Insect Models in Nutrition Research
Abstract
1. Introduction
2. Model Insects for Nutrition Research
2.1. Drosophila Melanogaster (Diptera: Drosophilidae)
2.2. Tribolium Castaneum (Coleoptera: Tenebrionidae)
2.3. Galleria Mellonella (Lepidoptera: Pyralidae)
2.4. Other Insects
3. Comparison of the Vertebrate and Invertebrate Gut Microbiome
4. Insects in Food Toxicological Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AKH | adipokinetic hormone |
| AMP | antimicrobial peptide |
| BS | brain stem |
| CAT | catalase |
| CR | caloric restriction |
| DMD | pheromone 4,8-dimethyl decanal |
| DR | dietary restriction |
| H | hypothalamus |
| HFD | high fat diet |
| HSD | high sugar diet |
| P:C | protein:carbohydrate |
| PCC | propionyl-CoA carboxylase |
| PD | Parkinson’s disease |
| PFJ | palm fruit juice |
| PG | pituitary gland |
| PI | pars intercerebralis |
| PL | pars lateralis |
| PSPA | purple sweet potato anthocyanin |
| RG | ring gland |
| RNAi | RNA interference |
| Rpn11 | ubiquitin carboxyl-terminal hydrolase |
| SC | spinal cord |
| SOD | superoxide dismutase |
| SZ | subesophageal zone |
| TAG | triacylglycerol |
| T2DM | type 2 diabetes mellitus |
| VNC | ventral nerve cord |
References
- Sandner, G.; König, A.; Wallner, M.; Weghuber, J. Alternative Model Organisms for Toxicological Fingerprinting of Relevant Parameters in Food and Nutrition. Crit. Rev. Food Sci. Nutr. 2022, 62, 5965–5982. [Google Scholar] [CrossRef] [PubMed]
- Levy, N. The Use of Animal as Models: Ethical Considerations. Int. J. Stroke 2012, 7, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Mikulak, E.; Gliniewicz, A.; Przygodzka, M.; Solecka, J. Galleria mellonella L. as Model Organism Used in Biomedical and Other Studies. Prz. Epidemiol. 2018, 72, 57–73. [Google Scholar]
- Jans, K.; Lüersen, K.; Rimbach, G. Drosophila melanogaster as a Model Organism to Study Lithium and Boron Bioactivity. Int. J. Mol. Sci. 2021, 22, 11710. [Google Scholar] [CrossRef] [PubMed]
- Renwick, J.; Kavanagh, K. Insects as models for studying the virulence of fungal pathogens of humans. In New Insights in Medical Mycology; Springer: Dordrecht, The Netherlands, 2007; pp. 45–67. [Google Scholar]
- Abdelli, N.; Peng, L.; Keping, C. Silkworm, Bombyx Mori, as an Alternative Model Organism in Toxicological Research. Environ. Sci. Pollut. Res. Int. 2018, 25, 35048–35054. [Google Scholar] [CrossRef]
- Meng, X.; Zhu, F.; Chen, K. Silkworm: A Promising Model Organism in Life Science. J. Insect Sci. 2017, 17, 97. [Google Scholar] [CrossRef]
- Rösner, J.; Wellmeyer, B.; Merzendorfer, H. Tribolium Castaneum: A Model for Investigating the Mode of Action of Insecticides and Mechanisms of Resistance. Curr. Pharm. Des. 2020, 26, 3554–3568. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yoshida, H. Drosophila as a Model Organism. Adv. Exp. Med. Biol. 2018, 1076, 1–10. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Teixeira, F.; Silva, S.; Madureira, A.R.; Pintado, M.E. Potential Prebiotic Activity of Tenebrio Molitor Insect Flour Using an Optimized in Vitro Gut Microbiota Model. Food Funct. 2019, 10, 3909–3922. [Google Scholar] [CrossRef]
- Gershman, A.; Romer, T.G.; Fan, Y.; Razaghi, R.; Smith, W.A.; Timp, W. De Novo Genome Assembly of the Tobacco Hornworm Moth (Manduca sexta). G3 Genes|Genomes|Genet 2021, 11, jkaa047. [Google Scholar] [CrossRef]
- Jo, Y.H.; Lee, J.H.; Patnaik, B.B.; Keshavarz, M.; Lee, Y.S.; Han, Y.S. Autophagy in Tenebrio Molitor Immunity: Conserved Antimicrobial Functions in Insect Defenses. Front. Immunol. 2021, 12, 667664. [Google Scholar] [CrossRef] [PubMed]
- Lozoya-Pérez, N.E.; García-Carnero, L.C.; Martínez-Álvarez, J.A.; Martínez-Duncker, I.; Mora-Montes, H.M. Tenebrio Molitor as an Alternative Model to Analyze the Sporothrix Species Virulence. Infect. Drug Resist. 2021, 14, 2059–2072. [Google Scholar] [CrossRef] [PubMed]
- Lyons, N.; Softley, I.; Balfour, A.; Williamson, C.; O’Brien, H.E.; Shetty, A.C.; Bruno, V.M.; Diezmann, S. Tobacco Hornworm (Manduca sexta) Caterpillars as a Novel Host Model for the Study of Fungal Virulence and Drug Efficacy. Virulence 2020, 11, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Cao, X.; Jiang, H. Digestion-Related Proteins in the Tobacco Hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 2020, 126, 103457. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.Y.; Zhang, L.S.; Han, Y.H.; An, T.; Liu, Y.; Li, Y.Y.; Chen, H.Y. Proteomic Research on Diapause-Related Proteins in the Female Ladybird, Coccinella septempunctata L. Bull. Entomol. Res. 2016, 106, 168–174. [Google Scholar] [CrossRef]
- Rubio-Aliaga, I. Model Organisms in Molecular Nutrition Research. Mol. Nutr. Food Res. 2012, 56, 844–853. [Google Scholar] [CrossRef]
- Piper, M.D.W.; Blanc, E.; Leitão-Gonçalves, R.; Yang, M.; He, X.; Linford, N.J.; Hoddinott, M.P.; Hopfen, C.; Soultoukis, G.A.; Niemeyer, C.; et al. A Holidic Medium for Drosophila melanogaster. Nat. Methods 2014, 11, 100–105. [Google Scholar] [CrossRef]
- Gimeno-Mallench, L.; Sanchez-Morate, E.; Parejo-Pedrajas, S.; Mas-Bargues, C.; Inglés, M.; Sanz-Ros, J.; Román-Domínguez, A.; Olaso, G.; Stromsnes, K.; Gambini, J. The Relationship between Diet and Frailty in Aging. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1373–1382. [Google Scholar] [CrossRef]
- Staats, S.; Lüersen, K.; Wagner, A.E.; Rimbach, G. Drosophila melanogaster as a Versatile Model Organism in Food and Nutrition Research. J. Agric. Food Chem. 2018, 66, 3737–3753. [Google Scholar] [CrossRef]
- Brandt, A.; Vilcinskas, A. The Fruit Fly Drosophila melanogaster as a Model for Aging Research. Adv. Biochem. Eng. Biotechnol. 2013, 135, 63–77. [Google Scholar] [CrossRef]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The Genome Sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.M.; Yandell, M.D.; Wortman, J.R.; Gabor Miklos, G.L.; Nelson, C.R.; Hariharan, I.K.; Fortini, M.E.; Li, P.W.; Apweiler, R.; Fleischmann, W.; et al. Comparative Genomics of the Eukaryotes. Science 2000, 287, 2204–2215. [Google Scholar] [CrossRef] [PubMed]
- Parks, A.L.; Cook, K.R.; Belvin, M.; Dompe, N.A.; Fawcett, R.; Huppert, K.; Tan, L.R.; Winter, C.G.; Bogart, K.P.; Deal, J.E.; et al. Systematic Generation of High-Resolution Deletion Coverage of the Drosophila melanogaster Genome. Nat. Genet. 2004, 36, 288–292. [Google Scholar] [CrossRef] [PubMed]
- De Nobrega, A.K.; Lyons, L.C. Aging and the Clock: Perspective from Flies to Humans. Eur. J. Neurosci. 2020, 51, 454–481. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.W.; Thomas, J.; Liu, J.; Li, T.; Moran, T.H. From Fat Fruitfly to Human Obesity. Physiol. Behav. 2014, 136, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Gáliková, M.; Klepsatel, P. Obesity and Aging in the Drosophila Model. Int. J. Mol. Sci. 2018, 19, 1896. [Google Scholar] [CrossRef]
