Effects of β2 Integrins on Osteoclasts, Macrophages, Chondrocytes, and Synovial Fibroblasts in Osteoarthritis
Abstract
1. Introduction
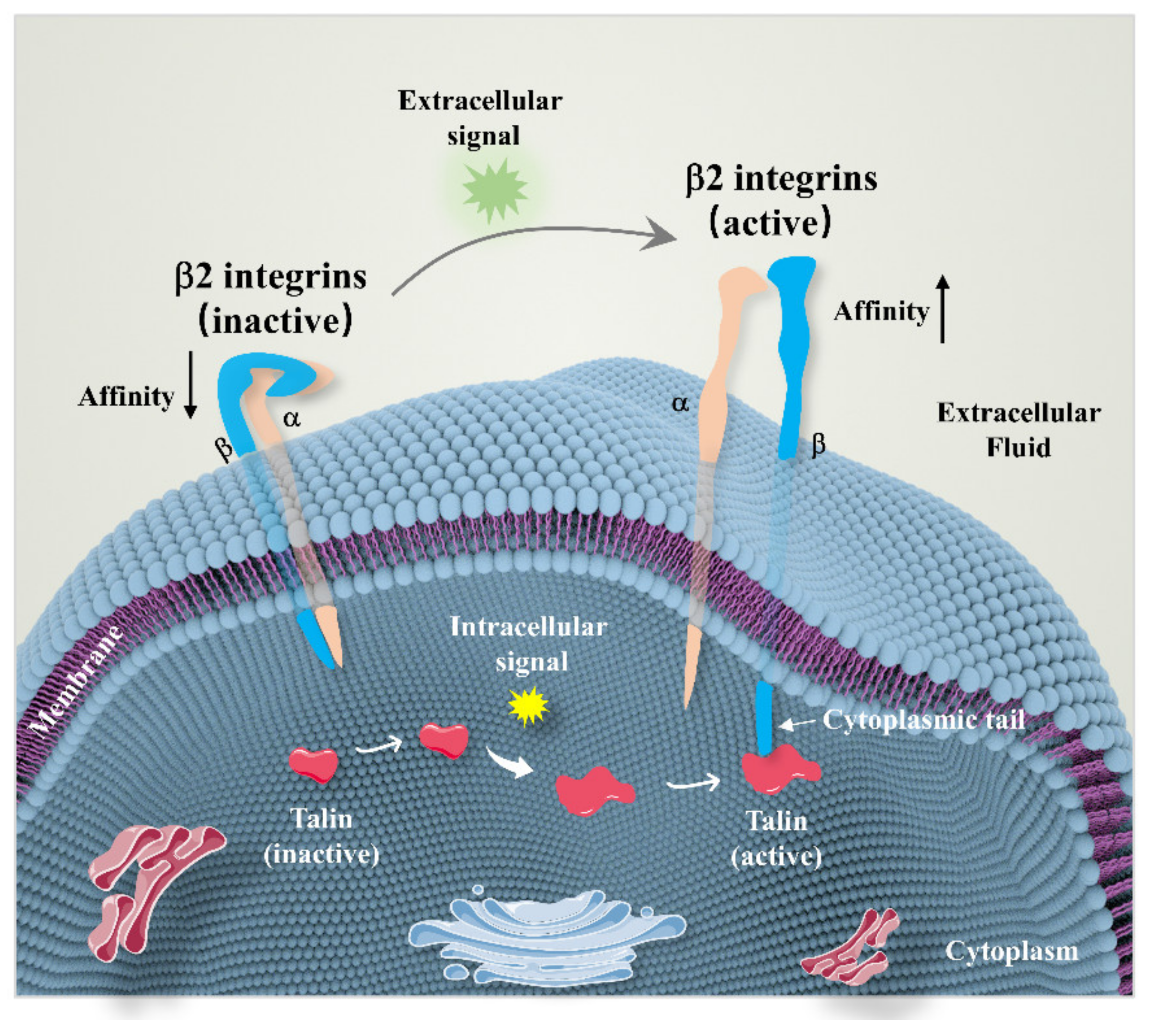
2. Overview of β2 Integrins
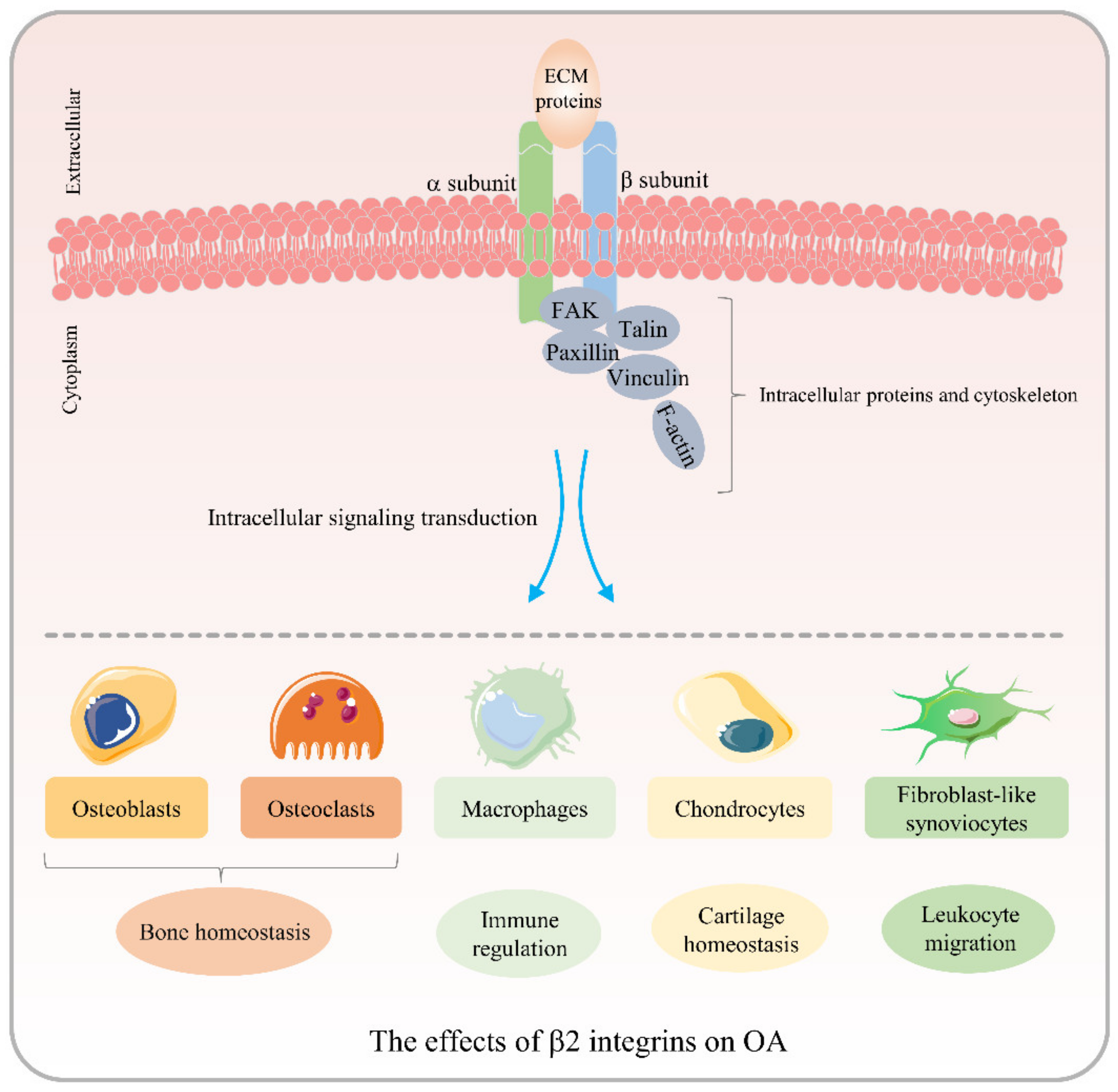
3. Role of β2 Integrins in OA Pathogenesis
3.1. Effects of β2 Integrins on OCs
3.2. Effects of β2 Integrins on Macrophages
3.3. Effects of β2 Integrins on Chondrocytes
3.4. Effects of β2 Integrins on FLS
4. The Potential of β2 Integrin Antagonists Serving as a Therapeutic Target of OA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568. [Google Scholar] [CrossRef] [PubMed]
- Franco-Trepat, E.; Alonso-Pérez, A.; Guillán-Fresco, M.; Jorge-Mora, A.; Crespo-Golmar, A.; López-Fagúndez, M.; Pazos-Pérez, A.; Gualillo, O.; Belén Bravo, S.; Gómez Bahamonde, R. Amitriptyline blocks innate immune responses mediated by toll-like receptor 4 and IL-1 receptor: Preclinical and clinical evidence in osteoarthritis and gout. Br. J. Pharmacol. 2022, 179, 270–286. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Fujita, M.; Kawasaki, S.; Sanaki, T.; Yoshioka, T.; Higashino, K.; Tofukuji, S.; Yoneda, S.; Takahashi, T.; Koda, K.; et al. Contribution of synovial macrophages to rat advanced osteoarthritis pain resistant to cyclooxygenase inhibitors. Pain 2019, 160, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Ni, J.; Witherel, C.E.; Yang, M.; Burdick, J.A.; Wen, C.; Wong, S.H.D. Harnessing Tissue-derived Extracellular Vesicles for Osteoarthritis Theranostics. Theranostics 2022, 12, 207–231. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Layseca, P.; Icha, J.; Hamidi, H.; Ivaska, J. Integrin trafficking in cells and tissues. Nat. Cell Biol. 2019, 21, 122–132. [Google Scholar] [CrossRef]
- Adair, B.D.; Alonso, J.L.; van Agthoven, J.; Hayes, V.; Ahn, H.S.; Yu, I.-S.; Lin, S.-W.; Xiong, J.-P.; Poncz, M.; Arnaout, M.A. Structure-guided design of pure orthosteric inhibitors of αIIbβ3 that prevent thrombosis but preserve hemostasis. Nat. Commun. 2020, 11, 398. [Google Scholar] [CrossRef]
- Bachmann, M.; Kukkurainen, S.; Hytönen, V.P.; Wehrle-Haller, B. Cell Adhesion by Integrins. Physiol. Rev. 2019, 99, 1655–1699. [Google Scholar] [CrossRef]
- Geoghegan, I.P.; Hoey, D.A.; McNamara, L.M. Integrins in Osteocyte Biology and Mechanotransduction. Curr. Osteoporos. Rep. 2019, 17, 195–206. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Wang, M.; Zhao, S.; Zhao, Z.; Fang, J. Mechanotransduction pathways in the regulation of cartilage chondrocyte homoeostasis. J. Cell Mol. Med. 2020, 24, 5408–5419. [Google Scholar] [CrossRef]
- Nowatzky, J.; Manches, O.; Khan, S.A.; Godefroy, E.; Bhardwaj, N. Modulation of human Th17 cell responses through complement receptor 3 (CD11 b/CD18) ligation on monocyte-derived dendritic cells. J. Autoimmun. 2018, 92, 57–66. [Google Scholar] [CrossRef]
