Structural–Functional Relationship of the Ribonucleolytic Activity of aIF5A from Sulfolobus solfataricus
Abstract
1. Introduction
2. Materials and Methods
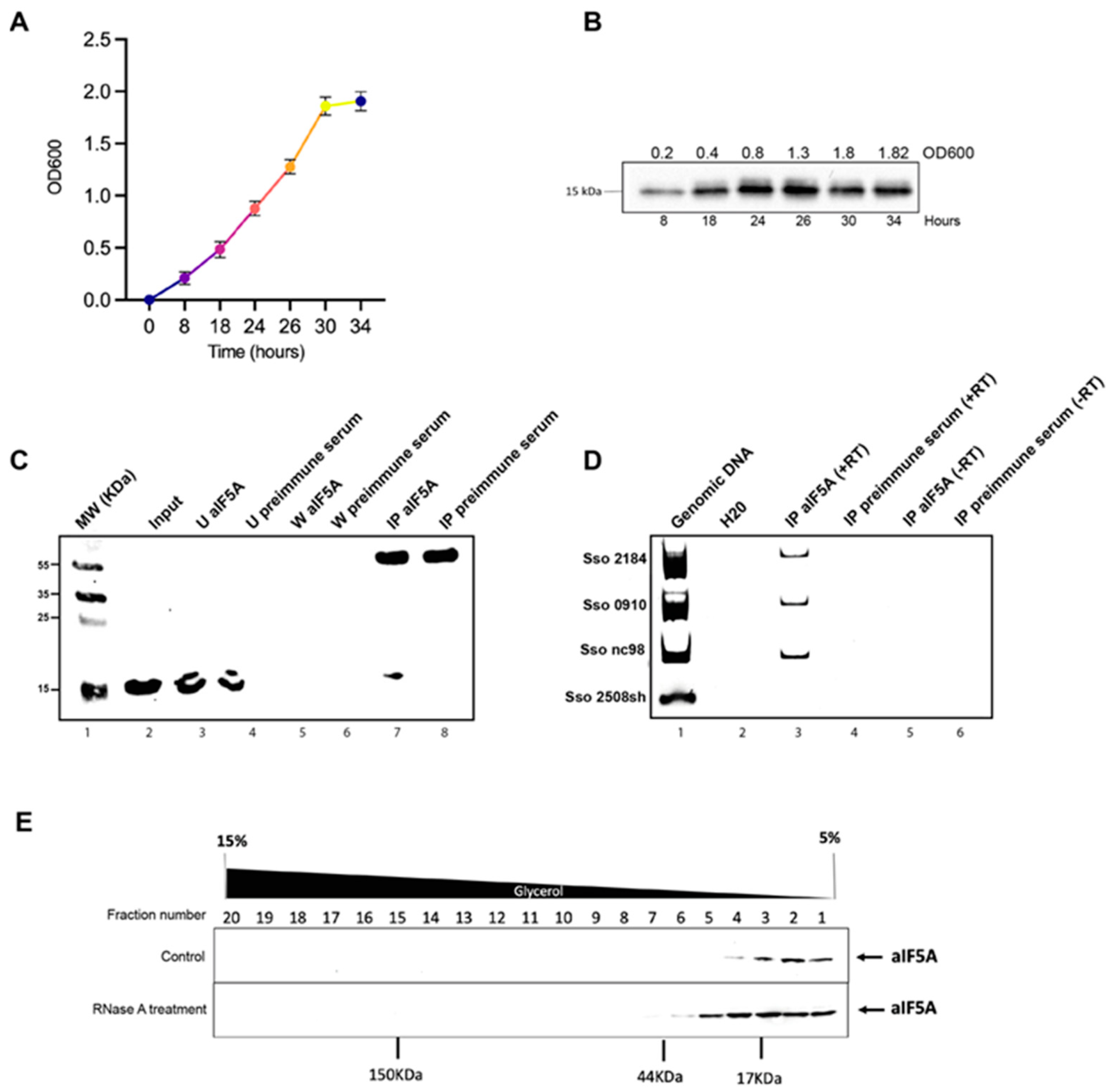
2.1. Cell Growth and S100 Lysate Preparation
2.2. Immunoprecipitation of Native aIF5A from S. solfataricus Lysate and RNA Extraction
2.3. Reverse Transcriptase-PCR
2.4. Density-Based Separation of Native aIF5A by 5–15% Glycerol Gradient Centrifugation
2.5. Recombinant aIF5A Purification
2.6. S. solfataricus Total rRNA and tRNA Extraction
2.7. rRNA 23S and 16S Isolation and Degradation Assays
2.8. In Vitro Transcription and RNase Activity Assay of aIF5A
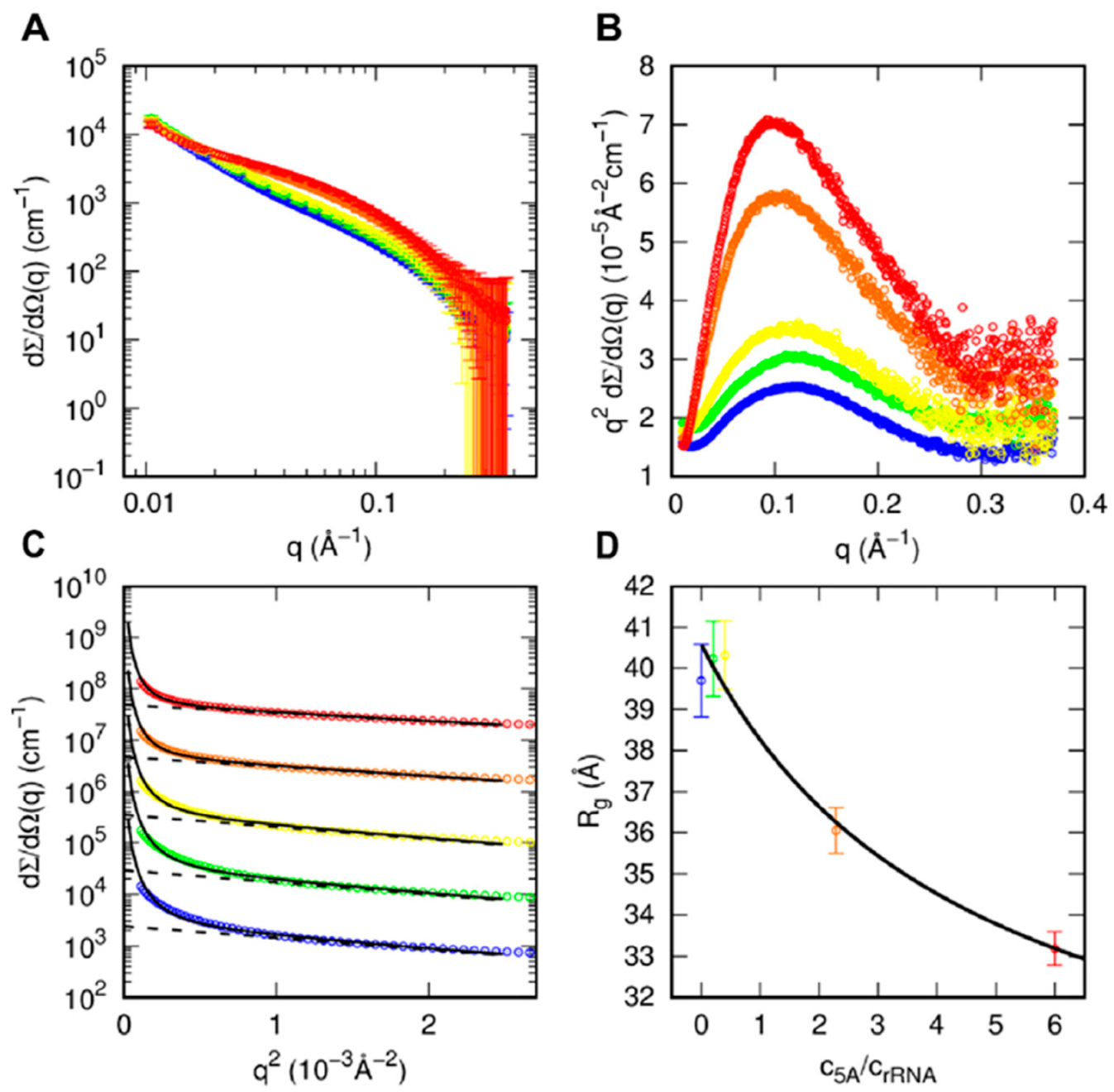
2.9. Small-Angle X-ray Scattering (SAXS)
2.10. SAXS Data Analysis
2.11. Computational Analysis
3. Results
3.1. A Specific Subset of RNA Molecules Is Associated with S. solfataricus aIF5A In Vivo
3.2. S. solfataricus aIF5A Exerts Its Ribonucleolytic Activity on Long and Structured RNA
3.3. Structural Characterization of S. solfataricus aIF5A in Solution by SAXS
3.4. SAXS Analysis of aIF5A in the Presence of RNA Substrate
3.5. Structural and Functional Features of S. solfataricus aIF5A by InterPro Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kyrpides, N.C.; Woese, C.R. Universally Conserved Translation Initiation Factors. Proc. Natl. Acad. Sci. USA 1998, 95, 224–228. [Google Scholar] [CrossRef]
- Benelli, D.; Londei, P. Begin at the Beginning: Evolution of Translational Initiation. Res. Microbiol. 2009, 160, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Dever, T.E.; Gutierrez, E.; Shin, B.S. The Hypusine-Containing Translation Factor EIF5A. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Dias, C.A.O.; Lee, S.B.; Valentini, S.R.; Sokabe, M.; Fraser, C.S.; Park, M.H. Production of Active Recombinant EIF5A: Reconstitution in E.Coli of Eukaryotic Hypusine Modification of EIF5A by Its Coexpression with Modifying Enzymes. Protein Eng. Des. Sel. 2011, 24, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Golubev, A.A.; Validov, S.Z.; Usachev, K.S.; Yusupov, M.M. Elongation Factor P: New Mechanisms of Function and an Evolutionary Diversity of Translation Regulation. Mol. Biol. 2019, 53, 501–512. [Google Scholar] [CrossRef]
- Wolff, E.C.; Kang, K.R.; Kim, Y.S.; Park, M.H. Posttranslational Synthesis of Hypusine: Evolutionary Progression and Specificity of the Hypusine Modification. Amino Acids 2007, 33, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Bartig, D.; Lemkemeier, K.; Frank, J.; Lottspeich, F.; Klink, F. The Archaebacterial Hypusine-Containing Protein. Structural Features Suggest Common Ancestry with Eukaryotic Translation Initiation Factor 5A. Eur. J. Biochem. 1992, 204, 751–758. [Google Scholar] [CrossRef]
