Evaluation of B7-H3 Targeted Immunotherapy in a 3D Organoid Model of Craniopharyngioma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Samples
2.2. Immunohistochemistry
2.3. Cell Culture and Lentivirus Packaging
2.4. Monoclonal Antibody Generation, Affinity, and Epitope Determination
2.5. Immunofluorescence Staining and Flow Cytometry
2.6. B7-H3 CAR-T Cell Generation
2.7. Preparation of B7-H3-Targeted Antibody Conjugate
2.8. Three-Dimensional (3D) Culture of CP Organoids
2.9. In Vitro Tumor Killing and Inhibition Assays
2.10. Statistics
3. Results
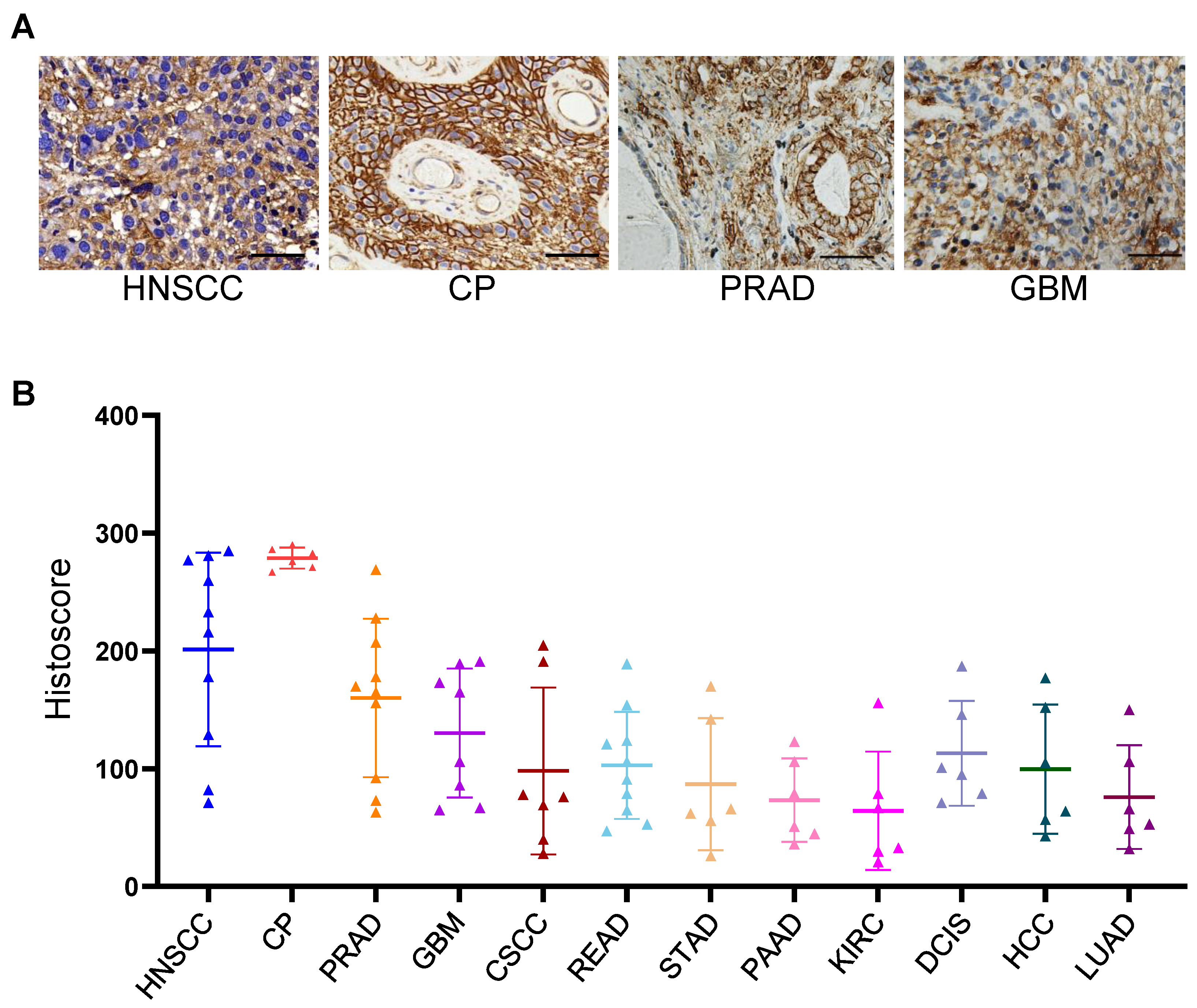
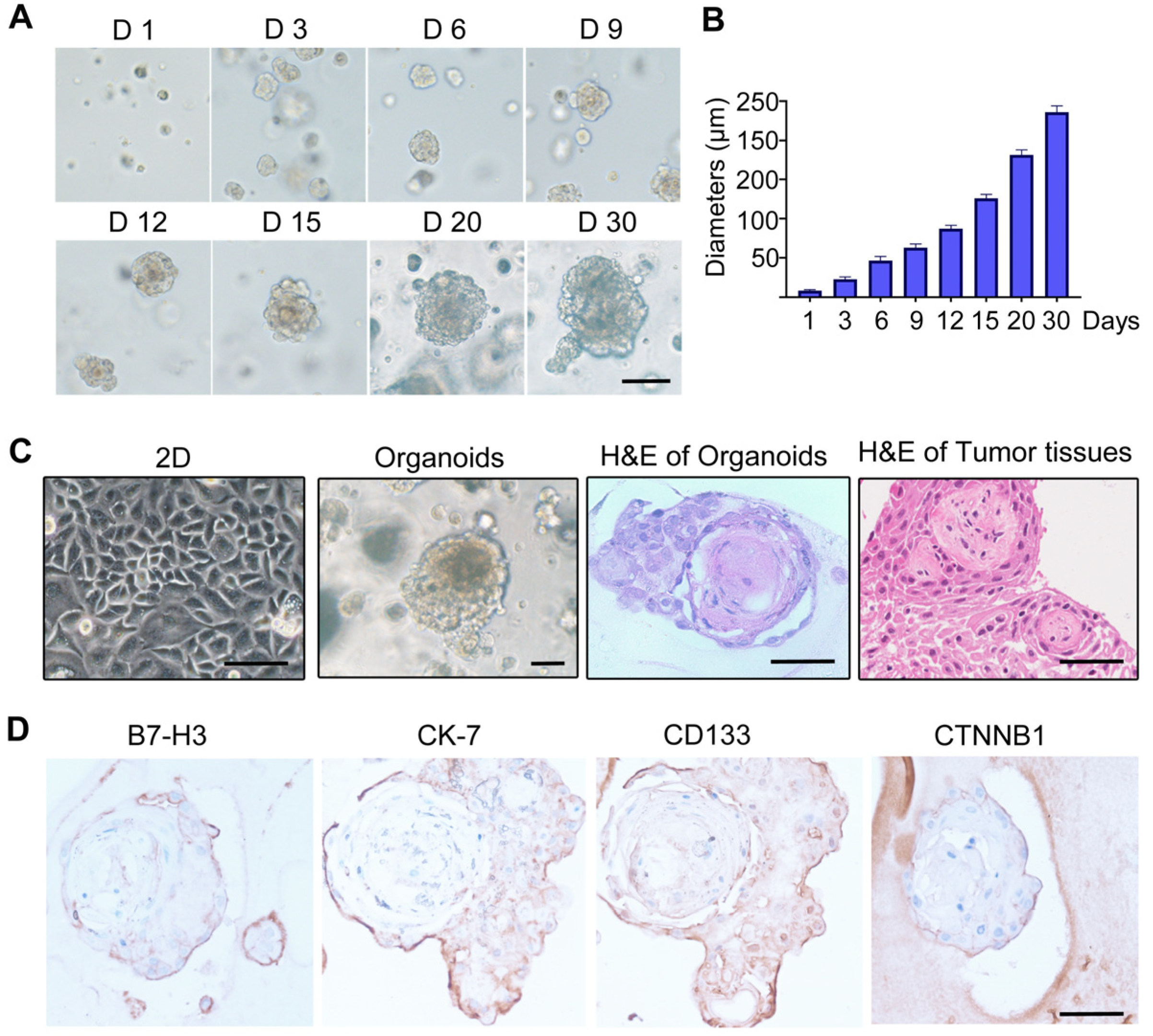
3.1. IHC Staining Analysis of B7-H3 in Clinical Tumor Tissue Samples
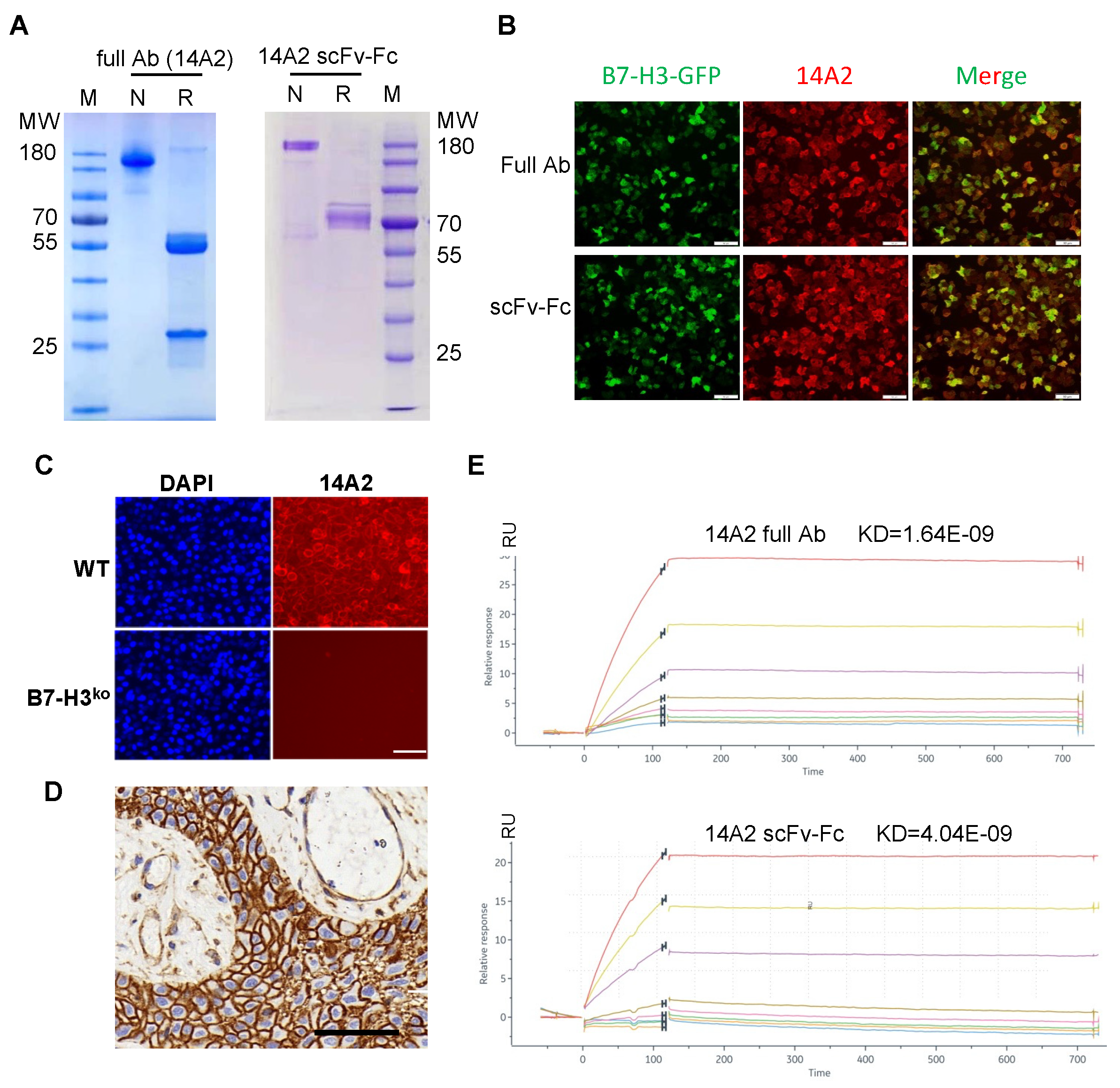
3.2. Generation and Characterization of B7-H3-Specific Monoclonal Antibody and scFv
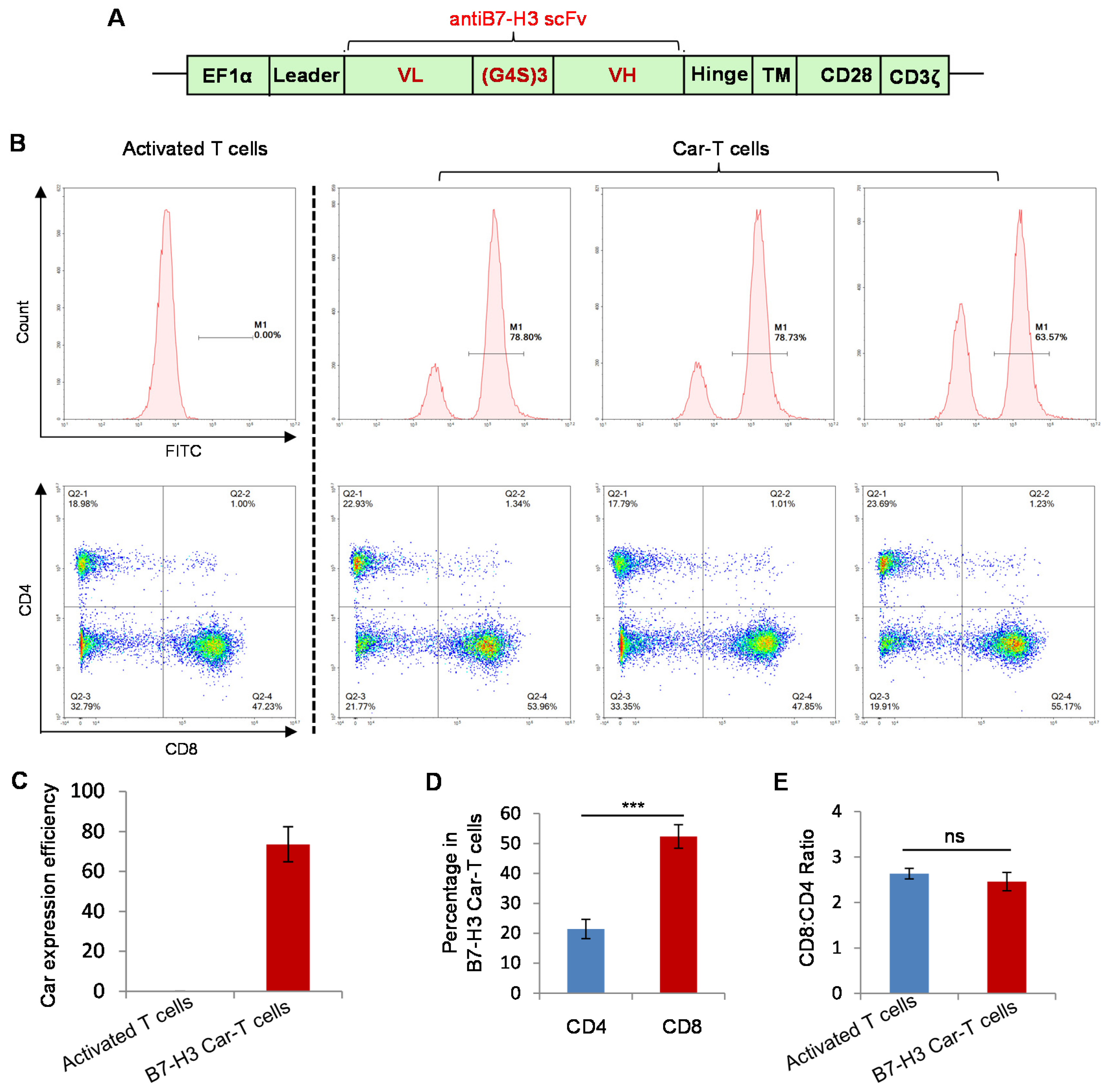
3.3. Preparation of B7-H3 Targeted CAR-T Cells
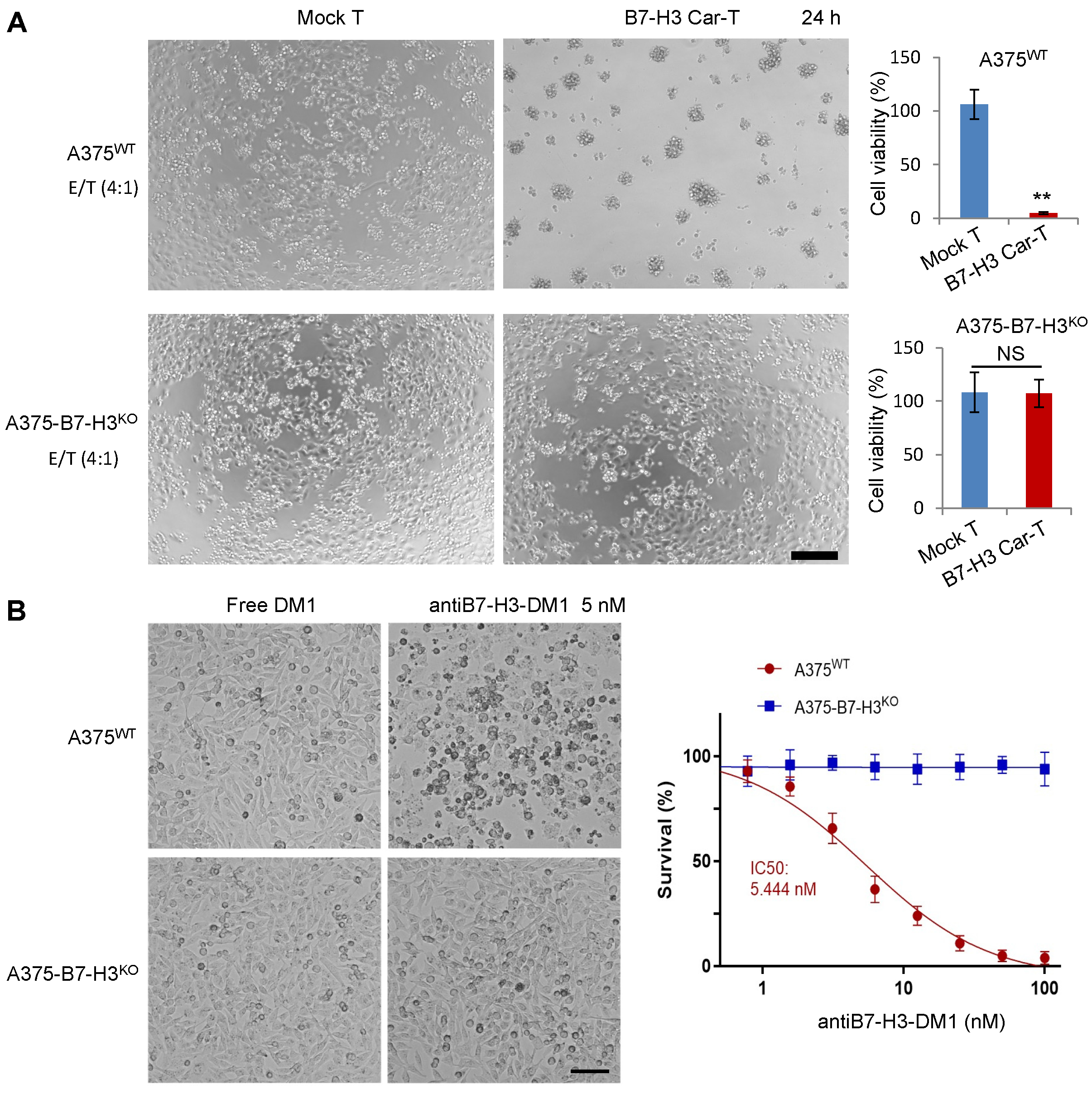
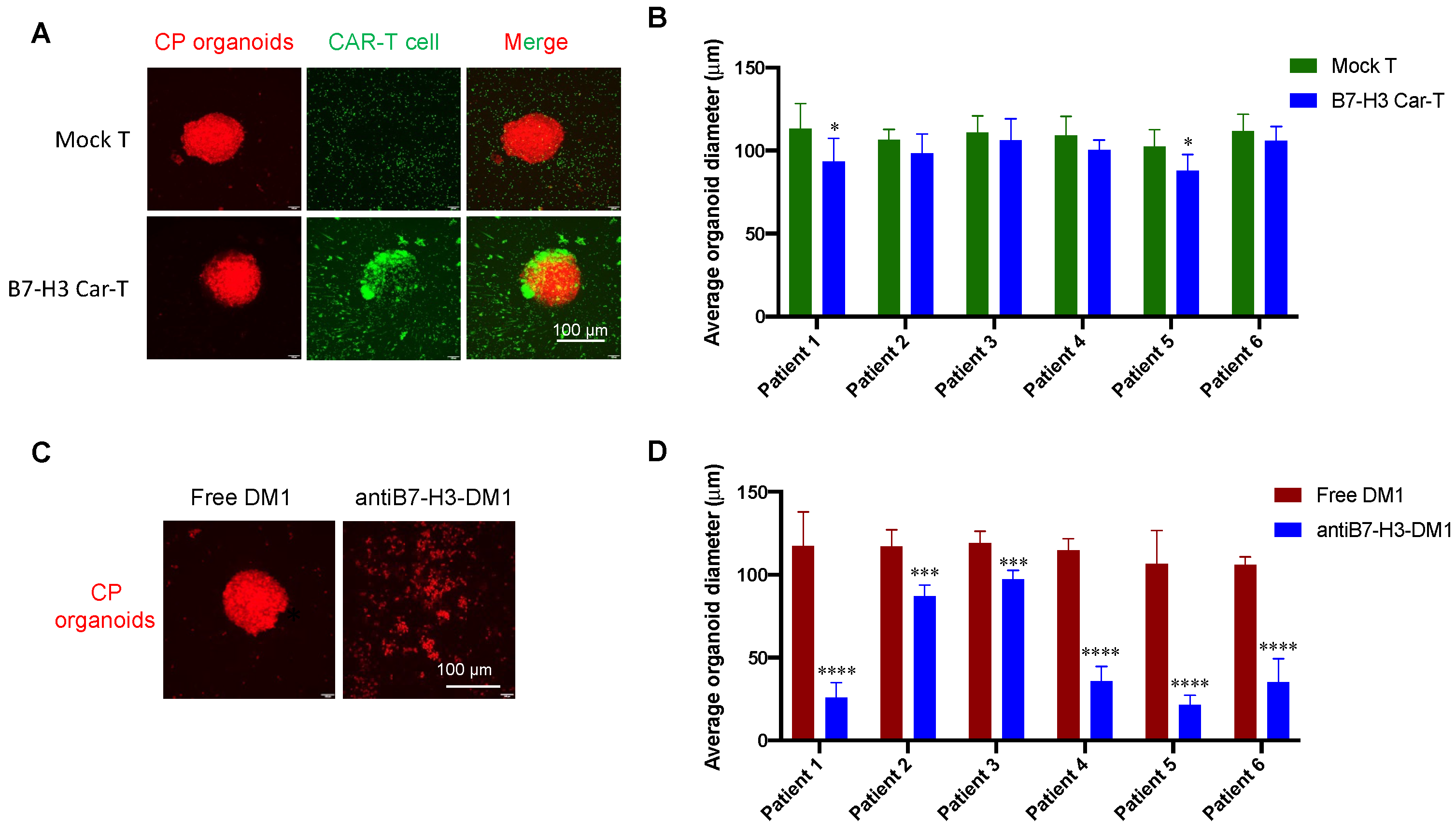
3.4. In Vitro Specific Antitumor Effects of B7-H3-Targeted CAR-T Cells and ADC
3.5. Establishment of Craniopharyngioma 3D Organoid Model