- Kragstrup, T.W.; Jalilian, B.; Keller, K.K.; Zhang, X.; Laustsen, J.K.; Stengaard-Pedersen, K.; Hetland, M.L.; Hørslev-Petersen, K.; Junker, P.; Østergaard, M.; et al. Changes in soluble CD18 in murine autoimmune arthritis and rheumatoid arthritis reflect disease establishment and treatment response. PLoS ONE 2016, 11, e0148486. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Jiang, S.; Wang, R.; Zhang, Y.; Dong, J.; Li, Y. Mechanistic Insight Into the Roles of Integrins in Osteoarthritis. Front. Cell Dev. Biol. 2021, 9, 693484. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Satoh, M.; Inoue, G.; Onuma, K.; Miyagi, M.; Iwabuchi, K.; Takaso, M. CD11c(+) macrophages and levels of TNF-α and MMP-3 are increased in synovial and adipose tissues of osteoarthritic mice with hyperlipidaemia. Clin. Exp. Immunol. 2015, 180, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Qi, W.; Lv, M.; Yuan, C.; Tian, K.; Zhang, F. Combined bioinformatics analysis reveals gene expression and DNA methylation patterns in osteoarthritis. Mol. Med. Rep. 2018, 17, 8069–8078. [Google Scholar] [CrossRef] [PubMed]
- Blythe, E.N.; Weaver, L.C.; Brown, A.; Dekaban, G.A. β2 Integrin CD11d/CD18: From Expression to an Emerging Role in Staged Leukocyte Migration. Front. Immunol. 2021, 12, 775447. [Google Scholar] [CrossRef]
- LaFlamme, S.E.; Mathew-Steiner, S.; Singh, N.; Colello-Borges, D.; Nieves, B. Integrin and microtubule crosstalk in the regulation of cellular processes. Cell Mol. Life Sci. CMLS 2018, 75, 4177–4185. [Google Scholar] [CrossRef]
- Walling, B.L.; Kim, M. LFA-1 in T Cell Migration and Differentiation. Front. Immunol. 2018, 9, 952. [Google Scholar] [CrossRef]
- Gahmberg, C.G.; Grönholm, M.; Madhavan, S.; Jahan, F.; Mikkola, E.; Viazmina, L.; Koivunen, E. Regulation of cell adhesion: A collaborative effort of integrins, their ligands, cytoplasmic actors, and phosphorylation. Q. Rev. Biophys. 2019, 52, e10. [Google Scholar] [CrossRef]
- Lahti, M.; Heino, J.; Käpylä, J. Leukocyte integrins αLβ2, αMβ2 and αXβ2 as collagen receptors–receptor activation and recognition of GFOGER motif. Int. J. Biochem. Cell Biol. 2013, 45, 1204–1211. [Google Scholar] [CrossRef]
- Sreeramkumar, V.; Adrover, J.M.; Ballesteros, I.; Cuartero, M.I.; Rossaint, J.; Bilbao, I.; Nácher, M.; Pitaval, C.; Radovanovic, I.; Fukui, Y.; et al. Neutrophils scan for activated platelets to initiate inflammation. Science 2014, 346, 1234–1238. [Google Scholar] [CrossRef]
- Shao, B.; Yago, T.; Panicker, S.R.; Zhang, N.; Liu, Z.; McEver, R.P. Th1 Cells Rolling on Selectins Trigger DAP12-Dependent Signals That Activate Integrin αLβ2. J. Immunol. 2020, 204, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Merino-Cortés, S.V.; Gardeta, S.R.; Roman-Garcia, S.; Martínez-Riaño, A.; Pineau, J.; Liebana, R.; Merida, I.; Dumenil, A.-M.L.; Pierobon, P.; Husson, J.; et al. Diacylglycerol kinase ζ promotes actin cytoskeleton remodeling and mechanical forces at the B cell immune synapse. Sci. Signal. 2020, 13, eaaw8214. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.; Tohme, M.; Butts, J.; Giguere, S.; Sage, P.T.; Velázquez, F.E.; Kam, C.; Milin, E.; Das, M.; Sobh, A.; et al. DOCK8 is essential for LFA-1-dependent positioning of T follicular helper cells in germinal centers. JCI Insight 2020, 5, e134508. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Hyun, Y.-M.; Lambert-Emo, K.; Topham, D.J.; Kim, M. Visualization of integrin Mac-1 in vivo. J. Immunol. Methods 2015, 426, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Lukácsi, S.; Nagy-Baló, Z.; Erdei, A.; Sándor, N.; Bajtay, Z. The role of CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in complement-mediated phagocytosis and podosome formation by human phagocytes. Immunol. Lett. 2017, 189, 144–145. [Google Scholar] [CrossRef]