- Bassani, F.; Romagnoli, A.; Cacciamani, T.; Amici, A.; Benelli, D.; Londei, P.; Märtens, B.; Bläsi, U.; La Teana, A. Modification of Translation Factor AIF5A from Sulfolobus Solfataricus. Extremophiles 2018, 22, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Kuroshu, R.; Zanelli, C.F.; Valentini, S.R. EIF5A and EF-P: Two Unique Translation Factors Are Now Traveling the Same Road. Wiley Interdiscip. Rev. RNA 2014, 5, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Hummels, K.R.; Kearns, D.B. Translation Elongation Factor P (EF-P). FEMS Microbiol. Rev. 2020, 44, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, E.; Shin, B.-S.; Woolstenhulme, C.J.; Kim, J.-R.; Saini, P.; Buskirk, A.R.; Dever, T.E. EIF5A Promotes Translation of Polyproline Motifs. Mol. Cell 2013, 51, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Ude, S.; Lassak, J.; Starosta, A.L.; Kraxenberger, T.; Wilson, D.N.; Jung, K. Translation Elongation Factor EF-P Alleviates Ribosome Stalling at Polyproline Stretches. Science 2013, 339, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Doerfel, L.K.; Wohlgemuth, I.; Kothe, C.; Peske, F.; Urlaub, H.; Rodnina, M.V. EF-P Is Essential for Rapid Synthesis of Proteins Containing Consecutive Proline Residues. Science 2013, 339, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Bassani, F.; Zink, I.A.; Pribasnig, T.; Wolfinger, M.T.; Romagnoli, A.; Resch, A.; Schleper, C.; Bläsi, U.; La Teana, A. Indications for a Moonlighting Function of Translation Factor AIF5A in the Crenarchaeum Sulfolobus Solfataricus. RNA Biol. 2019, 16, 675–685. [Google Scholar] [CrossRef]
- Schuller, A.P.; Wu, C.C.-C.; Dever, T.E.; Buskirk, A.R.; Green, R. EIF5A Functions Globally in Translation Elongation and Termination. Mol. Cell 2017, 66, 194–205.e5. [Google Scholar] [CrossRef] [PubMed]
- Mathews, M.B.; Hershey, J.W.B. The Translation Factor EIF5A and Human Cancer. Biochim. Biophys. Acta 2015, 1849, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, H.; Zhang, H.; Rehfeld, F.; Han, J.; Chang, T.-C.; Mendell, J.T. Suppression of Ribosomal Pausing by EIF5A Is Necessary to Maintain the Fidelity of Start Codon Selection. Cell Rep. 2019, 29, 3134–3146.e6. [Google Scholar] [CrossRef] [PubMed]
- Pelechano, V.; Alepuz, P. EIF5A Facilitates Translation Termination Globally and Promotes the Elongation of Many Non Polyproline-Specific Tripeptide Sequences. Nucleic Acids Res. 2017, 45, 7326–7338. [Google Scholar] [CrossRef] [PubMed]
