Abstract
Gangliosides are molecules widely present in the plasma membranes of mammalian cells, participating in a variety of processes, including protein organization, transmembrane signalling and cell adhesion. Gangliosides are abundant in the grey matter of the brain, where they are critically involved in postnatal neural development and function. The common precursor of the majority of brain gangliosides, GM3, is formed by the sialylation of lactosylceramide, and four derivatives of its a- and b-series, GM1, GD1a, GD1b and GT1b, constitute 95% of all the brain gangliosides. Impairments in ganglioside metabolism due to genetic abnormalities of GM-synthases are associated with severe neurological disorders. Apart from that, the latest genome-wide association and translational studies suggest a role of genes involved in brain ganglioside synthesis in less pervasive psychiatric disorders. Remarkably, the most recent animal studies showed that abnormal ganglioside functions result in dysregulated neuroinflammation, aberrant myelination and altered insulin receptor signalling. At the same time, these molecular features are well established as accompanying developmental psychiatric disorders such as attention-deficit hyperactivity disorder (ADHD) and autism spectrum disorders (ASD). This led us to hypothesize a role of deficient ganglioside function in developmental neuropsychiatric disorders and warrants further gene association clinical studies addressing this question. Here, we critically review the literature to discuss this hypothesis and focus on the recent studies on ST3GAL5-deficient mice. In addition, we elaborate on the therapeutic potential of various anti-inflammatory remedies for treatment of developmental neuropsychiatric conditions related to aberrant ganglioside functions.
1. Introduction
Gangliosides or sialo-glycolipids are molecules consisting of glycosphingolipid and one or more sialic acid residues. They are ubiquitous in cell membranes in all vertebrates and are involved in many key cellular processes [1]. Gangliosides of the CNS play critical roles in development and function [2,3,4], facilitating neuronal membrane protein organization, signalling and cell adhesion [4,5]. Brain gangliosides also regulate microglia and cytokine-mediated immune responses, including microglial activation, myelination and platelet activation [2,6,7].
The majority of brain gangliosides consist of derivatives of ganglioside GM3 [8]. The ganglioside GM3, which is the precursor of the principal brain gangliosides including GM1, GD1a, GD1b, GD3, GT1b and GQ1b, is generated by alpha-2,3-sialyltransferase 5 (ST3GAL5) or GM3-synthase [8]. Outside of the brain, GM3 is also known to play a role in membrane microdomain functionality of insulin receptors and in the induction of insulin resistance [9]. Serum GM3 levels were found to be significantly elevated in type 2 diabetes patients with severe obesity [10].
Here, we critically review the recent clinical and pre-clinical evidence of the involvement of aberrations in ganglioside metabolism in a range of neuropsychiatric and CNS pathologies, including attention-deficit hyperactivity disorder (ADHD) and autism spectrum disorders (ASD), and review the possible underlying mechanisms, such as neuroinflammation and oxidative stress, insulin signalling dysregulation and myelination abnormalities, as possible pathophysiological patterns resulting from central ganglioside deficiency.
2. Impairment of Ganglioside Metabolism in CNS Disorders
Genetic impairments of ganglioside metabolism are associated with several human disorders, most of which affect ganglioside catabolism, and only few families with disruptions of ganglioside biosynthesis have been reported [4]. Aberrant ganglioside catabolism causes lysosomal ganglioside storage diseases, comprising GM1-gangliosidoses and three forms of GM2-gangliosidoses, which are a group of inherited metabolic diseases caused by a deficiency of the different proteins that break down gangliosides [11]. Under normal conditions, gangliosides are catabolized by a group of lysosomal hydrolases, β-hexosaminidases A and B, encoded by genes HEXA and HEXB, respectively [11]. Besides hexosaminidases, ganglioside GM2 catabolism also depends on GM2 activator protein (GM2-AP), encoded by the GM2A gene, which also provides GM2 for hexosaminidases [12].
Depending on the affected gene, HEXA, HEXB or GM2A, three variants of GM2 gangliosidoses have been identified: Tay-Sachs’s disease, Sandhoff disease and AB Variant GM2 gangliosidoses [13]. The former two are child-onset disorders, usually rapidly progressing and leading to child death before the age of four [11,14,15]. Tay-Sachs’s disease, or GM2 gangliosidosis variants B and B1, is characterized by acute onset at infantile age with manifestations such as seizures, axial hypotonia and regressions in developmental milestones [16,17,18]. In the case of Sandhoff disease, or variant 0, subacute juvenile onset of ataxia, myoclonus, motor regression, psychotic episodes, intellectual disability and progressive clumsiness are usually observed [18,19]. The AB Variant is an adult-onset chronic disease, characterized by dysphagia, muscle atrophy, cerebellar ataxia, dysarthric speech, muscle weakness, manic depression and psychotic episodes [11,20,21]. GM1 gangliosidosis, in turn, is caused by a deficiency of acid β-galactosidase, usually with infantile onset and clinical hallmarks such as dysmorphic face, severe dysostosis, hepatomegaly, inability to sit and muscle hypotonia, and later on, spasticity, visual failure, foam cells in bone marrow, oligo-sacchariduria and muco-polysacchariduria [12]. The substrates of acid β-galactosidase include not only GM1, but also specific oligosaccharides, so that this disease is marked not only by ganglioside storage but also by oligosaccharidosis and mucopolysaccharidosis, with extra-neuronal clinical involvement due to them [12].
Disruptions of ganglioside biosynthesis are found much less frequently and lead to catastrophic neurological deficits, including severe cognitive impairments, seizures and motor and sensory dysfunctions [22,23]. These ganglioside biosynthesis disorders arise from mutations in genes encoding GM3-synthase (ST3GAL5) [2,24,25,26] or GM2/GD2 synthase (B4GALNT1) [27,28]. Loss-of-function mutations in either of the proteins lead to the complete absence of major brain gangliosides [23,29]. Loss of B4GALNT1 activity leads to hereditary spastic paraplegia, for which the main clinical hallmark is a child-onset slowly progressing central demyelination with axon loss and lower extremity spasticity with muscle weakness accompanied by cognitive impairments [27,28,30]. Abnormalities in the ST3GAL5 gene disrupt GM3 synthesis, leading to intellectual disability, microcephaly, seizures, blindness and deafness, somatic growth failure and metabolic syndrome [23,31]. Notably, it was recently shown that, in families with mutations in B4GALNT1 that do not lead to the complete loss of the protein activity, the neurological phenotype was much milder than in those with complete loss of function [32]. These observations suggest that, besides complete loss of function of ganglioside-synthesizing proteins, there may be intermediate phenotypes with milder symptoms, indicating that alterations in ganglioside metabolism may be overlooked as contributors to other diseases besides gangliosidoses and severe neurologic conditions.
Indeed, emerging evidence suggests that ganglioside deficiency may have other effects in addition to the severe neuropathology observed in individuals with ST3GAL5 and/or B4GALNT1 deficiency. Recent genome-wide association studies (GWAS) have reported an association between SNPs in gene encoding alpha-2,3-sialyltransferase-III (ST3GAL3) and factors regulating ganglioside function and the incidence of schizophrenia, attention-deficit/hyperactivity disorder (ADHD) or autism spectrum disorders (ASD). In particular, in a large-scale integrative analysis of GWAS, comprised of 20,183 ADHD cases and 35,191 controls, using a DEPICT analysis of gene prioritization, pathway and tissue/cell type enrichment analysis, ST3GAL3 was the top gene associated with ADHD (P = 1.19 × 10−2) [33]. Furthermore, the homozygous loss-of-function mutation of SLC39A8, a well-established schizophrenia biomarker, was shown to result in serum manganese (Mn) abnormalities, a causal factor of glycosyltransferases’ dysfunction. This mechanism was suggested to underlie the pathophysiology of schizophrenia [34]. A more recent GWAS also revealed a relationship between the increased expression of a ST3GAL3 transcript in the human foetal brain and a risk for ADHD and schizophrenia [35]. Moreover, children with ASD often displayed increased anti-ganglioside antibody levels [36,37], and changes in ganglioside expression have also been proposed as a biomarker for schizophrenia [38].
However, changes in the ganglioside profile in the brain were also associated with Alzheimer’s disease and amyloidosis [39]. In the hippocampi of an APPswe/PS1dE9 transgenic mouse model of Alzheimer’s disease, the progressive downregulation of major gangliosides and upregulation of acetylated and N-acetyl-galactosaminylated gangliosides were observed with the development of the disease from the early to late stages. The authors speculated that such changes are attributed to the inhibition of GD3-synthase activity [39]. GM1 and other gangliosides were shown to stabilize amyloid fibrils by changing their secondary structure [40,41,42].
Recently, a deficiency of gangliosides GM1 and GD1a in peripheral blood mononuclear cells of patients with Parkinson’s disease was found [43]. A deficiency of gangliosides GM1 and GD1a was also found in the brains of patients with Parkinson’s disease; in particular, this deficit was shown in the substantia nigra [44]. Moreover, reduced synthesis of GM1 in fibroblasts was also revealed in patients with Huntington’s disease [45], and distinct ganglioside content changes were found in the n. caudatus, putamen and cerebellum of post-mortem Huntington’s brains [46]. Elevated levels of gangliosides GM1 and GM3 were found in the spinal cords of amyotrophic lateral sclerosis (ALS) patients, along with increased activities of enzymes mediating their hydrolysis [47]. Anti-ganglioside antibodies were found in a patient with ALS attributed to the P525L mutation in the fused in sarcoma (FUS) gene [48]. Thus, alterations of ganglioside metabolism accompany the much broader spectrum of neurological and psychiatric disorders that was accepted until recently. This warrants further clinical and pre-clinical studies on this question; the use of animal models can be of particular value in this context.