- Lin, Z.; Schmidt, C.Q.; Koutsogiannaki, S.; Ricci, P.; Risitano, A.M.; Lambris, J.D.; Ricklin, D. Complement C3dg-mediated erythrophagocytosis: Implications for paroxysmal nocturnal hemoglobinuria. Blood 2015, 126, 891–894. [Google Scholar] [CrossRef]
- Skoberne, M.; Somersan, S.; Almodovar, W.; Truong, T.; Petrova, K.; Henson, P.M.; Bhardwaj, N. The apoptotic-cell receptor CR3, but not alphavbeta5, is a regulator of human dendritic-cell immunostimulatory function. Blood 2006, 108, 947–955. [Google Scholar] [CrossRef]
- Sebé-Pedrós, A.; Roger, A.J.; Lang, F.B.; King, N.; Ruiz-Trillo, I. Ancient origin of the integrin-mediated adhesion and signaling machinery. Proc. Natl. Acad. Sci. USA 2010, 107, 10142–10147. [Google Scholar] [CrossRef]
- Thome, S.; Begandt, D.; Pick, R.; Salvermoser, M.; Walzog, B. Intracellular β integrin (CD11/CD18) interacting partners in neutrophil trafficking. Eur. J. Clin. Investig. 2018, 48 (Suppl. S2), e12966. [Google Scholar] [CrossRef]
- Nayal, A.; Webb, D.J.; Horwitz, A.F. Talin: An emerging focal point of adhesion dynamics. Curr. Opin. Cell Biol. 2004, 16, 94–98. [Google Scholar] [CrossRef]
- Calderwood, D.A.; Campbell, I.D.; Critchley, D.R. Talins and kindlins: Partners in integrin-mediated adhesion. Nat. Rev. Mol. Cell Biol. 2013, 14, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Ye, F.; Hu, X.; Ginsberg, M.H. Talin activates integrins by altering the topology of the β transmembrane domain. J. Cell Biol. 2012, 197, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Melchionna, R.; Trono, P.; Tocci, A.; Nisticò, P. Actin cytoskeleton and regulation of TGFβ signaling: Exploring their links. Biomolecules 2021, 11, 336. [Google Scholar] [CrossRef]
- Kong, L.; Wang, B.; Yang, X.; He, B.; Hao, D.; Yan, L. Integrin-associated molecules and signalling cross talking in osteoclast cytoskeleton regulation. J. Cell Mol. Med. 2020, 24, 3271–3281. [Google Scholar] [CrossRef] [PubMed]
- Michael, M.; Parsons, M. New perspectives on integrin-dependent adhesions. Curr. Opin. Cell Biol. 2020, 63, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, B.; Tsykin, A.; Findlay, D.M.; Fazzalari, N.L. Microarray gene expression profiling of osteoarthritic bone suggests altered bone remodelling, WNT and transforming growth factor-beta/bone morphogenic protein signalling. Arthritis Res. Ther. 2007, 9, R100. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, J.; Yang, Y.; Lin, J.; Wu, Z. Genome-wide expression and methylation profiling reveal candidate genes in osteoarthritis. Clin. Exp. Rheumatol. 2017, 35, 983–990. [Google Scholar]
- Sun, Y.; Mauerhan, D.R.; Honeycutt, P.R.; Kneisl, J.S.; Norton, J.H.; Hanley, E.N.; Gruber, H.E. Analysis of meniscal degeneration and meniscal gene expression. BMC Musculoskelet. Disord. 2010, 11, 19. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Malengier-Devlies, B.; Yu, K.; Vandendriessche, S.; Yserbyt, J.; Matthys, P.; De Somer, L.; Wouters, C.; Proost, P. Synovial fluid neutrophils from patients with juvenile idiopathic arthritis display a hyperactivated phenotype. Arthritis Rheumatol. 2021, 73, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Schittenhelm, L.; Robertson, J.; Pratt, A.G.; Hilkens, C.M.; Morrison, V.L. Dendritic cell integrin expression patterns regulate inflammation in the rheumatoid arthritis joint. Rheumatology 2021, 60, 1533–1542. [Google Scholar] [CrossRef]
