Primary Cilia: The New Face of Craniofacial Research
Abstract
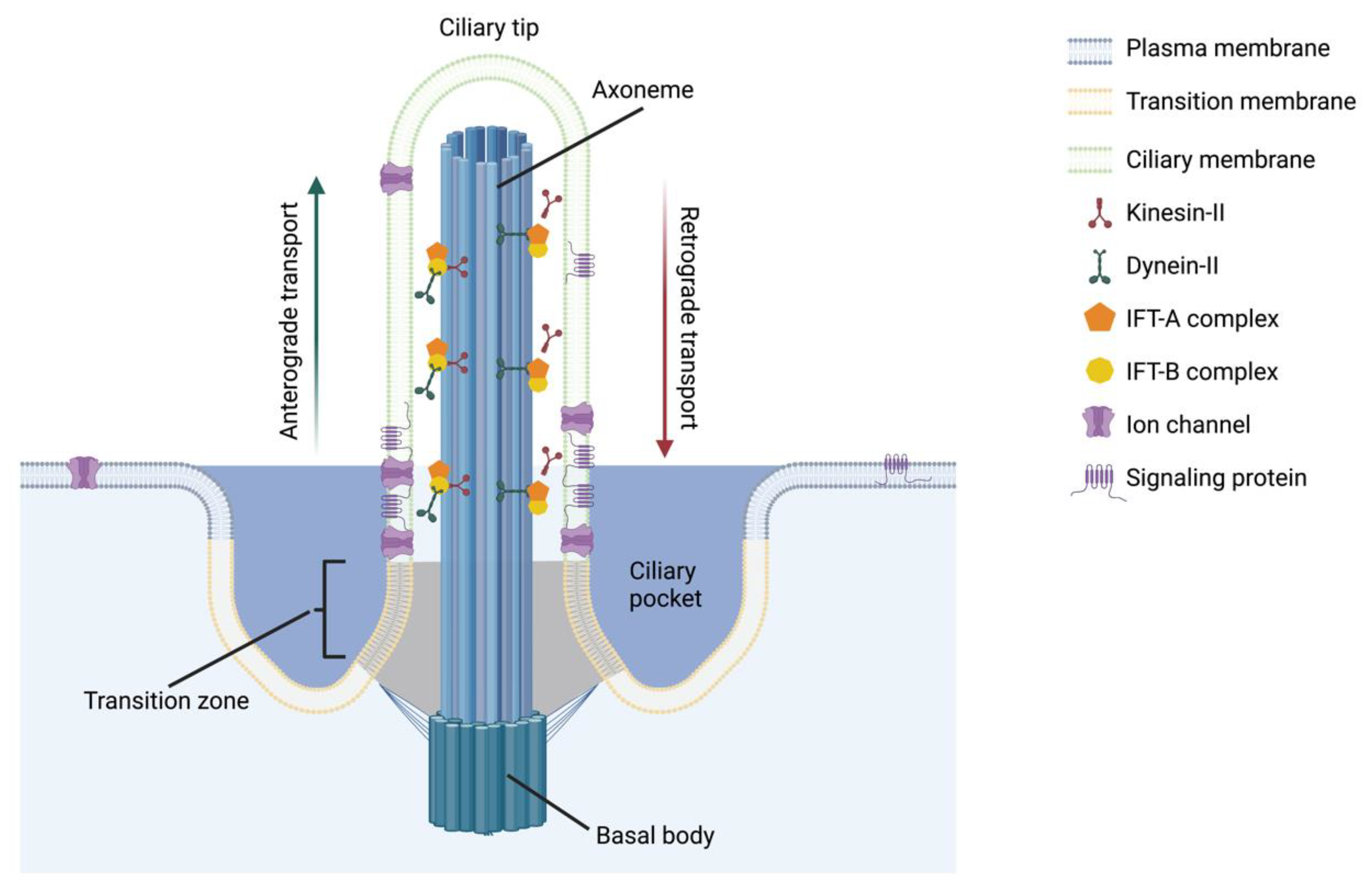
:1. Introduction
2. Craniofacial Defects Associated with Ciliopathies
3. Primary Cilia in Dental Cells
4. Primary Cilia in Craniofacial Development
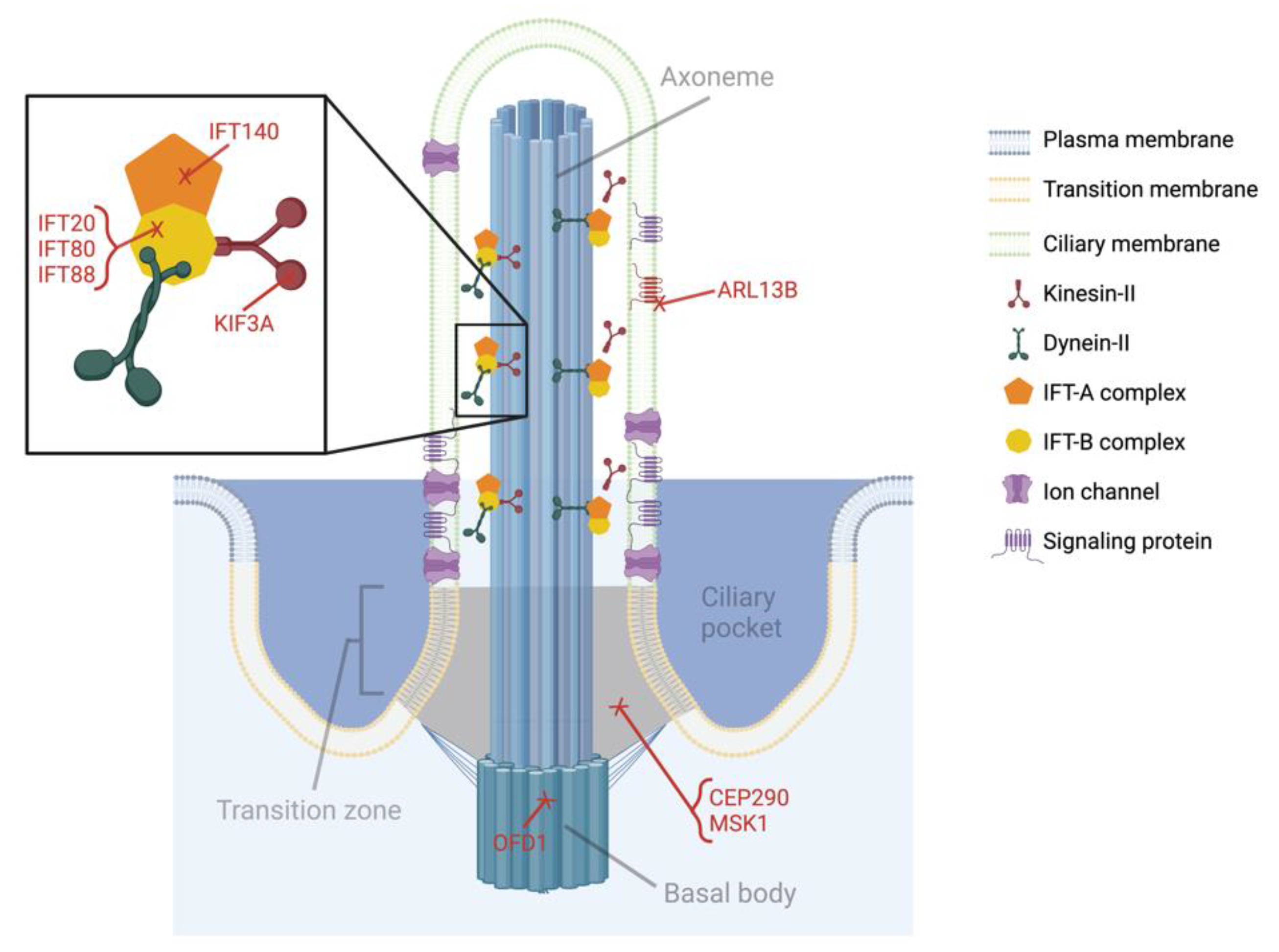
4.1. Kinesin-like Protein Subunit 3a (KIF3A)
4.2. Intraflagellar Transport Protein 88 (IFT88)
4.3. Intraflagellar Transport Protein 80 (IFT80)
4.4. Intraflagellar Transport Protein 140 (IFT140)
4.5. Other Primary Ciliary Genes Associated with Craniofacial Abnormalities
5. Primary Cilia in Dental Repair
6. The Future of Primary Cilia Research in Craniofacial Tissues
6.1. Mechanotransduction
6.2. Calcium Signaling
6.3. Innervation and Pain
6.4. The Primary Cilium as a Signaling Nexus
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Xin, Z.; Wang, J.; Li, S.; Lechtreck, K.; Pan, J. IFT54 directly interacts with kinesin-II and IFT dynein to regulate anterograde intraflagellar transport. EMBO J. 2021, 40, e105781. [Google Scholar]
- Nakayama, K.; Katoh, Y. Ciliary protein trafficking mediated by IFT and BBSome complexes with the aid of kinesin-2 and dynein-2 motors. J. Biochem. 2018, 163, 155–164. [Google Scholar] [CrossRef]
- Zaghloul, N.A.; Brugmann, S.A. The Emerging Face of Primary Cilia. Genesis 2011, 49, 231–246. [Google Scholar] [CrossRef] [Green Version]
- Brugmann, S.A.; Cordero, D.R.; Helms, J.A. Craniofacial Ciliopathies: A New Classification for Craniofacial Disorders. Am. J. Med. Genet. A 2010, 152A, 2995–3006. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, H.; Sun, Y. Primary Cilia in Hard Tissue Development and Diseases. Front. Med. 2021, 15, 657–678. [Google Scholar] [CrossRef] [PubMed]
- Pawlaczyk-Kamieńska, T.; Winiarska, H.; Kulczyk, T.; Cofta, S. Dental Anomalies in Rare, Genetic Ciliopathic Disorder–A Case Report and Review of Literature. Int. J. Environ. Res. Public Health 2020, 17, 4337. [Google Scholar] [CrossRef] [PubMed]
- Hampl, M.; Cela, P.; Szabo-Rogers, H.L.; Bosakova, M.K.; Dosedelova, H.; Krejci, P.; Buchtova, M. Role of Primary Cilia in Odontogenesis. J. Dent. Res. 2017, 96, 965–974. [Google Scholar] [CrossRef] [Green Version]
