Seasonal Changes in the Biochemical Constituents of Green Seaweed Chaetomorpha antennina from Covelong, India
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site and Collection
2.2. Photosynthetic Pigments
2.3. Metabolite Analysis
2.4. Redox State Estimation
2.5. Estimation of Minerals
2.6. Statistics
3. Results
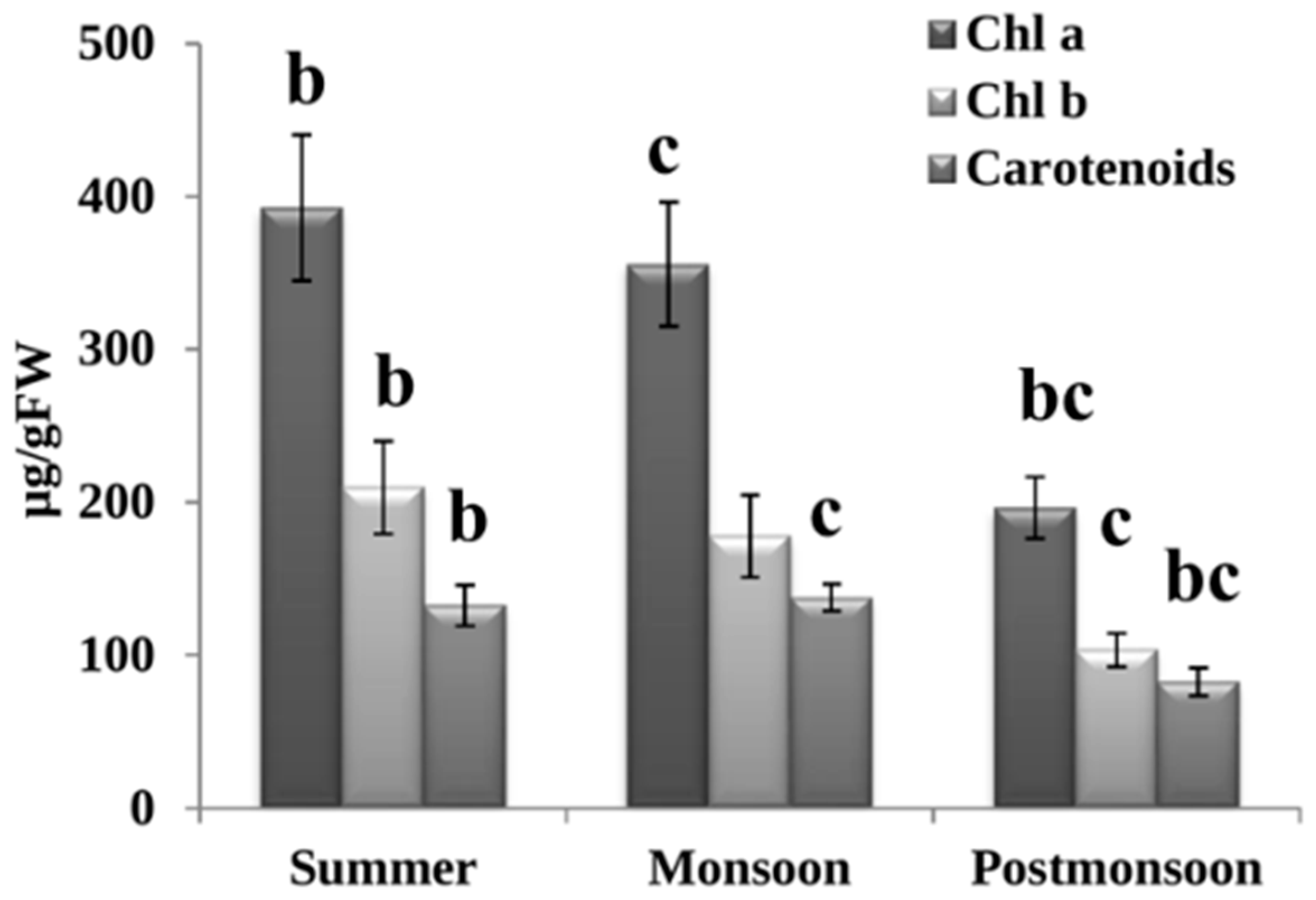
3.1. Photosynthetic Pigments
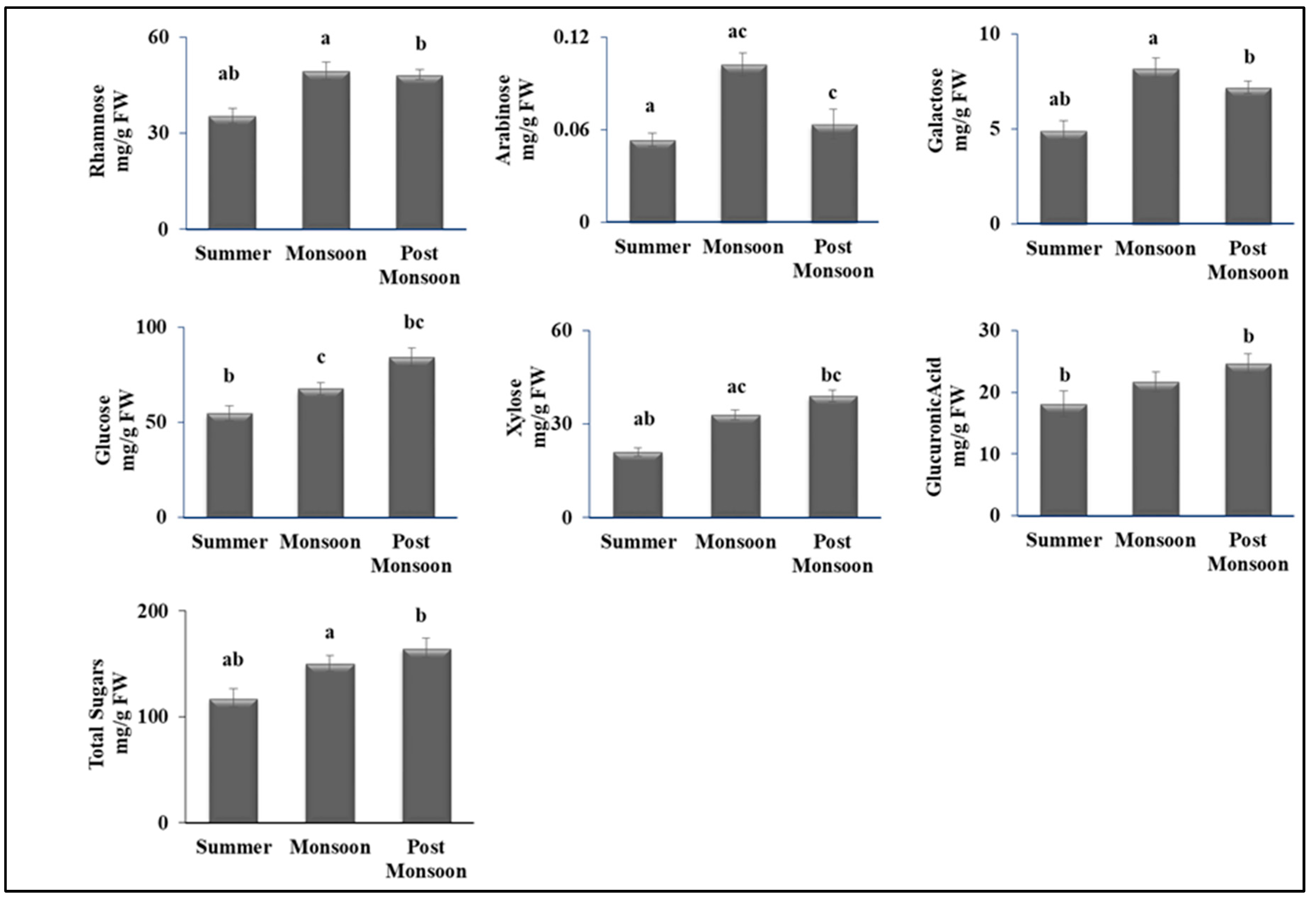
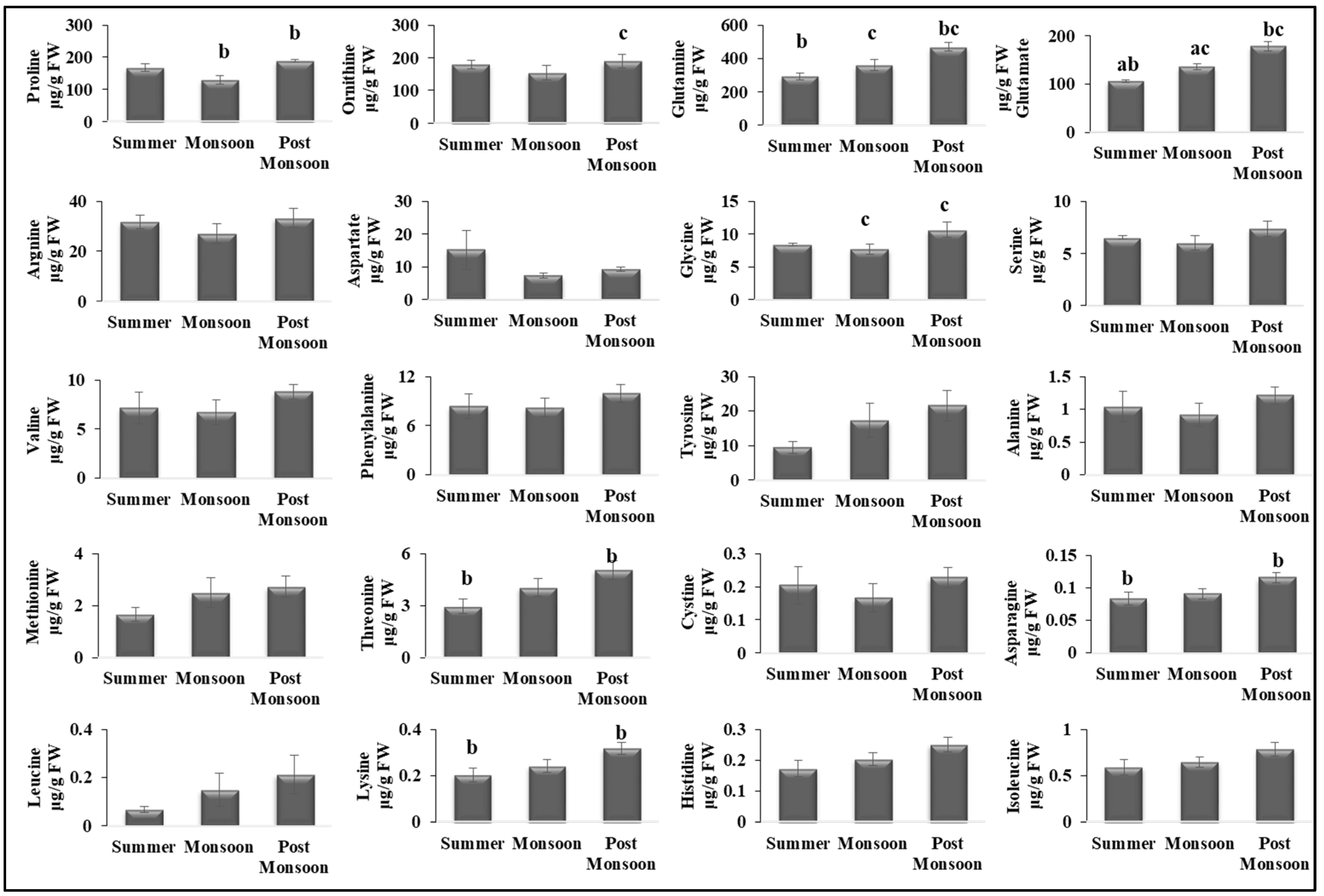
3.2. Seasonal Variability Affected Primary Metabolites
3.3. Elemental Composition
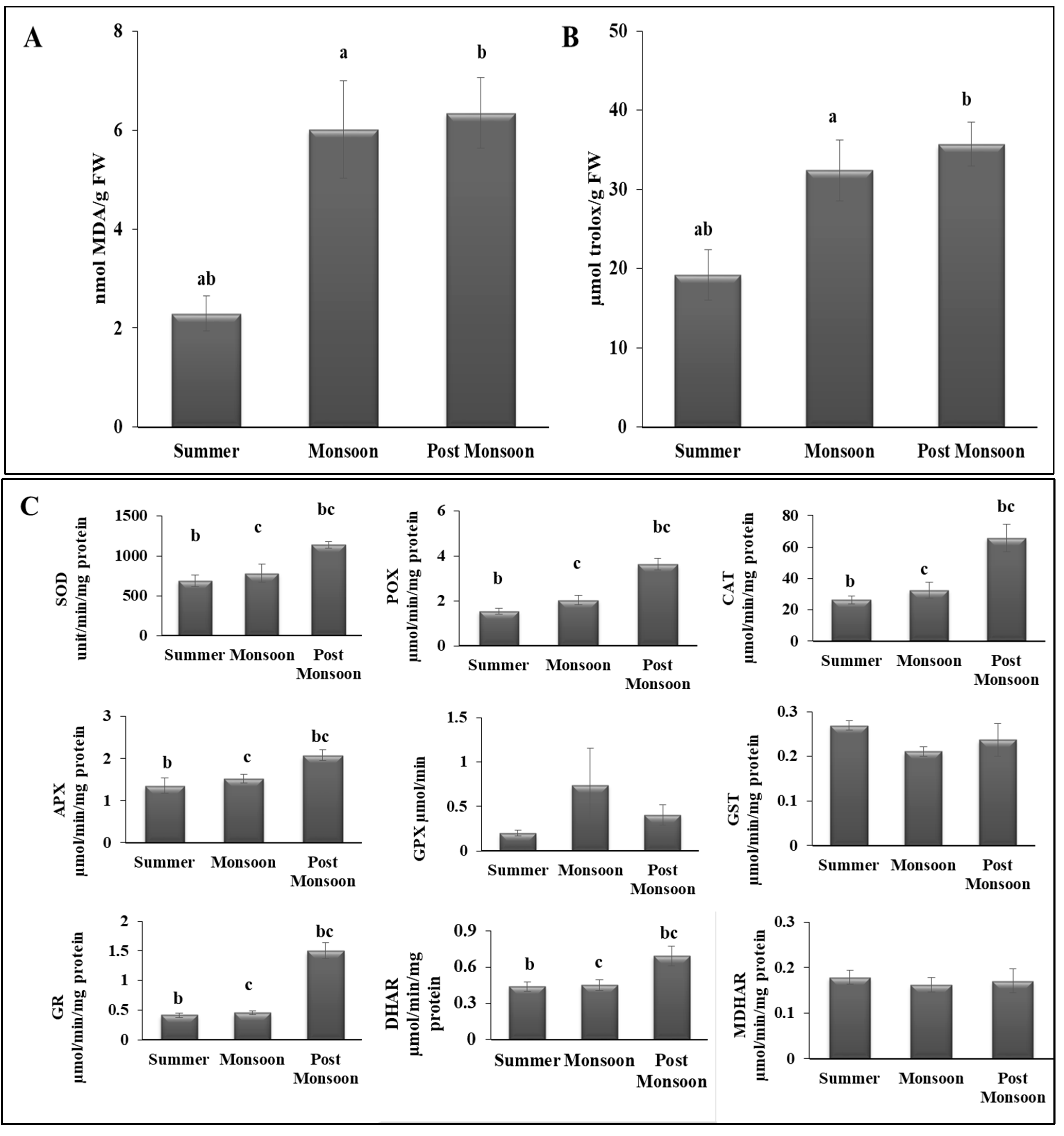
3.4. Redox Status
3.5. Global Change in the Metabolites and Mineral Composition of C. antennina in Different Seasons
4. Discussion
4.1. Seasonal Changes Affected Photosynthetic Pigments of C. antennina
4.2. Seasonal Changes Altered the Primary Metabolism of C. antennina
4.3. Seasonal Changes Altered the Antioxidant Properties of C. antennina
4.4. Variations in Minerals and Trace Elements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lalegerie, F.; Gager, L.; Stiger-Pouvreau, V.; Connan, S. The stressful life of red and brown seaweeds on the temperate intertidal zone: Effect of abiotic and biotic parameters on the physiology of macroalgae and content variability of particular metabolites. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 95, pp. 247–287. [Google Scholar]
- Britton, D.; Schmid, M.; Revill, A.T.; Virtue, P.; Nichols, P.D.; Hurd, C.L.; Mundy, C.N. Seasonal and site-specific variation in the nutritional quality of temperate seaweed assemblages: Implications for grazing invertebrates and the commercial exploitation of seaweeds. J. Appl. Phycol. 2021, 33, 603–616. [Google Scholar] [CrossRef]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Al-Hafedh, Y.S.; Alam, A.; Buschmann, A.H. Bioremediation potential, growth and biomass yield of the green seaweed, Ulva lactuca in an integrated marine aquaculture system at the Red Sea coast of Saudi Arabia at different stocking densities and effluent flow rates. Rev. Aquac. 2015, 7, 161–171. [Google Scholar] [CrossRef]
- Mansour, A.T.; Alprol, A.E.; Abualnaja, K.M.; El-Beltagi, H.S.; Ramadan, K.M.A.; Ashour, M. Dried Brown Seaweed’s Phytoremediation Potential for Methylene Blue Dye Removal from Aquatic Environments. Polymers 2022, 14, 1375. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.; Hassan, S.M.; Elshobary, M.E.; Ammar, G.A.G.; Gaber, A.; Alsanie, W.F.; Mansour, A.T.; El-Shenody, R. Impact of Commercial Seaweed Liquid Extract (TAM®) Biostimulant and Its Bioactive Molecules on Growth and Antioxidant Activities of Hot Pepper (Capsicum annuum). Plants 2021, 10, 1045. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.A.; Ghanem, S.M. Seasonal variation in the growth responses of some chlorophytic algal flora of the Red Sea. Egypt. J. Aquat. Res. 2017, 43, 129–134. [Google Scholar] [CrossRef]