3.6. Tumor Inhibition Evaluation with CP Organoid Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Müller, H.L.; Merchant, T.E.; Warmuth-Metz, M.; Martinez-Barbera, J.P.; Puget, S. Craniopharyngioma. Nat. Rev. Dis. Primers 2019, 5, 75. [Google Scholar] [CrossRef]
- Müller, H.L. Paediatrics: Surgical strategy and quality of life in craniopharyngioma. Nat. Rev. Endocrinol. 2013, 9, 447–449. [Google Scholar] [CrossRef]
- Müller, H.L. Childhood craniopharyngioma–current concepts in diagnosis, therapy and follow-up. Nat. Rev. Endocrinol. 2010, 6, 609–618. [Google Scholar] [CrossRef]
- Müller, H.L.; Merchant, T.E.; Puget, S.; Martinez-Barbera, J.P. New outlook on the diagnosis, treatment and follow-up of childhood-onset craniopharyngioma. Nat. Rev. Endocrinol. 2017, 13, 299–312. [Google Scholar] [CrossRef]
- Erfurth, E.M.; Holmer, H.; Fjalldal, S.B. Mortality and morbidity in adult craniopharyngioma. Pituitary 2012, 16, 46–55. [Google Scholar] [CrossRef]
- Sekine, S.; Shibata, T.; Kokubu, A.; Morishita, Y.; Noguchi, M.; Nakanishi, Y.; Sakamoto, M.; Hirohashi, S. Craniopharyngiomas of adamantinomatous type harbor beta-catenin gene mutations. Am. J. Pathol. 2002, 161, 1997–2001. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Taylor-Weiner, A.; Manley, P.E.; Jones, R.T.; Dias-Santagata, D.; Thorner, A.R.; Lawrence, M.S.; Rodriguez, F.J.; ABernardo, L.; Schubert, L.; et al. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat. Genet. 2014, 46, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Prieto, R.; Pascual, J.M. Can tissue biomarkers reliably predict the biological behavior of craniopharyngiomas? A comprehensive overview. Pituitary 2018, 21, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Apps, J.R.; Martinez-Barbera, J.P. Molecular pathology of adamantinomatous craniopharyngioma: Review and opportunities for practice. Neurosurg. Focus 2016, 41, E4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aylwin, S.J.B.; Bodi, I.; Beaney, R. Pronounced response of papillary craniopharyngioma to treatment with vemurafenib, a BRAF inhibitor. Pituitary 2015, 19, 544–546. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Zhong, K.; Jiang, C.; Wang, L.; Yuan, Z.; Nie, C.; Xu, J.; Guo, G.; Zhou, L.; et al. Frequent B7-H3 overexpression in craniopharyngioma. Biochem. Biophys. Res. Commun. 