- Walczak-Sztulpa, J.; Eggenschwiler, J.; Osborn, D.; Brown, D.A.; Emma, F.; Klingenberg, C.; Hennekam, R.C.; Torre, G.; Garshasbi, M.; Tzschach, A.; et al. Cranioectodermal Dysplasia, Sensenbrenner Syndrome, Is a Ciliopathy Caused by Mutations in the IFT122 Gene. Am. J. Hum. Genet. 2010, 86, 949–956. [Google Scholar] [CrossRef] [Green Version]
- Brugmann, S.A.; Allen, N.C.; James, A.W.; Mekonnen, Z.; Madan, E.; Helms, J.A. A Primary Cilia-Dependent Etiology for Midline Facial Disorders. Hum. Mol. Genet. 2010, 19, 1577–1592. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.F.; Kowal, T.J.; Ning, K.; Koo, E.B.; Wu, A.Y.; Mahajan, V.B.; Sun, Y. Review of Ocular Manifestations of Joubert Syndrome. Genes 2018, 9, 605. [Google Scholar] [CrossRef] [Green Version]
- Cantagrel, V.; Silhavy, J.L.; Bielas, S.L.; Swistun, D.; Marsh, S.E.; Bertrand, J.Y.; Audollent, S.; Attié-Bitach, T.; Holden, K.R.; Dobyns, W.B.; et al. Mutations in the Cilia Gene ARL13B Lead to the Classical Form of Joubert Syndrome. Am. J. Hum. Genet. 2008, 83, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Juric-Sekhar, G.; Adkins, J.; Doherty, D.; Hevner, R.F. Joubert Syndrome: Brain and Spinal Cord Malformations in Genotyped Cases and Implications for Neurodevelopmental Functions of Primary Cilia. Acta Neuropathol. 2012, 123, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Yang, S. Primary Cilia and Intraflagellar Transport Proteins in Bone and Cartilage. J. Dent. Res. 2016, 95, 1341–1349. [Google Scholar] [CrossRef] [Green Version]
- Reiter, J.F.; Leroux, M.R. Genes and Molecular Pathways Underpinning Ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Anoop, U.R.; Verma, K.; Narayanan, K. Primary Cilia in the Pathogenesis of Dentigerous Cyst: A New Hypothesis Based on Role of Primary Cilia in Autosomal Dominant Polycystic Kidney Disease. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 608–617. [Google Scholar] [CrossRef]
- Nadar Singarayan, J.M.; Rooban, T.; Joshua, E.; Rao, U.M.; Ranganathan, K. Immunohistochemical study of polycystin-1 in dentigerous cysts. Indian J. Dent Res. 2014, 25, 762–766. [Google Scholar]