3. Modelling of Ganglioside Deficiency in Animals
Several animal models of ganglioside deficiency have been generated to date. One of them is B4galnt1 knockout (−/−) mice [32]. They synthesize GM3 and GD3 but lack all of the GalNAc-bound gangliosides [1]. B4galnt1−/− mice display deficits in hindlimb reflex, balance, coordination and muscle strength [49]. They also show axonal degeneration, which resembles the pattern of human spastic paraplegia, caused by B4GALNT1 mutations [23]. Mice with heterozygous knockout (+/−) of B4galnt1, which leads to only partial ganglioside deficiency, display a Parkinson’s disease-like phenotype, which includes motor impairments, dysfunctions in short-term memory and cardiac and gastrointestinal symptoms [50].
Another animal model of ganglioside biosynthesis disruptions is St3gal5−/− mice [51]. Unlike B4galnt1−/− mice, these mutants only partly re-capitulate clinical abnormalities, which may be attributable to the compensatory synthesis of 0-series gangliosides GD1α and GM1b [52]. However, St3gal5−/− mice do lack the major CNS gangliosides GM3, GM1, GD1a, GD3, GT1b and GQ1b and display motor hyperactivity, impulsivity and inattentiveness [53], enhanced insulin sensitivity [51] and platelet activation and neuronal damage following brain trauma [6,54], so this model provides the opportunity to explore the consequences of the brain’s ganglioside deficiency rather than its complete absence [53]. The severest pathology is observed in double knockout St3gal5−/−/B4galnt1−/− mice. They are devoid of any ganglioside derivatives of LacCer and soon after birth develop severe neurodegeneration with impaired axon-glia interactions, hind limb weakness, ataxia and tremors, and die before two months of age. Such deficiency is also associated with increased inflammatory reactions [55].
Recently, we studied the baseline behaviour of St3gal5−/− mice, as data in the available literature was scarce [56]. We have found substantial increases in dominant and neutral social behaviours in male mutants and decreased neutral sociability of female St3gal5−/− mice, as well as other behavioural alterations resembling ASD-like and ASD-accompanying features, i.e., repetitive grooming behaviour and increased anxiety. Naïve St3gal5−/− male mice exhibited a substantial increase in dominant behaviour, and a similar trend was also found in St3gal5−/− female mice. Behavioural alterations were accompanied by increased mRNA expression of pro-inflammatory cytokine interleukin-1β (Il-1β) and tumour necrosis factor (Tnf), both in the prefrontal cortex (PFC) and the spleen of St3gal5−/− animals (Figure 1, for methods see [57]).
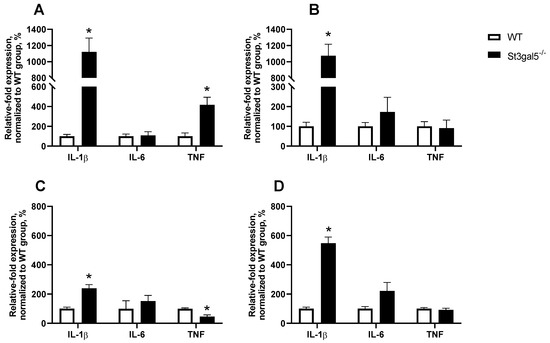
Figure 1.
Expression of pro-inflammatory cytokines Il-1β, Il-6 and TNF mRNA in (A) brains of male and (B) female St3gal5−/− mice and (C) spleens of male and (D) female St3gal5−/− mice. The expression of Il-1β is significantly elevated in both the brain and spleen of both genotypes. Differential mRNA expression of TNF is observed in male and female mutants’ brains, with a significant increase in male mutants compared to controls and the opposite effect in females. Male and female St3gal5−/− mice and their wild type littermates, n = 6–10 per group, aged 2–3 months, were housed under standard conditions. Mice were euthanized, perfused with saline, and their brains and spleens were dissected and frozen at −70 °C for qPCR assay. Full description of the study can be found in [57]; for primer sequences, see [56]. WT—wild-type mice. * p < 0.05 vs. control (wild-type) mice. Data are presented as Mean ± SEM.
Additionally, we investigated whether LPS administration causes a different inflammatory response in St3gal5−/− mice compared to wild-type controls. We demonstrated profound sex differences in the behavioural reaction to LPS administration. While systemic inflammation led to a substantial increase in dominant and aggressive behaviours in St3gal5−/− males, in female mice, an increase in those behaviours was observed in wild-type control mice [56]. Sex differences were also found in the cytokine expression response to LPS administration. In general, compared to wild-type controls, St3gal5−/− mice of both sexes had a lower degree of increase in the expression of the pro-inflammatory cytokines in the brain and a higher degree in the periphery. However, the LPS-induced increase of Il-1β expression both in the brain and in the periphery was significantly higher in St3gal5−/− females than in wild-type controls, while in male wild-type control mice the rise of Il-1β expression was higher than in St3gal5−/− males [56]. Thus, there are remarkable sex-specific effects of both the St3gal5 knockout alone and its interaction with inflammatory stress on social and aggressive behaviour. Notably, sex-specific differences in the severity of outcomes were shown for many genetic rodent models of neurodevelopmental disorders [58,59].
As there is a substantial body of evidence suggesting links between brain gangliosides and myelination, we also assessed the mRNA expression of myelination-related proteins, myelin basic protein (MBP), proteolipid protein 1 (PLP1), myelin-associated glycoprotein (MAG) and myelin oligodendrocyte glycoprotein (MOG) in the PFC of naïve St3gal5−/− mice. In mutants of both sexes, we showed a nearly two-fold decrease of expression of PLP1 both in mRNA and protein levels. No changes in the expression of the other three myelin proteins were found [56]. Interestingly, a mutation in the Plp1 gene leading to its decreased expression was found in rabbits with paralytic tremor disease and myelin lipid content in these animals was altered [60]. It was shown that Plp1−/− mice, as well as patients with a lack of PLP1, develop length-dependent axonal degeneration progressing with age, with no histological signs of demyelination [61]. Local deficits of axonal transport were also found in the internodes myelinated by PLP1-deficient oligodendrocytes [62]. Given the abovementioned results, we may suggest that decreased expression of PLP1 in the PFC of St3gal5−/− mice may arise from aberrant regulation of oligodendrocytes in the lack of major brain gangliosides. A decrease in PLP1 may be a factor contributing to white matter abnormalities affecting brain circuits involved in the regulation of social and aggressive behaviours.
In another study we found increased locomotor activity in mutants of both sexes, as well as elevated anxiety-like behaviour and decreased exploratory behaviour [57]. These changes were accompanied by alterations in the expression of insulin receptor (IR) isoforms in the spleen and liver; in male mice, mRNA expression of both the A and B isoforms of IR was increased in wild type animals compared to mutants in both the liver and spleen, while in females, IR-B expression was increased in the livers of mutants. The body weight of male mutants was significantly increased compared to male control mice. Additionally, we found an increased number of high-amplitude EEG spikes [57], which we speculated to be representative of seizures observed in some of the patients with ganglioside metabolism disorders.
Notably, St3gal3−/− mice, which do not lack major brain gangliosides, were shown to have a significantly decreased ratio of myelinated to non-myelinated axons in corpus callosum, as well as reduced MBP protein content [63]. St3gal3−/− mice also demonstrated poorer performance in the rotarod test, motor hyperactivity in the open field associated with increased exploratory behaviour and decreased learning in passive avoidance task [63]. Recently, some of the pathological features were also observed in St3gal3+/− mice. These mice were shown to have lowered MBP protein expression in the PFC, as well as cognitive deficits in male St3gal3+/− mice, and increased activity and enhanced cognitive control in female St3gal3+/− mice [64].
It is believed that, while one of the major functions of ST3GAL3 protein is sialylation of glycoproteins, it also participates in synthesis of a minor ganglioside of lacto-series, sialyl-lactotetraosylceramide (sialyl-Lc4) [65], which is found in the brain in concentrations much lower than major brain gangliosides [66]. Additionally, ST3GAL3 also accepts ganglioside GM1 as a substrate [67]. Thus, findings in the St3gal3 knockout mice together with the suggestion that ST3GAL3 may contribute to the brain minor ganglioside synthesis, further supports the view on possible role of subtle ganglioside metabolism alterations in neuropsychiatric pathology.
4. Gangliosides in Neuroinflammation
Neuroinflammation is known to play a significant role in the pathogenesis of many neuropsychiatric and neurodegenerative disorders. It was implicated as a pathological mechanism in ASD where increases in reactive microglia and astrocytes are found in patients’ brains as well as increased pro-inflammatory cytokines such as IL-1β, IL-6, TNF in brain tissue, cerebrospinal fluid and blood serum [68,69,70,71,72]. For ADHD, developmental exposure to inflammation is considered a risk factor [73] and, in a number of studies, elevated levels of microglia activation markers, pro-inflammatory cytokines and autoantibodies against nervous system cell types were found in ADHD patients, as reviewed in [74]. Developmental neuroinflammation is also viewed as a risk factor for schizophrenia [75,76]. Neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases [77,78] and ALS [79] are also accompanied by microglial activation and a pro-inflammatory cytokine profile. In turn, gangliosides are known to participate in the regulation of microglia and cytokine-mediated immune responses and platelet activation [2,6,7]. This may suggest a link between ganglioside deficiency and the pathology to which it may contribute.