- Poetini, M.R.; Araujo, S.M.; Trindade de Paula, M.; Bortolotto, V.C.; Meichtry, L.B.; Polet de Almeida, F.; Jesse, C.R.; Kunz, S.N.; Prigol, M. Hesperidin Attenuates Iron-Induced Oxidative Damage and Dopamine Depletion in Drosophila melanogaster Model of Parkinson’s Disease. Chem.-Biol. Interact. 2018, 279, 177–186. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Thirumurugan, K. Longevity-Promoting Efficacies of Rutin in High Fat Diet Fed Drosophila melanogaster. Biogerontology 2020, 21, 653–668. [Google Scholar] [CrossRef]
- Savola, E.; Montgomery, C.; Waldron, F.M.; Monteith, K.M.; Vale, P.; Walling, C. Testing Evolutionary Explanations for the Lifespan Benefit of Dietary Restriction in Fruit Flies (Drosophila melanogaster). Evolution 2021, 75, 450–463. [Google Scholar] [CrossRef]
- Kharat, P.; Sarkar, P.; Mouliganesh, S.; Tiwary, V.; Priya, V.B.R.; Sree, N.Y.; Annapoorna, H.V.; Saikia, D.K.; Mahanta, K.; Thirumurugan, K. Ellagic Acid Prolongs the Lifespan of Drosophila melanogaster. GeroScience 2020, 42, 271–285. [Google Scholar] [CrossRef]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health Benefits of Resveratrol Administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekara, K.T.; Shakarad, M.N. Aloe Vera or Resveratrol Supplementation in Larval Diet Delays Adult Aging in the Fruit Fly, Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Staats, S.; Wagner, A.E.; Kowalewski, B.; Rieck, F.T.; Soukup, S.T.; Kulling, S.E.; Rimbach, G. Dietary Resveratrol Does Not Affect Life Span, Body Composition, Stress Response, and Longevity-Related Gene Expression in Drosophila melanogaster. Int. J. Mol. Sci. 2018, 19, 223. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Jiang, C.; Liu, L.; Ordovas, J.M.; Lai, C.-Q.; Shen, L. Curcumin Supplementation Increases Survival and Lifespan in Drosophila under Heat Stress Conditions. BioFactors 2018, 44, 577–587. [Google Scholar] [CrossRef]
- Pitsouli, C.; Perrimon, N. Developmental Biology: Our Fly Cousins’ Gut. Nature 2008, 454, 592–593. [Google Scholar] [CrossRef]
- Bader, R.; Colomb, J.; Pankratz, B.; Schröck, A.; Stocker, R.F.; Pankratz, M.J. Genetic Dissection of Neural Circuit Anatomy Underlying Feeding Behavior in Drosophila: Distinct Classes of Hugin-Expressing Neurons. J. Comp. Neurol. 2007, 502, 848–856. [Google Scholar] [CrossRef]
- Canavoso, L.E.; Jouni, Z.E.; Karnas, K.J.; Pennington, J.E.; Wells, M.A. Fat Metabolism in Insects. Annu. Rev. Nutr. 2001, 21, 23–46. [Google Scholar] [CrossRef]
- Gutierrez, E.; Wiggins, D.; Fielding, B.; Gould, A.P. Specialized Hepatocyte-like Cells Regulate Drosophila Lipid Metabolism. Nature 2007, 445, 275–280. [Google Scholar] [CrossRef]
- Lemaitre, B.; Miguel-Aliaga, I. The Digestive Tract of Drosophila melanogaster. Annu. Rev. Genet. 2013, 47, 377–404. [Google Scholar] [CrossRef]
- Miguel-Aliaga, I.; Jasper, H.; Lemaitre, B. Anatomy and Physiology of the Digestive Tract of Drosophila melanogaster. Genetics 2018, 210, 357–396. [Google Scholar] [CrossRef] [PubMed]
- Musselman, L.P.; Kühnlein, R.P. Drosophila as a Model to Study Obesity and Metabolic Disease. J. Exp. Biol. 2018, 221 (Suppl. S1), jeb163881. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Ansah, E.; Perrimon, N. Modeling Metabolic Homeostasis and Nutrient Sensing in Drosophila: Implications for Aging and Metabolic Diseases. Dis. Models Mech. 2014, 7, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Doane, W.W. Developmental Physiology of the Mutant Female Sterile(2)Adipose of Drosophila melanogaster. III. Corpus Allatum-Complex and Ovarian Transplantations. J. Exp. Zool. 1961, 146, 275–298. [Google Scholar] [CrossRef] [PubMed]
- Doane, W.W. Developmental Physiology of the Mutant Female Sterile(2)Adipose of Drosophila melanogaster. I. Adult Morphology, Longevity, Egg Production, and Egg Lethality. J. Exp. Zool. 1960, 145, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Häder, T.; Müller, S.; Aguilera, M.; Eulenberg, K.G.; Steuernagel, A.; Ciossek, T.; Kühnlein, R.P.; Lemaire, L.; Fritsch, R.; Dohrmann, C.; et al. Control of Triglyceride Storage by a WD40/TPR-Domain Protein. EMBO Rep. 2003, 4, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-Q.; Parnell, L.D.; Arnett, D.K.; García-Bailo, B.; Tsai, M.Y.; Kabagambe, E.K.; Straka, R.J.; Province, M.A.; An, P.; Borecki, I.B.; et al. WDTC1, the Ortholog of Drosophila Adipose Gene, Associates With Human Obesity, Modulated by MUFA Intake. Obesity 2009, 17, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.M.; Zeve, D.; McKay, R.; Seo, J.; Salo, Z.; Li, R.; Wang, M.; Graff, J.M. Adipose Is a Conserved Dosage-Sensitive Antiobesity Gene. Cell Metab. 2007, 6, 195–207. [Google Scholar] [CrossRef]
- Liao, S.; Amcoff, M.; Nässel, D.R. Impact of High-Fat Diet on Lifespan, Metabolism, Fecundity and Behavioral Senescence in Drosophila. Insect Biochem. Mol. Biol. 2021, 133, 103495. [Google Scholar] [CrossRef]
- Baenas, N.; Wagner, A.E. Drosophila melanogaster as a Model Organism for Obesity and Type-2 Diabetes Mellitus by Applying High-Sugar and High-Fat Diets. Biomolecules 2022, 12, 307. [Google Scholar] [CrossRef]
- Al-Anzi, B.; Sapin, V.; Waters, C.; Zinn, K.; Wyman, R.J.; Benzer, S. Obesity-Blocking Neurons in Drosophila. Neuron 2009, 64, 290–291. [Google Scholar] [CrossRef]
- Carvalho, M.; Sampaio, J.L.; Palm, W.; Brankatschk, M.; Eaton, S.; Shevchenko, A. Effects of Diet and Development on the Drosophila Lipidome. Mol. Syst. Biol. 2012, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, R.; Varacallo, M. Biochemistry, Glycolysis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mastrototaro, L.; Roden, M. Insulin Resistance and Insulin Sensitizing Agents. Metabolism 2021, 125, 154892. [Google Scholar] [CrossRef] [PubMed]
- Palanker Musselman, L.; Fink, J.L.; Narzinski, K.; Ramachandran, P.V.; Sukumar Hathiramani, S.; Cagan, R.L.; Baranski, T.J. A High-Sugar Diet Produces Obesity and Insulin Resistance in Wild-Type Drosophila. Dis. Models Mech. 2011, 4, 842–849. [Google Scholar] [CrossRef]
- Birse, R.T.; Choi, J.; Reardon, K.; Rodriguez, J.; Graham, S.; Diop, S.; Ocorr, K.; Bodmer, R.; Oldham, S. High-Fat-Diet-Induced Obesity and Heart Dysfunction Are Regulated by the TOR Pathway in Drosophila. Cell Metab. 2010, 12, 533–544. [Google Scholar] [CrossRef]
- Oldham, S. Obesity and Nutrient Sensing TOR Pathway in Flies and Vertebrates: Functional Conservation of Genetic Mechanisms. Trends Endocrinol. Metab. 2011, 22, 45–52. [Google Scholar] [CrossRef]