- Kulak, N.A.; Pichler, G.; Paron, I.; Nagaraj, N.; Mann, M. Minimal, Encapsulated Proteomic-Sample Processing Applied to Copy-Number Estimation in Eukaryotic Cells. Nat. Methods 2014, 11, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Chen, K.Y. Hypusine Is Required for a Sequence-Specific Interaction of Eukaryotic Initiation Factor 5A with Postsystematic Evolution of Ligands by Exponential Enrichment RNA. J. Biol. Chem. 2001, 276, 2555–2561. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Jao, D.L.-E.; Chen, K.Y. Identification of MRNA That Binds to Eukaryotic Initiation Factor 5A by Affinity Co-Purification and Differential Display. Biochem. J. 2004, 384, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Zuk, D.; Jacobson, A. A Single Amino Acid Substitution in Yeast EIF-5A Results in MRNA Stabilization. EMBO J. 1998, 17, 2914–2925. [Google Scholar] [CrossRef]
- Valentini, S.R.; Casolari, J.M.; Oliveira, C.C.; Silver, P.A.; McBride, A.E. Genetic Interactions of Yeast Eukaryotic Translation Initiation Factor 5A (EIF5A) Reveal Connections to Poly(A)-Binding Protein and Protein Kinase C Signaling. Genetics 2002, 160, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Schrader, R.; Young, C.; Kozian, D.; Hoffmann, R.; Lottspeich, F. Temperature-Sensitive EIF5A Mutant Accumulates Transcripts Targeted to the Nonsense-Mediated Decay Pathway. J. Biol. Chem. 2006, 281, 35336–35346. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Klug, G. An Archaeal Protein with Homology to the Eukaryotic Translation Initiation Factor 5A Shows Ribonucleolytic Activity. J. Biol. Chem. 2007, 282, 13966–13976. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.A.O.; Garcia, W.; Zanelli, C.F.; Valentini, S.R. EIF5A Dimerizes Not Only in Vitro but Also in Vivo and Its Molecular Envelope Is Similar to the EF-P Monomer. Amino Acids 2013, 44, 631–644. [Google Scholar] [CrossRef]
- Hanawa-Suetsugu, K.; Sekinet, S.I.; Sakai, H.; Hori-Takemoto, C.; Terada, T.; Unzai, S.; Tame, J.R.H.; Kuramitsu, S.; Shirouzu, M.; Yokoyama, S. Crystal Structure of Elongation Factor P from Thermus Thermophilus HB8. Proc. Natl. Acad. Sci. USA 2004, 101, 9595–9600. [Google Scholar] [CrossRef]
- Melnikov, S.; Mailliot, J.; Shin, B.-S.; Rigger, L.; Yusupova, G.; Micura, R.; Dever, T.E.; Yusupov, M. Crystal Structure of Hypusine-Containing Translation Factor EIF5A Bound to a Rotated Eukaryotic Ribosome. J. Mol. Biol. 2016, 428, 3570–3576. [Google Scholar] [CrossRef]