There is strong evidence of participation of gangliosides in regulation of immune responses in which they have an anti-inflammatory effect [3,80]. The knockout of B4galnt1 in mice led to elevated infiltrating microglia, and double knockout of B4galnt1 and GD3-synthase (St8sia1) led to the upregulation of inflammation-related genes in the brain, including Il-1β and TNF [55]. The function of the latter cytokine was also shown to be regulated by gangliosides [81]. Neuro-statin (O-acetyl GD1b) [82] and several other O-acetylated gangliosides were shown to reduce inducible nitric oxide synthase (iNOS) and Il-6 expression in LPS-activated microglial culture and promote neuronal survival in co-cultures [83], and for microglia treated with lipopolysaccharide (LPS) or IL-1β, the application of gangliosides exerted anti-inflammatory effects [3]. In rats, GM1 administration relieved negative consequences of developmental lead exposure, reverting cognitive deficit and cell death in the hippocampus via an antioxidant effect, as well as activation of the SIRT1/cAMP response element-binding protein (CREB)/brain-derived neurotrophic factor (BDNF) and anti-apoptotic pathways [84,85]. In addition, gangliosides affect nitric oxide synthase (NOS2) and ICAM-1 and MCP-1-mediated signalling [7,86]. On the other hand, major brain gangliosides were shown to play a role in platelet activation in the case of traumatic brain injury or neurodegeneration, leading to enhanced inflammation upon disruption of the blood-brain barrier [54,87]. Thus, while major evidence suggests a strong anti-inflammatory effect of CNS gangliosides, their action seems to be rather complex.
5. Gangliosides and Myelination in CNS Pathologies
Evidence links both ganglioside deficiency and neuroinflammation to deficits in myelination. Such deficits are observed in numerous neurological and neuropsychiatric disorders. In addition to well-known demyelinating diseases such as multiple sclerosis and leukodystrophies, neuro-visualization studies found myelination deficits in late-onset neurodegenerative diseases such as Alzheimer’s disease [78,88,89], Parkinson’s disease [78,90] and ALS [91], neurodevelopmental disorders such as ASD and ADHD [92,93,94], and schizophrenia [95]. In addition, factors such as prenatal selective serotonin reuptake inhibitors exposure or social isolation, which are detrimental for CNS development and function, lead to myelination abnormalities, as well as inflammatory disease, e.g., necrotizing enterocolitis in children [96]. Gangliosides are known to participate in myelination in several ways.
One of the major myelination proteins, myelin-associated glycoprotein (MAG), is located on the surface of myelinating glial cells and interacts with neuronal membrane gangliosides to inhibit neurite outgrowth [97]. Gangliosides GD1a and GT1b, which are ligands of MAG, are absent in B4GALNT1 deficiency, and B4galnt1 knockout mice display axonal degeneration in the CNS and PNS, as well as motor deficits similar to those in Mag knockout mice [98]. Finally, the interaction of ganglioside GM1 and MBP stimulates Schwann cell proliferation in the PNS [99]. Recently, we have found decreased mRNA expression of myelination marker Plp1 in St3gal5−/− mice, which have a prominent neuropsychiatric phenotype, providing additional evidence of myelination alterations in ganglioside deficiency [56].
6. Gangliosides in Insulin Signalling
Gangliosides are also known to regulate IR function and dysregulation of IR was shown to be associated with increased incidence of ADHD and ASD [100,101]. Ganglioside GM3 was shown to regulate IR activation in membrane microdomains by suppressing its phosphorylation [9] and may mediate the inhibitory effects of TNF on the IR [81].
In our recent study, we found decreased expression of IRs in the spleen and the liver of St3gal5−/− male mice accompanied by an increase in body weight, suggesting metabolic changes [57]. Previously, changes in IR signalling in adipose tissue and skeletal muscle were also found in these mice [51]. Given that insulin signalling changes are implicated in neuropsychiatric disorders in multiple pathways, including neuroinflammation and oxidative stress [100,102,103], such changes may also contribute to pathology caused by ganglioside deficiency. It was also shown that insulin signalling may regulate the myelination process. Mice with insulin-resistant Schwann cells due to Schwann cell-specific knockout of IR and insulin-like growth factor receptor 1 (IGFR1) showed thinner myelination and the authors hypothesized that insulin resistance in myelinating cells is one of the pathological contributors to diabetic neuropathy [104].
Thus, the available literature suggests that in ganglioside deficiency, interrelated mechanisms such as neuroinflammation, aberrant insulin signalling and impaired myelination occur (Figure 2).
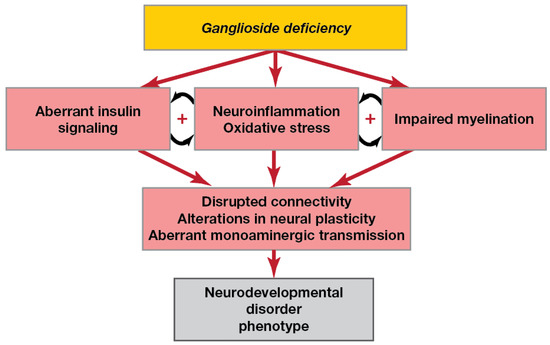
Figure 2.
Pathological pathways in brain ganglioside deficiency. Ganglioside deficiency may lead to both neuroinflammation, aberrant insulin signalling and myelination deficits, which are known to be interrelated. Impaired myelination causes neuroinflammation: myelin debris, products of neuron elimination due to necrosis and apoptosis and dysregulation of activity-dependent astrocytes may activate microglia and macrophages. Pro-inflammatory cytokines and dysregulation of glia by inflammation negatively affect myelination. Aberrant signal propagation, axon degradation and neuronal death due to a lack of metabolic support from myelin sheaths disrupt brain connectivity. Additionally, neuroinflammation contributes to alterations in monoaminergic transmission and neural plasticity.
Oxidative stress, caused by reactive oxygen species, is tightly interconnected with neuroinflammation. Oxidative stress in connection with neuroinflammation was also implicated in animal models of various pathologies, such as autism [105], fragile X syndrome [106], Alzheimer’s disease [77,107], amyotrophic lateral sclerosis [79,108] and myelination abnormalities in schizophrenia [109] and other conditions [110].
In patients with autism, antioxidant enzymes have decreased activity in the brain, which is associated with increased levels of lipid peroxidation products [111,112]. In turn, several studies have shown that gangliosides prevent lipid peroxidation product accumulation in synaptosomes and protect synaptosomal cAMP phosphodiesterase and Na+, K+-ATPase from deactivation caused by lipid peroxidation [111,113]. Increased catalase (CAT) activity was also shown in GM1-treated rat cortex ex vivo, which also may lower oxidative stress [114]. Also, levels of CAT, superoxide dismutase (SOD) and glutathione peroxidase (GSH POD) were increased in primary and peri-ischemic rat cortex treated with GM1 [115]. Exogenously added gangliosides were shown to reduce ROS levels, which are associated with oxidative stress in isolated cell cultures of the heart [116], hepatocytes [117], spermatozoa [118] and PC12 cell culture [119,120]. The aforementioned studies included gangliosides downstream of ST3GAL5 and B4GALNT1. Together, these data suggest that in ganglioside deficits, including those caused by the dysfunction of ST3GAL5 and/or B4GALNT1, the ability to fight inflammation and oxidative stress is compromised.
7. Perspectives of Antioxidative Stress and Anti-Inflammatory Manipulation in the Treatment of Ganglioside Deficit-Related Disorders
Since gangliosides have antioxidant and anti-inflammatory effects; the medication that increases the level of ganglioside may be efficient in conditions to which pathogenesis neuroinflammation contributes. One example is resveratrol, a phytoestrogen with antioxidant, anti-inflammatory and neuroprotective effects, which are properties that were studied in the rat model of global cerebral ischemia that leads to an increase in lipid peroxidation and ROS formation. In a model of global cerebral ischemia in rats, a significant decrease in ganglioside, phospholipids and cholesterol in the hippocampus and cortex was observed. Resveratrol administration seven days prior to ischemia prevented the reduction in the total ganglioside content in the hippocampus and cerebral cortex, thus stabilizing disrupted antioxidant defences [121]. Another example of a medication acting on gangliosides is ferulic acid, which was shown to exert an antioxidative effect in Fe2+-induced oxidative injury in rat brains. The induction of oxidative injury with FeSO4 led to the complete depletion of ganglioside GT3 and ganglioside GD2 and treatment with ferulic acid led to the restoration of GT3 levels [122].
An alternative drug strategy for neuroinflammation regulation could be using gangliosides themselves as a therapy. It was shown in a model of B4galnt1+/− mice, which have a partial deficit of major brain gangliosides and demonstrate some of the motor and cognitive features of Parkinson’s disease. Daily intraperitoneal administration of E. coli derived GM1 ganglioside alleviated both motor and cognitive deficits in these mice [50]. In addition, authors also demonstrated GM1 uptake by the brain of B4galnt1−/− mice upon intraperitoneal administration [50], which suggests that the compound may penetrate the blood–brain barrier and act in the CNS directly with no additional delivery system. Moreover, it was shown that administration of GM1 decreases oxidative stress. In rats in a hypobaric hypoxic (HH) environment, GM1 ganglioside treatment led to the cessation of increased ROS production, which was otherwise observed in this environment without treatment. GM1 also suppressed the accumulation of a marker of oxidative stress, malondialdehyde, and increased levels of SOD and glutathione. Simultaneously, GM1 administration counteracted enhanced inflammation in HH-exposed rats by muting pro-inflammatory cytokines IL-1β, TNF-α and IL-6 levels in serum and brain tissues [123]. Another study in a focal cortical ischemia rat model investigated the changes in the levels of the antioxidant enzymes SOD, GSH POD and CAT. Intramuscular GM1 injection within 10 min of the ischemia increased levels of SOD, CAT and GSH POD, which remained significantly high even at 2 weeks after ischemia [115].
