Rosmarinic Acid and Related Dietary Supplements: Potential Applications in the Prevention and Treatment of Cancer
Abstract
1. Introduction
2. Methodology
3. Culture Techniques, Extraction Processes, and Anti-Tumor Applications of RA-Rich Plants
| Source | Biotechnological Application for Production and Extraction Process | RA Content | Anti-tumor Effect | Ref |
|---|---|---|---|---|
| Rosmarinus officinalis L. | Aqueous extract of leaves | 45.64 mg/g | Cervical cancer Breast cancer T-cell leukemia | [16] |
| Leaves removed the lipidic phase using hexane. Then, extracted in ethyl acetate | Approximately 50.11% w/w RA | Colorectal cancer | [17] | |
| Dried leaves of Rosmarinus officinalis L. were extracted with 70% (v/v) ethyl alcohol overnight at 22 °C on a shaker. The stock solutions were collected from the supernatant | - | Ovarian carcinoma | [18] | |
| Perilla frutescens (L.) Britt. | Fresh Perilla leaves were extracted with 1% w/v citric acid at 90 °C for 30 min, then mixed with n-butanol, dried, and dissolved in water. Elution with 0.1% w/v TFA containing 80% v/v methanol on Diaion HP2MG column | 68% w/w RA of freeze-dried powder | Skin carcinogenesis | [20] |
| The dried leaves were chopped, boiled in 1 L of distilled water for 1 h, and filtered. The supernatant was lyophilized. | - | HCC | [19] | |
| The seed meal was extracted in 70% ethanol and dried, then dissolved in ethyl acetate | 600.32–647.68 mg/g | Lung cancer | [21,22] | |
| Melissa officinalis L. | 50% ethanolic extracts of leaves | N.A. | Colorectal cancer | [23] |
| Ethanolic extracts of dry leaves | 184.4 ± 0.3 mg/g | Lung cancer | [8] | |
| Ethanolic extract | Approximately 18% | Photoaging and skin cancer | [24] | |
| Ocimum tenuiflorum L. | Leaves were soaked in 95% ethanol for two weeks, then filtered and dried | Approximately 7.86 mg/g | HNSCC | [27] |
| Ocimum basilicum L. | 99% methanol extracts of dry leaves contained RA 3.01 mg/g | 3.01 mg/g | Cervical cancer Breast cancer T-cell leukemia | [26] |
| Callus of basil supplemented with 5 mg/L BAP and 1 mg/L NAA and extracted using 100% ethanol | 7.4 mg/g | - | [28] | |
| Callus of basil grown on medium supplemented with 10 mg/L CuO-NPs, then extracted using 99.9% methanol | 11.4 mg/g | - | [29] | |
| Callus of basil grown on with LED irradiation (24 h, 660 nm), then extracted using methanol | 96.0 mg/g | - | [30] | |
| Origanum vulgare L. | The aqueous part of the plant was chromatographed on silica gel and eluted with hexane | 0.15 mg/g RA/dry plant | Glioma Cervical cancer | [31] |
| Herb was ground and sieved using a 125-μm sieve. The powder was extracted with hot reflux in 90% (v/v) ethanol at 95 °C for 4 h | Approximately 36 mg/g | Glioma Breast cancer | [32] | |
| Thymus vulgaris L. | Dried callus was extracted by Soxhlet continuous extraction device | 5.67 mg/g | Breast cancer | [33] |
| Thymus longicaulis C.Presl | The leaves were collected in October using 50% methanol for ultrasonic extraction | 3.03 mg/mL | Leukemia Glioma Breast cancer Colorectal cancer | [34] |
| Salvia officinalis L. and Salvia fruticosa Mill. | Aqueous extracts | 52.0 and 71.5 μg/mL RA of water extract | Colorectal cancer | [35] |
| Salvia officinalis L. | The seedlings were irrigated with 1 mM sodium silicate, 200 μM sodium nitroprusside, and 200 μM CuSO4 | 0.62 mg/g | - | [37] |
| Salvia miltiorrhiza Bunge | Ground powder was enzymatically incubated and extracted with Cellulase A, Protamex (1:1), and distilled water at 30 °C for 2 h with stirring. | 28.23 mg/g | - | [40] |
| Prunella laciniata (L.) L. | 60% ethanol extract of leaves | 2.31 mg/g | Lung cancer | [43,44] |
| Gastrocotyle hispida (Forssk.) Bunge | 80% methanol extracts from leaves | - | HCC Breast cancer | [45] |
| Glechoma hederacea L. | The whole plants were extracted in distilled water (3 hr at 100 °C) at a dilution of 1:50 (w/v), then extracted with ethyl acetate | 174.10 ± 5.80 mg/g | HCC | [46] |
| Ehretia tinifolia L. | The juice in the fruit was applied onto an Amberlite XAD-7 column and eluted with methanol | - | Cervical cancer Breast cancer Colorectal cancer | [47] |