- Fleurence, J.; Levine, I. Seaweed in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Davison, I.R.; Pearson, G.A. Stress tolerance in intertidal seaweeds. J. Phycol. 1996, 32, 197–211. [Google Scholar] [CrossRef]
- Lüning, K. Seaweeds: Their Environment, Biogeography, and Ecophysiology; John Wiley & Sons: Hoboken, NJ, USA, 1991. [Google Scholar]
- Schmid, M.; Guihéneuf, F.; Stengel, D.B. Ecological and commercial implications of temporal and spatial variability in the composition of pigments and fatty acids in five Irish macroalgae. Mar. Biol. 2017, 164, 1–18. [Google Scholar] [CrossRef]
- Chappuis, E.; Terradas, M.; Cefalì, M.E.; Mariani, S.; Ballesteros, E. Vertical zonation is the main distribution pattern of littoral assemblages on rocky shores at a regional scale. Estuar. Coast. Shelf Sci. 2014, 147, 113–122. [Google Scholar] [CrossRef]
- Olsson, J.; Toth, G.B.; Albers, E. Biochemical composition of red, green and brown seaweeds on the Swedish west coast. J. Appl. Phycol. 2020, 32, 3305–3317. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Fonseca, P.; Carneiro, M.; Moreira, W. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour. Technol. 2006, 97, 2402–2406. [Google Scholar] [CrossRef] [PubMed]
- Harley, C.D.; Anderson, K.M.; Demes, K.W.; Jorve, J.P.; Kordas, R.L.; Coyle, T.A.; Graham, M.H. Effects of climate change on global seaweed communities. J. Phycol. 2012, 48, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, K.H.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P. Metabolites from algae with economical impact. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef]
- Aguilera, J.; Dummermuth, A.; Karsten, U.; Schriek, R.; Wiencke, C. Enzymatic defences against photooxidative stress induced by ultraviolet radiation in Arctic marine macroalgae. Polar Biol. 2002, 25, 432–441. [Google Scholar] [CrossRef]
- Kumar, M.; Gupta, V.; Trivedi, N.; Kumari, P.; Bijo, A.; Reddy, C.; Jha, B. Desiccation induced oxidative stress and its biochemical responses in intertidal red alga Gracilaria corticata (Gracilariales, Rhodophyta). Environ. Exp. Bot. 2011, 72, 194–201. [Google Scholar] [CrossRef]
- Liu, F.; Pang, S.J. Stress tolerance and antioxidant enzymatic activities in the metabolisms of the reactive oxygen species in two intertidal red algae Grateloupia turuturu and Palmaria palmata. J. Exp. Mar. Biol. Ecol. 2010, 382, 82–87. [Google Scholar] [CrossRef]
- Maharana, D.; Das, P.B.; Verlecar, X.N.; Pise, N.M.; Gauns, M. Oxidative stress tolerance in intertidal red seaweed Hypnea musciformis (Wulfen) in relation to environmental components. Environ. Sci. Pollut. Res. 2015, 22, 18741–18749. [Google Scholar] [CrossRef]