2019, 514, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Suh, W.-K.; Gajewska, B.U.; Okada, H.; Gronski, M.; Bertram, E.M.; Dawicki, W.; Duncan, G.S.; Bukczynski, J.; Plyte, S.; Elia, A.J.; et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1–mediated immune responses. Nat. Immunol. 2003, 4, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.; Jia, L.; Kim, J.K.; Li, J.; Deng, P.; Zhang, W.; Krebsbach, P.H.; Wang, C.-Y. CD276 expression enables squamous cell carcinoma stem cells to evade immune surveillance. Cell Stem Cell 2021, 28, 1597–1613.e7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, W.; Phillips, J.B.; Arora, R.; McClellan, S.; Li, J.; Kim, J.-H.; Sobol, R.W.; Tan, M. Immunoregulatory protein B7-H3 regulates cancer stem cell enrichment and drug resistance through MVP-mediated MEK activation. Oncogene 2018, 38, 88–102. [Google Scholar] [CrossRef]
- Nunes-Xavier, C.E.; Kildal, W.; Kleppe, A.; Danielsen, H.E.; Wæhre, H.; Llarena, R.; Mælandsmo, G.M.; Fodstad, Ø.; Pulido, R.; López, J.I. Immune checkpoint B7-H3 protein expression is associated with poor outcome and androgen receptor status in prostate cancer. Prostate 2021, 81, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Yerrabelli, R.S.; He, P.; Fung, E.K.; Kramer, K.; Zanzonico, P.B.; Humm, J.L.; Guo, H.; Pandit-Taskar, N.; Larson, S.M.; Cheung, N.-K.V. IntraOmmaya compartmental radioimmunotherapy using 131I-omburtamab—pharmacokinetic modeling to optimize therapeutic index. Eur. J. Nucl. Med. 2020, 48, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, Y.; Yang, N.; Mu, M.; Wu, Z.; Li, H.; Tang, X.; Zhong, K.; Zhang, Z.; Huang, C.; et al. Genome-scale CRISPR–Cas9 screen reveals novel regulators of B7-H3 in tumor cells. J. Immunother. Cancer 2022, 10, e004875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, C.; Liu, Z.; Yang, M.; Tang, X.; Wang, Y.; Zheng, M.; Huang, J.; Zhong, K.; Zhao, S.; et al. B7-H3-Targeted CAR-T Cells Exhibit Potent Antitumor Effects on Hematologic and Solid Tumors. Mol. Ther.-Oncolytics 2020, 17, 180–189. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, S.; Zhang, Y.; Wang, Y.; Zhang, Z.; Yang, M.; Zhu, Y.; Zhang, G.; Guo, G.; Tong, A.; et al. B7-H3 as a Novel CAR-T Therapeutic Target for Glioblastoma. Mol. Ther.