| Dracocephalum kotschyi Boiss. | Transformed roots were influenced by 50 mg/L tTiO2 NPs for 24 h exposure time and incubated for one week. The transformed roots were harvested and extracted under 80% methanol ultrasound | 530.5 μg/g | - | [48] |
| In vitro grown leaves were co-cultivated with Agrobacterium rhizogenes strain to mediate hairy root. Hairy roots were exposed to 75 mg/L Fe NP for 24h, then harvested and extracted under 80% methanol ultrasound | 1194 μg/g | - | [49] | |
| Leonotis nepetifolia (L.) R.Br. | Young seedlings were infected with Rhizobium rhizogenes strain A4, then harvested and extracted under 80% methanol ultrasound | 2643 µg/g | Lung cancer Breast cancer T-cell leukemia | [12] |
| Satureja khuzistanica Jamzad | Cell suspension cultures of plants supplemented with 100 μM MeJA for 21 days Methanol extraction | 3.9 g/L RA in cell suspension cultures | - | [50] |
| Cell suspension cultures of plants elicited with 1 µM coronatine | 2.67 g/L RA in cell suspension cultures | Breast cancer | [51] | |
| Lactobacillus plantarum | Fresh grape skins were vacuum-cooled and powdered, fermented by Lactobacillus plantarum KFY02 for 96 h | - | HCC | [52] |
4. Improvement of Bioaccessibility and Bioavailability—Novel Technologies
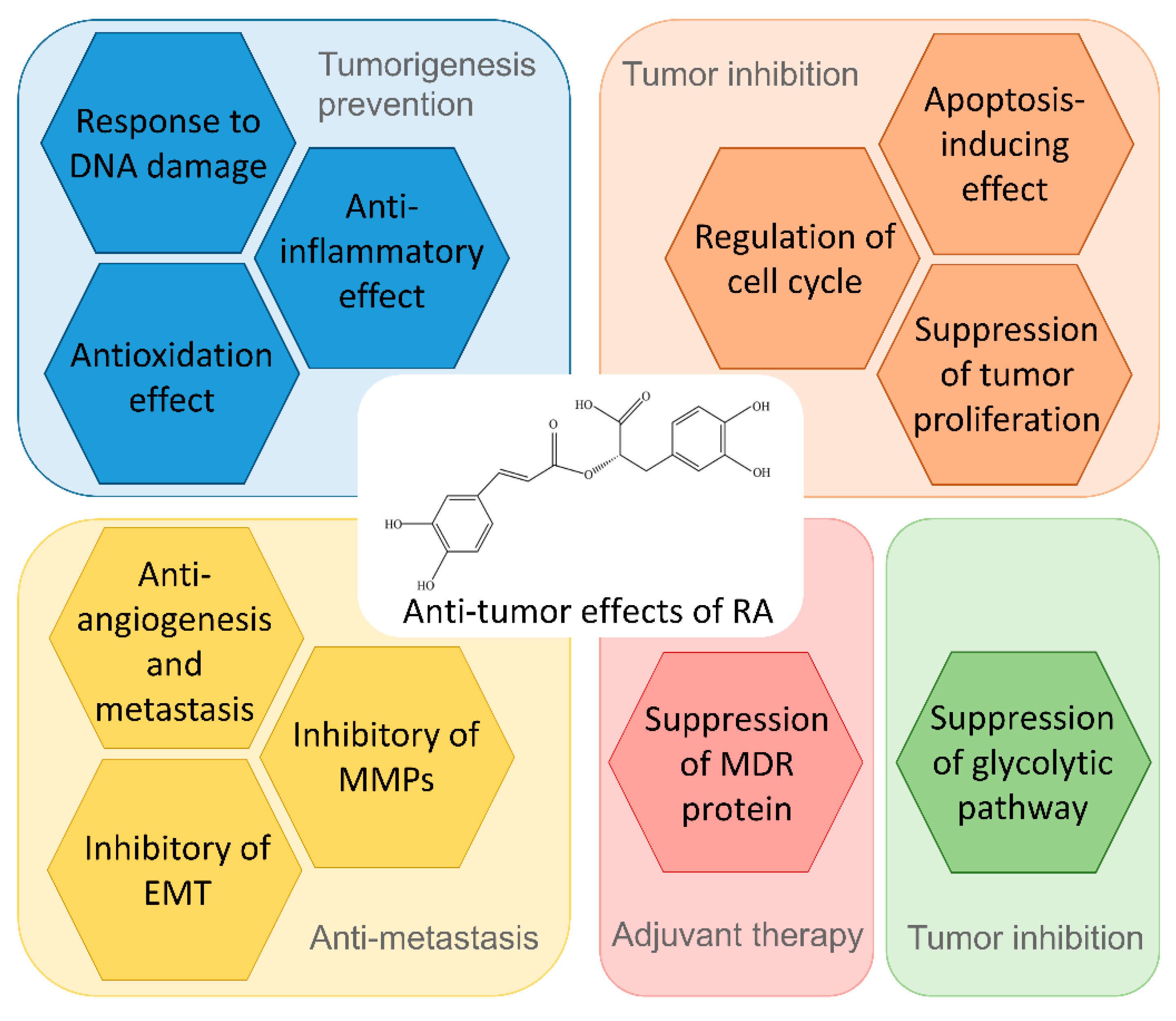
5. Biological Processes and Mechanism of Action of RA in Tumor Prevention and Treatment
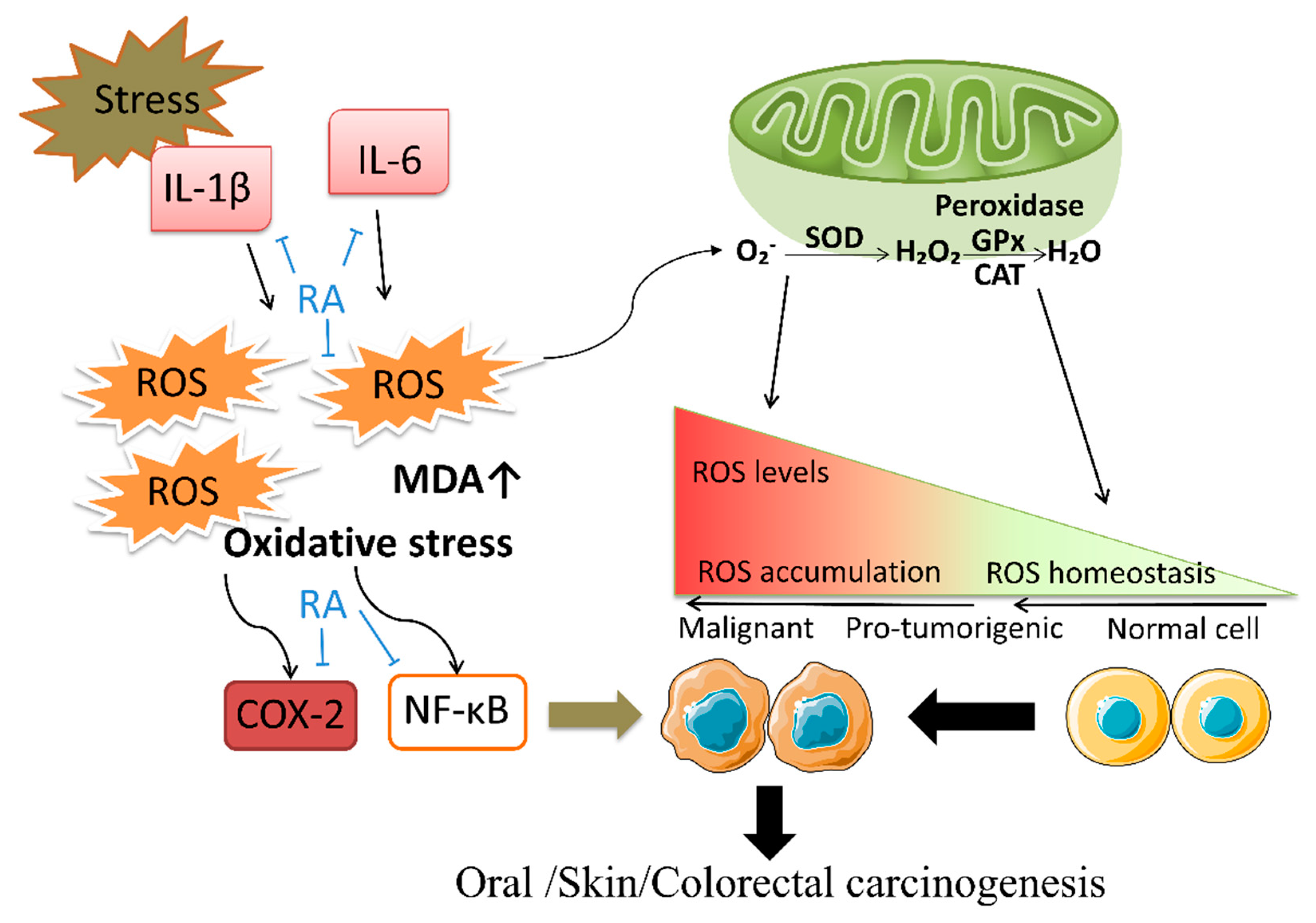
5.1. Antioxidation and Anti-Inflammatory Effect
5.2. Response to DNA Damage
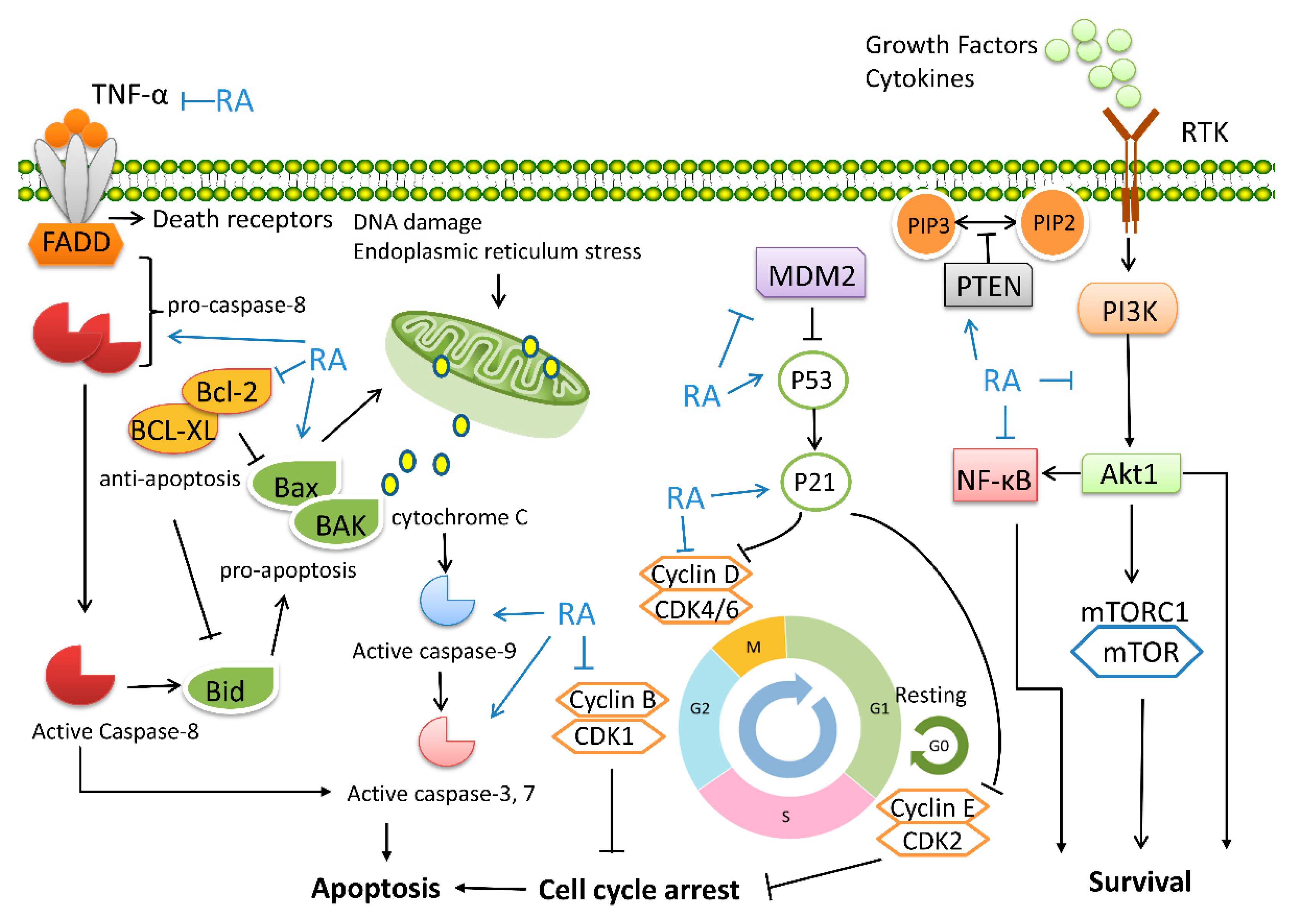
5.3. Regulation of Cell Cycle and Tumor Proliferation
5.4. Apoptosis-Inducing Effect
5.5. Suppression of Multidrug Resistance (MDR) Proteins
5.6. Suppression of Glycolytic Pathway
5.7. EMT Inhibition
5.8. Anti-Angiogenesis and Metastasis
6. Prevention of RA in Tumorigenesis
7. The Therapeutic Effect of RA on Cancer
8. Chemosensitivity Effect of RA on Tumor Therapy
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Emery, J.; Butow, P.; Lai-Kwon, J.; Nekhlyudov, L.; Rynderman, M.; Jefford, M. Management of common clinical problems experienced by survivors of cancer. Lancet 2022, 399, 1537–1550. [Google Scholar] [CrossRef]
- Sardana, R.K.; Chhikara, N.; Tanwar, B.; Panghal, A. Dietary impact on esophageal cancer in humans: A review. Food Funct. 2018, 9, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Du, B.; Chen, J. Effects and mechanisms of dietary bioactive compounds on breast cancer prevention. Pharmacol. Res. 2022, 178, 105974. [Google Scholar] [CrossRef] [PubMed]
- Crowder, S.L.; Playdon, M.C.; Gudenkauf, L.M.; Ose, J.; Gigic, B.; Greathouse, L.; Peoples, A.R.; Sleight, A.G.; Jim, H.S.L.; Figueiredo, J.C. A Molecular Approach to Understanding the Role of Diet in Cancer-Related Fatigue: Challenges and Future Opportunities. Nutrients 2022, 14, 1496. [Google Scholar] [CrossRef]
- Khatoon, E.; Banik, K.; Harsha, C.; Sailo, B.L.; Thakur, K.K.; Khwairakpam, A.D.; Vikkurthi, R.; Devi, T.B.; Gupta, S.C.; Kunnumakkara, A.B. Phytochemicals in cancer cell chemosensitization: Current knowledge and future perspectives. Semin. Cancer Biol. 2022, 80, 306–339. [Google Scholar] [CrossRef]
- Nunes, S.; Madureira, A.R.; Campos, D.; Sarmento, B.; Gomes, A.M.; Pintado, M.; Reis, F. Therapeutic and nutraceutical potential of rosmarinic acid-Cytoprotective properties and pharmacokinetic profile. Crit. Rev. Food Sci. Nutr. 2017, 57, 1799–1806. [Google Scholar] [CrossRef]
- Magalhães, D.B.; Castro, I.; Lopes-Rodrigues, V.; Pereira, J.M.; Barros, L.; Ferreira, I.; Xavier, C.P.R.; Vasconcelos, M.H. Melissa officinalis L. ethanolic extract inhibits the growth of a lung cancer cell line by interfering with the cell cycle and inducing apoptosis. Food Funct. 2018, 9, 3134–3142. [Google Scholar] [CrossRef]
- Jin, B.R.; Chung, K.S.; Hwang, S.; Hwang, S.N.; Rhee, K.J.; Lee, M.; An, H.J. Rosmarinic acid represses colitis-associated colon cancer: A pivotal involvement of the TLR4-mediated NF-κB-STAT3 axis. Neoplasia 2021, 23, 561–573. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Tang, H.; Pan, Y.; Hu, B.; Huang, G. Rosmarinic acid inhibits cell proliferation, migration, and invasion and induces apoptosis in human glioma cells. Int. J. Mol. Med. 2021, 47, 1–11. [Google Scholar] [CrossRef]