- Vinuganesh, A.; Kumar, A.; Prakash, S.; Alotaibi, M.O.; Saleh, A.M.; Mohammed, A.E.; Beemster, G.T.; AbdElgawad, H. Influence of seawater acidification on biochemical composition and oxidative status of green algae Ulva compressa. Sci. Total Environ. 2022, 806, 150445. [Google Scholar] [CrossRef]
- Banerjee, K.; Ghosh, R.; Homechaudhuri, S.; Mitra, A. Seasonal variation in the biochemical composition of red seaweed (Catenella repens) from Gangetic delta, northeast coast of India. J. Earth Syst. Sci. 2009, 118, 497–505. [Google Scholar] [CrossRef]
- Suresh Kumar, K.; Ganesan, K.; Subba Rao, P. Seasonal variation in nutritional composition of Kappaphycus alvarezii (Doty) Doty—An edible seaweed. J. Food Sci. Technol. 2015, 52, 2751–2760. [Google Scholar] [CrossRef] [PubMed]
- Aroyehun, A.Q.; Palaniveloo, K.; Ghazali, F.; Rizman-Idid, M.; Abdul Razak, S. Effects of seasonal variability on the physicochemical, biochemical, and nutritional composition of Western Peninsular Malaysia Gracilaria manilaensis. Molecules 2019, 24, 3298. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.; Barroso, S.; Ferreira, A.S.; Adão, P.; Mendes, S.; Gil, M.M. Seasonal Evaluation of Phlorotannin-Enriched Extracts from Brown Macroalgae Fucus spiralis. Molecules 2021, 26, 4287. [Google Scholar] [CrossRef] [PubMed]
- Celis-Plá, P.S.; Bouzon, Z.L.; Hall-Spencer, J.M.; Schmidt, E.C.; Korbee, N.; Figueroa, F.L. Seasonal biochemical and photophysiological responses in the intertidal macroalga Cystoseira tamariscifolia (Ochrophyta). Mar. Environ. Res. 2016, 115, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Seasonal variability of the biochemical composition and antioxidant properties of Fucus spiralis at two Azorean Islands. Mar. Drugs 2018, 16, 248. [Google Scholar] [CrossRef] [PubMed]
- Vilg, J.V.; Nylund, G.M.; Werner, T.; Qvirist, L.; Mayers, J.J.; Pavia, H.; Undeland, I.; Albers, E. Seasonal and spatial variation in biochemical composition of Saccharina latissima during a potential harvesting season for Western Sweden. Bot. Mar. 2015, 58, 435–447. [Google Scholar] [CrossRef]
- Bolton, J.; Oyieke, H.; Gwada, P. The seaweeds of Kenya: Checklist, history of seaweed study, coastal environment, and analysis of seaweed diversity and biogeography. S. Afr. J. Bot. 2007, 73, 76–88. [Google Scholar] [CrossRef]
- Littler, M.M.; Martz, D.R.; Littler, D.S. Effects of recurrent sand deposition on rocky intertidal organisms: Importance of substrate heterogeneity in a fluctuating environment. Mar. Ecol. Prog. Ser. Oldendorf 1983, 11, 129–139. [Google Scholar] [CrossRef]