-Oncolytics 2019, 14, 279–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Wang, Y.; Wu, Y.; Jiang, X.; Tao, Y.; Yao, Y.; Peng, Y.; Chen, X.; Fu, Y.; Yu, L.; et al. Therapeutic potential of an anti-HER2 single chain antibody–DM1 conjugates for the treatment of HER2-positive cancer. Signal Transduct. Target. Ther. 2017, 2, 17015. [Google Scholar] [CrossRef] [PubMed]
- Driehuis, E.; Kolders, S.; Spelier, S.; Lõhmussaar, K.; Willems, S.M.; Devriese, L.A.; de Bree, R.; de Ruiter, E.J.; Korving, J.; Begthel, H.; et al. Oral Mucosal Organoids as a Potential Platform for Personalized Cancer Therapy. Cancer Discov. 2019, 9, 852–871. [Google Scholar] [CrossRef] [PubMed]
- Hölsken, A.; Gebhardt, M.; Buchfelder, M.; Fahlbusch, R.; Blümcke, I.; Buslei, R. EGFR Signaling Regulates Tumor Cell Migration in Craniopharyngiomas. Clin. Cancer Res. 2011, 17, 4367–4377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, M.; Taghvaei, M.; Yu, S.; Sathe, A.; Collopy, S.; Prashant, G.N.; Evans, J.J.; Karsy, M. Targeted Therapy in the Management of Modern Craniopharyngiomas. Front. Biosci. 2022, 27, 136. [Google Scholar] [CrossRef]