These findings raise the question of whether anti-inflammatory and antioxidant therapies, as well as treatment with gangliosides per se, may be beneficial in disorders associated with or caused by ganglioside deficiency. Clinical data suggests such an approach is viable. Gangliosides combined with mouse nerve growth factor (NGF) showed high therapeutic efficacy in infants with hypoxic-ischemic encephalopathy (HIE). Severe hypoxia in infants with HIE can cause vascular endothelial cells and brain neuron damage by exposure to free radical oxidative stress and release of inflammatory factors, cytokines and immune molecules such as sICAM-1, TNF and interleukins. This study demonstrated the decline of pro-inflammatory factor levels and free radical levels in the treated group, indicating that ganglioside combined with NGF could enhance immune function, improve vascular injury and promote inflammation regression [124]. In a recent meta-analysis of antioxidative interventions in autism, the authors analysed 12 publications where effects of antioxidants such as n-Acetyl-cystein, melatonin, folic acid, arachidonic acid, docosahexaenoic acid, resveratrol, palmitoyl-ethanolamide and sulforaphane were analysed. They concluded that, while additional research is needed, their analysis suggested a potential role of antioxidants in the management of some symptoms of ASD [125]. This suggests potential benefits of a broad range of treatments with anti-oxidant activity, e.g., thiamine compounds and phyto anti-oxidants [126,127,128,129,130]. However, none of the analysed papers included an analysis of ganglioside deficiency. Based on the present critical literature review that evidences a connection between gangliosides, oxidative stress and psychiatric disorders, we may speculate that antioxidant and anti-inflammatory therapies, as well as treatment with gangliosides, should be studied in pathologies associated with ganglioside deficiency.
8. Conclusions
Together, clinical and preclinical data suggest that ganglioside metabolism alterations may be involved in a broader spectrum of disorders than was thought previously. A “triad” of inter-related molecular mechanisms of neuroinflammation, myelination and IR signalling might underlie the consequences of compromised ganglioside metabolism contributing to the pathophysiology of neuropsychiatric disorders and might be considered a pattern response to a ganglioside-associated deficit. These findings warrant further studies exploring the role of gangliosides in the diagnosis and pathophysiology and treatment of neuropsychiatric and CNS disorders, including those for which the role of ganglioside dysfunction is not known.
Author Contributions
The draft was written and edited by E.S. (Evgeniy Svirin), E.S. (Elisaveta Sheveleva), J.d.M. and A.U.; A.V.K., A.S., S.M. and S.W. reviewed and edited the manuscript; E.S. and T.S. wrote the manuscript and supervised the entire work. All authors agree to be responsible for the publication. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Eat2beNice EU framework (2018–2023, to T.S.), by PhytoAPP EU framework (2021–2025, to E.S., S.L. and T.S.), Swiss-RF program-2020 (to T.S., S.W. and E.S.), FGFU-2022-0012 and FGFU-2022-0013 (to E.S., S.M., E.S. (Elisaveta Sheveleva), T.S.), and Priority Program-2030 (to E.S., A.U., E.S. (Elisaveta Sheveleva), A.S., T.S.). The Eat2beNICE project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 728018 and the PhytoAPP project has received funding from the European Union’s HORIZON 2020 research and innovation programme under the Marie Sklodowvska-Curie grant agreement 101007642. This publication reflects only the author’s views and the European Commission is not liable for any use that may be made of the information contained therein.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schnaar, R.L. Gangliosides of the Vertebrate Nervous System. J. Mol. Biol. 2016, 428, 3325–3336. [Google Scholar] [CrossRef]
- Bowser, L.E.; Young, M.; Wenger, O.K.; Ammous, Z.; Brigatti, K.W.; Carson, V.J.; Moser, T.; Deline, J.; Aoki, K.; Morlet, T.; et al. Recessive GM3 Synthase Deficiency: Natural History, Biochemistry, and Therapeutic Frontier. Mol. Genet. Metab. 2019, 126, 475–488. [Google Scholar] [CrossRef]
- Galleguillos, D.; Wang, Q.; Steinberg, N.; Zaidi, A.; Shrivastava, G.; Dhami, K.; Daskhan, G.C.; Schmidt, E.N.; Dworsky-Fried, Z.; Giuliani, F.; et al. Anti-Inflammatory Role of GM1 and Other Gangliosides on Microglia. J. Neuroinflamm. 2022, 19, 1–18. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic Acids in the Brain: Gangliosides and Polysialic Acid in Nervous System Development, Stability, Disease, and Regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef]
- Lopez, P.H.H.; Báez, B.B. Gangliosides in Axon Stability and Regeneration. Prog. Mol. Biol. Transl. Sci. 2018, 156, 383–412. [Google Scholar] [CrossRef] [PubMed]
- Dukhinova, M.; Kuznetsova, I.; Kopeikina, E.; Veniaminova, E.; Yung, A.W.Y.; Veremeyko, T.; Levchuk, K.; Barteneva, N.S.; Wing-Ho, K.K.; Yung, W.H.; et al. Platelets Mediate Protective Neuroinflammation and Promote Neuronal Plasticity at the Site of Neuronal Injury. Brain Behav. Immun. 2018, 74, 7–27. [Google Scholar] [CrossRef]
- Kim, O.S.; Park, E.J.; Joe, E.; Jou, I. JAK-STAT Signaling Mediates Gangliosides-Induced Inflammatory Responses in Brain Microglial Cells. J. Biol. Chem. 2002, 277, 40594–40601. [Google Scholar] [CrossRef]
- Ishii, A.; Ohta, M.; Watanabe, Y.; Matsuda, K.; Ishiyama, K.; Sakoe, K.; Nakamura, M.; Inokuchi, J.I.; Sanai, Y.; Saito, M. Expression Cloning and Functional Characterization of Human CDNA for Ganglioside G(M3) Synthase. J. Biol. Chem. 1999, 273, 31652–31655. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, J.; Inamori, K.; Kabayama, K.; Nagafuku, M.; Uemura, S.; Go, S.; Suzuki, A.; Ohno, I.; Kanoh, H.; Shishido, F. Biology of GM3 Ganglioside. Prog. Mol. Biol. Transl. Sci. 2018, 156, 151–195. [Google Scholar] [CrossRef] [PubMed]
- Senn, H.-J.; Orth, M.; Fitzke, E.; Wieland, H.; Gerok, W. Gangliosides in Normal Human Serum. Concentration, Pattern and Transport by Lipoproteins. Eur. J. Biochem. 1989, 181, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Cachon-Gonzalez, M.B.; Zaccariotto, E.; Cox, T.M. Genetics and Therapies for GM2 Gangliosidosis. Curr. Gene Ther. 2018, 18, 68–89. [Google Scholar] [CrossRef]
- Sandhoff, K.; Harzer, K. Gangliosides and Gangliosidoses: Principles of Molecular and Metabolic Pathogenesis. J. Neurosci. 2013, 33, 10195–10208. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.F.; Benincore-Flórez, E.; Solano-Galarza, D.; Jaramillo, R.G.G.; Echeverri-Peña, O.Y.; Suarez, D.A.; Alméciga-Díaz, C.J.; Espejo-Mojica, A.J. Gm2 Gangliosidoses: Clinical Features, Pathophysiological Aspects, and Current Therapies. Int. J. Mol. Sci. 2020, 21, 6213. [Google Scholar] [CrossRef] [PubMed]
- Filho, J.A.F.; Shapiro, B.E. Tay-Sachs Disease. Arch. Neurol. 2004, 61, 1466. [Google Scholar] [CrossRef]
- Sandhoff, K. Variation of β-N-Acetylhexosaminidase-Pattern in Tay-Sachs Disease. FEBS Lett. 1969, 4, 351–354. [Google Scholar] [CrossRef]
- Bley, A.E.; Giannikopoulos, O.A.; Hayden, D.; Kubilus, K.; Tifft, C.J.; Eichler, F.S. Natural History of Infantile G(M2) Gangliosidosis. Pediatrics 2011, 128, e1233–e1241. [Google Scholar] [CrossRef]
- Gort, L.; de Olano, N.; Macías-Vidal, J.; Coll, M.J. GM2 Gangliosidoses in Spain: Analysis of the HEXA and HEXB Genes in 34 Tay-Sachs and 14 Sandhoff Patients. Gene 2012, 506, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Er, E.; Canda, E.; Yazıcı, H.; Eraslan, C.; Sözmen, E.; Kalkan Uçar, S.; Çoker, M. An Evalution of the Demographic and Clinical Characterictics of Patients with GM2 Gangliosidosis. J. Pediatr. Res. 2018, 5, 12–16. [Google Scholar] [CrossRef]