- Uchida, K.; Naruse, K.; Satoh, M.; Onuma, K.; Ueno, M.; Takano, S.; Urabe, K.; Takaso, M. Increase of circulating CD11b(+)Gr1(+) cells and recruitment into the synovium in osteoarthritic mice with hyperlipidemia. Exp. Anim. 2013, 62, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Watts, G.M.; Beurskens, F.J.M.; Martin-Padura, I.; Ballantyne, C.M.; Klickstein, L.B.; Brenner, M.B.; Lee, D.M. Manifestations of inflammatory arthritis are critically dependent on LFA-1. J. Immunol. 2005, 174, 3668–3675. [Google Scholar] [CrossRef] [PubMed]
- Zieba, J.T.; Chen, Y.-T.; Lee, B.H.; Bae, Y. Notch signaling in skeletal development, homeostasis and pathogenesis. Biomolecules 2020, 10, 332. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhu, J.; Zhen, G.; Hu, Y.; An, S.; Li, Y.; Zheng, Q.; Chen, Z.; Yang, Y.; Wan, M.; et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J. Clin. Investig. 2019, 129, 1076–1093. [Google Scholar] [CrossRef]
- Hügle, T.; Geurts, J. What drives osteoarthritis?-synovial versus subchondral bone pathology. Rheumatology 2017, 56, 1461–1471. [Google Scholar] [CrossRef]
- Park, D.R.; Kim, J.; Kim, G.M.; Lee, H.; Kim, M.; Hwang, D.; Lee, H.; Kim, H.-S.; Kim, W.; Park, M.C.; et al. Osteoclast-associated receptor blockade prevents articular cartilage destruction via chondrocyte apoptosis regulation. Nat. Commun. 2020, 11, 4343. [Google Scholar] [CrossRef]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef]
- Lavigne, P.; Benderdour, M.; Lajeunesse, D.; Shi, Q.; Fernandes, J.C. Expression of ICAM-1 by osteoblasts in healthy individuals and in patients suffering from osteoarthritis and osteoporosis. Bone 2004, 35, 463–470. [Google Scholar] [CrossRef]
- Kurachi, T.; Morita, I.; Murota, S. Involvement of adhesion molecules LFA-1 and ICAM-1 in osteoclast development. Biochim. Biophys. Acta 1993, 1178, 259–266. [Google Scholar] [CrossRef]
- Tanaka, Y.; Maruo, A.; Fujii, K.; Nomi, M.; Nakamura, T.; Eto, S.; Minami, Y. Intercellular adhesion molecule 1 discriminates functionally different populations of human osteoblasts: Characteristic involvement of cell cycle regulators. J. Bone Miner. Res. 2000, 15, 1912–1923. [Google Scholar] [CrossRef]
- Sanadgol, N.; Mostafaie, A.; Mansouri, K.; Bahrami, G. Effect of palmitic acid and linoleic acid on expression of ICAM-1 and VCAM-1 in human bone marrow endothelial cells (HBMECs). Arch. Med. Sci. AMS 2012, 8, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Bays, J.L.; DeMali, K.A. Vinculin in cell–cell and cell–matrix adhesions. Cell Mol. Life Sci. 2017, 74, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.B.; Wei, L.; Castellot, J.J. The matricellular protein CCN5 regulates podosome function via interaction with integrin αvβ 3. J. Cell Commun. Signal. 2014, 8, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Rafiq, N.B.M.; Krishnasamy, A.; Hartman, K.L.; Jones, G.E.; Bershadsky, A.D.; Sheetz, M.P. Integrin-matrix clusters form podosome-like adhesions in the absence of traction forces. Cell Rep. 2013, 5, 1456–1468. [Google Scholar] [CrossRef] [PubMed]