- Coy, S.; Rashid, R.; Lin, J.R.; Du, Z.; Donson, A.M.; Hankinson, T.C.; Foreman, N.K.; Manley, P.E.; Kieran, M.W.; Reardon, D.A.; et al. Multiplexed immunofluorescence reveals potential PD-1/PD-L1 pathway vulnerabilities in craniopharyngioma. Neuro Oncol. 2018, 20, 1101–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Deng, J.; Wang, L.; Zhou, T.; Yang, J.; Tian, Z.; Yang, J.; Chen, H.; Tang, X.; Zhao, S.; et al. Expression and clinical significance of PD-L1, B7-H3, B7-H4 and VISTA in craniopharyngioma. J. Immunother. Cancer 2020, 8, e000406. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef] [Green Version]
- Tarantino, P.; Pestana, R.C.; Corti, C.; Modi, S.; Bardia, A.; Tolaney, S.M.; Cortes, J.; Soria, J.; Curigliano, G. Antibody–drug conjugates: Smart chemotherapy delivery across tumor histologies. CA A Cancer J. Clin. 2021, 72, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, Y.H. Trastuzumab deruxtecan for HER2+ advanced breast cancer. Futur. Oncol. 2022, 18, 7–19. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.; Chen, C.; Wang, G.; Wang, Y.; Zhang, Z.; Li, H.; Lu, Q.; Wang, Z.; Zhao, S.; Yang, C.; et al. Evaluation of B7-H3 Targeted Immunotherapy in a 3D Organoid Model of Craniopharyngioma. Biomolecules 2022, 12, 1744. https://doi.org/10.3390/biom12121744
Tang M, Chen C, Wang G, Wang Y, Zhang Z, Li H, Lu Q, Wang Z, Zhao S, Yang C, et al. Evaluation of B7-H3 Targeted Immunotherapy in a 3D Organoid Model of Craniopharyngioma. Biomolecules. 2022; 12(12):1744. https://doi.org/10.3390/biom12121744
Chicago/Turabian StyleTang, Mei, Caili Chen, Guoqing Wang, Yuelong Wang, Zongliang Zhang, Hexian Li, Qizhong Lu, Zeng Wang, Shasha Zhao, Chen Yang, and et al. 2022. "Evaluation of B7-H3 Targeted Immunotherapy in a 3D Organoid Model of Craniopharyngioma" Biomolecules 12, no. 12: 1744. https://doi.org/10.3390/biom12121744
APA StyleTang, M., Chen, C., Wang, G., Wang, Y., Zhang, Z., Li, H., Lu, Q., Wang, Z., Zhao, S., Yang, C., Zhong, K., Zhang, R., Guo, L., Yuan, Z., Nie, C., & Tong, A. (2022). Evaluation of B7-H3 Targeted Immunotherapy in a 3D Organoid Model of Craniopharyngioma. Biomolecules, 12(12), 1744. https://doi.org/10.3390/biom12121744