- Liao, X.Z.; Gao, Y.; Sun, L.L.; Liu, J.H.; Chen, H.R.; Yu, L.; Chen, Z.Z.; Chen, W.H.; Lin, L.Z. Rosmarinic acid reverses non-small cell lung cancer cisplatin resistance by activating the MAPK signaling pathway. Phytother. Res. 2020, 34, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Merecz-Sadowska, A.; Rijo, P.; Isca, V.; Picot, L.; Wielanek, M.; Śliwiński, T.; Sitarek, P. Preliminary Phytochemical Analysis and Evaluation of the Biological Activity of Leonotis nepetifolia (L.) R. Br Transformed Roots Extracts Obtained through Rhizobium rhizogenes-Mediated Transformation. Cells 2021, 10, 1242. [Google Scholar] [CrossRef] [PubMed]
- Achour, M.; Bravo, L.; Sarriá, B.; Ben Fredj, M.; Nouira, M.; Mtiraoui, A.; Saguem, S.; Mateos, R. Bioavailability and nutrikinetics of rosemary tea phenolic compounds in humans. Food Res. Int. 2021, 139, 109815. [Google Scholar] [CrossRef] [PubMed]
- Kamal, Z.; Bairage, J.J.; Moniruzzaman, P.R.D.; Tabibul, M.; Islam, M.O.F.; Islam, M.R.; Paul, P.K.; Mou, S.M.; Rahmatullah, M.J.W.J.o.P.; Sciences, P. Folk medicinal uses of some plants in Tangail district, Bangladesh. World J. Pharm. Pharm. Sci. 2014, 3, 52–63. [Google Scholar]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Elansary, H.O.; Mahmoud, E.A. Egyptian herbal tea infusions’ antioxidants and their antiproliferative and cytotoxic activities against cancer cells. Nat. Prod. Res. 2015, 29, 474–479. [Google Scholar] [CrossRef]
- Amar, Y.; Meddah, B.; Bonacorsi, I.; Costa, G.; Pezzino, G.; Saija, A.; Cristani, M.; Boussahel, S.; Ferlazzo, G.; Meddah, A.T. Phytochemicals, Antioxidant and Antiproliferative Properties of Rosmarinus officinalis L on U937 and CaCo-2 Cells. Iran. J. Pharm. Res. IJPR 2017, 16, 315–327. [Google Scholar]
- Tai, J.; Cheung, S.; Wu, M.; Hasman, D. Antiproliferation effect of Rosemary (Rosmarinus officinalis) on human ovarian cancer cells in vitro. Phytomedicine 2012, 19, 436–443. [Google Scholar] [CrossRef]
- Lin, C.S.; Kuo, C.L.; Wang, J.P.; Cheng, J.S.; Huang, Z.W.; Chen, C.F. Growth inhibitory and apoptosis inducing effect of Perilla frutescens extract on human hepatoma HepG2 cells. J. Ethnopharmacol. 2007, 112, 557–567. [Google Scholar] [CrossRef]
- Osakabe, N.; Yasuda, A.; Natsume, M.; Yoshikawa, T. Rosmarinic acid inhibits epidermal inflammatory responses: Anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis 2004, 25, 549–557. [Google Scholar] [CrossRef]
- Tantipaiboonwong, P.; Chaiwangyen, W.; Suttajit, M.; Kangwan, N.; Kaowinn, S.; Khanaree, C.; Punfa, W.; Pintha, K. Molecular Mechanism of Antioxidant and Anti-Inflammatory Effects of Omega-3 Fatty Acids in Perilla Seed Oil and Rosmarinic Acid Rich Fraction Extracted from Perilla Seed Meal on TNF-α Induced A549 Lung Adenocarcinoma Cells. Molecules 2021, 26, 6757. [Google Scholar] [CrossRef] [PubMed]
- Pintha, K.; Chaiwangyen, W.; Yodkeeree, S.; Suttajit, M.; Tantipaiboonwong, P. Suppressive Effects of Rosmarinic Acid Rich Fraction from Perilla on Oxidative Stress, Inflammation and Metastasis Ability in A549 Cells Exposed to PM via C-Jun, P-65-Nf-Κb and Akt Signaling Pathways. Biomolecules 2021, 11, 1090. [Google Scholar] [CrossRef] [PubMed]
- Encalada, M.A.; Hoyos, K.M.; Rehecho, S.; Berasategi, I.; de Ciriano, M.G.; Ansorena, D.; Astiasarán, I.; Navarro-Blasco, I.; Cavero, R.Y.; Calvo, M.I. Anti-proliferative effect of Melissa officinalis on human colon cancer cell line. Plant Foods Hum. Nutr. 2011, 66, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Castillo, J.; Micol, V. Lemon balm extract (Melissa officinalis, L.) promotes melanogenesis and prevents UVB-induced oxidative stress and DNA damage in a skin cell model. J. Dermatol. Sci. 2016, 84, 169–177. [Google Scholar] [CrossRef]
- Mansouri, M.; Mohammadi, F. Transcriptome analysis to identify key genes involved in terpenoid and rosmarinic acid biosynthesis in lemon balm (Melissa officinalis). Gene 2021, 773, 145417. [Google Scholar] [CrossRef]