- Eickelberg, V.; Lüersen, K.; Staats, S.; Rimbach, G. Phenotyping of Drosophila melanogaster-A Nutritional Perspective. Biomolecules 2022, 12, 221. [Google Scholar] [CrossRef]
- Tatar, M. Diet Restriction in Drosophila melanogaster. Design and Analysis. Interdiscip. Top Gerontol. 2007, 35, 115–136. [Google Scholar] [CrossRef]
- Burger, J.M.S.; Buechel, S.D.; Kawecki, T.J. Dietary Restriction Affects Lifespan but Not Cognitive Aging in Drosophila melanogaster. Aging Cell 2010, 9, 327–335. [Google Scholar] [CrossRef]
- Pletcher, S.D.; Libert, S.; Skorupa, D. Flies and Their Golden Apples: The Effect of Dietary Restriction on Drosophila Aging and Age-Dependent Gene Expression. Ageing Res. Rev. 2005, 4, 451–480. [Google Scholar] [CrossRef]
- Budzynska, B.; Faggio, C.; Kruk-Slomka, M.; Samec, D.; Nabavi, S.F.; Sureda, A.; Devi, K.P.; Nabavi, S.M. Rutin as Neuroprotective Agent: From Bench to Bedside. Curr. Med. Chem. 2019, 26, 5152–5164. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.S. A Review on Plant-Based Rutin Extraction Methods and Its Pharmacological Activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Motallebi, M.; Khorsandi, K.; Sepahy, A.A.; Chamani, E.; Hosseinzadeh, R. Effect of Rutin as Flavonoid Compound on Photodynamic Inactivation against P. Aeruginosa and S. Aureus. Photodiagn. Photodyn. Ther. 2020, 32, 102074. [Google Scholar] [CrossRef]
- Negahdari, R.; Bohlouli, S.; Sharifi, S.; Maleki Dizaj, S.; Rahbar Saadat, Y.; Khezri, K.; Jafari, S.; Ahmadian, E.; Gorbani Jahandizi, N.; Raeesi, S. Therapeutic Benefits of Rutin and Its Nanoformulations. Phytother. Res. 2021, 35, 1719–1738. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Yoon, Y.; Yoon, H.; Park, H.-M.; Song, S.; Yeum, K.-J. Dietary Anthocyanins against Obesity and Inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.M.; Lei, L.; Liu, Y.; Wang, X.; Ma, K.Y.; Zhang, C.; Zhu, H.; Zhao, Y.; Chen, Z.-Y. Purple Sweet Potato Anthocyanin Attenuates Fat-Induced Mortality in Drosophila melanogaster. Exp. Gerontol. 2016, 82, 95–103. [Google Scholar] [CrossRef]
- Bass, T.M.; Weinkove, D.; Houthoofd, K.; Gems, D.; Partridge, L. Effects of Resveratrol on Lifespan in Drosophila melanogaster and Caenorhabditis Elegans. Mech. Ageing Dev. 2007, 128, 546–552. [Google Scholar] [CrossRef]
- Leow, S.-S.; Luu, A.; Shrestha, S.; Hayes, K.C.; Sambanthamurthi, R. Drosophila Larvae Fed Palm Fruit Juice (PFJ) Delay Pupation via Expression Regulation of Hormetic Stress Response Genes Linked to Ageing and Longevity. Exp. Gerontol. 2018, 106, 198–221. [Google Scholar] [CrossRef]
- Wang, C.; Wheeler, C.T.; Alberico, T.; Sun, X.; Seeberger, J.; Laslo, M.; Spangler, E.; Kern, B.; de Cabo, R.; Zou, S. The Effect of Resveratrol on Lifespan Depends on Both Gender and Dietary Nutrient Composition in Drosophila melanogaster. Age 2013, 35, 69–81. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Draayer, K.; Kalman, D.S. Palm Fruit Bioactive Complex (PFBc), a Source of Polyphenols, Demonstrates Potential Benefits for Inflammaging and Related Cognitive Function. Nutrients 2021, 13, 1127. [Google Scholar] [CrossRef]
- Syarifah-Noratiqah, S.-B.; Zulfarina, M.S.; Ahmad, S.U.; Fairus, S.; Naina-Mohamed, I. The Pharmacological Potential of Oil Palm Phenolics (OPP) Individual Components. Int. J. Med. Sci. 2019, 16, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Bolsinger, J.; Pronczuk, A.; Sambanthamurthi, R.; Hayes, K.C. Anti-Diabetic Effects of Palm Fruit Juice in the Nile Rat (Arvicanthis niloticus). J. Nutr. Sci. 2014, 3, e5. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.; Sen, S.; Chatterjee, R.; Roy, D.; James, J.; Thirumurugan, K. Context- and Dose-Dependent Modulatory Effects of Naringenin on Survival and Development of Drosophila melanogaster. Biogerontology 2016, 17, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aquino, E.; Muriel, P. Beneficial Effects of Naringenin in Liver Diseases: Molecular Mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef]
- Wang, Q.; Ou, Y.; Hu, G.; Wen, C.; Yue, S.; Chen, C.; Xu, L.; Xie, J.; Dai, H.; Xiao, H.; et al. Naringenin Attenuates Non-Alcoholic Fatty Liver Disease by down-Regulating the NLRP3/NF-ΚB Pathway in Mice. Br. J. Pharmacol. 2020, 177, 1806–1821. [Google Scholar] [CrossRef]
- Lushchak, O.V.; Gospodaryov, D.V.; Rovenko, B.M.; Yurkevych, I.S.; Perkhulyn, N.V.; Lushchak, V.I. Specific Dietary Carbohydrates Differentially Influence the Life Span and Fecundity of Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 3–12. [Google Scholar] [CrossRef]
- Lushchak, O.V.; Gospodaryov, D.V.; Rovenko, B.M.; Glovyak, A.D.; Yurkevych, I.S.; Klyuba, V.P.; Shcherbij, M.V.; Lushchak, V.I. Balance between Macronutrients Affects Life Span and Functional Senescence in Fruit Fly Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 118–125. [Google Scholar] [CrossRef]
- LeMone, P. Vitamins and Minerals. J. Obstet. Gynecol. Neonatal Nurs. 1999, 28, 520–533. [Google Scholar] [CrossRef]
- Capozzi, A.; Scambia, G.; Lello, S. Calcium, Vitamin D, Vitamin K2, and Magnesium Supplementation and Skeletal Health. Maturitas 2020, 140, 55–63. [Google Scholar] [CrossRef]
- Granger, M.; Eck, P. Dietary Vitamin C in Human Health. Adv. Food Nutr. Res. 2018, 83, 281–310. [Google Scholar] [CrossRef]
- Smith, A.D.; Warren, M.J.; Refsum, H. Vitamin B12. Adv. Food Nutr. Res. 2018, 83, 215–279. [Google Scholar] [CrossRef] [PubMed]
- Contestabile, R.; di Salvo, M.L.; Bunik, V.; Tramonti, A.; Vernì, F. The Multifaceted Role of Vitamin B6 in Cancer: Drosophila as a Model System to Investigate DNA Damage. Open Biol. 2020, 10, 200034. [Google Scholar] [CrossRef] [PubMed]
- Ernst, I.M.A.; Pallauf, K.; Bendall, J.K.; Paulsen, L.; Nikolai, S.; Huebbe, P.; Roeder, T.; Rimbach, G. Vitamin E Supplementation and Lifespan in Model Organisms. Ageing Res. Rev. 2013, 12, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Gnocchini, E.; Pilesi, E.; Schiano, L.; Vernì, F. Vitamin B6 Deficiency Promotes Loss of Heterozygosity (LOH) at the Drosophila&Nbsp;Warts (Wts) Locus. Int. J. Mol. Sci. 2022, 23, 6087. [Google Scholar] [CrossRef] [PubMed]
- Merigliano, C.; Mascolo, E.; La Torre, M.; Saggio, I.; Vernì, F. Protective Role of Vitamin B6 (PLP) against DNA Damage in Drosophila Models of Type 2 Diabetes. Sci. Rep. 2018, 8, 11432. [Google Scholar] [CrossRef]
- Nan, Y.; Lin, J.; Cui, Y.; Yao, J.; Yang, Y.; Li, Q. Protective Role of Vitamin B6 against Mitochondria Damage in Drosophila Models of SCA3. Neurochem. Int. 2021, 144, 104979. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Sambrook, J. Biotin. Cold Spring Harb. Protoc. 2021, 10, 373–375. [Google Scholar] [CrossRef]