- Ajzenberg, H.; Slaats, G.G.; Stokman, M.F.; Arts, H.H.; Logister, I.; Kroes, H.Y.; Renkema, K.Y.; van Haelst, M.M.; Terhal, P.A.; van Rooij, I.A.; et al. Non-Invasive Sources of Cells with Primary Cilia from Pediatric and Adult Patients. Cilia 2015, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Magloire, H.; Couble, M.L.; Romeas, A.; Bleicher, F. Odontoblast Primary Cilia: Facts and Hypotheses. Cell Biol. Int. 2004, 28, 93–99. [Google Scholar] [CrossRef]
- Thivichon-Prince, B.; Couble, M.L.; Giamarchi, A.; Delmas, P.; Franco, B.; Romio, L.; Struys, T.; Lambrichts, I.; Ressnikoff, D.; Magloire, H.; et al. Primary Cilia of Odontoblasts: Possible Role in Molar Morphogenesis. J. Dent. Res. 2009, 88, 910–915. [Google Scholar] [CrossRef]
- Ohazama, A.; Haycraft, C.J.; Seppala, M.; Blackburn, J.; Ghafoor, S.; Cobourne, M.; Martinelli, D.C.; Fan, C.M.; Peterkova, R.; Lesot, H.; et al. Primary Cilia Regulate Shh Activity in the Control of Molar Tooth Number. Development 2009, 136, 897–903. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Liu, M.; Cao, X.; Yang, S. Ciliary IFT80 Regulates Dental Pulp Stem Cells Differentiation by FGF/FGFR1 and Hh/BMP2 Signaling. Int. J. Biol. Sci. 2019, 15, 2087–2099. [Google Scholar] [CrossRef]
- Martínez, C.; Smith, P.C.; Rodriguez, J.P.; Palma, V. Sonic Hedgehog Stimulates Proliferation of Human Periodontal Ligament Stem Cells. J. Dent. Res. 2011, 90, 483–488. [Google Scholar] [CrossRef]
- Yuan, X.; Cao, X.; Yang, S. IFT80 Is Required for Stem Cell Proliferation, Differentiation, and Odontoblast Polarization during Tooth Development. Cell Death Dis. 2019, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Hisamoto, M.; Goto, M.; Muto, M.; Nio-Kobayashi, J.; Iwanaga, T.; Yokoyama, A. Developmental Changes in Primary Cilia in the Mouse Tooth Germ and Oral Cavity. Biomed. Res. 2016, 37, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Feng, J.; Li, J.; Ho, T.V.; Yuan, Y.; Liu, Y.; Brindopke, F.; Figueiredo, J.C.; Magee, W.; Sanchez-Lara, P.A.; et al. Intraflagellar Transport 88 (IFT88) Is Crucial for Craniofacial Development in Mice and Is a Candidate Gene for Human Cleft Lip and Palate. Hum. Mol. Genet. 2017, 26, 860–872. [Google Scholar] [CrossRef]
- Kero, D.; Novakovic, J.; Vukojevic, K.; Petricevic, J.; Kalibovic Govorko, D.; Biocina-Lukenda, D.; Saraga-Babic, M. Expression of Ki-67, Oct-4, γ-Tubulin and α-Tubulin in Human Tooth Development. Arch. Oral Biol. 2014, 59, 1119–1129. [Google Scholar] [CrossRef]