- Bonnelye, E.; Saltel, F.; Chabadel, A.; Zirngibl, R.A.; Aubin, J.E.; Jurdic, P. Involvement of the orphan nuclear estrogen receptor-related receptor α in osteoclast adhesion and transmigration. J. Mol. Endocrinol. 2010, 45, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, L.; Wang, Y.-X.; Li, Z.-L.; Wang, Q.; Zhao, Z.-D.; Zhao, S.; Wang, H.; Wu, C.-T.; Mao, N.; et al. Skeletal stem cell-mediated suppression on inflammatory osteoclastogenesis occurs via concerted action of cell adhesion molecules and osteoprotegerin. Stem. Cells Transl. Med. 2020, 9, 261–272. [Google Scholar] [CrossRef]
- Hayashi, H.; Nakahama, K.; Sato, T.; Tuchiya, T.; Asakawa, Y.; Maemura, T.; Tanaka, M.; Morita, M.; Morita, I. The role of Mac-1 (CD11b/CD18) in osteoclast differentiation induced by receptor activator of nuclear factor-kappaB ligand. FEBS Lett. 2008, 582, 3243–3248. [Google Scholar] [CrossRef]
- Yang, G.; Chen, X.; Yan, Z.; Zhu, Q.; Yang, C. CD11b promotes the differentiation of osteoclasts induced by RANKL through the spleen tyrosine kinase signalling pathway. J. Cell. Mol. Med. 2017, 21, 3445–3452. [Google Scholar] [CrossRef]
- Li, X.; Akiyama, M.; Nakahama, K.; Koshiishi, T.; Takeda, S.; Morita, I. Role of intercellular adhesion molecule-2 in osteoclastogenesis. Genes Cells 2012, 17, 568–575. [Google Scholar] [CrossRef]
- Ohtsuji, M.; Lin, Q.; Okazaki, H.; Takahashi, K.; Amano, H.; Yagita, H.; Nishimura, H.; Hirose, S. Anti-CD11b antibody treatment suppresses the osteoclast generation, inflammatory cell infiltration, and autoantibody production in arthritis-prone FcγRIIB-deficient mice. Arthritis Res. Ther. 2018, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Park-Min, K.-H.; Lee, E.Y.; Moskowitz, N.K.; Lim, E.; Lee, S.-K.; Lorenzo, J.A.; Huang, C.; Melnick, A.M.; Purdue, P.E.; Goldring, S.R.; et al. Negative regulation of osteoclast precursor differentiation by CD11b and β2 integrin-B-cell lymphoma 6 signaling. J. Bone Miner. Res. 2013, 28, 135–149. [Google Scholar] [CrossRef]
- Stevanin, M.; Busso, N.; Chobaz, V.; Pigni, M.; Ghassem-Zadeh, S.; Zhang, L.; Acha-Orbea, H.; Ehirchiou, D. CD11b regulates the Treg/Th17 balance in murine arthritis via IL-6. Eur. J. Immunol. 2017, 47, 637–645. [Google Scholar] [CrossRef]
- Gallois, A.; Lachuer, J.; Yvert, G.; Wierinckx, A.; Brunet, F.; Rabourdin-Combe, C.; Delprat, C.; Jurdic, P.; Mazzorana, M. Genome-wide expression analyses establish dendritic cells as a new osteoclast precursor able to generate bone-resorbing cells more efficiently than monocytes. J. Bone Miner. Res. 2010, 25, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.M.J.; Delaissé, J.-M.; Olesen, J.B.; Madsen, J.S.; Canto, L.M.; Bechmann, T.; Rogatto, S.R.; Søe, K. Aging and menopause reprogram osteoclast precursors for aggressive bone resorption. Bone Res. 2020, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Ruef, N.; Dolder, S.; Aeberli, D.; Seitz, M.; Balani, D.; Hofstetter, W. Granulocyte-macrophage colony-stimulating factor-dependent CD11c-positive cells differentiate into active osteoclasts. Bone 2017, 97, 267–277. [Google Scholar] [CrossRef]
- Tsukasaki, M.; Huynh, N.C.-N.; Okamoto, K.; Muro, R.; Terashima, A.; Kurikawa, Y.; Komatsu, N.; Pluemsakunthai, W.; Nitta, T.; Abe, T.; et al. Stepwise cell fate decision pathways during osteoclastogenesis at single-cell resolution. Nat. Metab. 2020, 2, 1382–1390. [Google Scholar] [CrossRef]