- Landenberger, A.; Kabil, H.; Harshman, L.G.; Zempleni, J. Biotin Deficiency Decreases Life Span and Fertility but Increases Stress Resistance in Drosophila melanogaster. J. Nutr. Biochem. 2004, 15, 591–600. [Google Scholar] [CrossRef]
- León-Del-Río, A. Biotin in Metabolism, Gene Expression, and Human Disease. J. Inherit. Metab. Dis. 2019, 42, 647–654. [Google Scholar] [CrossRef]
- Mock, D.M. Biotin: From Nutrition to Therapeutics. J. Nutr. 2017, 147, 1487–1492. [Google Scholar] [CrossRef]
- Wongkittichote, P.; Mew, N.A.; Chapman, K.A. Propionyl-CoA Carboxylase—A Review. Mol. Genet. Metab. 2017, 122, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Mathers, J.C. Nutrigenomics in the Modern Era. Proc. Nutr. Soc. 2017, 76, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Peña-Romero, A.C.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. The Future of Nutrition: Nutrigenomics and Nutrigenetics in Obesity and Cardiovascular Diseases. Crit. Rev. Food. Sci. Nutr. 2018, 58, 3030–3041. [Google Scholar] [CrossRef]
- Ye, J.; Cui, X.; Loraine, A.; Bynum, K.; Kim, N.C.; White, G.; De Luca, M.; Garfinkel, M.D.; Lu, X.; Ruden, D.M. Methods for Nutrigenomics and Longevity Studies in Drosophila: Effects of Diets High in Sucrose, Palmitic Acid, Soy, or Beef. Methods Mol. Biol. 2007, 371, 111–141. [Google Scholar] [CrossRef] [PubMed]
- Ming, Q.-L.; Cheng, C. Influence of Nutrition on Male Development and Reproduction in Tribolium Castaneum. J. Econ. Entomol. 2012, 105, 1471–1476. [Google Scholar] [CrossRef]
- Grünwald, S.; Adam, I.V.; Gurmai, A.-M.; Bauer, L.; Boll, M.; Wenzel, U. The Red Flour Beetle Tribolium Castaneum as a Model to Monitor Food Safety and Functionality. Adv. Biochem. Eng. Biotechnol. 2013, 135, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Schröder, R.; Beermann, A.; Wittkopp, N.; Lutz, R. From Development to Biodiversity--Tribolium Castaneum, an Insect Model Organism for Short Germband Development. Dev. Genes Evol. 2008, 218, 119–126. [Google Scholar] [CrossRef]
- Tribolium Genome Sequencing Consortium; Richards, S.; Gibbs, R.A.; Weinstock, G.M.; Brown, S.J.; Denell, R.; Beeman, R.W.; Gibbs, R.; Beeman, R.W.; Brown, S.J.; et al. The Genome of the Model Beetle and Pest Tribolium Castaneum. Nature 2008, 452, 949–955. [Google Scholar] [CrossRef]
- Grünwald, S.; Stellzig, J.; Adam, I.V.; Weber, K.; Binger, S.; Boll, M.; Knorr, E.; Twyman, R.M.; Vilcinskas, A.; Wenzel, U. Longevity in the Red Flour Beetle Tribolium Castaneum Is Enhanced by Broccoli and Depends on Nrf-2, Jnk-1 and Foxo-1 Homologous Genes. Genes Nutr. 2013, 8, 439–448. [Google Scholar] [CrossRef]
- Cheng, S.-Q.; Xia, Y.-Y.; He, J.-L.; Liu, X.-Q.; Chen, X.-M.; Ding, Y.-B.; Wang, Y.-X.; Peng, B.; Tu, B.-J. Neurotoxic Effect of Subacute Benzo(a)Pyrene Exposure on Gene and Protein Expression in Sprague-Dawley Rats. Environ. Toxicol. Pharmacol. 2013, 36, 648–658. [Google Scholar] [CrossRef]
- Ming, Q.-L.; Lewis, S.M. Pheromone Production by Male Tribolium Castaneum (Coleoptera: Tenebrionidae) Is Influenced by Diet Quality. J. Econ. Entomol. 2010, 103, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, Prebiotics and Synbiotics: Safe Options for next-Generation Therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Grau, T.; Vilcinskas, A.; Joop, G. Probiotic Enterococcus Mundtii Isolate Protects the Model Insect Tribolium Castaneum against Bacillus Thuringiensis. Front. Microbiol. 2017, 8, 1261. [Google Scholar] [CrossRef]
- Piras, C.; Roncada, P.; Rodrigues, P.M.; Bonizzi, L.; Soggiu, A. Proteomics in Food: Quality, Safety, Microbes, and Allergens. Proteomics 2016, 16, 799–815. [Google Scholar] [CrossRef]
- Jorjão, A.L.; Oliveira, L.D.; Scorzoni, L.; Figueiredo-Godoi, L.M.A.; Prata, M.C.A.; Jorge, A.O.C.; Junqueira, J.C. From Moths to Caterpillars: Ideal Conditions for Galleria mellonella Rearing for in Vivo Microbiological Studies. Virulence 2018, 9, 383–389. [Google Scholar] [CrossRef]
- Kwadha, C.A.; Ong’amo, G.O.; Ndegwa, P.N.; Raina, S.K.; Fombong, A.T. The Biology and Control of the Greater Wax Moth, Galleria mellonella. Insects 2017, 8, 61. [Google Scholar] [CrossRef]
- Firacative, C.; Khan, A.; Duan, S.; Ferreira-Paim, K.; Leemon, D.; Meyer, W. Rearing and Maintenance of Galleria mellonella and Its Application to Study Fungal Virulence. J. Fungi 2020, 6, 130. [Google Scholar] [CrossRef]
- Cé, R.; Silva, R.C.; Trentin, D.S.; Marchi, J.G.B.D.; Paese, K.; Guterres, S.S.; Macedo, A.J.; Pohlmann, A.R. Galleria mellonella Larvae as an In Vivo Model to Evaluate the Toxicity of Polymeric Nanocapsules. J. Nanosci. Nanotechnol. 2020, 20, 1486–1494. [Google Scholar] [CrossRef]
- Junqueira, J.C.; Mylonakis, E.; Borghi, E. Galleria mellonella Experimental Model: Advances and Future Directions. Pathog. Dis. 2021, 79, ftab021. [Google Scholar] [CrossRef]
- Ménard, G.; Rouillon, A.; Cattoir, V.; Donnio, P.-Y. Galleria mellonella as a Suitable Model of Bacterial Infection: Past, Present and Future. Front. Cell. Infect. Microbiol. 2021, 11, 782733. [Google Scholar] [CrossRef] [PubMed]
- Singkum, P.; Suwanmanee, S.; Pumeesat, P.; Luplertlop, N. A Powerful in Vivo Alternative Model in Scientific Research: Galleria mellonella. Acta Microbiol. Immunol. Hung. 2019, 66, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.Q.; Casadevall, A. Fungal Immunity and Pathogenesis in Mammals versus the Invertebrate Model Organism Galleria mellonella. Pathog. Dis. 2021, 79, ftab013. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Duma, L.; Rossez, Y. Galleria mellonella as a Good Model to Study Acinetobacter Baumannii Pathogenesis. Pathogens 2021, 10, 1483. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.; Beier, S.; Huson, D.H.; Parusel, R.; Iglauer, F.; Frick, J.-S. Genome Sequence of Galleria mellonella (Greater Wax Moth). Genome Announc. 2018, 6, e01220-17. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Palika, R.; Ismail, A.; Pullakhandam, R.; Reddy, G.B. Nutrigenomics: Opportunities & Challenges for Public Health Nutrition. Indian J. Med. Res. 2018, 148, 632–641. [Google Scholar] [CrossRef]
- Kangassalo, K.; Valtonen, T.M.; Sorvari, J.; Kecko, S.; Pölkki, M.; Krams, I.; Krama, T.; Rantala, M.J. Independent and Interactive Effects of Immune Activation and Larval Diet on Adult Immune Function, Growth and Development in the Greater Wax Moth (Galleria mellonella). J. Evol. Biol. 2018, 31, 1485–1497. [Google Scholar] [CrossRef]