- Dosedelová, H.; Dumková, J.; Lesot, H.; Glocová, K.; Kunová, M.; Tucker, A.S.; Veselá, I.; Krejčí, P.; Tichý, F.; Hampl, A.; et al. Fate of the Molar Dental Lamina in the Monophyodont Mouse. PLoS ONE 2015, 10, e0127543. [Google Scholar] [CrossRef] [Green Version]
- Lehman, J.M.; Laag, E.; Michaud, E.J.; Yoder, B.K. An Essential Role for Dermal Primary Cilia in Hair Follicle Morphogenesis. J. Investig. Dermatol. 2009, 129, 438–448. [Google Scholar] [CrossRef] [Green Version]
- Yuan, G.; Singh, G.; Chen, S.; Perez, K.C.; Wu, Y.; Liu, B.; Helms, J.A. Cleft Palate and Aglossia Result from Perturbations in Wnt and Hedgehog Signaling. Cleft Palate. Craniofac. J. 2017, 54, 269–280. [Google Scholar] [CrossRef]
- Kudo, T.; Kawasaki, M.; Kawasaki, K.; Meguro, F.; Nihara, J.; Honda, I.; Kitamura, M.; Fujita, A.; Osawa, K.; Ichikawa, K.; et al. Ift88 Regulates Enamel Formation via Involving Shh Signaling. Oral Dis. 2022, 00, 1–10. [Google Scholar] [CrossRef]
- Li, G.; Liu, M.; Zhang, S.; Wan, H.; Zhang, Q.; Yue, R.; Yan, X.; Wang, X.; Wang, Z.; Sun, Y. Essential Role of IFT140 in Promoting Dentinogenesis. J. Dent. Res. 2018, 97, 423–431. [Google Scholar] [CrossRef]
- Liu, B.; Chen, S.; Cheng, D.; Jing, W.; Helms, J.A. Primary Cilia Integrate Hedgehog and Wnt Signaling during Tooth Development. J. Dent. Res. 2014, 93, 475–482. [Google Scholar] [CrossRef]
- Thesleff, I. Epithelial-Mesenchymal Signalling Regulating Tooth Morphogenesis. J. Cell Sci. 2003, 116, 1647–1648. [Google Scholar] [CrossRef] [Green Version]
- Corbit, K.C.; Shyer, A.E.; Dowdle, W.E.; Gaulden, J.; Singla, V.; Reiter, J.F. Kif3a Constrains β-Catenin-Dependent Wnt Signalling through Dual Ciliary and Non-Ciliary Mechanisms. Nat. Cell Biol. 2008, 10, 70–76. [Google Scholar] [CrossRef]
- Kim, M.; Suh, Y.A.; Oh, J.H.; Lee, B.R.; Kim, J.; Jang, S.J. KIF3A Binds to β-Arrestin for Suppressing Wnt/β-Catenin Signalling Independently of Primary Cilia in Lung Cancer. Sci. Rep. 2016, 6, 32770. [Google Scholar] [CrossRef] [Green Version]