- Elansary, H.O.; Mahmoud, E.A. In vitro antioxidant and antiproliferative activities of six international basil cultivars. Nat. Prod. Res. 2015, 29, 2149–2154. [Google Scholar] [CrossRef]
- Utispan, K.; Niyomtham, N.; Yingyongnarongkul, B.E.; Koontongkaew, S. Ethanolic Extract of Ocimum sanctum Leaves Reduced Invasion and Matrix Metalloproteinase Activity of Head and Neck Cancer Cell Lines. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 363–370. [Google Scholar] [CrossRef]
- Nazir, S.; Jan, H.; Tungmunnithum, D.; Drouet, S.; Zia, M.; Hano, C.; Abbasi, B.H. Callus Culture of Thai Basil Is an Effective Biological System for the Production of Antioxidants. Molecules 2020, 25, 4859. [Google Scholar] [CrossRef]
- Nazir, S.; Jan, H.; Zaman, G.; Khan, T.; Ashraf, H.; Meer, B.; Zia, M.; Drouet, S.; Hano, C.; Abbasi, B.H. Copper oxide (CuO) and manganese oxide (MnO) nanoparticles induced biomass accumulation, antioxidants biosynthesis and abiotic elicitation of bioactive compounds in callus cultures of Ocimum basilicum (Thai basil). Artif. Cells Nanomed. Biotechnol. 2021, 49, 626–634. [Google Scholar] [CrossRef]
- Nadeem, M.; Abbasi, B.H.; Younas, M.; Ahmad, W.; Zahir, A.; Hano, C. LED-enhanced biosynthesis of biologically active ingredients in callus cultures of Ocimum basilicum. J. Photochem. Photobiology. B Biol. 2019, 190, 172–178. [Google Scholar] [CrossRef]
- Erenler, R.; Sen, O.; Aksit, H.; Demirtas, I.; Yaglioglu, A.S.; Elmastas, M.; Telci, İ. Isolation and identification of chemical constituents from Origanum majorana and investigation of antiproliferative and antioxidant activities. J. Sci. Food Agric. 2016, 96, 822–836. [Google Scholar] [CrossRef] [PubMed]
- Baranauskaite, J.; Kubiliene, A.; Marksa, M.; Petrikaite, V.; Vitkevičius, K.; Baranauskas, A.; Bernatoniene, J. The Influence of Different Oregano Species on the Antioxidant Activity Determined Using HPLC Postcolumn DPPH Method and Anticancer Activity of Carvacrol and Rosmarinic Acid. BioMed Res. Int. 2017, 2017, 1681392. [Google Scholar] [CrossRef] [PubMed]
- Darw, H.Y.; Abdelmigid, H.; Albogami, S.; Alotaibi, S.; El-De, A.N.; Alnefaie, A. Induction of Biosynthetic Genes Related to Rosmarinic Acid in Plant Callus Culture and Antiproliferative Activity Against Breast Cancer Cell Line. Pak. J. Biol. Sci. 2020, 23, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Galasso, S.; Pacifico, S.; Kretschmer, N.; Pan, S.P.; Marciano, S.; Piccolella, S.; Monaco, P.; Bauer, R. Influence of seasonal variation on Thymus longicaulis C. Presl chemical composition and its antioxidant and anti-inflammatory properties. Phytochemistry 2014, 107, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.P.R.; Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Salvia Fruticosa, Salvia Officinalis, and Rosmarinic Acid Induce Apoptosis and Inhibit Proliferation of Human Colorectal Cell Lines: The Role in MAPK/ERK Pathway. Nutr. Cancer 2009, 61, 564–571. [Google Scholar] [CrossRef]
- Irtegun Kandemir, S.; Fidan, H.S.; Yener, I.; Mete, N.; Ertas, A.; Topcu, G.; Kolak, U. Investigation of cytotoxic and apoptotic effects of 63 compounds obtained from Salvia species: Promising anticancer agents. J. Food Biochem. 2022, 46, e14226. [Google Scholar] [CrossRef]
- Pirooz, P.; Amooaghaie, R.; Ahadi, A.; Sharififar, F.; Torkzadeh-Mahani, M. Silicon and nitric oxide synergistically modulate the production of essential oil and rosmarinic acid in Salvia officinalis under Cu stress. Protoplasma 2021, 259, 905–916. [Google Scholar] [CrossRef]
- Deng, C.; Wang, Y.; Huang, F.; Lu, S.; Zhao, L.; Ma, X.; Kai, G. SmMYB2 promotes salvianolic acid biosynthesis in the medicinal herb Salvia miltiorrhiza. J. Integr. Plant Biol. 2020, 62, 1688–1702. [Google Scholar] [CrossRef]
- Hou, Z.; Li, Y.; Su, F.; Chen, J.; Zhang, X.; Xu, L.; Yang, D.; Liang, Z. Application of (1)H-NMR combined with qRT-PCR technology in the exploration of rosmarinic acid biosynthesis in hair roots of Salvia miltiorrhiza Bunge and Salvia castanea f. tomentosa Stib. Planta 2020, 253, 2. [Google Scholar] [CrossRef]