- Maegawa, G.H.B.; Stockley, T.; Tropak, M.; Banwell, B.; Blaser, S.; Kok, F.; Giugliani, R.; Mahuran, D.; Clarke, J.T.R. The Natural History of Juvenile or Subacute GM2 Gangliosidosis: 21 New Cases and Literature Review of 134 Previously Reported. Pediatrics 2006, 118, 118. [Google Scholar] [CrossRef]
- Neudorfer, O.; Pastores, G.M.; Zeng, B.J.; Gianutsos, J.; Zaroff, C.M.; Kolodny, E.H. Late-Onset Tay-Sachs Disease: Phenotypic Characterization and Genotypic Correlation in 21 Affected Patients. Genet. Med. 2005, 7, 119–123. [Google Scholar] [CrossRef]
- Masingue, M.; Dufour, L.; Lenglet, T.; Saleille, L.; Goizet, C.; Ayrignac, X.; Ory-Magne, F.; Barth, M.; Lamari, F.; Mandia, D.; et al. Natural History of Adult Patients with GM2 Gangliosidosis. Ann. Neurol. 2020, 87, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Indellicato, R.; Parini, R.; Domenighini, R.; Malagolini, N.; Iascone, M.; Gasperini, S.; Masera, N.; Dall’Olio, F.; Trinchera, M. Total Loss of GM3 Synthase Activity by a Normally Processed Enzyme in a Novel Variant and in All ST3GAL5 Variants Reported to Cause a Distinct Congenital Disorder of Glycosylation. Glycobiology 2019, 29, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Trinchera, M.; Parini, R.; Indellicato, R.; Domenighini, R.; dall’Olio, F. Diseases of Ganglioside Biosynthesis: An Expanding Group of Congenital Disorders of Glycosylation. Mol. Genet. Metab. 2018, 124, 230–237. [Google Scholar] [CrossRef]
- Lee, J.S.; Yoo, Y.; Lim, B.C.; Kim, K.J.; Song, J.; Choi, M.; Chae, J.H. GM3 Synthase Deficiency Due to ST3GAL5 Variants in Two Korean Female Siblings: Masquerading as Rett Syndrome-like Phenotype. Am. J. Med. Genet. A 2016, 170, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.A.; Cross, H.; Proukakis, C.; Priestman, D.A.; Neville, D.C.A.; Reinkensmeier, G.; Wang, H.; Wiznitzer, M.; Gurtz, K.; Verganelaki, A.; et al. Infantile-Onset Symptomatic Epilepsy Syndrome Caused by a Homozygous Loss-of-Function Mutation of GM3 Synthase. Nat. Genet. 2004, 36, 1225–1229. [Google Scholar] [CrossRef]
- Boccuto, L.; Aoki, K.; Flanagan-Steet, H.; Chen, C.F.; Fan, X.; Bartel, F.; Petukh, M.; Pittman, A.; Saul, R.; Chaubey, A.; et al. A Mutation in a Ganglioside Biosynthetic Enzyme, ST3GAL5, Results in Salt & Pepper Syndrome, a Neurocutaneous Disorder with Altered Glycolipid and Glycoprotein Glycosylation. Hum. Mol. Genet. 2014, 23, 418–433. [Google Scholar] [CrossRef]
- Boukhris, A.; Schule, R.; Loureiro, J.L.; Lourenço, C.M.; Mundwiller, E.; Gonzalez, M.A.; Charles, P.; Gauthier, J.; Rekik, I.; Lebrigio, R.F.A.; et al. Alteration of Ganglioside Biosynthesis Responsible for Complex Hereditary Spastic Paraplegia. Am. J. Hum. Genet. 2013, 93, 118–123. [Google Scholar] [CrossRef]
- Harlalka, G.V.; Lehman, A.; Chioza, B.; Baple, E.L.; Maroofian, R.; Cross, H.; Sreekantan-Nair, A.; Priestman, D.A.; Al-Turki, S.; McEntagart, M.E.; et al. Mutations in B4GALNT1 (GM2 Synthase) Underlie a New Disorder of Ganglioside Biosynthesis. Brain 2013, 136, 3618–3624. [Google Scholar] [CrossRef]
- Schnaar, R.L. Brain Gangliosides in Axon-Myelin Stability and Axon Regeneration. FEBS Lett. 2010, 584, 1741–1747. [Google Scholar] [CrossRef]
- Wakil, S.M.; Monies, D.M.; Ramzan, K.; Hagos, S.; Bastaki, L.; Meyer, B.F.; Bohlega, S. Novel B4GALNT1 Mutations in a Complicated Form of Hereditary Spastic Paraplegia. Clin. Genet. 2014, 86, 500–501. [Google Scholar] [CrossRef]
- Gordon-Lipkin, E.; Cohen, J.S.; Srivastava, S.; Soares, B.P.; Levey, E.; Fatemi, A. ST3GAL5-Related Disorders: A Deficiency in Ganglioside Metabolism and a Genetic Cause of Intellectual Disability and Choreoathetosis. J. Child. Neurol. 2018, 33, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, R.H.; Ohmi, Y.; Ohkawa, Y.; Zhang, P.; Takano, M.; Hashimoto, N.; Okajima, T.; Furukawa, K.; Furukawa, K. Loss of Enzyme Activity in Mutated B4GALNT1 Gene Products in Patients with Hereditary Spastic Paraplegia Results in Relatively Mild Neurological Disorders: Similarity with Phenotypes of B4galnt1 Knockout Mice. Neuroscience 2019, 397, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liang, X.; Zhu, F.; Wen, Y.; Xu, J.; Yang, J.; Ding, M.; Cheng, B.; Ma, M.; Zhang, L.; et al. A Large-Scale Integrative Analysis of GWAS and Common MeQTLs across Whole Life Course Identifies Genes, Pathways and Tissue/Cell Types for Three Major Psychiatric Disorders. Neurosci. Biobehav. Rev. 2018, 95, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Mealer, R.G.; Jenkins, B.G.; Chen, C.Y.; Daly, M.J.; Ge, T.; Lehoux, S.; Marquardt, T.; Palmer, C.D.; Park, J.H.; Parsons, P.J.; et al. The Schizophrenia Risk Locus in SLC39A8 Alters Brain Metal Transport and Plasma Glycosylation. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.S.; Pain, O.; O’Brien, H.E.; Anney, R.; Walters, J.T.R.; Owen, M.J.; O’Donovan, M.C.; Bray, N.J. Cis-Effects on Gene Expression in the Human Prenatal Brain Associated with Genetic Risk for Neuropsychiatric Disorders. Mol. Psychiatry 2021, 26, 2082–2088. [Google Scholar] [CrossRef]
- Mostafa, G.A.; Al-Ayadhi, L.Y. Increased Serum Levels of Anti-Ganglioside M1 Auto-Antibodies in Autistic Children: Relation to the Disease Severity. J. Neuroinflamm. 2011, 8, 39. [Google Scholar] [CrossRef]
- Yang, X.; Liang, S.; Wang, L.; Han, P.; Jiang, X.; Wang, J.; Hao, Y.; Wu, L. Sialic Acid and Anti-Ganglioside Antibody Levels in Children with Autism Spectrum Disorders. Brain Res. 2018, 1678, 273–277. [Google Scholar] [CrossRef]
- Sarbu, M.; Vukelić, Ž.; Clemmer, D.E.; Zamfir, A.D. Ion Mobility Mass Spectrometry Provides Novel Insights into the Expression and Structure of Gangliosides in the Normal Adult Human Hippocampus. Analyst 2018, 143, 5234–5246. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Wang, Z.; Xie, Y.; Yang, L.; Zhao, Y.; Tian, R. Mass Spectrometry-Based Ganglioside Profiling Provides Potential Insights into Alzheimer’s Disease Development. J. Chromatogr. A 2022, 1676, 463196. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Stefani, M.; Melki, R.; Zecchi-Orlandini, S.; Nosi, D. The Amphipathic GM1 Molecule Stabilizes Amyloid Aggregates, Preventing Their Cytotoxicity. Biophys. J. 2020, 119, 326–336. [Google Scholar] [CrossRef]
- Rawal, P.; Zhao, L. Sialometabolism in Brain Health and Alzheimer’s Disease. Front Neurosci. 2021, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yang, Z.; Chen, X.; Li, W. The Significance of Sialylation on the Pathogenesis of Alzheimer’s Disease. Brain Res. Bull. 2021, 173, 116–123. [Google Scholar] [CrossRef]
- Alselehdar, S.K.; Chakraborty, M.; Chowdhury, S.; Alcalay, R.N.; Surface, M.; Ledeen, R. Subnormal GM1 in PBMCs: Promise for Early Diagnosis of Parkinson’s Disease? Int. J. Mol. Sci. 2021, 22, 1522. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S. A Critical Role for GM1 Ganglioside in the Pathophysiology and Potential Treatment of Parkinson’s Disease. Glycoconj. J. 2022, 39, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Maglione, V.; Marchi, P.; di Pardo, A.; Lingrell, S.; Horkey, M.; Tidmarsh, E.; Sipione, S. Impaired Ganglioside Metabolism in Huntington’s Disease and Neuroprotective Role of GM1. J. Neurosci. 2010, 30, 4072–4080. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.R.; Saville, J.T.; Hancock, S.E.; Brown, S.H.J.; Jenner, A.M.; McLean, C.; Fuller, M.; Newell, K.A.; Mitchell, T.W. The Long and the Short of Huntington’s Disease: How the Sphingolipid Profile Is Shifted in the Caudate of Advanced Clinical Cases. Brain Commun. 2022, 4, fcab303. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.C.; Treleaven, C.M.; Pacheco, J.; Cooper, S.; Bao, C.; Abraham, M.; Cromwell, M.; Sardi, S.P.; Chuang, W.L.; Sidman, R.L.; et al. Glycosphingolipids Are Modulators of Disease Pathogenesis in Amyotrophic Lateral Sclerosis. Proc. Natl. Acad. Sci. USA 2015, 112, 8100–8105. [Google Scholar] [CrossRef]