- Xie, J.; Huang, Z.; Yu, X.; Zhou, L.; Pei, F. Clinical implications of macrophage dysfunction in the development of osteoarthritis of the knee. Cytokine Growth Factor Rev. 2019, 46, 36–44. [Google Scholar] [CrossRef]
- Esen, I.; Jiemy, W.F.; van Sleen, Y.; van der Geest, K.S.M.; Sandovici, M.; Heeringa, P.; Boots, A.M.H.; Brouwer, E. Functionally Heterogenous Macrophage Subsets in the Pathogenesis of Giant Cell Arteritis: Novel Targets for Disease Monitoring and Treatment. J. Clin. Med. 2021, 10, 4958. [Google Scholar] [CrossRef]
- Xie, J.W.; Wang, Y.; Xiao, K.; Xu, H.; Luo, Z.Y.; Li, L.; Pei, F.X.; Kraus, V.B.; Huang, Z.Y. Alpha defensin-1 attenuates surgically induced osteoarthritis in association with promoting M1 to M2 macrophage polarization. Osteoarthr. Cartil. 2021, 29, 1048–1059. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, M.; Zhao, J.; Zheng, M.; Yang, H. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp. Ther. Med. 2018, 16, 5009–5014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, J.; Xiang, X.; Zhou, B.; Zhao, C.; Wei, Q.; Sun, Y.; Chen, J.; Lai, B.; Luo, Z.; et al. Tert-butylhydroquinone attenuates osteoarthritis by protecting chondrocytes and inhibiting macrophage polarization. Bone Jt. Res. 2021, 10, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Chiang, C.-F.; Kuo, F.-C.; Su, S.-C.; Huang, C.-L.; Liu, J.-S.; Lu, C.-H.; Hsieh, C.-H.; Wang, C.-C.; Lee, C.-H.; et al. High-Molecular-Weight Hyaluronic Acid Inhibits IL-1β-Induced Synovial Inflammation and Macrophage Polarization through the GRP78-NF-κB Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 11917. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Xie, Y.; Li, K.; Hu, T.; Lu, X.; Cao, Y.; Zheng, X. Unveiling the Mechanism of Surface Hydrophilicity-Modulated Macrophage Polarization. Adv. Healthc. Mater. 2018, 7, e1800675. [Google Scholar] [CrossRef]
- Gao, J.X.; Issekutz, A.C. Mac-1 (CD11b/CD18) is the predominant beta 2 (CD18) integrin mediating human neutrophil migration through synovial and dermal fibroblast barriers. Immunology 1996, 88, 463–470. [Google Scholar] [CrossRef]
- Eyles, J.L.; Hickey, M.J.; Norman, M.U.; Croker, B.A.; Roberts, A.W.; Drake, S.F.; James, W.G.; Metcalf, D.; Campbell, I.K.; Wicks, I.P. A key role for G-CSF-induced neutrophil production and trafficking during inflammatory arthritis. Blood 2008, 112, 5193–5201. [Google Scholar] [CrossRef]
- LaPointe, V.L.S.; Verpoorte, A.; Stevens, M.M. The changing integrin expression and a role for integrin β8 in the chondrogenic differentiation of mesenchymal stem cells. PLoS ONE 2013, 8, e82035. [Google Scholar] [CrossRef]
- Loeser, R.F. Integrins and chondrocyte-matrix interactions in articular cartilage. Matrix Biol. 2014, 39, 11–16. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, F.-J.; Lei, G.-H. Role of integrins and their ligands in osteoarthritic cartilage. Rheumatol. Int. 2015, 35, 787–798. [Google Scholar] [CrossRef]
- Ehirchiou, D.; Bernabei, I.; Chobaz, V.; Castelblanco, M.; Hügle, T.; So, A.; Zhang, L.; Busso, N.; Nasi, S. CD11b Signaling Prevents Chondrocyte Mineralization and Attenuates the Severity of Osteoarthritis. Front. Cell Dev. Biol. 2020, 8, 611757. [Google Scholar] [CrossRef]