- Su, C.H.; Pham, T.T.T.; Cheng, H.H. Aqueous enzymatic extraction of rosmarinic acid from Salvia officinalis: Optimisation using response surface methodology. Phytochem. Anal. PCA 2020, 31, 575–582. [Google Scholar] [CrossRef]
- Ramos, A.A.; Pedro, D.; Collins, A.R.; Pereira-Wilson, C. Protection by Salvia extracts against oxidative and alkylation damage to DNA in human HCT15 and CO115 cells. J. Toxicol. Environ. Health. Part A 2012, 75, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Ru, M.; Wang, K.; Bai, Z.; Peng, L.; He, S.; Wang, Y.; Liang, Z. A tyrosine aminotransferase involved in rosmarinic acid biosynthesis in Prunella vulgaris L. Sci. Rep. 2017, 7, 4892. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Jia, X.; Zhu, M.-M.; Chen, Y.; Shi, F. Antioxidant Activities of Total Phenols of Prunella vulgaris L. in Vitro and in Tumor-bearing Mice. Molecules 2010, 15, 9145–9156. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Jia, X.-B.; Jiang, J.; Zhu, M.-M.; Chen, Y.; Tan, X.-B.; Shi, F. Combination of Active Components Enhances the Efficacy of Prunella in Prevention and Treatment of Lung Cancer. Molecules 2010, 15, 7893–7906. [Google Scholar] [CrossRef]
- Shahat, A.A.; Hidayathulla, S.; Khan, A.A.; Alanazi, A.M.; Al Meanazel, O.T.; Alqahtani, A.S.; Alsaid, M.S.; Hussein, A.A. Phytochemical profiling, antioxidant and anticancer activities of Gastrocotyle hispida growing in Saudi Arabia. Acta Trop. 2019, 191, 243–247. [Google Scholar] [CrossRef]
- Chao, W.W.; Liou, Y.J.; Ma, H.T.; Chen, Y.H.; Chou, S.T. Phytochemical composition and bioactive effects of ethyl acetate fraction extract (EAFE) of Glechoma hederacea L. J. Food Biochem. 2021, 45, e13815. [Google Scholar] [CrossRef]
- Monroy-García, I.N.; Carranza-Torres, I.E.; Carranza-Rosales, P.; Oyón-Ardoiz, M.; García-Estévez, I.; Ayala-Zavala, J.F.; Morán-Martínez, J.; Viveros-Valdez, E. Phenolic Profiles and Biological Activities of Extracts from Edible Wild Fruits Ehretia tinifolia and Sideroxylon lanuginosum. Foods 2021, 10, 2710. [Google Scholar] [CrossRef]
- Nourozi, E.; Hosseini, B.; Maleki, R.; Abdollahi Mandoulakani, B. Inductive effect of titanium dioxide nanoparticles on the anticancer compounds production and expression of rosmarinic acid biosynthesis genes in Dracocephalum kotschyi transformed roots. Plant Physiol. Biochem. PPB 2021, 167, 934–945. [Google Scholar] [CrossRef]
- Nourozi, E.; Hosseini, B.; Maleki, R.; Abdollahi Mandoulakani, B. Iron oxide nanoparticles: A novel elicitor to enhance anticancer flavonoid production and gene expression in Dracocephalum kotschyi hairy-root cultures. J. Sci. Food Agric. 2019, 99, 6418–6430. [Google Scholar] [CrossRef]
- Khojasteh, A.; Mirjalili, M.H.; Palazon, J.; Eibl, R.; Cusido, R.M. Methyl jasmonate enhanced production of rosmarinic acid in cell cultures of Satureja khuzistanica in a bioreactor. Eng. Life Sci. 2016, 16, 740–749. [Google Scholar] [CrossRef]
- Khojasteh, A.; Metón, I.; Camino, S.; Cusido, R.M.; Eibl, R.; Palazon, J. In Vitro Study of the Anticancer Effects of Biotechnological Extracts of the Endangered Plant Species Satureja Khuzistanica. Int. J. Mol. Sci. 2019, 20, 2400. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, F.; Liu, X.; Yi, R.; Zhao, X. Exploring the Antioxidant Effects and Periodic Regulation of Cancer Cells by Polyphenols Produced by the Fermentation of Grape Skin by Lactobacillus plantarum KFY02. Biomolecules 2019, 9, 575. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Bi, H.; Zhuang, Y.; Liu, S.; Liu, T.; Ma, Y. Engineered synthesis of rosmarinic acid in Escherichia coli resulting production of a new intermediate, caffeoyl-phenyllactate. Biotechnol. Lett. 2016, 38, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Gangopadhyay, M.; Das, U.; Sahu, R.; Khanra, R. Enhanced rosmarinic acid biosynthesis in Solenostemon scutellarioides culture: A precursor-feeding strategy. Nat. Prod. Res. 2014, 28, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Busch, T.; Petersen, M. Identification and biochemical characterisation of tyrosine aminotransferase from Anthoceros agrestis unveils the conceivable entry point into rosmarinic acid biosynthesis in hornworts. Planta 2021, 253, 98. [Google Scholar] [CrossRef]