- Tanemoto, M.; Hisahara, S.; Ikeda, K.; Yokokawa, K.; Manabe, T.; Tsuda, R.; Yamamoto, D.; Matsushita, T.; Matsumura, A.; Suzuki, S.; et al. Sporadic Amyotrophic Lateral Sclerosis Due to a Fus P525l Mutation with Asymmetric Muscle Weakness and Anti-Ganglioside Antibodies. Intern. Med. 2021, 60, 1949–1953. [Google Scholar] [CrossRef]
- Chiavegatto, S.; Sun, J.; Nelson, R.J.; Schnaar, R.L. A Functional Role for Complex Gangliosides: Motor Deficits in GM2/GD2 Synthase Knockout Mice. Exp. Neurol. 2000, 166, 227–234. [Google Scholar] [CrossRef]
- Wu, G.; Lu, Z.; Seo, J.H.; Alselehdar, S.K.; DeFrees, S.; Ledeen, R.W. Mice Deficient in GM1 Manifest Both Motor and Non-Motor Symptoms of Parkinson’s Disease; Successful Treatment with Synthetic GM1 Ganglioside. Exp. Neurol. 2020, 329, 113284. [Google Scholar] [CrossRef]
- Yamashita, T.; Hashiramoto, A.; Haluzik, M.; Mizukami, H.; Beck, S.; Norton, A.; Kono, M.; Tsuji, S.; Daniotti, J.L.; Werth, N.; et al. Enhanced Insulin Sensitivity in Mice Lacking Ganglioside GM3. Proc. Natl. Acad. Sci. USA 2003, 100, 3445–3449. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Wu, Y.P.; Sandhoff, R.; Werth, N.; Mizukami, H.; Ellis, J.M.; Dupreell, J.L.; Geyer, R.; Sandhoff, K.; Proia, R.L. Interruption of Ganglioside Synthesis Produces Central Nervous System Degeneration and Altered Axon-Glial Interactions. Proc. Natl. Acad. Sci. USA 2005, 102, 2725–2730. [Google Scholar] [CrossRef] [PubMed]
- Niimi, K.; Nishioka, C.; Miyamoto, T.; Takahashi, E.; Miyoshi, I.; Itakura, C.; Yamashita, T. Impairment of Neuropsychological Behaviors in Ganglioside GM3-Knockout Mice. Biochem. Biophys. Res. Commun. 2011, 406, 524–528. [Google Scholar] [CrossRef]
- Kopeikina, E.; Dukhinova, M.; Yung, A.W.Y.; Veremeyko, T.; Kuznetsova, I.S.; Lau, T.Y.B.; Levchuk, K.; Ponomarev, E.D. Platelets Promote Epileptic Seizures by Modulating Brain Serotonin Level, Enhancing Neuronal Electric Activity, and Contributing to Neuroinflammation and Oxidative Stress. Prog. Neurobiol. 2020, 188, 101783. [Google Scholar] [CrossRef]
- Ohmi, Y.; Tajima, O.; Ohkawa, Y.; Yamauchi, Y.; Sugiura, Y.; Furukawa, K.; Furukawa, K. Gangliosides Are Essential in the Protection of Inflammation and Neurodegeneration via Maintenance of Lipid Rafts: Elucidation by a Series of Ganglioside-Deficient Mutant Mice. J. Neurochem. 2011, 116, 926–935. [Google Scholar] [CrossRef]
- Strekalova, T.; Svirin, E.; Veniaminova, E.; Kopeikina, E.; Veremeyko, T.; Yung, A.W.Y.; Proshin, A.; Walitza, S.; Anthony, D.C.; Lim, L.W.; et al. ASD-like Behaviors, a Dysregulated Inflammatory Response and Decreased Expression of PLP1 Characterize Mice Deficient for Sialyltransferase ST3GAL5. Brain Behav. Immun. Health 2021, 16, 100306. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Veniaminova, E.; Svirin, E.; Kopeikina, E.; Veremeyko, T.; Yung, A.W.Y.; Proshin, A.; Tan, S.Z.K.; Khairuddin, S.; Lim, L.W.; et al. Sex-Specific ADHD-Like Behaviour, Altered Metabolic Functions, and Altered EEG Activity in Sialyltransferase ST3GAL5-Deficient Mice. Biomolecules 2021, 11, 1759. [Google Scholar] [CrossRef]
- Mossa, A.; Manzini, M.C. Molecular Causes of Sex-Specific Deficits in Rodent Models of Neurodevelopmental Disorders. J. Neurosci. Res. 2021, 99, 37–56. [Google Scholar] [CrossRef]
- Jung, H.; Park, H.; Choi, Y.; Kang, H.; Lee, E.; Kweon, H.; Roh, J.D.; Ellegood, J.; Choi, W.; Kang, J.; et al. Sexually Dimorphic Behavior, Neuronal Activity, and Gene Expression in Chd8-Mutant Mice. Nat. Neurosci. 2018, 21, 1218–1228. [Google Scholar] [CrossRef]
- Sypecka, J.; Domańska-Janik, K. Rabbit Paralytic Tremor Phenotype-A Plp1 Gene Mutation as a Model of Human Pelizaeus-Merzbacher Disease. Acta Neurobiol. Exp. 2005, 65, 221–229. [Google Scholar]
- Garbern, J.Y.; Yool, D.A.; Moore, G.J.; Wilds, I.B.; Faulk, M.W.; Klugmann, M.; Nave, K.A.; Sistermans, E.A.; van der Knaap, M.S.; Bird, T.D.; et al. Patients Lacking the Major CNS Myelin Protein, Proteolipid Protein 1, Develop Length-Dependent Axonal Degeneration in the Absence of Demyelination and Inflammation. Brain 2002, 125, 551–561. [Google Scholar] [CrossRef]
- Gruenenfelder, F.I.; Thomson, G.; Penderis, J.; Edgar, J.M. Axon-Glial Interaction in the CNS: What We Have Learned from Mouse Models of Pelizaeus-Merzbacher Disease. J. Anat. 2011, 219, 33–43. [Google Scholar] [CrossRef]
- Yoo, S.W.; Motari, M.G.; Susuki, K.; Prendergast, J.; Mountney, A.; Hurtado, A.; Schnaar, R.L. Sialylation Regulates Brain Structure and Function. FASEB J. 2015, 29, 3040–3053. [Google Scholar] [CrossRef]
- Rivero, O.; Alhama-Riba, J.; Ku, H.P.; Fischer, M.; Ortega, G.; Álmos, P.; Diouf, D.; van den Hove, D.; Lesch, K.P. Haploinsufficiency of the Attention-Deficit/Hyperactivity Disorder Risk Gene St3gal3 in Mice Causes Alterations in Cognition and Expression of Genes Involved in Myelination and Sialylation. Front Genet. 2021, 12, 688488. [Google Scholar] [CrossRef]
- Indellicato, R.; Domenighini, R.; Malagolini, N.; Cereda, A.; Mamoli, D.; Pezzani, L.; Iascone, M.; Dall’olio, F.; Trinchera, M. A Novel Nonsense and Inactivating Variant of ST3GAL3 in Two Infant Siblings Suffering Severe Epilepsy and Expressing Circulating CA19.9. Glycobiology 2020, 30, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Molin, K.; Mansson, J.-E.; Fredman, P.; Svennerholm, L. Sialosyllactotetraosylceramide, 3′-IsoLM1, a Ganglioside of the Lactotetraose Series Isolated from Normal Human Infant Brain. J. Neurochem. 1987, 49, 216–219. [Google Scholar] [CrossRef]
- Sturgill, E.R.; Aoki, K.; Lopez, P.H.H.; Colacurcio, D.; Vajn, K.; Lorenzini, I.; Majić, S.; Yang, W.H.; Heffer, M.; Tiemeyer, M.; et al. Biosynthesis of the Major Brain Gangliosides GD1a and GT1b. Glycobiology 2012, 22, 1289–1301. [Google Scholar] [CrossRef]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The Influence of Neuroinflammation in Autism Spectrum Disorder. Brain Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial Activation and Neuroinflammation in the Brain of Patients with Autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef]
- Napolioni, V.; Ober-Reynolds, B.; Szelinger, S.; Corneveaux, J.J.; Pawlowski, T.; Ober-Reynolds, S.; Kirwan, J.; Persico, A.M.; Melmed, R.D.; Craig, D.W.; et al. Plasma Cytokine Profiling in Sibling Pairs Discordant for Autism Spectrum Disorder. J. Neuroinflamm. 2013, 10, 813. [Google Scholar] [CrossRef]
- Gevezova, M.; Sarafian, V.; Anderson, G.; Maes, M. Inflammation and Mitochondrial Dysfunction in Autism Spectrum Disorder. CNS Neurol. Disord. Drug Targets 2020, 19, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Siniscalco, D.; Schultz, S.; Brigida, A.; Antonucci, N. Inflammation and Neuro-Immune Dysregulations in Autism Spectrum Disorders. Pharmaceuticals 2018, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.A.; Nigg, J.T.; Sullivan, E.L. Neuroinflammation as a Risk Factor for Attention Deficit Hyperactivity Disorder. Pharmacol. Biochem. Behav. 2019, 182, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Corona, J.C. Role of Oxidative Stress and Neuroinflammation in Attention-Deficit/Hyperactivity Disorder. Antioxidants 2020, 9, 1039. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U. Developmental Neuroinflammation and Schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 42, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.; Santos, S.G.; Almeida, M.I.; Coelho, R.; Barbosa, M.A. Bridging Autism Spectrum Disorders and Schizophrenia through Inflammation and Biomarkers-Pre-Clinical and Clinical Investigations. J. Neuroinflamm. 2017, 14, 179. [Google Scholar] [CrossRef]