- Buschauer, R.; Matsuo, Y.; Sugiyama, T.; Chen, Y.-H.; Alhusaini, N.; Sweet, T.; Ikeuchi, K.; Cheng, J.; Matsuki, Y.; Nobuta, R.; et al. The Ccr4-Not Complex Monitors the Translating Ribosome for Codon Optimality. Science 2020, 368, eaay6912. [Google Scholar] [CrossRef]
- Kim, K.K.; Hung, L.W.; Yokota, H.; Kim, R.; Kim, S.H. Crystal Structures of Eukaryotic Translation Initiation Factor 5A from Methanococcus Jannaschii at 1.8 A Resolution. Proc. Natl. Acad. Sci. USA 1998, 95, 10419–10424. [Google Scholar] [CrossRef]
- Gentz, P.M.; Blatch, G.L.; Dorrington, R.A. Dimerization of the Yeast Eukaryotic Translation Initiation Factor 5A Requires Hypusine and Is RNA Dependent. FEBS J. 2009, 276, 695–706. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.; Romagnoli, A.; Di Marino, D.; La Teana, A. Insights in the Binding Mechanism of GC7 in Sulfolobus Solfataricus: Toward the Design of New Inhibitors of the Deoxyhypusine Synthase. Biophys. J. 2020, 118, 361a. [Google Scholar] [CrossRef]
- Londei, P.; Altamura, S.; Cammarano, P.; Petrucci, L. Differential Features of Ribosomes and of Poly(U)-Programmed Cell-Free Systems Derived from Sulphur-Dependent Archaebacterial Species. Eur. J. Biochem. 1986, 157, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Benelli, D.; Londei, P. In Vitro Studies of Archaeal Translational Initiation. Methods Enzymol. 2007, 430, 79–109. [Google Scholar] [CrossRef] [PubMed]
- Benelli, D.; La Teana, A.; Londei, P. RNA Metabolism and Gene Expression in Archaea. RNA Metab. Gene Expr. Archaea 2017, 32, 71–88. [Google Scholar] [CrossRef]
- Hasenöhrl, D.; Lombo, T.; Kaberdin, V.; Londei, P.; Bläsi, U. Translation Initiation Factor a/EIF2(-Gamma) Counteracts 5’ to 3’ MRNA Decay in the Archaeon Sulfolobus Solfataricus. Proc. Natl. Acad. Sci. USA 2008, 105, 2146–2150. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making Protein Folding Accessible to All. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Blum, M.; Chang, H.-Y.; Chuguransky, S.; Grego, T.; Kandasaamy, S.; Mitchell, A.; Nuka, G.; Paysan-Lafosse, T.; Qureshi, M.; Raj, S.; et al. The InterPro Protein Families and Domains Database: 20 Years On. Nucleic Acids Res. 2021, 49, D344–D354. [Google Scholar] [CrossRef]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and Sequence Analysis Tools Services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef]
