeem, M.; Aguis, L.; Avdjian, A.C.; Bouget, M.; Atashi, A.; Brovko, L.; Rochefort, D.; Griffiths, M.W. Development of prototypes of bioactive packaging materials based on immobilized bacteriophages for control of growth of bacterial pathogens in foods. Int. J. Food Microbiol. 2016, 217, 49–58. [Google Scholar] [CrossRef]
- O’Connell, L.; Marcoux, P.R.; Roupioz, Y. Strategies for Surface Immobilization of Whole Bacteriophages: A Review. ACS Biomater. Sci. Eng. 2021, 7, 1987–2014. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, R.; Fernandes, M.; Fangueiro, R. Biopolymers in medical implants: A brief review. Procedia Eng. 2017, 20, 236–243. [Google Scholar] [CrossRef]
- Stipniece, L.; Salma-Ancane, K.; Rjabovs, V.; Juhnevica, I.; Turks, M.; Narkevica, I.; Berzina-Cimdina, L. Development of functionalized hydroxyapatite/poly(vinyl alcohol) composites. J. Cryst. Growth 2016, 444, 14–20. [Google Scholar] [CrossRef]
- Hosseinidoust, Z.; Olsson, A.L.J.; Tufenkji, N. Going viral: Designing bioactive surfaces with bacteriophage. Colloids Surf. B Biointerfaces 2014, 124, 2–16. [Google Scholar] [CrossRef] [Green Version]
- Munoz-Bonilla, A.; Echeverria, C.; Sonseca, A.; Arrieta, M.P.; Fernandez-Garcia, M. Bio-based polymers with antimicrobial properties towards sustainable development. Materials 2019, 12, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szekalska, M.; Pucilowska, A.; Szymanska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef] [Green Version]
- Rotman, S.G.; Sumrall, E.; Ziadlou, R.; Grijpma, D.W.; Richards, R.G.; Eglin, D.; Moriarty, T.F. Local bacteriophage delivery for treatment and prevention of bacterial infections. Front. Microbiol. 2020, 11, 538060. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Hu, J.J. Sub-100-micron calcium-alginate microspheres: Preparation by nitrogen flow focusing, dependence of spherical shape on gas streams and a drug carrier using acetaminophen as a model drug. Carbohydr. Polym. 2021, 269, 118262. [Google Scholar] [CrossRef]
- Kim, H.Y.; Chang, R.Y.K.; Morales, S.; Chan, H.K. Bacteriophage-delivering hydrogels: Current progress in combating antibiotic resistant bacterial infection. Antibiotics 2021, 10, 130. [Google Scholar] [CrossRef]
- Arciola, C.R.; An, Y.H.; Campoccia, D.; Donati, M.E.; Montanaro, L. Etiology of implant orthopedic infections: A survey on 1027 clinical isolates. Int. J. Artif. Organs 2005, 28, 1091–1100. [Google Scholar] [CrossRef]
- Kaplan, S.L. Recent lessons for the management of bone and joint infections. J. Infect. 2014, 68, S51–S56. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, P.C. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2006, 12, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abatángelo, V.; Bacci, N.P.; Boncompain, C.A.; Amadio, A.F.; Carrasco, S.; Suarez, C.A.; Morbidoni, H.R. Broad-range lytic bacteriophages that kill Staphylococcus aureus local field strains. PLoS ONE 2017, 12, e0187387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, G.; Wolz, C. Phages of Staphylococcus aureus and their impact on host evolution. Infect. Genet. Evol. 2014, 21, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.H.; Tanji, Y. Peculiarities of Staphylococcus aureus phages and their possible application in phage therapy. Appl. Microb. Biotechnol. 2019, 103, 4279–4289. [Google Scholar] [CrossRef]
- Fokine, A.; Rossmann, M.G. Molecular architecture of tailed double-stranded DNA phages. Bacteriophage 2014, 4, e28281. [Google Scholar] [CrossRef] [Green Version]
- Bourdin, G.; Navarro, A.; Sarker, S.A.; Pittet, A.C.; Qadri, F.; Sultana, S.; Cravioto, A.; Talukder, K.A.; Reuteler, G.; Brussow, H. Coverage of diarrhoea-associated Escherichia coli isolates from different origins with two types of phage cocktails. Microb. Biotechnol. 2014, 7, 165–176. [Google Scholar] [CrossRef]
- IR Spectrum Table & Chart. Available online: https://www.sigmaaldrich.com/LV/en/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 27 October 2022).