- Krams, I.; Kecko, S.; Kangassalo, K.; Moore, F.R.; Jankevics, E.; Inashkina, I.; Krama, T.; Lietuvietis, V.; Meija, L.; Rantala, M.J. Effects of Food Quality on Trade-Offs among Growth, Immunity and Survival in the Greater Wax Moth Galleria mellonella. Insect Sci. 2015, 22, 431–439. [Google Scholar] [CrossRef]
- Banville, N.; Browne, N.; Kavanagh, K. Effect of Nutrient Deprivation on the Susceptibility of Galleria mellonella Larvae to Infection. Virulence 2012, 3, 497–503. [Google Scholar] [CrossRef]
- Dimofski, P.; Meyre, D.; Dreumont, N.; Leininger-Muller, B. Consequences of Paternal Nutrition on Offspring Health and Disease. Nutrients 2021, 13, 2818. [Google Scholar] [CrossRef]
- Rossoni, R.D.; Fuchs, B.B.; de Barros, P.P.; Velloso, M.D.S.; Jorge, A.O.C.; Junqueira, J.C.; Mylonakis, E. Lactobacillus Paracasei Modulates the Immune System of Galleria mellonella and Protects against Candida Albicans Infection. PLoS ONE 2017, 12, e0173332. [Google Scholar] [CrossRef] [PubMed]
- Scalfaro, C.; Iacobino, A.; Nardis, C.; Franciosa, G. Galleria mellonella as an in vivo model for assessing the protective activity of probiotics against gastrointestinal bacterial pathogens. FEMS Microbiol. Lett. 2017, 364, fnx064. [Google Scholar] [CrossRef]
- Wollowski, I.; Rechkemmer, G.; Pool-Zobel, B.L. Protective Role of Probiotics and Prebiotics in Colon Cancer. Am. J. Clin. Nutr. 2001, 73 (Suppl. S2), 451S–455S. [Google Scholar] [CrossRef]
- Grounta, A.; Harizanis, P.; Mylonakis, E.; Nychas, G.-J.E.; Panagou, E.Z. Investigating the Effect of Different Treatments with Lactic Acid Bacteria on the Fate of Listeria Monocytogenes and Staphylococcus Aureus Infection in Galleria mellonella Larvae. PLoS ONE 2016, 11, e0161263. [Google Scholar] [CrossRef]
- Jorjão, A.L.; de Oliveira, F.E.; Leão, M.V.P.; Jorge, A.O.C.; de Oliveira, L.D. Effect of Lactobacillus Rhamnosus on the Response of Galleria mellonella against Staphylococcus Aureus and Escherichia Coli Infections. Arch. Microbiol. 2018, 200, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.C.; de Barros, P.P.; Rossoni, R.D.; Junqueira, J.C.; Jorge, A.O.C. Lactobacillus Rhamnosus Inhibits Candida Albicans Virulence Factors in Vitro and Modulates Immune System in Galleria mellonella. J. Appl. Microbiol. 2017, 122, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Rossoni, R.D.; Dos Santos Velloso, M.; Figueiredo, L.M.A.; Martins, C.P.; Jorge, A.O.C.; Junqueira, J.C. Clinical Strains of Lactobacillus Reduce the Filamentation of Candida Albicans and Protect Galleria mellonella against Experimental Candidiasis. Folia Microbiol. 2018, 63, 307–314. [Google Scholar] [CrossRef]
- Vilela, S.F.G.; Barbosa, J.O.; Rossoni, R.D.; Santos, J.D.; Prata, M.C.A.; Anbinder, A.L.; Jorge, A.O.C.; Junqueira, J.C. Lactobacillus Acidophilus ATCC 4356 Inhibits Biofilm Formation by C. Albicans and Attenuates the Experimental Candidiasis in Galleria mellonella. Virulence 2015, 6, 29–39. [Google Scholar] [CrossRef]
- Dhinaut, J.; Balourdet, A.; Teixeira, M.; Chogne, M.; Moret, Y. A Dietary Carotenoid Reduces Immunopathology and Enhances Longevity through an Immune Depressive Effect in an Insect Model. Sci. Rep. 2017, 7, 12429. [Google Scholar] [CrossRef]
- Chang, M.X.; Xiong, F. Astaxanthin and Its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A Review of Its Chemistry and Applications. Crit Rev Food. Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential Anti-Atherosclerotic Properties of Astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxid. Med. Cell. Longev. 2019, 2019, 3849692. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.; Kattel, R.; Bhatia, B.; Petherwick, A.; Chapman, T. The Effect of Diet, Sex and Mating Status on Longevity in Mediterranean Fruit Flies (Ceratitis Capitata), Diptera: Tephritidae. Exp. Gerontol. 2005, 40, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.-H.; Wei, D.; Wei, D.-D.; Yuan, G.-R.; Wang, J.-J. The Effect of Dietary Restriction on Longevity, Fecundity, and Antioxidant Responses in the Oriental Fruit Fly, Bactrocera Dorsalis (Hendel) (Diptera: Tephritidae). J. Insect Physiol. 2013, 59, 1008–1016. [Google Scholar] [CrossRef]
- Duregon, E.; Pomatto-Watson, L.C.D.D.; Bernier, M.; Price, N.L.; de Cabo, R. Intermittent Fasting: From Calories to Time Restriction. GeroScience 2021, 43, 1083–1092. [Google Scholar] [CrossRef]
- Fontana, L. The Scientific Basis of Caloric Restriction Leading to Longer Life. Curr. Opin. Gastroenterol. 2009, 25, 144–150. [Google Scholar] [CrossRef]
- Fanson, B.G.; Weldon, C.W.; Pérez-Staples, D.; Simpson, S.J.; Taylor, P.W. Nutrients, Not Caloric Restriction, Extend Lifespan in Queensland Fruit Flies (Bactrocera tryoni). Aging Cell 2009, 8, 514–523. [Google Scholar] [CrossRef]
- Schatral, A. Diet Influences Male-Female Interactions in the BushcricketRequena Verticalis (Orthoptera: Tettigoniidae). J. Insect Behav. 1993, 6, 379–388. [Google Scholar] [CrossRef]
- Droney, D.C. The Influence of the Nutritional Content of the Adult Male Diet on Testis Mass, Body Condition and Courtship Vigour in a Hawaiian Drosophila. Funct. Ecol. 1998, 12, 920–928. [Google Scholar] [CrossRef]
- Stoffolano, J.G., Jr.; Tobin, E.N.; Wilson, J.; Yin, C.-M. Diet Affects Insemination and Sexual Activity in Male Phormia Regina (Diptera: Calliphoridae). Ann. Entomol. Soc. Am. 1995, 88, 240–246. [Google Scholar] [CrossRef]
- Engqvist, L.; Sauer, K.P. Influence of Nutrition on Courtship and Mating in the Scorpionfly Panorpa Cognata (Mecoptera, Insecta). Ethology 2003, 109, 911–928. [Google Scholar] [CrossRef]
- Angel, T.; Aryal, U. Impact of Gut Microbiota on Host by Exploring Proteomics. In Gut Microbiome and Its Impact on Health and Diseases; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Arias-Rojas, A.; Iatsenko, I. The Role of Microbiota in Drosophila melanogaster Aging. Front. Aging 2022, 3, 909509. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Vidal, H. Impact of Gut Microbiota on Host Glycemic Control. Front. Endocrinol. 2019, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Gould, A.L.; Zhang, V.; Lamberti, L.; Jones, E.W.; Obadia, B.; Korasidis, N.; Gavryushkin, A.; Carlson, J.M.; Beerenwinkel, N.; Ludington, W.B. Microbiome Interactions Shape Host Fitness. Proc. Natl. Acad. Sci. USA 2018, 115, E11951–E11960. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Lee, S.-H.; Lee, J.-H.; Lee, W.-J.; Min, K.-J. The Role of Commensal Microbes in the Lifespan of Drosophila melanogaster. Aging 2019, 11, 4611–4640. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Sun, E.W.; Rogers, G.B.; Keating, D.J. The Influence of the Gut Microbiome on Host Metabolism Through the Regulation of Gut Hormone Release. Front. Physiol. 2019, 10, 428. [Google Scholar] [CrossRef]