- Bastianoni, S.; Coppola, F.; Tiezzi, E.; Colacevich, A.; Borghini, F.; Focardi, S. Biofuel potential production from the Orbetello lagoon macroalgae: A comparison with sunflower feedstock. Biomass Bioenergy 2008, 32, 619–628. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Wang, G. Two-stage Hydrolysis of Invasive Algal Feedstock for Ethanol Fermentation F. J. Integr. Plant Biol. 2011, 53, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, M.; Trivedi, N.; Gupta, V.; Madhav, S.V.; Reddy, C.R.; Levine, I.A. Seaweed resources in India–current status of diversity and cultivation: Prospects and challenges. Bot. Mar. 2019, 62, 463–482. [Google Scholar] [CrossRef]
- Roy, S. Seaweed diversity within intertidal zone of Olaikuda and Vadakkadu, Rameshwaram, southeast coast of India. Heliyon 2020, 6, e04585. [Google Scholar] [CrossRef] [PubMed]
- Sahayaraj, K.; Rajesh, S.; Asha, A.; Rathi, J.; Raja, P. Distribution and diversity assessment of the marine macroalgae at four southern districts of Tamil Nadu, India. Indian J. Geo-Mar. Sci. 2014, 43, 607–617. [Google Scholar]
- UshaKiran, B.; Treasa, M.; Sathianandan, T.; Kaladharan, P. Marine macroalgal resources from nine beaches along the Kerala coast, India. J. Mar. Biol. Assoc. India 2017, 59, 73–81. [Google Scholar] [CrossRef]
- Babu, M.Y.; Palanikumar, L.; Nagarani, N.; Devi, V.J.; Kumar, S.R.; Ramakritinan, C.; Kumaraguru, A. Cadmium and copper toxicity in three marine macroalgae: Evaluation of the biochemical responses and DNA damage. Environ. Sci. Pollut. Res. 2014, 21, 9604–9616. [Google Scholar] [CrossRef] [PubMed]
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs 2012, 10, 403–416. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Vijayakumar, S.; Mukherjee, A.; Chandrasekaran, N.; Thomas, J. Antioxidant and antibacterial activity of Chaetomorpha antennina against shrimp pathogen Vibrio parahaemolyticus. Aquaculture 2014, 433, 467–475. [Google Scholar] [CrossRef]
- Unnikrishnan, P.; Jayasri, M. Antidiabetic studies of Chaetomorpha antennina extract using experimental models. J. Appl. Phycol. 2017, 29, 1047–1056. [Google Scholar] [CrossRef]
- Desikachary, T.V.; Krishnamurthy, V.; Balakrishnan, M.S. Rhodophyta: Volume II-Part-II B; Madras Science Foundation: Madras, India, 1998. [Google Scholar]
- Krishnamurthy, V. Algae of India and Neighbouring Countries; Science Publishers: New York, NY, USA, 2000. [Google Scholar]
- Rao, M.U. Key for identification of economically important seaweeds. CMFRI Bull. 1987, 41, 19–25. [Google Scholar]
- Lichtenthaler, H.K. [34] Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Alasalvar, C.; Shahidi, F.; Liyanapathirana, C.M.; Ohshima, T. Turkish tombul hazelnut (Corylus avellana L.). 1. Compositional characteristics. J. Agric. Food Chem. 2003, 51, 3790–3796. [Google Scholar] [CrossRef] [PubMed]
- Torras-Claveria, L.; Berkov, S.; Jáuregui, O.; Caujapé, J.; Viladomat, F.; Codina, C.; Bastida, J. Metabolic profiling of bioactive Pancratium canariense extracts by GC-MS. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2010, 21, 80–88. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. [2] Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 15–27. [Google Scholar]