- Agostinho, P.; Cunha, R.A.; Oliveira, C. Neuroinflammation, Oxidative Stress and the Pathogenesis of Alzheimer’s Disease. Curr. Pharm. Des. 2010, 16, 2766–2778. [Google Scholar] [CrossRef]
- Han, F.; Perrin, R.J.; Wang, Q.; Wang, Y.; Perlmutter, J.S.; Morris, J.C.; Benzinger, T.L.S.; Xu, J. Neuroinflammation and Myelin Status in Alzheimer’s Disease, Parkinson’s Disease, and Normal Aging Brains: A Small Sample Study. Parkinson’s Dis. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador, R.; Estrela, J.M.; López-Blanch, R.; Jihad-Jebbar, A.; Vallés, S.L. Oxidative Stress, Neuroinflammation and Mitochondria in the Pathophysiology of Amyotrophic Lateral Sclerosis. Antioxidants 2020, 9, 901. [Google Scholar] [CrossRef]
- Sipione, S.; Monyror, J.; Galleguillos, D.; Steinberg, N.; Kadam, V. Gangliosides in the Brain: Physiology, Pathophysiology and Therapeutic Applications. Front Neurosci. 2020, 14, 572965. [Google Scholar] [CrossRef]
- Tagami, S.; Inokuchi, J.I.; Kabayama, K.; Yoshimura, H.; Kitamura, F.; Uemura, S.; Ogawa, C.; Ishii, A.; Saito, M.; Ohtsuka, Y.; et al. Ganglioside GM3 Participates in the Pathological Conditions of Insulin Resistance. J. Biol. Chem. 2002, 277, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Abad-Rodríguez, J.; Bernabé, M.; Romero-Ramírez, L.; Vallejo-Cremades, M.; Fernández-Mayoralas, A.; Nieto-Sampedro, M. Purification and Structure of Neurostatin, an Inhibitor of Astrocyte Division of Mammalian Brain. J. Neurochem. 2002, 74, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Yanguas-casás, N.; Ojalvo-sanz, A.C.; Martínez-vázquez, A.; Goneau, M.; Gilbert, M.; Nieto-sampedro, M.; Romero-ramírez, L. Neurostatin and Other O-Acetylated Gangliosides Show Anti- Neuroin Fl Ammatory Activity Involving the NF κ B Pathway. Toxicol. Appl. Pharmacol. 2019, 377, 114627. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, C.C.; Yang, Y.; Liu, J.W.; Yan, C.H. GM1 Ameliorates Lead-Induced Cognitive Deficits and Brain Damage Through Activating the SIRT1/CREB/BDNF Pathway in the Developing Male Rat Hippocampus. Biol. Trace Elem. Res. 2019, 190, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Wang, L.; He, J.; Wang, Z. The Protective Effect of Gangliosides on Lead (Pb)-Induced Neurotoxicity Is Mediated by Autophagic Pathways. Int. J. Environ. Res. Public Health 2016, 13, 365. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; Shin, W.H.; Kim, J.; Joe, E.H.; Lee, Y.B.; Cho, K.G.; Oh, Y.J.; Kim, S.U.; Jin, B.K. Trisialoganglioside GT1b Induces in Vivo Degeneration of Nigral Dopaminergic Neurons: Role of Microglia. Glia 2002, 38, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Dukhinova, M.; Veremeyko, T.; Yung, A.W.Y.; Kuznetsova, I.S.; Lau, T.Y.B.; Kopeikina, E.; Chan, A.M.L.; Ponomarev, E.D. Fresh Evidence for Major Brain Gangliosides as a Target for the Treatment of Alzheimer’s Disease. Neurobiol. Aging 2019, 77, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Bartzokis, G. Alzheimer’s Disease as Homeostatic Responses to Age-Related Myelin Breakdown. Neurobiol. Aging 2011, 32, 1341–1371. [Google Scholar] [CrossRef]
- Nasrabady, S.E.; Rizvi, B.; Goldman, J.E.; Brickman, A.M. White Matter Changes in Alzheimer’s Disease: A Focus on Myelin and Oligodendrocytes. Acta Neuropathol. Commun. 2018, 6, 22. [Google Scholar] [CrossRef]
- Dean, D.C.; Sojkova, J.; Hurley, S.; Kecskemeti, S.; Okonkwo, O.; Bendlin, B.B.; Theisen, F.; Johnson, S.C.; Alexander, A.L.; Gallagher, C.L. Alterations of Myelin Content in Parkinson’s Disease: A Cross-Sectional Neuroimaging Study. PLoS ONE 2016, 11, 1–20. [Google Scholar] [CrossRef]
- Kolind, S.; Sharma, R.; Knight, S.; Johansen-Berg, H.; Talbot, K.; Turner, M.R. Myelin Imaging in Amyotrophic and Primary Lateral Sclerosis. Amyotroph. Lateral Scler. Frontotemp. Degener. 2013, 14, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Gozzi, M.; Nielson, D.M.; Lenroot, R.K.; Ostuni, J.L.; Luckenbaugh, D.A.; Thurm, A.E.; Giedd, J.N.; Swedo, S.E. A Magnetization Transfer Imaging Study of Corpus Callosum Myelination in Young Children with Autism. Biol. Psychiatry 2012, 72, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.P. Editorial: Can Dysregulated Myelination Be Linked to ADHD Pathogenesis and Persistence? J. Child. Psychol. Psychiatry 2019, 60, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Onnink, A.M.H.; Zwiers, M.P.; Hoogman, M.; Mostert, J.C.; Dammers, J.; Kan, C.C.; Vasquez, A.A.; Schene, A.H.; Buitelaar, J.; Franke, B. Deviant White Matter Structure in Adults with Attention-Deficit/Hyperactivity Disorder Points to Aberrant Myelination and Affects Neuropsychological Performance. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 63, 14–22. [Google Scholar] [CrossRef]
- Takahashi, N.; Sakurai, T.; Davis, K.L.; Buxbaum, J.D. Linking Oligodendrocyte and Myelin Dysfunction to Neurocircuitry Abnormalities in Schizophrenia. Prog. Neurobiol. 2011, 93, 13–24. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, E.K.; Yoo, H.; Choi, Y.H.; Kim, S.; Lee, B.K.; Jung, Y.H.; Kim, H.Y.; Kim, H.S.; Choi, J.H. Surgical Necrotizing Enterocolitis versus Spontaneous Intestinal Perforation in White Matter Injury on Brain Magnetic Resonance Imaging. Neonatology 2016, 110, 148–154. [Google Scholar] [CrossRef]
- Vinson, M.; Strijbos, P.J.L.M.; Rowles, A.; Facci, L.; Moore, S.E.; Simmons, D.L.; Walsh, F.S. Myelin-Associated Glycoprotein Interacts with Ganglioside GT1b. A Mechanism for Neurite Outgrowth Inhibition. J. Biol. Chem. 2001, 276, 20280–20285. [Google Scholar] [CrossRef]
- Pan, B.; Fromholt, S.E.; Hess, E.J.; Crawford, T.O.; Griffin, J.W.; Sheikh, K.A.; Schnaar, R.L. Myelin-Associated Glycoprotein and Complementary Axonal Ligands, Gangliosides, Mediate Axon Stability in the CNS and PNS: Neuropathology and Behavioral Deficits in Single- and Double-Null Mice. Exp. Neurol. 2005, 195, 208–217. [Google Scholar] [CrossRef]
- Tzeng, S.F.; Deibler, G.E.; DeVries, G.H. Myelin Basic Protein and Myelin Basic Protein Peptides Induce the Proliferation of Schwann Cells via Ganglioside GM1 and the FGF Receptor. Neurochem. Res. 1999, 24, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Pomytkin, I.; Costa-Nunes, J.P.; Kasatkin, V.; Veniaminova, E.; Demchenko, A.; Lyundup, A.; Lesch, K.-P.; Ponomarev, E.D.; Strekalova, T. Insulin Receptor in the Brain: Mechanisms of Activation and the Role in the CNS Pathology and Treatment. CNS Neurosci. Ther. 2018, 24, 763–774. [Google Scholar] [CrossRef]
- Landau, Z.; Pinhas-Hamiel, O. Attention Deficit/Hyperactivity, the Metabolic Syndrome, and Type 2 Diabetes. Curr. Diab. Rep. 2019, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Clemenzi, M.N.; Wellhauser, L.; Aljghami, M.E.; Belsham, D.D. Tumour Necrosis Factor α Induces Neuroinflammation and Insulin Resistance in Immortalised Hypothalamic Neurones through Independent Pathways. J. Neuroendocrinol. 2019, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, N.; Dwivedi, S.; Nath, C.; Hanif, K.; Shukla, R. Protection of Streptozotocin Induced Insulin Receptor Dysfunction, Neuroinflammation and Amyloidogenesis in Astrocytes by Insulin. Neuropharmacology 2014, 86, 337–352. [Google Scholar] [CrossRef]