- Wang, X.; Blizzard, L.; Halliday, A.; Han, W.; Jin, X.; Cicuttini, F.; Jones, G.; Ding, C. Association between MRI-detected knee joint regional effusion-synovitis and structural changes in older adults: A cohort study. Ann. Rheum. Dis. 2016, 75, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Aso, K.; Shahtaheri, S.M.; Hill, R.; Wilson, D.; McWilliams, D.F.; Walsh, D.A. Associations of Symptomatic Knee Osteoarthritis With Histopathologic Features in Subchondral Bone. Arthritis Rheumatol. 2019, 71, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Krishna, V.; Yin, X.; Song, Q.; Walsh, A.; Pocalyko, D.; Bachman, K.; Anderson, I.; Madakamutil, L.; Nagpal, S. Integration of the transcriptome and genome-wide landscape of BRD2 and BRD4 binding motifs identifies key superenhancer genes and reveals the mechanism of bet inhibitor action in rheumatoid arthritis synovial fibroblasts. J. Immunol. 2021, 206, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Kasama, T.; Shiozawa, F.; Kobayashi, K.; Yajima, N.; Hanyuda, M.; Takeuchi, H.T.; Mori, Y.; Negishi, M.; Ide, H.; Adachi, M. Vascular endothelial growth factor expression by activated synovial leukocytes in rheumatoid arthritis: Critical involvement of the interaction with synovial fibroblasts. Arthritis Rheum. 2001, 44, 2512–2524. [Google Scholar] [CrossRef]
- Hanaoka, R.; Kasama, T.; Muramatsu, M.; Yajima, N.; Shiozawa, F.; Miwa, Y.; Negishi, M.; Ide, H.; Miyaoka, H.; Uchida, H.; et al. A novel mechanism for the regulation of IFN-gamma inducible protein-10 expression in rheumatoid arthritis. Arthritis Res. Ther. 2003, 5, R74–R81. [Google Scholar] [CrossRef]
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef]
- Hanyuda, M.; Kasama, T.; Isozaki, T.; Matsunawa, M.M.; Yajima, N.; Miyaoka, H.; Uchida, H.; Kameoka, Y.; Ide, H.; Adachi, M. Activated leucocytes express and secrete macrophage inflammatory protein-1alpha upon interaction with synovial fibroblasts of rheumatoid arthritis via a beta2-integrin/ICAM-1 mechanism. Rheumatology 2003, 42, 1390–1397. [Google Scholar] [CrossRef]
- Singh, K.; Colmegna, I.; He, X.; Weyand, C.M.; Goronzy, J.J. Synoviocyte stimulation by the LFA-1-intercellular adhesion molecule-2-Ezrin-Akt pathway in rheumatoid arthritis. J. Immunol. 2008, 180, 1971–1978. [Google Scholar] [CrossRef]
- Huang, D.; Zhao, Q.; Liu, H.; Guo, Y.; Xu, H. PPAR-α agonist WY-14643 inhibits LPS-Induced inflammation in synovial fibroblasts via NF-kB pathway. J. Mol. Neurosci. MN 2016, 59, 544–553. [Google Scholar] [CrossRef]
- Lee, K.-T.; Su, C.-H.; Liu, S.-C.; Chen, B.-C.; Chang, J.-W.; Tsai, C.-H.; Huang, W.-C.; Hsu, C.-J.; Chen, W.-C.; Wu, Y.-C.; et al. Cordycerebroside A inhibits ICAM-1-dependent M1 monocyte adhesion to osteoarthritis synovial fibroblasts. J. Food Biochem. 2022, 46, e14108. [Google Scholar] [CrossRef]
- Han, D.; Fang, Y.; Tan, X.; Jiang, H.; Gong, X.; Wang, X.; Hong, W.; Tu, J.; Wei, W. The emerging role of fibroblast-like synoviocytes-mediated synovitis in osteoarthritis: An update. J. Cell. Mol. Med. 2020, 24, 9518–9532. [Google Scholar] [CrossRef] [PubMed]