- Lehman, J.M.; Michaud, E.J.; Schoeb, T.R.; Aydin-Son, Y.; Miller, M.; Yoder, B.K. The Oak Ridge Polycystic Kidney Mouse: Modeling Ciliopathies of Mice and Men. Dev. Dyn. 2008, 237, 1960–1971. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, H.; Terajima, M.; Kitami, M.; Wang, J.; He, L.; Saeki, M.; Yamauchi, M.; Komatsu, Y. IFT20 is critical for collagen biosynthesis in craniofacial bone formation. Biochem. Biophys. Res. Commun. 2020, 533, 739–744. [Google Scholar] [CrossRef]
- Noda, K.; Kitami, M.; Kitami, K.; Kaku, M.; Komatsu, Y. Canonical and noncanonical intraflagellar transport regulates craniofacial skeletal development. Proc. Natl. Acad. Sci. USA 2016, 113, E2589–E2597. [Google Scholar] [CrossRef] [Green Version]
- Ferrante, M.I.; Zullo, A.; Adriano, B.; Bimonte, S.; Messaddeq, N.; Studer, M.; Dollé, P.; Franco, B. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat. Gen. 2006, 38, 112–117. [Google Scholar] [CrossRef]
- Bengueddach, H.; Lemullois, M.; Aubusson-Fleury, A.; Koll, F. Basal body positioning and anchoring in the multiciliated cell Paramecium tetraurelia: Roles of OFD1 and VFL3. Cilia 2017, 6, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Gigante, E.D.; Taylor, M.R.; Ivanova, A.A.; Kahn, R.A.; Caspary, T. ARL13B regulates Sonic hedgehog signaling from outside primary cilia. eLife 2020, 9, e50434. [Google Scholar] [CrossRef]
- Zhou, S.; Li, G.; Zhou, T.; Zhang, S.; Xue, H.; Geng, J.; Liu, W.; Sun, Y. The Role of IFT140 in Early Bone Healing of Tooth Extraction Sockets. Oral Dis. 2022, 28, 1188–1197. [Google Scholar] [CrossRef]
- Moore, E.R.; Michot, B.; Erdogan, O.; Ba, A.; Gibbs, J.L.; Yang, Y. CGRP and Shh Mediate the Dental Pulp Cell Response to Neuron Stimulation. J. Dent. Res. 2022, 101, 1119–1126. [Google Scholar] [CrossRef]
- Moore, E.R.; Mathews, O.A.; Yao, Y.; Yang, Y. Prx1-Expressing Cells Contributing to Fracture Repair Require Primary Cilia for Complete Healing in Mice. Bone 2020, 143, 115738. [Google Scholar] [CrossRef]
- Moore, E.R.; Chen, J.C.; Jacobs, C.R. Prx1-Expressing Progenitor Primary Cilia Mediate Bone Formation in Response to Mechanical Loading in Mice. Stem Cells Int. 2019, 2019, 3094154. [Google Scholar] [CrossRef]
- Lee, K.L.; Hoey, D.A.; Spasic, M.; Tang, T.; Hammond, H.K.; Jacobs, C.R. Adenylyl Cyclase 6 Mediates Loading-Induced Bone Adaptation in Vivo. FASEB J. 2014, 28, 1157–1165. [Google Scholar] [CrossRef] [Green Version]