- Hackett, A.R.; Strickland, A.; Milbrandt, J. Disrupting Insulin Signaling in Schwann Cells Impairs Myelination and Induces a Sensory Neuropathy. Glia 2020, 68, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, H.; Zou, M.; Li, L.; Li, Q.; Sun, C.; Xia, W.; Cao, Y.; Wu, L. Folic Acid Improves Abnormal Behavior via Mitigation of Oxidative Stress, Inflammation, and Ferroptosis in the BTBR T+ Tf/J Mouse Model of Autism. J. Nutr. Biochem. 2019, 71, 98–109. [Google Scholar] [CrossRef] [PubMed]
- de Diego-Otero, Y.; Romero-Zerbo, Y.; el Bekay, R.; Decara, J.; Sanchez, L.; de Fonseca, F.R.; del Arco-Herrera, I. α-Tocopherol Protects against Oxidative Stress in the Fragile X Knockout Mouse: An Experimental Therapeutic Approach for the Fmr1 Deficiency. Neuropsychopharmacology 2009, 34, 1011–1026. [Google Scholar] [CrossRef]
- Gao, H.-M.; Zhou, H.; Hong, J.-S. Oxidative Stress, Neuroinflammation, and Neurodegeneration. In Neuroinflammation and Neurodegeneration; Peterson, P.K., Toborek, M., Eds.; Springer: New York, NY, USA, 2014; pp. 81–104. [Google Scholar]
- Hensley, K.; Mhatre, M.; Mou, S.; Pye, Q.N.; Stewart, C.; West, M.; Williamson, K.S. On the Relation of Oxidative Stress to Neuroinflammation: Lessons Learned from the G93A-SOD1 Mouse Model of Amyotrophic Lateral Sclerosis. Antioxid. Redox Signal. 2006, 8, 2075–2087. [Google Scholar] [CrossRef]
- Filiou, M.D.; Teplytska, L.; Otte, D.M.; Zimmer, A.; Turck, C.W. Myelination and Oxidative Stress Alterations in the Cerebellum of the G72/G30 Transgenic Schizophrenia Mouse Model. J. Psychiatr. Res. 2012, 46, 1359–1365. [Google Scholar] [CrossRef]
- Reid, M.V.; Murray, K.A.; Marsh, E.D.; Golden, J.A.; Simmons, R.A.; Grinspan, J.B. Delayed Myelination in an Intrauterine Growth Retardation Model Is Mediated by Oxidative Stress Upregulating Bone Morphogenetic Protein 4. J. Neuropathol. Exp. Neurol. 2012, 71, 640–653. [Google Scholar] [CrossRef]
- Söǧüt, S.; Zoroǧlu, S.S.; Özyurt, H.; Yilmaz, H.R.; Özuǧurlu, F.; Sivasli, E.; Yetkin, Ö.; Yanik, M.; Tutkun, H.; Savaş, H.A.; et al. Changes in Nitric Oxide Levels and Antioxidant Enzyme Activities May Have a Role in the Pathophysiological Mechanisms Involved in Autism. Clin. Chim. Acta 2003, 331, 111–117. [Google Scholar] [CrossRef]
- Meguid, N.A.; Dardir, A.A.; Abdel-Raouf, E.R.; Hashish, A. Evaluation of Oxidative Stress in Autism: Defective Antioxidant Enzymes and Increased Lipid Peroxidation. Biol. Trace Elem. Res. 2011, 143, 58–65. [Google Scholar] [CrossRef]
- Tyurina, Y.Y.; Tyurin, V.A.; Avrova, N.F. Ganglioside GM1 Protects CAMP 3′ 5′: Phosphodiesterase from Inactivation Caused by Lipid Peroxidation in Brain Synaptosomes of Rats. Mol. Chem. Neuropathol. 1993, 19, 205–217. [Google Scholar] [CrossRef]
- Fighera, M.R.; Bonini, J.S.; Frussa-Filho, R.; Dutra-Filho, C.S.; Kienzle Hagen, M.E.; Rubin, M.A.; Mello, C.F. Monosialoganglioside Increases Catalase Activity in Cerebral Cortex of Rats. Free Radic. Res. 2004, 38, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Mahadik, S.P.; Makar, T.K.; Murthy, J.N.; Ortiz, A.; Wakade, C.G.; Karpiak, S.E. Temporal Changes in Superoxide Dismutase, Glutathione Peroxidase, and Catalase Levels in Primary and Peri-Ischemic Tissue-Monosialoganglioside (GM1) Treatment Effects. Mol. Chem. Neuropathol. 1993, 18, 1–14. [Google Scholar] [CrossRef]
- Maulik, N.; Das, D.K.; Gogineni, M.; Cordis, G.A.; Avrova, N.; Denisova, N. Reduction of Myocardial Ischemic Reperfusion Injury by Sialylated Glycosphingolipids, Gangliosides. J. Cardiovasc. Pharmacol. 1993, 22, 74–81. [Google Scholar] [CrossRef]
- Sergent, O.; Pereira, M.; Belhomme, C.; Chevanne, M.; Huc, L.; Lagadic-Gossmann, D. Role for Membrane Fluidity in Ethanol-Induced Oxidative Stress of Primary Rat Hepatocytes. J. Pharmacol. Exp. Ther. 2005, 313, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Gavella, M.; Lipovac, V. Protective Effects of Exogenous Gangliosides on ROS-Induced Changes in Human Spermatozoa. Asian J. Androl. 2013, 15, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, Y.A.; Zakharova, I.O.; Sokolova, T.I.; Furaev, V.V.; Rychkova, M.P.; Avrova, N.F. Role of Tyrosine Kinase of Trk-Receptors in Realization of Antioxidant Effect of Ganglioside GM1 in PC12 Cells. J. Evol. Biochem. Physiol. 2009, 45, 562–570. [Google Scholar] [CrossRef]
- Zakharova, I.O.; Sokolova, T.V.; Furaev, V.V.; Rychkova, M.P.; Avrova, N.F. Effects of Oxidative Stress Inducers, Neurotoxins, and Ganglioside GM1 on Na+, K+-ATPase in PC12 and Brain Synaptosomes. Z. Evol. Biokhim. Fiziol. 2007, 43, 148–154. [Google Scholar] [PubMed]
- Simão, F.; Matté, A.; Breier, A.C.; Kreutz, F.; Trindade, V.M.T.; Netto, C.A.; Salbego, C.G. Resveratrol Prevents Global Cerebral Ischemiainduced Decrease in Lipid Content. Neurol. Res. 2013, 35, 59–64. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Ibeji, C.U.; Olasehinde, T.A.; Koorbanally, N.A.; Islam, M.S. Ferulic Acid Modulates Dysfunctional Metabolic Pathways and Purinergic Activities, While Stalling Redox Imbalance and Cholinergic Activities in Oxidative Brain Injury. Neurotox. Res. 2020, 37, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Yin, L.; Yuan, L.; Sui, D.; Sun, Y.; Fu, H.; Chen, L.; Wang, X. Ganglioside GM1 Protects against High Altitude Cerebral Edema in Rats by Suppressing the Oxidative Stress and Inflammatory Response via the PI3K/AKT-Nrf2 Pathway. Mol. Immunol. 2018, 95, 91–98. [Google Scholar] [CrossRef]
- Ding, W.; Liu, R.; Shan, R. Effect of Gangliosides Combined with Mouse NGF on the Expression of Serum HIF-1α, NSE, and SICAM-1 Levels in Neonates with HIE. Am. J. Transl. Res. 2021, 13, 10570–10577. [Google Scholar] [PubMed]
- Liu, Y.; Yang, Z.; Du, Y.; Shi, S.; Cheng, Y. Antioxidant Interventions in Autism Spectrum Disorders: A Meta-Analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 113, 110476. [Google Scholar] [CrossRef] [PubMed]
- Gorlova, A.; Pavlov, D.; Anthony, D.C.; Ponomarev, E.D.; Sambon, M.; Proshin, A.; Shafarevich, I.; Babaevskaya, D.; Lesch, K.P.; Bettendorff, L.; et al. Thiamine and Benfotiamine Counteract Ultrasound-Induced Aggression, Normalize AMPA Receptor Expression and Plasticity Markers, and Reduce Oxidative Stress in Mice. Neuropharmacology 2019, 156, 107543. [Google Scholar] [CrossRef]
- Costa-Nunes, J.P.; Gorlova, A.; Pavlov, D.; Cespuglio, R.; Gorovaya, A.; Proshin, A.; Umriukhin, A.; Ponomarev, E.D.; Kalueff, A.V.; Strekalova, T.; et al. Ultrasound Stress Compromises the Correlates of Emotional-like States and Brain AMPAR Expression in Mice: Effects of Antioxidant and Anti-Inflammatory Herbal Treatment. Stress 2020, 23, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, D.; Gorlova, A.; Bettendorff, L.; Kalueff, A.A.; Umriukhin, A.; Proshin, A.; Lysko, A.; Landgraf, R.; Anthony, D.C.; Strekalova, T. Enhanced Conditioning of Adverse Memories in the Mouse Modified Swim Test Is Associated with Neuroinflammatory Changes—Effects That Are Susceptible to Antidepressants. Neurobiol. Learn Mem. 2020, 172, 107227. [Google Scholar] [CrossRef]
- de Munter, J.; Pavlov, D.; Gorlova, A.; Sicker, M.; Proshin, A.; Kalueff, A.V.; Svistunov, A.; Kiselev, D.; Nedorubov, A.; Morozov, S.; et al. Increased Oxidative Stress in the Prefrontal Cortex as a Shared Feature of Depressive- and PTSD-Like Syndromes: Effects of a Standardized Herbal Antioxidant. Front Nutr. 2021, 8, 661455. [Google Scholar] [CrossRef]
- Schapovalova, O.; Gorlova, A.; de Munter, J.; Sheveleva, E.; Eropkin, M.; Gorbunov, N.; Sicker, M.; Umriukhin, A.; Lybchik, S.; Lesch, K.P.; et al. Immunomodulatory Effects of New Phytotherapy on Human Macrophages and TLR4- and TLR7/8-Mediated Viral-like Inflammation in Mice. Front Med. 2022, 9, 952977. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).