- Fan, X.; Gaur, U.; Yang, M. Intestinal Homeostasis and Longevity: Drosophila Gut Feeling. Adv. Exp. Med. Biol. 2018, 1086, 157–168. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef]
- Chiang, M.-H.; Ho, S.-M.; Wu, H.-Y.; Lin, Y.-C.; Tsai, W.-H.; Wu, T.; Lai, C.-H.; Wu, C.-L. Drosophila Model for Studying Gut Microbiota in Behaviors and Neurodegenerative Diseases. Biomedicines 2022, 10, 596. [Google Scholar] [CrossRef]
- Royet, J. Epithelial Homeostasis and the Underlying Molecular Mechanisms in the Gut of the Insect Model Drosophila melanogaster. Cell. Mol. Life Sci. 2011, 68, 3651–3660. [Google Scholar] [CrossRef] [PubMed]
- Kitani-Morii, F.; Friedland, R.P.; Yoshida, H.; Mizuno, T. Drosophila as a Model for Microbiota Studies of Neurodegeneration. J. Alzheimers Dis. 2021, 84, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wang, L.; Jiang, B. The Role of Gut Microbiota in Aging and Aging Related Neurodegenerative Disorders: Insights from Drosophila Model. Life 2021, 11, 855. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Wagner, A.E. Drosophila melanogaster as an Alternative Model Organism in Nutrigenomics. Genes Nutr. 2019, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Erkosar, B.; Leulier, F. Transient Adult Microbiota, Gut Homeostasis and Longevity: Novel Insights from the Drosophila Model. FEBS Lett. 2014, 588, 4250–4257. [Google Scholar] [CrossRef] [PubMed]
- Lesperance, D.N.A.; Broderick, N.A. Gut Bacteria Mediate Nutrient Availability in Drosophila Diets. Appl. Environ. Microbiol. 2020, 87, e01401-20. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.I.; Walker, D.W. Role of Gut Microbiota in Aging-Related Health Decline: Insights from Invertebrate Models. Cell. Mol. Life Sci. 2018, 75, 93–101. [Google Scholar] [CrossRef]
- Maynard, C.; Weinkove, D. The Gut Microbiota and Ageing. Subcell. Biochem. 2018, 90, 351–371. [Google Scholar] [CrossRef]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive Decline in Parkinson Disease. Nat. Rev. Neurol. 2017, 13, 217–231. [Google Scholar] [CrossRef]
- Jellinger, K.A. Dementia with Lewy Bodies and Parkinson’s Disease-Dementia: Current Concepts and Controversies. J. Neural Transm. 2018, 125, 615–650. [Google Scholar] [CrossRef]
- Soria Lopez, J.A.; González, H.M.; Léger, G.C. Alzheimer’s Disease. Handb. Clin. Neurol. 2019, 167, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.; Budson, A. Current Understanding of Alzheimer’s Disease Diagnosis and Treatment. F1000Research 2018, 7, 1161. [Google Scholar] [CrossRef]
- Tan, F.H.P.; Liu, G.; Lau, S.-Y.A.; Jaafar, M.H.; Park, Y.-H.; Azzam, G.; Li, Y.; Liong, M.-T. Lactobacillus Probiotics Improved the Gut Microbiota Profile of a Drosophila melanogaster Alzheimer’s Disease Model and Alleviated Neurodegeneration in the Eye. Benef. Microbes 2020, 11, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Tan, F.H.-P.; Lau, S.-Y.A.; Jaafar, M.H.; Chung, F.Y.-L.; Azzam, G.; Liong, M.-T.; Li, Y. Lactic Acid Bacteria Feeding Reversed the Malformed Eye Structures and Ameliorated Gut Microbiota Profiles of Drosophila melanogaster Alzheimer’s Disease Model. J. Appl. Microbiol. 2022, 132, 3155–3167. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Lee, W.-J. Role of DUOX in Gut Inflammation: Lessons from Drosophila Model of Gut-Microbiota Interactions. Front. Cell. Infect. Microbiol. 2014, 3, 116. [Google Scholar] [CrossRef]
- Charroux, B.; Royet, J. Gut-Microbiota Interactions in Non-Mammals: What Can We Learn from Drosophila? Semin. Immunol. 2012, 24, 17–24. [Google Scholar] [CrossRef]
- Agarwal, A.; Agashe, D. The Red Flour Beetle Tribolium Castaneum: A Model for Host-Microbiome Interactions. PLoS ONE 2020, 15, e0239051. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, Z.; Chen, X.; Hong, Z.; Lin, W.; Mu, X.; Hu, X.; Zheng, H. High-Fat Diets with Differential Fatty Acids Induce Obesity and Perturb Gut Microbiota in Honey Bee. Int. J. Mol. Sci. 2021, 22, 834. [Google Scholar] [CrossRef]
- Allonsius, C.N.; Van Beeck, W.; De Boeck, I.; Wittouck, S.; Lebeer, S. The Microbiome of the Invertebrate Model Host Galleria mellonella Is Dominated by Enterococcus. Anim. Microbiome 2019, 1, 7. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Sweeteners as Food Additives in the XXI Century: A Review of What Is Known, and What Is to Come. Food. Chem. Toxicol. 2017, 107, 302–317. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Emerging Point-of-Care Technologies for Food Safety Analysis. Sensors 2019, 19, E817. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Shaffer, R.M.; Sathyanarayana, S.; COUNCIL ON ENVIRONMENTAL HEALTH. Food Additives and Child Health. Pediatrics 2018, 142, e20181408. [Google Scholar] [CrossRef] [PubMed]
- Stadler, R.H.; Alfred, S. Acrylamide formation mechanisms. In Acrylamide in Food Analysis, Content and Potential Health Effects; Gökmen, V., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 1–17. [Google Scholar]
- Michalak, J.; Czarnowska-Kujawska, M.; Klepacka, J.; Gujska, E. Effect of Microwave Heating on the Acrylamide Formation in Foods. Molecules 2020, 25, 4140. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Aalami, M.; Shoeibi, S.; Kashaninejad, M.; Ghorbani, M.; Delavar, M. Effects of Different Roasting Methods on Formation of Acrylamide in Pistachio. Food Sci. Nutr. 2020, 8, 2875–2881. [Google Scholar] [CrossRef] [PubMed]
- Bušová, M.; Bencko, V.; Kromerová, K.; Nadjo, I.; Babjaková, J. Occurrence of Acrylamide in Selected Food Products. Cent. Eur. J. Public Health 2020, 28, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, S.; Niedermeier, J.; Wenzel, U. Hormesis Is Induced in the Red Flour Beetle Tribolium Castaneum through Ingestion of Charred Toast. Eur. J. Nutr. 2015, 54, 535–541. [Google Scholar] [CrossRef]
- Knight, M.; McWilliam, S.; Peck, S.; Koutsidis, G.; Chope, G.; Puddephat, I.; Wedzicha, B. Kinetic Modelling of Acrylamide Formation during the Frying of Potato Chips. Food Chem. 2021, 352, 129305. [Google Scholar] [CrossRef]
- Rifai, L.; Saleh, F.A. A Review on Acrylamide in Food: Occurrence, Toxicity, and Mitigation Strategies. Int. J. Toxicol. 2020, 39, 93–102. [Google Scholar] [CrossRef]
- Singh, L.; Varshney, J.G.; Agarwal, T. Polycyclic Aromatic Hydrocarbons’ Formation and Occurrence in Processed Food. Food Chem. 2016, 199, 768–781. [Google Scholar] [CrossRef]
- EU European Commission Regulation of 20 November 2017 Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food (2017/2158). Available online: https://eur-lex.europa.eu/eli/reg/2017/2158/oj (accessed on 20 September 2022).