- Potters, G.; Horemans, N.; Bellone, S.; Caubergs, R.J.; Trost, P.; Guisez, Y.; Asard, H. Dehydroascorbate influences the plant cell cycle through a glutathione-independent reduction mechanism. Plant Physiol. 2004, 134, 1479–1487. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Siebert, K.J. Modeling the flavor thresholds of organic acids in beer as a function of their molecular properties. Food Qual. Prefer. 1999, 10, 129–137. [Google Scholar] [CrossRef]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Microplate quantification of enzymes of the plant ascorbate–glutathione cycle. Anal. Biochem. 2008, 383, 320–322. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Drotar, A.; Phelps, P.; Fall, R. Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci. 1985, 42, 35–40. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Agusa, T.; Kunito, T.; Iwata, H.; Monirith, I.; Tana, T.S.; Subramanian, A.; Tanabe, S. Mercury contamination in human hair and fish from Cambodia: Levels, specific accumulation and risk assessment. Environ. Pollut. 2005, 134, 79–86. [Google Scholar] [CrossRef]
- Kumar, A.; Vinuganesh, A.; Prakash, S. An assessment of marine and coastal diversity of Covelong, Chennai, India. Reg. Stud. Mar. Sci. 2021, 48, 102034. [Google Scholar] [CrossRef]
- Pereira, D.C.; Trigueiro, T.G.; Colepicolo, P.; Marinho-Soriano, E. Seasonal changes in the pigment composition of natural population of Gracilaria domingensis (Gracilariales, Rhodophyta). Rev. Bras. De Farmacogn. 2012, 22, 874–880. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. Carotenoid composition in sun and shade leaves of plants with different life forms. Plant Cell Environ. 1992, 15, 411–419. [Google Scholar] [CrossRef]
- Gu, J.; Zhou, Z.; Li, Z.; Chen, Y.; Wang, Z.; Zhang, H.; Yang, J. Photosynthetic properties and potentials for improvement of photosynthesis in pale green leaf rice under high light conditions. Front. Plant Sci. 2017, 8, 1082. [Google Scholar] [CrossRef] [PubMed]
- Eismann, A.I.; Reis, R.P.; da Silva, A.F.; Cavalcanti, D.N. Ulva spp. carotenoids: Responses to environmental conditions. Algal Res. 2020, 48, 101916. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, Z.; Zou, D.; Gao, K. Ecophysiological responses of marine macroalgae to climate change factors. J. Appl. Phycol. 2016, 28, 2953–2967. [Google Scholar] [CrossRef]
- Khairy, H.M.; El-Shafay, S.M. Seasonal variations in the biochemical composition of some common seaweed species from the coast of Abu Qir Bay, Alexandria, Egypt. Oceanologia 2013, 55, 435–452. [Google Scholar] [CrossRef]