- Temiyasathit, S.; Tang, W.J.; Leucht, P.; Anderson, C.T.; Monica, S.D.; Castillo, A.B.; Helms, J.A.; Stearns, T.; Jacobs, C.R. Mechanosensing by the Primary Cilium: Deletion of Kif3A Reduces Bone Formation Due to Loading. PLoS ONE 2012, 7, e33368. [Google Scholar] [CrossRef] [Green Version]
- Spasic, M.; Jacobs, C. Lengthening Primary Cilia Enhances Cellular Mechanosensitivity. Eur. Cells Mater. 2017, 33, 158–168. [Google Scholar] [CrossRef]
- Spasic, M.; Duffy, M.P.; Jacobs, C.R. Fenoldopam Sensitizes Primary Cilia-Mediated Mechanosensing to Promote Osteogenic Intercellular Signaling and Whole Bone Adaptation. J. Bone Miner. Res. 2022, 37, 972–982. [Google Scholar] [CrossRef]
- Pintado, P.; Seixas, C.; Barral, D.C.; Lopes, S.S. Arl13b Interferes with α-Tubulin Acetylation. Cilia 2015, 4, P73. [Google Scholar] [CrossRef] [Green Version]
- Moore, E.R.; Ryu, H.S.; Zhu, Y.X.; Jacobs, C.R. Adenylyl Cyclases and TRPV4 Mediate Ca2+/CAMP Dynamics to Enhance Fluid Flow-Induced Osteogenesis in Osteocytes. J. Mol. Biochem. 2018, 7, 48–59. [Google Scholar]
- Nauli, S.M.; Alenghat, F.J.; Luo, Y.; Williams, E.; Vassilev, P.; Li, X.; Elia, A.E.H.; Lu, W.; Brown, E.M.; Quinn, S.J.; et al. Polycystins 1 and 2 Mediate Mechanosensation in the Primary Cilium of Kidney Cells. Nat. Genet. 2003, 33, 129–137. [Google Scholar] [CrossRef]
- Nauli, S.M.; Kawanabe, Y.; Kaminski, J.J.; Pearce, W.J.; Ingber, D.E.; Zhou, J. Endothelial Cilia Are Fluid Shear Sensors That Regulate Calcium Signaling and Nitric Oxide Production Through Polycystin-1. Circulation 2008, 117, 1161–1171. [Google Scholar] [CrossRef]
- Nguyen, A.M.; Jacobs, C.R. Emerging Role of Primary Cilia as Mechanosensors in Osteocytes. Bone 2013, 54, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Moore, E.R.; Yang, Y.; Jacobs, C.R. Primary Cilia Are Necessary for Prx1-Expressing Cells to Contribute to Postnatal Skeletogenesis. J. Cell Sci. 2018, 131, jcs217828. [Google Scholar] [CrossRef] [Green Version]
- Moore, E.R.; Zhu, Y.X.; Ryu, H.S.; Jacobs, C.R. Periosteal Progenitors Contribute to Load-Induced Bone Formation in Adult Mice and Require Primary Cilia to Sense Mechanical Stimulation. Stem Cell Res. Ther. 2018, 9, 190. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, A.; Sugimoto, A.; Yoshizaki, K.; Kawarabayashi, K.; Iwata, K.; Kurogoushi, R.; Kitamura, T.; Otsuka, K.; Hasegawa, T.; Akazawa, Y.; et al. Coordination of WNT Signaling and Ciliogenesis during Odontogenesis by Piezo Type Mechanosensitive Ion Channel Component 1. Sci. Rep. 2019, 9, 14762. [Google Scholar] [CrossRef] [Green Version]