- EU European Commission Recommendation of 7 November 2019 on the Monitoring of the Presence of Acrylamide in Certain Foods (2019/1888/EU). Commission Recommendation. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019H1888 (accessed on 20 September 2022).
- Grünwald, S.; Gurmai, A.-M.; Schuierer, K.; Boll, M.; Wenzel, U. The Red Flour Beetle Tribolium Castaneum Allows for the Convenient Determination of Fitness and Survival as a Measure of Toxic Effects of the Food Contaminant Acrylamide. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 1826–1833. [Google Scholar] [CrossRef]
- Fu, L.-L.; Zhao, X.-Y.; Ji, L.-D.; Xu, J. Okadaic Acid (OA): Toxicity, Detection and Detoxification. Toxicon 2019, 160, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Louzao, M.C.; Vieytes, M.R.; Botana, L.M. Effect of Okadaic Acid on Glucose Regulation. Mini Rev. Med. Chem. 2005, 5, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Valdiglesias, V.; Prego-Faraldo, M.V.; Pásaro, E.; Méndez, J.; Laffon, B. Okadaic Acid: More than a Diarrheic Toxin. Mar. Drugs 2013, 11, 4328–4349. [Google Scholar] [CrossRef] [PubMed]
- Coates, C.J.; Lim, J.; Harman, K.; Rowley, A.F.; Griffiths, D.J.; Emery, H.; Layton, W. The Insect, Galleria mellonella, Is a Compatible Model for Evaluating the Toxicology of Okadaic Acid. Cell Biol. Toxicol. 2019, 35, 219–232. [Google Scholar] [CrossRef]
- Maguire, R.; Duggan, O.; Kavanagh, K. Evaluation of Galleria mellonella Larvae as an in Vivo Model for Assessing the Relative Toxicity of Food Preservative Agents. Cell Biol. Toxicol. 2016, 32, 209–216. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Gresnigt, M.S.; Romani, L.; Netea, M.G.; Latgé, J.-P. Aspergillus Fumigatus Morphology and Dynamic Host Interactions. Nat. Rev. Microbiol. 2017, 15, 661–674. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, W.; Fan, J.; Jin, C.; Lu, L.; Fang, W. Aspergillus Fumigatus Mitochondrial Acetyl Coenzyme A Acetyltransferase as an Antifungal Target. Appl. Environ. Microbiol. 2020, 86, e02986-19. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. Filamentous Fungal Human Pathogens from Food Emphasising Aspergillus, Fusarium and Mucor. Microorganisms 2017, 5, 44. [Google Scholar] [CrossRef]
- Durieux, M.-F.; Melloul, É.; Jemel, S.; Roisin, L.; Dardé, M.-L.; Guillot, J.; Dannaoui, É.; Botterel, F. Galleria mellonella as a Screening Tool to Study Virulence Factors of Aspergillus Fumigatus. Virulence 2021, 12, 818–834. [Google Scholar] [CrossRef]
- Renwick, J.; Daly, P.; Reeves, E.P.; Kavanagh, K. Susceptibility of Larvae of Galleria mellonella to Infection by Aspergillus Fumigatus Is Dependent upon Stage of Conidial Germination. Mycopathologia 2006, 161, 377–384. [Google Scholar] [CrossRef]
- Sheehan, G.; Clarke, G.; Kavanagh, K. Characterisation of the Cellular and Proteomic Response of Galleria mellonella Larvae to the Development of Invasive Aspergillosis. BMC Microbiol. 2018, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Fallon, J.P.; Reeves, E.P.; Kavanagh, K. The Aspergillus Fumigatus Toxin Fumagillin Suppresses the Immune Response of Galleria mellonella Larvae by Inhibiting the Action of Haemocytes. Microbiology 2011, 157, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Gorgus, E.; Hittinger, M.; Schrenk, D. Estimates of Ethanol Exposure in Children from Food Not Labeled as Alcohol-Containing. J. Anal. Toxicol. 2016, 40, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Le Daré, B.; Lagente, V.; Gicquel, T. Ethanol and Its Metabolites: Update on Toxicity, Benefits, and Focus on Immunomodulatory Effects. Drug Metab. Rev. 2019, 51, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.A.; Innocent, L.V.; Martinez, D.J.; Huang, I.L.; Yang, J.L.; Eisen, M.B.; Ludington, W.B. Microbiome-by-Ethanol Interactions Impact Drosophila melanogaster Fitness, Physiology, and Behavior. iScience 2022, 25, 104000. [Google Scholar] [CrossRef]