- Afonso, C.; Correia, A.P.; Freitas, M.V.; Baptista, T.; Neves, M.; Mouga, T. Seasonal changes in the nutritional composition of Agarophyton vermiculophyllum (Rhodophyta, Gracilariales) from the center of Portugal. Foods 2021, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- Nichols, P.D.; Virtue, P.; Mooney, B.; Elliott, N.; Yearsley, G. Seafood the Good Food: The Oil (Fat) Content and Composition of Australian Commercial Fishes, Shellfishes and Crustaceans; CSIRO Div. of Marine Research//Fisheries Research & Development Corporation: Hobart, Australia, 1998. [Google Scholar]
- Sánchez-Machado, D.; López-Cervantes, J.; Lopez-Hernandez, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Ansari, A.A.; Ghanem, S.M. Growth attributes and biochemical composition of Padina pavonica (L.) from the Red Sea, in response to seasonal alterations of Tabuk, Saudi Arabia. Egypt. J. Aquat. Res. 2019, 45, 139–144. [Google Scholar] [CrossRef]
- Nordøy, A. Fish oils in clinical medicine. J. Intern. Med. 1989, 225, 145–146. [Google Scholar] [CrossRef]
- Khatkar, D.; Kuhad, M. Short-term salinity induced changes in two wheat cultivars at different growth stages. Biol. Plant. 2000, 43, 629–632. [Google Scholar] [CrossRef]
- Lesser, M.P. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Farasat, M.; Khavari-Nejad, R.-A.; Nabavi, S.M.B.; Namjooyan, F. Antioxidant properties of some filamentous green algae (Chaetomorpha Genus). Braz. Arch. Biol. Technol. 2013, 56, 921–927. [Google Scholar] [CrossRef]
- Marinho, G.S.; Sørensen, A.-D.M.; Safafar, H.; Pedersen, A.H.; Holdt, S.L. Antioxidant content and activity of the seaweed Saccharina latissima: A seasonal perspective. J. Appl. Phycol. 2019, 31, 1343–1354. [Google Scholar] [CrossRef]
- Rozema, J.; Björn, L.O.; Bornman, J.; Gaberščik, A.; Häder, D.-P.; Trošt, T.; Germ, M.; Klisch, M.; Gröniger, A.; Sinha, R. The role of UV-B radiation in aquatic and terrestrial ecosystems—An experimental and functional analysis of the evolution of UV-absorbing compounds. J. Photochem. Photobiol. B Biol. 2002, 66, 2–12. [Google Scholar] [CrossRef]
- Wu, S.-C.; Wang, F.-J.; Pan, C.-L. The comparison of antioxidative properties of seaweed oligosaccharides fermented by two lactic acid bacteria. J. Mar. Sci. Technol. 2010, 18, 8. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Stockdale, A.; Tipping, E.; Lofts, S.; Mortimer, R.J. Effect of ocean acidification on organic and inorganic speciation of trace metals. Environ. Sci. Technol. 2016, 50, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A. Minerals from macroalgae origin: Health benefits and risks for consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef]
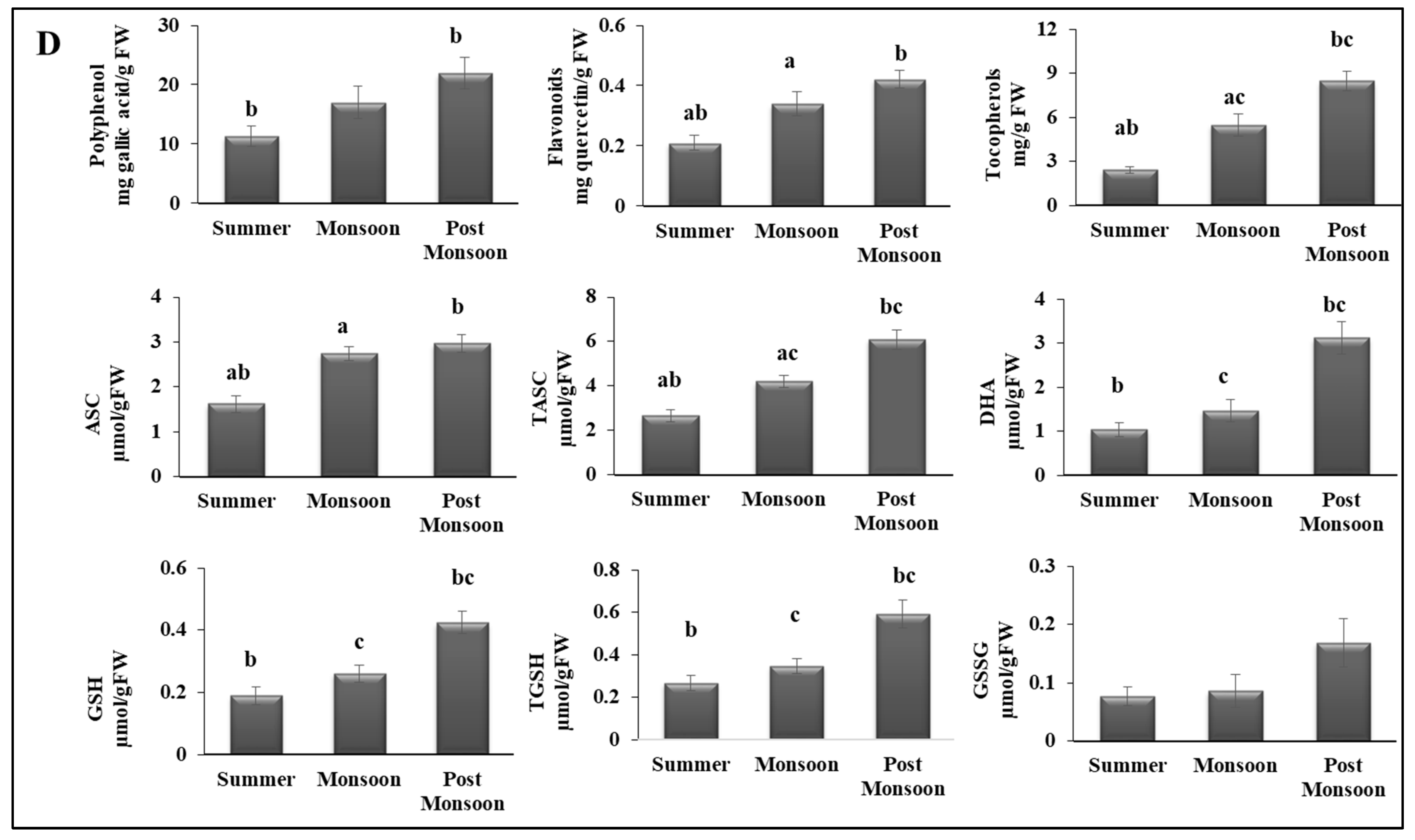
 represents pigments,
represents pigments,  antioxidants,
antioxidants,  minerals,
minerals,  sugars,
sugars,  amino acids, and
amino acids, and  fatty acids).