- Levsh, O.; Pluskal, T.; Carballo, V.; Mitchell, A.J.; Weng, J.K. Independent evolution of rosmarinic acid biosynthesis in two sister families under the Lamiids clade of flowering plants. J. Biol. Chem. 2019, 294, 15193–15205. [Google Scholar] [CrossRef]
- Bloch, S.E.; Schmidt-Dannert, C. Construction of a chimeric biosynthetic pathway for the de novo biosynthesis of rosmarinic acid in Escherichia coli. Chembiochem A Eur. J. Chem. Biol. 2014, 15, 2393–2401. [Google Scholar] [CrossRef]
- Yan, Y.; Jia, P.; Bai, Y.; Fan, T.P.; Zheng, X.; Cai, Y. Production of rosmarinic acid with ATP and CoA double regenerating system. Enzym. Microb. Technol. 2019, 131, 109392. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Zhang, H. Balancing the non-linear rosmarinic acid biosynthetic pathway by modular co-culture engineering. Metab. Eng. 2019, 54, 1–11. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, G.; Liu, L.; Xu, D.; Liu, J. Anti-invasion effect of rosmarinic acid via the extracellular signal-regulated kinase and oxidation-reduction pathway in Ls174-T cells. J. Cell Biochem. 2010, 111, 370–379. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, D.; Zhai, X.; Zhang, L.; Luo, X.; Fu, X. Oral Administration of Prunella vulgaris L Improves the Effect of Taxane on Preventing the Progression of Breast Cancer and Reduces Its Side Effects. Front Pharm. 2018, 9, 806. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Osakabe, N.; Natsume, M.; Yasuda, A.; Muto, Y.; Hiyoshi, K.; Takano, H.; Yoshikawa, T.; Terao, J. Absorption, metabolism, degradation and urinary excretion of rosmarinic acid after intake of Perilla frutescens extract in humans. Eur. J. Nutr. 2005, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Blažević, T.; Reznicek, G.; Ding, L.; Yang, G.; Haiss, P.; Heiss, E.H.; Dirsch, V.M.; Liu, R. Short Chain (≤C4) Esterification Increases Bioavailability of Rosmarinic Acid and Its Potency to Inhibit Vascular Smooth Muscle Cell Proliferation. Front Pharm. 2020, 11, 609756. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Xing, Y.; Xue, Z.; Ma, Z.; Zhang, B.; Peng, H.; Zhou, Q.T.; Liu, H.; Liu, Z.; Li, J. Pharmacokinetics of salvianolic acid B, rosmarinic acid and Danshensu in rat after pulmonary administration of Salvia miltiorrhiza polyphenolic acid solution. Biomed. Chromatogr. BMC 2019, 33, e4561. [Google Scholar] [CrossRef]
- Veras, K.S.; Fachel, F.N.S.; Pittol, V.; Garcia, K.R.; Bassani, V.L.; Dos Santos, V.; Henriques, A.T.; Teixeira, H.F.; Koester, L.S. Compatibility study of rosmarinic acid with excipients used in pharmaceutical solid dosage forms using thermal and non-thermal techniques. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2019, 27, 1138–1145. [Google Scholar] [CrossRef]
- Fuster, M.G.; Carissimi, G.; Montalbán, M.G.; Víllora, G. Antitumor Activity of Rosmarinic Acid-Loaded Silk Fibroin Nanoparticles on HeLa and MCF-7 Cells. Polymers 2021, 13, 3169. [Google Scholar] [CrossRef]
- Madureira, A.R.; Nunes, S.; Campos, D.A.; Fernandes, J.C.; Marques, C.; Zuzarte, M.; Gullón, B.; Rodríguez-Alcalá, L.M.; Calhau, C.; Sarmento, B.; et al. Safety profile of solid lipid nanoparticles loaded with rosmarinic acid for oral use: In vitro and animal approaches. Int. J. Nanomed. 2016, 11, 3621–3640. [Google Scholar] [CrossRef]
- Xue, X.; Ricci, M.; Qu, H.; Lindstrom, A.; Zhang, D.; Wu, H.; Lin, T.Y.; Li, Y. Iron-crosslinked Rososome with robust stability and high drug loading for synergistic cancer therapy. J. Control. Release 2021, 329, 794–804. [Google Scholar] [CrossRef]
- Huang, J.; Chen, P.X.; Rogers, M.A.; Wettig, S.D. Investigating the Phospholipid Effect on the Bioaccessibility of Rosmarinic Acid-Phospholipid Complex through a Dynamic Gastrointestinal in Vitro Model. Pharmaceutics 2019, 11, 156. [Google Scholar] [CrossRef]