- Gui, T.; Lin, Y.-K.; Huan, S.-W.; Li, Y.-H.; Wang, B.-H.; Yang, J.; Gu, R.-H.; Liu, Q.; Li, Z.-Y.; Li, S.-M.; et al. Elevated expression of ICAM-1 in synovium is associated with early inflammatory response for cartilage degeneration in type 2 diabetes mellitus. J. Cell Biochem. 2019, 120, 13177–13186. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Shimizu, S.; Ohhinata, K.; Naito, S.; Tokuyama, S.; Mori, Y.; Kiuchi, Y.; Yamamoto, T. Differential roles of ICAM-1 and E-selectin in polymorphonuclear leukocyte-induced angiogenesis. Am. J. Physiol. Cell Physiol. 2002, 282, C917–C925. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Panés, J. Integrin Inhibitors in Inflammatory Bowel Disease: From Therapeutic Antibodies to Small-Molecule Drugs. Gastroenterology 2021, 161, 1791–1793. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.-F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Cyrille, M.; Hansen, M.B.; Feagan, B.G.; Loftus, E.V.; Rogler, G.; Vermeire, S.; Cruz, M.L.; Yang, J.; Boedigheimer, M.J.; et al. Efficacy and Safety of Abrilumab in a Randomized, Placebo-Controlled Trial for Moderate-to-Severe Ulcerative Colitis. Gastroenterology 2019, 156, 946–957.e18. [Google Scholar] [CrossRef]
- Rubin, D.T.; Dotan, I.; DuVall, A.; Bouhnik, Y.; Radford-Smith, G.; Higgins, P.D.R.; Mishkin, D.S.; Arrisi, P.; Scalori, A.; Oh, Y.S.; et al. Etrolizumab versus adalimumab or placebo as induction therapy for moderately to severely active ulcerative colitis (HIBISCUS): Two phase 3 randomised, controlled trials. Lancet Gastroenterol. Hepatol. 2022, 7, 17–27. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Mattheakis, L.C.; Modi, N.B.; Pugatch, D.; Bressler, B.; Lee, S.; Bhandari, R.; Kanwar, B.; Shames, R.; D’Haens, G.; et al. PTG-100, an Oral α4β7 Antagonist Peptide: Preclinical Development and Phase 1 and 2a Studies in Ulcerative Colitis. Gastroenterology 2021, 161, 1853–1864.e10. [Google Scholar] [CrossRef]
- Yoshimura, N.; Watanabe, M.; Motoya, S.; Tominaga, K.; Matsuoka, K.; Iwakiri, R.; Watanabe, K.; Hibi, T. AJM300 Study Group Safety and Efficacy of AJM300, an Oral Antagonist of α4 Integrin, in Induction Therapy for Patients With Active Ulcerative Colitis. Gastroenterology 2015, 149, 1775–1783.e2. [Google Scholar] [CrossRef]
- Scheinfeld, N. Efalizumab: A review of events reported during clinical trials and side effects. Expert Opin. Drug Saf. 2006, 5, 197–209. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, T.; Zhang, Z.; Deng, C.; Ma, X.; Liu, X. Effects of β2 Integrins on Osteoclasts, Macrophages, Chondrocytes, and Synovial Fibroblasts in Osteoarthritis. Biomolecules 2022, 12, 1653. https://doi.org/10.3390/biom12111653
Hu T, Zhang Z, Deng C, Ma X, Liu X. Effects of β2 Integrins on Osteoclasts, Macrophages, Chondrocytes, and Synovial Fibroblasts in Osteoarthritis. Biomolecules. 2022; 12(11):1653. https://doi.org/10.3390/biom12111653
Chicago/Turabian StyleHu, Tiantian, Zhan Zhang, Chunbo Deng, Xun Ma, and Xueyong Liu. 2022. "Effects of β2 Integrins on Osteoclasts, Macrophages, Chondrocytes, and Synovial Fibroblasts in Osteoarthritis" Biomolecules 12, no. 11: 1653. https://doi.org/10.3390/biom12111653
APA StyleHu, T., Zhang, Z., Deng, C., Ma, X., & Liu, X. (2022). Effects of β2 Integrins on Osteoclasts, Macrophages, Chondrocytes, and Synovial Fibroblasts in Osteoarthritis. Biomolecules, 12(11), 1653. https://doi.org/10.3390/biom12111653