- Papadopoulos, J.S.; Agarwala, R. COBALT: Constraint-Based Alignment Tool for Multiple Protein Sequences. Bioinformatics 2007, 23, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS Biomolecular Solvation Software Suite. Protein Sci. 2018, 27, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Lassak, J.; Wilson, D.N.; Jung, K. Stall No More at Polyproline Stretches with the Translation Elongation Factors EF-P and IF-5A. Mol. Microbiol. 2016, 99, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, A.; Nakashima, T.; Taniguchi, M.; Hosaka, H.; Kimura, M.; Tanaka, I. The Three-Dimensional Structure of the RNA-Binding Domain of Ribosomal Protein L2; a Protein at the Peptidyl Transferase Center of the Ribosome. EMBO J. 1999, 18, 1459–1467. [Google Scholar] [CrossRef]
- Agrawal, V.; Kishan, K.V.R. OB-Fold: Growing Bigger with Functional Consistency. Curr. Protein Pept. Sci. 2003, 4, 195–206. [Google Scholar] [CrossRef]
- Thodkar, K.; Cazade, P.A.; Bergmann, F.; Lopez-Calle, E.; Thompson, D.; Heindl, D. Self-Assembled Pyrene Stacks and Peptide Monolayers Tune the Electronic Properties of Functionalized Electrolyte-Gated Graphene Field-Effect Transistors. ACS Appl. Mater. Interfaces 2021, 13, 9134–9142. [Google Scholar] [CrossRef]
- Mayer, B.J. SH3 Domains: Complexity in Moderation. J. Cell Sci. 2001, 114, 1253–1263. [Google Scholar] [CrossRef]
- Draper, D.E.; Reynaldo, L.P. RNA Binding Strategies of Ribosomal Proteins. Nucleic Acids Res. 1999, 27, 381–388. [Google Scholar] [CrossRef]
- Peat, T.S.; Newman, J.; Waldo, G.S.; Berendzen, J.; Terwilliger, T.C. Structure of Translation Initiation Factor 5A from Pyrobaculum Aerophilum at 1.75 A Resolution. Structure 1998, 6, 1207–1214. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Schubert, M.; Edge, R.E.; Lario, P.; Cook, M.A.; Strynadka, N.C.J.; Mackie, G.A.; McIntosh, L.P. Structural Characterization of the RNase E S1 Domain and Identification of Its Oligonucleotide-Binding and Dimerization Interfaces. J. Mol. Biol. 2004, 341, 37–54. [Google Scholar] [CrossRef]
- Arcus, V. OB-Fold Domains: A Snapshot of the Evolution of Sequence, Structure and Function. Curr. Opin. Struct. Biol. 2002, 12, 794–801. [Google Scholar] [CrossRef]
- Theobald, D.L.; Mitton-Fry, R.M.; Wuttke, D.S. Nucleic Acid Recognition by OB-Fold Proteins. Annu. Rev. Biophys. Biomol. Struct. 2003, 32, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Clouet-d’Orval, B.; Batista, M.; Bouvier, M.; Quentin, Y.; Fichant, G.; Marchfelder, A.; Maier, L.-K. Insights into RNA-Processing Pathways and Associated RNA-Degrading Enzymes in Archaea. FEMS Microbiol. Rev. 2018, 42, 579–613. [Google Scholar] [CrossRef]
- Glatter, O.; Kratky, O. Small Angle X-Ray Scattering. Acta Crystallogr. Sect. A 1983, 39, 500. [Google Scholar] [CrossRef]
- Spinozzi, F.; Ortore, M.G.; Nava, G.; Bomboi, F.; Carducci, F.; Amenitsch, H.; Bellini, T.; Sciortino, F.; Mariani, P. Gelling without Structuring: A SAXS Study of the Interactions among DNA Nanostars. Langmuir 2020, 36, 10387–10396. [Google Scholar] [CrossRef]