- Sánchez-Campillo, M.; Gabaldon, J.A.; Castillo, J.; Benavente-García, O.; Del Baño, M.J.; Alcaraz, M.; Vicente, V.; Alvarez, N.; Lozano, J.A. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2009, 47, 386–392. [Google Scholar] [CrossRef]
- Gui, H.; Jin, Y.; Lin, A.; Wang, P.; Wang, Y.; Zhu, H. Rosmarinic acid relieves cisplatin-induced ovary toxicity in female mice via suppression of oxidative stress and inflammation. J. Biochem. Mol. Toxicol. 2021, 35, e22839. [Google Scholar] [CrossRef] [PubMed]
- Helfinger, V.; Schröder, K. Redox control in cancer development and progression. Mol. Asp. Med. 2018, 63, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Anusuya, C.; Manoharan, S. Antitumor initiating potential of rosmarinic acid in 7,12-dimethylbenz(a)anthracene-induced hamster buccal pouch carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. Off. Organ Int. Soc. Environ. Toxicol. Cancer 2011, 30, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Gunasekaran, S.; Jesudoss, V.A.; Namasivayam, N. The effect of rosmarinic acid on 1,2-dimethylhydrazine induced colon carcinogenesis. Exp. Toxicol. Pathol. Off. J. Ges. Fur Toxikol. Pathol. 2013, 65, 409–418. [Google Scholar] [CrossRef]
- Karthikkumar, V.; Sivagami, G.; Vinothkumar, R.; Rajkumar, D.; Nalini, N. Modulatory efficacy of rosmarinic acid on premalignant lesions and antioxidant status in 1,2-dimethylhydrazine induced rat colon carcinogenesis. Env. Toxicol Pharm. 2012, 34, 949–958. [Google Scholar] [CrossRef]
- Karthikkumar, V.; Sivagami, G.; Viswanathan, P.; Nalini, N. Rosmarinic acid inhibits DMH-induced cell proliferation in experimental rats. J. Basic Clin. Physiol. Pharm. 2015, 26, 185–200. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Gunasekaran, S.; Namasivayam, N. Biochemical and molecular mechanisms underlying the chemopreventive efficacy of rosmarinic acid in a rat colon cancer. Eur. J. Pharm. 2016, 791, 37–50. [Google Scholar] [CrossRef]
- Rodríguez-Luna, A.; Ávila-Román, J.; Oliveira, H.; Motilva, V.; Talero, E. Fucoxanthin and Rosmarinic Acid Combination Has Anti-Inflammatory Effects through Regulation of NLRP3 Inflammasome in UVB-Exposed HaCaT Keratinocytes. Mar. Drugs 2019, 17, 451. [Google Scholar] [CrossRef]
- Ilhan, N.; Bektas, I.; Susam, S.; Ozercan, I.H. Protective effects of rosmarinic acid against azoxymethane-induced colorectal cancer in rats. J. Biochem. Mol. Toxicol. 2021, 36, e22961. [Google Scholar] [CrossRef]
- Waer, C.N.; Kaur, P.; Tumur, Z.; Hui, D.D.; Le, B.; Guerra, C.; Henson, B.; Seleem, D.; Lewis, J. Rosmarinic Acid/ Blue Light Combination Treatment Inhibits Head and Neck Squamous Cell Carcinoma In Vitro. Anticancer Res. 2020, 40, 751–758. [Google Scholar] [CrossRef]
- Saiko, P.; Steinmann, M.T.; Schuster, H.; Graser, G.; Bressler, S.; Giessrigl, B.; Lackner, A.; Grusch, M.; Krupitza, G.; Bago-Horvath, Z.; et al. Epigallocatechin gallate, ellagic acid, and rosmarinic acid perturb dNTP pools and inhibit de novo DNA synthesis and proliferation of human HL-60 promyelocytic leukemia cells: Synergism with arabinofuranosylcytosine. Phytomedicine 2015, 22, 213–222. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Peng, S.; Zhang, Y.; Qiao, Y. Rosmarinic Acid as a Candidate in a Phenotypic Profiling Cardio-/Cytotoxicity Cell Model Induced by Doxorubicin. Molecules 2020, 25, 836. [Google Scholar] [CrossRef] [PubMed]
- Jafaripour, L.; Naserzadeh, R.; Alizamani, E.; Javad Mashhadi, S.M.; Moghadam, E.R.; Nouryazdan, N.; Ahmadvand, H. Effects of Rosmarinic Acid on Methotrexate-induced Nephrotoxicity and Hepatotoxicity in Wistar Rats. Indian J. Nephrol. 2021, 31, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Rahbardar, M.G.; Eisvand, F.; Rameshrad, M.; Razavi, B.M.; Hosseinzadeh, H. In Vivo and In Vitro Protective Effects of Rosmarinic Acid against Doxorubicin-Induced Cardiotoxicity. Nutr. Cancer 2021, 74, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Scheckel, K.A.; Degner, S.C.; Romagnolo, D.F. Rosmarinic acid antagonizes activator protein-1-dependent activation of cyclooxygenase-2 expression in human cancer and nonmalignant cell lines. J. Nutr. 2008, 138, 2098–2105. [Google Scholar] [CrossRef]