- Deren, M.; Yang, X.; Guan, Y.; Chen, Q. Biological and Chemical Removal of Primary Cilia Affects Mechanical Activation of Chondrogenesis Markers in Chondroprogenitors and Hypertrophic Chondrocytes. Int. J. Mol. Sci. 2016, 17, 188. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.L.; Guevarra, M.D.; Nguyen, A.M.; Chua, M.C.; Wang, Y.; Jacobs, C.R. The Primary Cilium Functions as a Mechanical and Calcium Signaling Nexus. Cilia 2015, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, S.F.; Owsianik, G.; Nilius, B. TRP Channels: An Overview. Cell Calcium 2005, 38, 233–252. [Google Scholar] [CrossRef]
- Davidson, R.M.; Guo, L. Calcium Channel Current in Rat Dental Pulp Cells. J. Membr. Biol. 2000, 178, 21–30. [Google Scholar] [CrossRef]
- Guo, J.; Otis, J.M.; Suciu, S.K.; Catalano, C.; Xing, L.; Constable, S.; Wachten, D.; Gupton, S.; Lee, J.; Lee, A.; et al. Primary Cilia Signaling Promotes Axonal Tract Development and Is Disrupted in Joubert Syndrome-Related Disorders Models. Dev. Cell 2019, 51, 759–774.e5. [Google Scholar] [CrossRef]
- Green, J.A.; Mykytyn, K. Neuronal Primary Cilia: An Underappreciated Signaling and Sensory Organelle in the Brain. Neuropsychopharmacology 2014, 39, 244–245. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, J.; Seidel, K.; Shi, S.; Klein, O.; Sharpe, P.; Chai, Y. Secretion of Shh by a Neurovascular Bundle Niche Supports Mesenchymal Stem Cell Homeostasis in the Adult Mouse Incisor. Cell Stem Cell 2014, 14, 160–173. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-F.; Serra, R. Ift88 Regulates Hedgehog Signaling, Sfrp5 Expression, and β-Catenin Activity in Post-Natal Growth Plate. J. Orthop. Res. 2013, 31, 350–356. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Haycraft, C.J.; Seo, H.; Yoder, B.K.; Serra, R. Development of the Post-Natal Growth Plate Requires Intraflagellar Transport Proteins. Dev. Biol. 2007, 305, 202–216. [Google Scholar] [CrossRef] [Green Version]