- Demir, E.; Demir, F.T.; Marcos, R. Drosophila as a Suitable In Vivo Model in the Safety Assessment of Nanomaterials. Adv. Exp. Med. Biol. 2022, 1357, 275–301. [Google Scholar] [CrossRef]
- Chifiriuc, M.C.; Ratiu, A.C.; Popa, M.; Ecovoiu, A.A. Drosophotoxicology: An Emerging Research Area for Assessing Nanoparticles Interaction with Living Organisms. Int. J. Mol. Sci. 2016, 17, 36. [Google Scholar] [CrossRef]
- Pappus, S.A.; Mishra, M. A Drosophila Model to Decipher the Toxicity of Nanoparticles Taken Through Oral Routes. Adv. Exp. Med. Biol. 2018, 1048, 311–322. [Google Scholar] [CrossRef]
- Alaraby, M.; Annangi, B.; Hernández, A.; Creus, A.; Marcos, R. A Comprehensive Study of the Harmful Effects of ZnO Nanoparticles Using Drosophila melanogaster as an in Vivo Model. J. Hazard. Mater. 2015, 296, 166–174. [Google Scholar] [CrossRef]
- Barik, B.K.; Mishra, M. Nanoparticles as a Potential Teratogen: A Lesson Learnt from Fruit Fly. Nanotoxicology 2019, 13, 258–284. [Google Scholar] [CrossRef]
- Carmona, E.R.; Escobar, B.; Vales, G.; Marcos, R. Genotoxic Testing of Titanium Dioxide Anatase Nanoparticles Using the Wing-Spot Test and the Comet Assay in Drosophila. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 778, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Panda, M. Reactive Oxygen Species: The Root Cause of Nanoparticle-Induced Toxicity in Drosophila melanogaster. Free Radic. Res. 2021, 55, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yang, X.; Sun, L.; Han, X.; Xu, L.; Gu, W.; Zhang, M. Effects of Cadmium on Oxidative Stress and Cell Apoptosis in Drosophila melanogaster Larvae. Sci. Rep. 2022, 12, 4762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wolosker, M.B.; Zhao, Y.; Ren, H.; Lemos, B. Exposure to Microplastics Cause Gut Damage, Locomotor Dysfunction, Epigenetic Silencing, and Aggravate Cadmium (Cd) Toxicity in Drosophila. Sci. Total Environ. 2020, 744, 140979. [Google Scholar] [CrossRef] [PubMed]
- Sabat, D.; Patnaik, A.; Ekka, B.; Dash, P.; Mishra, M. Investigation of Titania Nanoparticles on Behaviour and Mechanosensory Organ of Drosophila melanogaster. Physiol. Behav. 2016, 167, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, B.; Cvetković, V.J.; Mitrović, T.L. Effects of Human Food Grade Titanium Dioxide Nanoparticle Dietary Exposure on Drosophila melanogaster Survival, Fecundity, Pupation and Expression of Antioxidant Genes. Chemosphere 2016, 144, 43–49. [Google Scholar] [CrossRef]
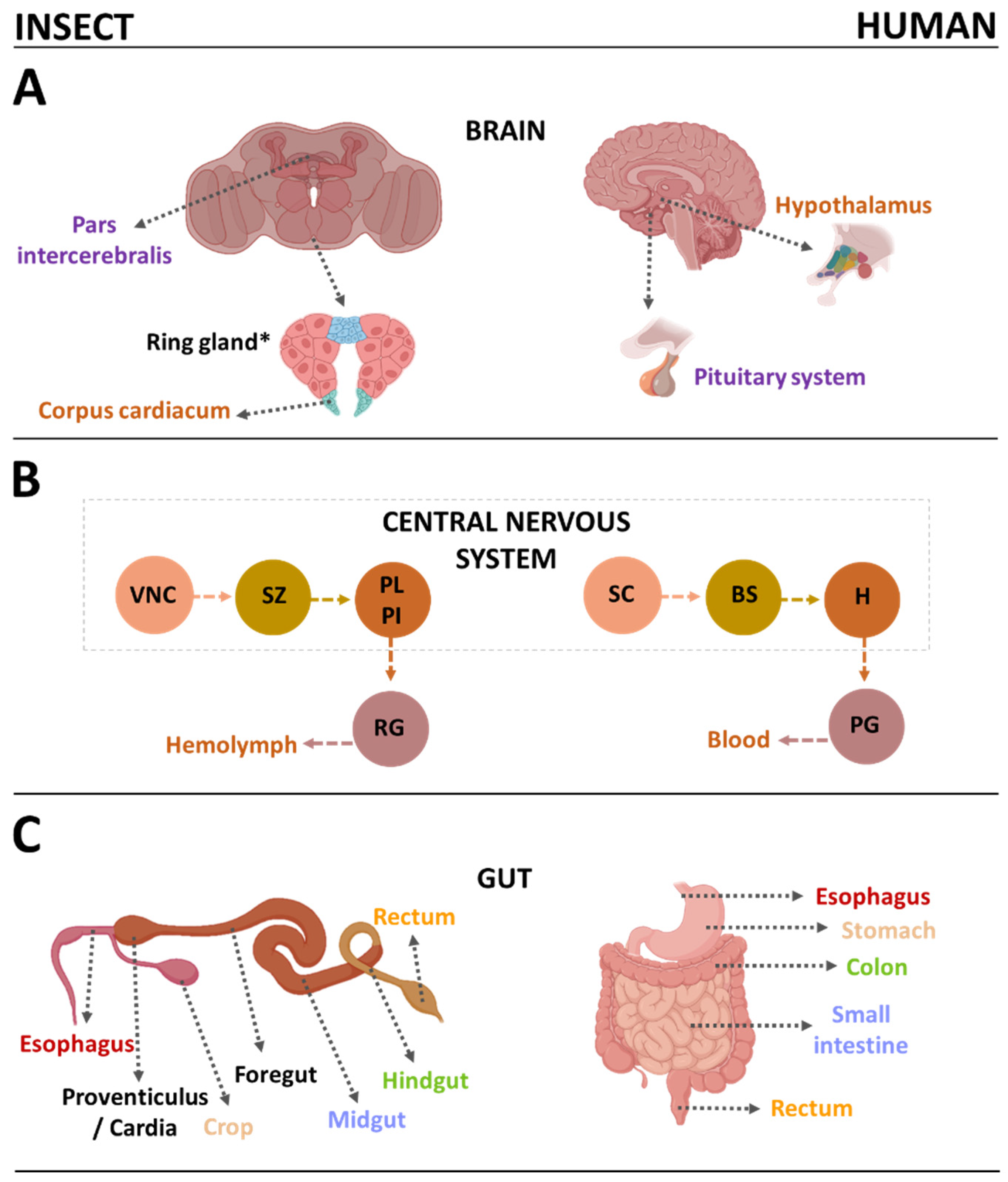

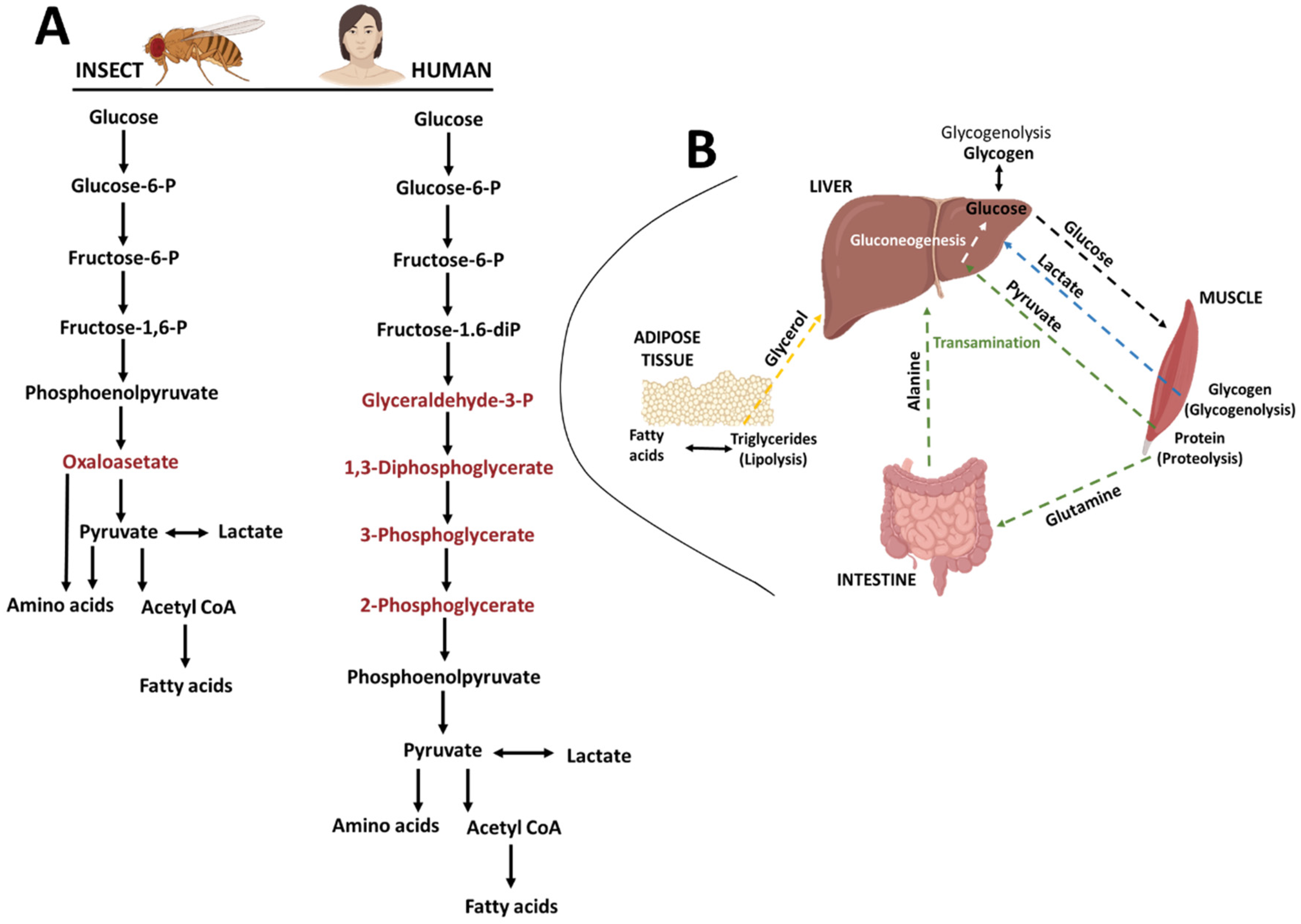
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonk-Rügen, M.; Vilcinskas, A.; Wagner, A.E. Insect Models in Nutrition Research. Biomolecules 2022, 12, 1668. https://doi.org/10.3390/biom12111668
Tonk-Rügen M, Vilcinskas A, Wagner AE. Insect Models in Nutrition Research. Biomolecules. 2022; 12(11):1668. https://doi.org/10.3390/biom12111668
Chicago/Turabian StyleTonk-Rügen, Miray, Andreas Vilcinskas, and Anika E. Wagner. 2022. "Insect Models in Nutrition Research" Biomolecules 12, no. 11: 1668. https://doi.org/10.3390/biom12111668
APA StyleTonk-Rügen, M., Vilcinskas, A., & Wagner, A. E. (2022). Insect Models in Nutrition Research. Biomolecules, 12(11), 1668. https://doi.org/10.3390/biom12111668