fatty acids).
 represents pigments,
represents pigments,  antioxidants,
antioxidants,  minerals,
minerals,  sugars,
sugars,  amino acids, and
amino acids, and  fatty acids).
fatty acids).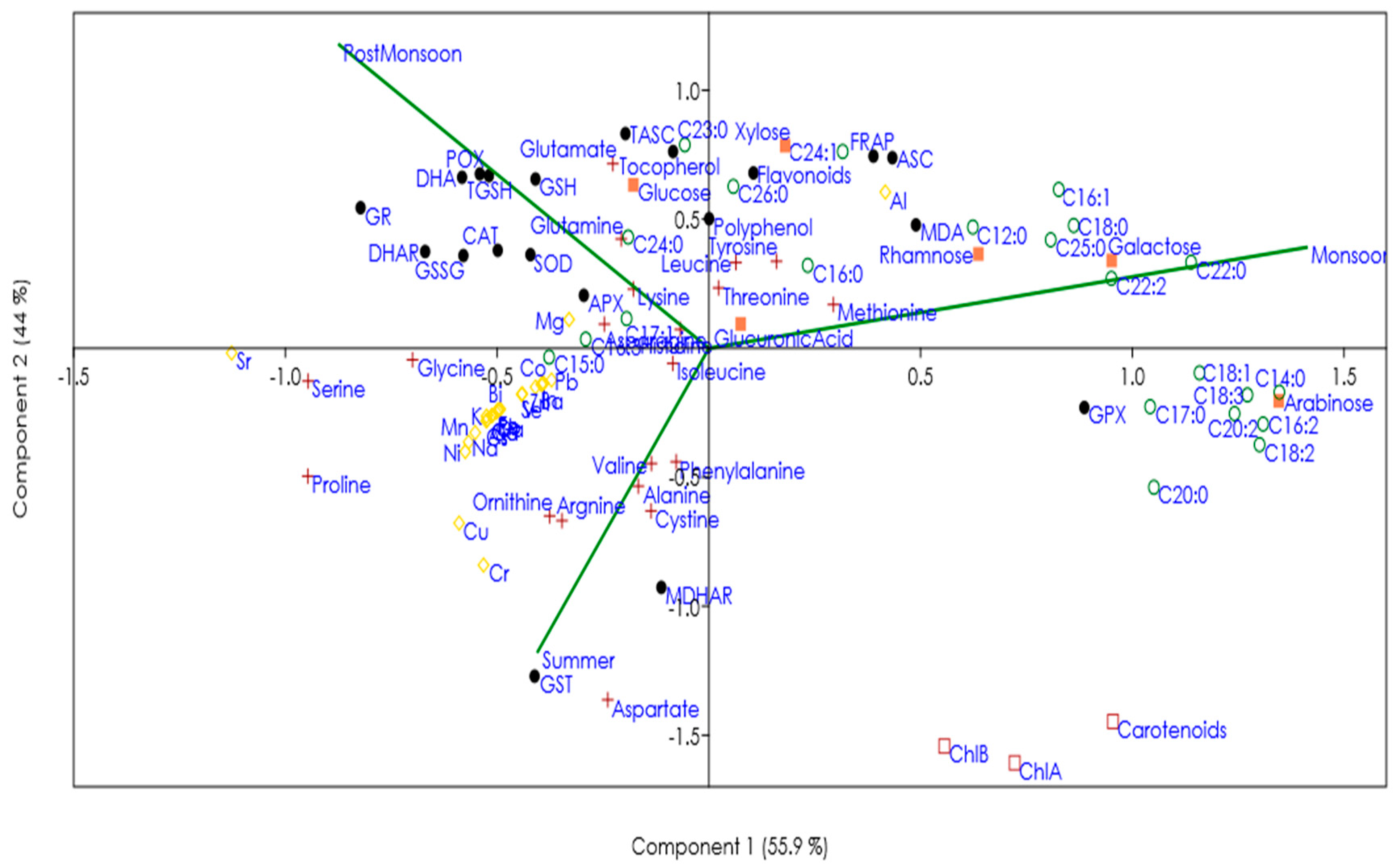
| Summer | Monsoon | Post-Monsoon | |
|---|---|---|---|
| C12:0 | 0.289 ± 0.023 ab | 0.573 ± 0.036 a | 0.562 ± 0.084 b |
| C14:0 | 0.011 ± 0.001 a | 0.022 ± 0.002 ac | 0.014 ± 0.002 c |
| C15:0 | 0.017 ± 0.002 | 0.031 ± 0.002 | 1.565 ± 1.543 |
| C16:0 | 8.481 ± 0.623 | 10.806 ± 0.536 | 11.742 ± 2.291 |
| C16:1 | 0.079 ± 0.005 ab | 0.128 ± 0.008 a | 0.121 ± 0.017 b |
| C16:2 | 0.007 ± 0.001 a | 0.012 ± 0.001 ac | 0.008 ± 0.001 c |
| C16:3 | 0.007 ± 0.001 | 0.013 ± 0.001 | 0.049 ± 0.04 |
| C17:0 | 0.225 ± 0.02 a | 0.32 ± 0.021 a | 0.253 ± 0.04 |
| C17:1 | 0.048 ± 0.005 | 0.087 ± 0.004 | 0.187 ± 0.128 |
| C18:0 | 0.751 ± 0.062 ab | 1.306 ± 0.084 a | 1.191 ± 0.061 b |
| C18:1 | 3.244 ± 0.251 a | 6.378 ± 0.649 ac | 4.337 ± 0.713 c |
| C18:2 | 0.185 ± 0.022 a | 0.328 ± 0.019 ac | 0.198 ± 0.032 c |
| C18:3 | 0.033 ± 0.002 a | 0.059 ± 0.005 ac | 0.039 ± 0.006 c |
| C20:0 | 0.018 ± 0.004 | 0.03 ± 0.004 | 0.017 ± 0.003 |
| C20:2 | 0.02 ± 0.002 a | 0.037 ± 0.004 ac | 0.024 ± 0.004 c |
| C22:0 | 0.011 ± 0.001 a | 0.02 ± 0.001 a | 0.016 ± 0.002 |
| C22:2 | 0.016 ± 0.002 ab | 0.029 ± 0.002 a | 0.024 ± 0.002 b |
| C23:0 | 0.007 ± 0.001 ab | 0.016 ± 0.004 a | 0.024 ± 0.002 b |
| C24:0 | 0.011 ± 0.001 b | 0.02 ± 0.002 | 0.034 ± 0.01 b |
| C24:1 | 0.039 ± 0.002 ab | 0.07 ± 0.007 a | 0.08 ± 0.006 b |
| C25:0 | 0.03 ± 0.002 ab | 0.061 ± 0.007 a | 0.055 ± 0.007 b |
| C26:0 | 0.0008 ± 0.0001 b | 0.0014 ± 0.0001 | 0.0018 ± 0.0001 b |
| Summer | Monsoon | Post-Monsoon | |
|---|---|---|---|
| Na | 18453.2 ± 509.5 | 17262.2 ± 1170.6 c | 20336.1 ± 567.3 c |
| Rb | 4.7 ± 0.2 | 4.5 ± 0.4 c | 5.4 ± 0.2 c |
| Sr | 897.2 ± 24.8 ab | 780.7 ± 21.2 ac | 1091.3 ± 30 bc |
| Cd | 0.283 ± 0.009 | 0.268 ± 0.021 c | 0.319 ± 0.01 c |
| In | 0.37 ± 0.02 | 0.36 ± 0.04 | 0.45 ± 0.02 |
| Cs | 0.53 ± 0.01 | 0.5 ± 0.04 c | 0.59 ± 0.02 c |
| Ba | 79.4 ± 3.6 | 77.4 ± 8.5 | 96.5 ± 3 |
| Pb | 5.2 ± 0.3 | 5.1 ± 0.6 | 6.4 ± 0.3 |