- Moore, E.R.; Jacobs, C.R. The Primary Cilium as a Signaling Nexus for Growth Plate Function and Subsequent Skeletal Development. J. Orthop. Res. 2017, 36, 533–545. [Google Scholar] [CrossRef]
- Jussila, M.; Thesleff, I. Signaling Networks Regulating Tooth Organogenesis and Regeneration, and the Specification of Dental Mesenchymal and Epithelial Cell Lineages. Cold Spring Harb. Perspect. Biol. 2012, 4, a008425. [Google Scholar] [CrossRef]


| Model | Mechanism of Disruption | Phenotype | Ref. |
|---|---|---|---|
| Wnt1Cre;Kif3afl/fl | IFT motor protein impaired in neural crest cells | cleft palate, cleft skull, bifid nasal septum, cranium occultum, agenesis of the corpus callosum, missing tongue, shorter and wider mandibles, facial widening that is exacerbated over time | [9,29] |
| Wnt1Cre;Ift88fl/fl | IFT-B complex impaired in neural crest cells | cleft lip and palate, tongue agenesis, wider facial midlines, indistinguishable palatine processes, smaller and misshapen mandibles, missing incisors, extra molar | [20,25] |
| Keratin14;Ift88fl/fl | IFT-B complex impaired in epithelial cells | chalky incisors, roughened enamel surfaces on molars, thinner enamel layers in all teeth | [30] |
| OsxCre;Ift80fl/fl | IFT-B complex impaired in odontoblasts, osteoblasts, and differentiating DPSCs | Delayed incisor eruption, incisor malocclusion, short molar roots, thin dentin layers, decreased bone mass in calvaria and alveolar bone | [23] |
| OsxCre;Ift140fl/fl | IFT-A complex impaired in odontoblasts, osteoblasts, and differentiating DPSCs | Short molar roots, thin dentin layers, reduced mineralization rate and dentin matrix protein production | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, E.R. Primary Cilia: The New Face of Craniofacial Research. Biomolecules 2022, 12, 1724. https://doi.org/10.3390/biom12121724
Moore ER. Primary Cilia: The New Face of Craniofacial Research. Biomolecules. 2022; 12(12):1724. https://doi.org/10.3390/biom12121724
Chicago/Turabian StyleMoore, Emily R. 2022. "Primary Cilia: The New Face of Craniofacial Research" Biomolecules 12, no. 12: 1724. https://doi.org/10.3390/biom12121724
APA StyleMoore, E. R. (2022). Primary Cilia: The New Face of Craniofacial Research. Biomolecules, 12(12), 1724. https://doi.org/10.3390/biom12121724