- Peretz, S.; Florea-Spiroiu, M.; Anghel, D.-F.; Bala, D.; Stoian, C.; Zgherea, G. Preparation of porous calcium alginate beads and their use for adsorption of O-nitrophenol from aqueous solutions. Microfluid Nanoeng. 2013, 22, 123–136. [Google Scholar]
- Pang, Y.; Xi, F.; Luo, J.; Liu, G.; Guo, T.; Zhang, C. An alginate film-based degradable triboelectric nanogenerator. RSC Adv. 2018, 8, 6719–6726. [Google Scholar] [CrossRef] [Green Version]
- Olszak, T.; Zarnowiec, P.; Kaca, W.; Danis-Wlodarczyk, K.; Augustyniak, D.; Drevinek, P.; de Soyza, A.; McClean, S.; Drulis-Kawa, Z. In vitro and in vivo antibacterial activity of environmental bacteriophages against Pseudomonas aeruginosa strains from cystic fibrosis patients. Appl. Microbiol. Biotechnol. 2015, 99, 6021–6033. [Google Scholar] [CrossRef] [Green Version]
- Jurczak-Kurek, A.; Gasior, T.; Nejman-Falenczyk, B.; Bloch, S.; Dydecka, A.; Topka, G.; Necel, A.; Jakubowska-Deredas, M.; Narajczyk, M.; Richert, M.; et al. Biodiversity of bacteriophages: Morphological and biological properties of a large group of phages isolated from urban sewage. Sci. Rep. 2016, 6, 34338. [Google Scholar] [CrossRef] [PubMed]
- Cobb, L.H.; Park, J.; Swanson, E.A.; Beard, M.C.; McCabe, E.M.; Rourke, A.S.; Seo, K.S.; Olivier, A.K.; Priddy, L.B. CRISPR-Cas9 modified bacteriophage for treatment of Staphylococcus aureus induced osteomyelitis and soft tissue infection. PLoS ONE 2019, 14, e0220421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Pacan, J.C.; Wang, Q.; Sabour, P.M.; Huang, X.; Xu, Y. Enhanced alginate microspheres as means of oral delivery of bacteriophage for reducing Staphylococcus aureus intestinal carriage. Food Hydrocoll. 2012, 26, 434–440. [Google Scholar] [CrossRef]
- Barros, J.A.R.; Melo, L.D.R.; Silva, R.A.R.D.; Ferraz, M.P.; Azeredo, J.C.V.R.; Pinheiro, V.M.C.; Colaco, B.J.A.; Fernandes, M.H.R.; Gomes, P.S.; Monteiro, F.J. Encapsulated bacteriophages in alginate-nanohydroxyapatite hydrogel as a novel delivery system to prevent orthopedic implant-associated infections. Nanomedicine 2020, 24, 102145. [Google Scholar] [CrossRef] [PubMed]

| Pyo Bacteriophages | Staph Bacteriophages | |||
|---|---|---|---|---|
| Titre, PFU/mL | Titre Reduction, Log Units | Titre, PFU/mL | Titre Reduction, Log Units | |
| Expected | 1.6 × 109 | 0.22 ± 0.01 | 5.0 × 108 | 0.30 ± 0.02 |
| Measured | (9.6 ± 0.3) × 108 | (2.5 ± 0.1) × 108 | ||
| Stock Solution, PFU/mL | Titre after Drying at 40 °C, PFU/mL | |||
|---|---|---|---|---|
| 5 h | 24 h | 144 h | ||
| Stock solution of Pyo bacteriophages | 4.8 × 108 | N/D * | N/D | N/D |
| Pyo bacteriophages/ Na-alginate | (2.2 ± 0.7) × 108 | (1.2 ± 0.2) × 108 | (1.3 ± 0.2) × 108 | 0 |
| Titre, PFU/mL | Titre Reduction, Log Units | ||
|---|---|---|---|
| Expected | 2.4 × 108 | 0.03 ± 0.01 | 0.05 ± 0.01 |
| Na-alginate solution | (2.2 ± 0.7) × 108 | ||
| 0.01 ± 0.01 | |||
| Ca-alginate hydrogel | (2.1 ± 0.2) × 108 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stipniece, L.; Rezevska, D.; Kroica, J.; Racenis, K. Effect of the Biopolymer Carrier on Staphylococcus aureus Bacteriophage Lytic Activity. Biomolecules 2022, 12, 1875. https://doi.org/10.3390/biom12121875
Stipniece L, Rezevska D, Kroica J, Racenis K. Effect of the Biopolymer Carrier on Staphylococcus aureus Bacteriophage Lytic Activity. Biomolecules. 2022; 12(12):1875. https://doi.org/10.3390/biom12121875
Chicago/Turabian StyleStipniece, Liga, Dace Rezevska, Juta Kroica, and Karlis Racenis. 2022. "Effect of the Biopolymer Carrier on Staphylococcus aureus Bacteriophage Lytic Activity" Biomolecules 12, no. 12: 1875. https://doi.org/10.3390/biom12121875
APA StyleStipniece, L., Rezevska, D., Kroica, J., & Racenis, K. (2022). Effect of the Biopolymer Carrier on Staphylococcus aureus Bacteriophage Lytic Activity. Biomolecules, 12(12), 1875. https://doi.org/10.3390/biom12121875