- Frauenfelder, H.; Hartmann, H.; Karplus, M.; Kuntz, I.D.J.; Kuriyan, J.; Parak, F.; Petsko, G.A.; Ringe, D.; Tilton, R.F.J.; Connolly, M.L. Thermal Expansion of a Protein. Biochemistry 1987, 26, 254–261. [Google Scholar] [CrossRef]
- Tarek, M.; Tobias, D.J. The Dynamics of Protein Hydration Water: A Quantitative Comparison of Molecular Dynamics Simulations and Neutron-Scattering Experiments. Biophys. J. 2000, 79, 3244–3257. [Google Scholar] [CrossRef]
- Spinozzi, F.; Beltramini, M. QUAFIT: A Novel Method for the Quaternary Structure Determination from Small-Angle Scattering Data. Biophys. J. 2012, 103, 511–521. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guinier, A.; Fournet, G. Small Angle Scattering of X-Rays. J. Polym. Sci. 1956, 19, 594. [Google Scholar] [CrossRef]
- Malmberg, C.G.; Maryott, A.A. Dielectric Constant of Water from 0 0 to 100 0 C. In Proceedings of the Physic; American Institute of Physics: Melville, NY, USA, 2011. [Google Scholar]
- Yao, M.; Ohsawa, A.; Kikukawa, S.; Tanaka, I.; Kimura, M. Crystal Structure of Hyperthermophilic Archaeal Initiation Factor 5A: A Homologue of Eukaryotic Initiation Factor 5A (EIF-5A). J. Biochem. 2003, 133, 75–81. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romagnoli, A.; Moretti, P.; D’Agostino, M.; Rexha, J.; Perta, N.; Piccinini, A.; Di Marino, D.; Spinozzi, F.; La Teana, A. Structural–Functional Relationship of the Ribonucleolytic Activity of aIF5A from Sulfolobus solfataricus. Biomolecules 2022, 12, 1432. https://doi.org/10.3390/biom12101432
Romagnoli A, Moretti P, D’Agostino M, Rexha J, Perta N, Piccinini A, Di Marino D, Spinozzi F, La Teana A. Structural–Functional Relationship of the Ribonucleolytic Activity of aIF5A from Sulfolobus solfataricus. Biomolecules. 2022; 12(10):1432. https://doi.org/10.3390/biom12101432
Chicago/Turabian StyleRomagnoli, Alice, Paolo Moretti, Mattia D’Agostino, Jesmina Rexha, Nunzio Perta, Astra Piccinini, Daniele Di Marino, Francesco Spinozzi, and Anna La Teana. 2022. "Structural–Functional Relationship of the Ribonucleolytic Activity of aIF5A from Sulfolobus solfataricus" Biomolecules 12, no. 10: 1432. https://doi.org/10.3390/biom12101432
APA StyleRomagnoli, A., Moretti, P., D’Agostino, M., Rexha, J., Perta, N., Piccinini, A., Di Marino, D., Spinozzi, F., & La Teana, A. (2022). Structural–Functional Relationship of the Ribonucleolytic Activity of aIF5A from Sulfolobus solfataricus. Biomolecules, 12(10), 1432. https://doi.org/10.3390/biom12101432