| Bi | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.16 ± 0.01 |
| Mg | 2917.6 ± 88.7 b | 2973.9 ± 45.6 c | 3280.9 ± 92.7 bc |
| Al | 97.7 ± 5.1 ab | 136.5 ± 10.3 a | 144.1 ± 4.5 b |
| Ca | 3600.9 ± 104.6 | 3400.5 ± 262.4 c | 4058.5 ± 110.5 c |
| V | 1.2 ± 0.05 | 1.15 ± 0.11 | 1.41 ± 0.05 |
| Cr | 9.9 ± 0.3 | 8.9 ± 0.3 | 9.9 ± 0.3 |
| Mn | 62.1 ± 1.7 | 57.8 ± 3.7 c | 67.7 ± 1.9 c |
| Fe | 182.5 ± 5.8 | 173.1 ± 14.2 c | 207.7 ± 6 c |
| Co | 0.29 ± 0.01 | 0.28 ± 0.03 | 0.36 ± 0.01 |
| Ni | 2.6 ± 0.1 | 2.4 ± 0.2 | 2.8 ± 0.1 |
| Cu | 7.3 ± 0.2 | 6.6 ± 0.3 | 7.5 ± 0.2 |
| Zn | 72.4 ± 3 | 70.2 ± 7.4 | 87 ± 2.7 |
| Ga | 0.063 ± 0.002 | 0.059 ± 0.005 c | 0.071 ± 0.002 c |
| K | 288 ± 13.6 | 268.8 ± 23.3 c | 330.2 ± 8.3 c |
| As | 25.5 ± 0.7 | 24 ± 1.8 c | 28.5 ± 0.8 c |
| Se | 4.9 ± 0.2 | 4.7 ± 0.4 c | 5.7 ± 0.2 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinuganesh, A.; Kumar, A.; Korany, S.M.; Alsherif, E.A.; Selim, S.; Prakash, S.; Beemster, G.T.S.; AbdElgawad, H. Seasonal Changes in the Biochemical Constituents of Green Seaweed Chaetomorpha antennina from Covelong, India. Biomolecules 2022, 12, 1475. https://doi.org/10.3390/biom12101475
Vinuganesh A, Kumar A, Korany SM, Alsherif EA, Selim S, Prakash S, Beemster GTS, AbdElgawad H. Seasonal Changes in the Biochemical Constituents of Green Seaweed Chaetomorpha antennina from Covelong, India. Biomolecules. 2022; 12(10):1475. https://doi.org/10.3390/biom12101475
Chicago/Turabian StyleVinuganesh, A., Amit Kumar, Shereen Magdy Korany, Emad A. Alsherif, Samy Selim, Sanjeevi Prakash, Gerrit T. S. Beemster, and Hamada AbdElgawad. 2022. "Seasonal Changes in the Biochemical Constituents of Green Seaweed Chaetomorpha antennina from Covelong, India" Biomolecules 12, no. 10: 1475. https://doi.org/10.3390/biom12101475
APA StyleVinuganesh, A., Kumar, A., Korany, S. M., Alsherif, E. A., Selim, S., Prakash, S., Beemster, G. T. S., & AbdElgawad, H. (2022). Seasonal Changes in the Biochemical Constituents of Green Seaweed Chaetomorpha antennina from Covelong, India. Biomolecules, 12(10), 1475. https://doi.org/10.3390/biom12101475