- Tao, L.; Wang, S.; Zhao, Y.; Sheng, X.; Wang, A.; Zheng, S.; Lu, Y. Phenolcarboxylic acids from medicinal herbs exert anticancer effects through disruption of COX-2 activity. Phytomedicine 2014, 21, 1473–1482. [Google Scholar] [CrossRef]
- Moon, D.O.; Kim, M.O.; Lee, J.D.; Choi, Y.H.; Kim, G.Y. Rosmarinic acid sensitizes cell death through suppression of TNF-alpha-induced NF-kappaB activation and ROS generation in human leukemia U937 cells. Cancer Lett. 2010, 288, 183–191. [Google Scholar] [CrossRef]
- Cao, W.; Hu, C.; Wu, L.; Xu, L.; Jiang, W. Rosmarinic acid inhibits inflammation and angiogenesis of hepatocellular carcinoma by suppression of NF-κB signaling in H22 tumor-bearing mice. J. Pharm. Sci. 2016, 132, 131–137. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Okda, T.M.; Omran, G.A.; Abd-Alhaseeb, M.M. Rosmarinic acid suppresses inflammation, angiogenesis, and improves paclitaxel induced apoptosis in a breast cancer model via NF3 κB-p53-caspase-3 pathways modulation. J. Appl. Biomed. 2021, 19, 202–209. [Google Scholar] [CrossRef]
- Wu, C.F.; Hong, C.; Klauck, S.M.; Lin, Y.L.; Efferth, T. Molecular mechanisms of rosmarinic acid from Salvia miltiorrhiza in acute lymphoblastic leukemia cells. J. Ethnopharmacol. 2015, 176, 55–68. [Google Scholar] [CrossRef]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Furtado, R.A.; Oliveira, B.R.; Silva, L.R.; Cleto, S.S.; Munari, C.C.; Cunha, W.R.; Tavares, D.C. Chemopreventive effects of rosmarinic acid on rat colon carcinogenesis. Eur. J. Cancer Prev. 2015, 24, 106–112. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, Y.; Huang, R.; Zheng, X. Rosmarinic Acid Combined with Adriamycin Induces Apoptosis by Triggering Mitochondria-Mediated Signaling Pathway in HepG2 and Bel-7402 Cells. Med. Sci. Monit. 2018, 24, 7898–7908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, M.; Liu, L.; Cheng, X.L.; Cai, J.; Zhou, J.; Wang, T. Anticancer effects of Rosmarinic acid in OVCAR-3 ovarian cancer cells are mediated via induction of apoptosis, suppression of cell migration and modulation of lncRNA MALAT-1 expression. J. BU ON. Off. J. Balk. Union Oncol. 2018, 23, 763–768. [Google Scholar]
- Ingham, M.; Schwartz, G.K. Cell-Cycle Therapeutics Come of Age. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 2949–2959. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.G.; Hwang, K.A.; Choi, K.C. Rosmarinic Acid, a Component of Rosemary Tea, Induced the Cell Cycle Arrest and Apoptosis through Modulation of HDAC2 Expression in Prostate Cancer Cell Lines. Nutrients 2018, 10, 1784. [Google Scholar] [CrossRef] [PubMed]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F.A. Rosmarinic acid-induced apoptosis and cell cycle arrest in triple-negative breast cancer cells. Eur. J. Pharm. 2020, 885, 173419. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, W.; Li, Z.; Chen, L.; Wen, C.; Ruan, Q.; Xu, Z.; Liu, R.; Xu, J.; Bai, Y.; et al. Rosmarinic Acid Decreases the Malignancy of Pancreatic Cancer Through Inhibiting Gli1 Signaling. Phytomedicine 2022, 95, 153861. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.T.; Ho, B.Y.; Tai, Y.T.; Huang, C.J.; Chao, W.W. Bidirect effects from cisplatin combine with rosmarinic acid (RA) or hot water extracts of Glechoma hederacea (HWG) on renal cancer cells. Chin. Med. 2020, 15, 77. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, Z.; Xu, X.; Qi, H.; Cheng, Z.; Chen, L. Anticancer effects of rosmarinic acid in human oral cancer cells is mediated via endoplasmic reticulum stress, apoptosis, G2/M cell cycle arrest and inhibition of cell migration. J. BU ON. Off. J. Balk. Union Oncol. 2020, 25, 1245–1250. [Google Scholar]
- Huang, L.; Chen, J.; Quan, J.; Xiang, D. Rosmarinic acid inhibits proliferation and migration, promotes apoptosis and enhances cisplatin sensitivity of melanoma cells through inhibiting ADAM17/EGFR/AKT/GSK3β axis. Bioengineered 2021, 12, 3065–3076. [Google Scholar] [CrossRef] [PubMed]
- Erenler, R.; Yildiz, I.; Aydin, A.; Genc, N. Antiproliferative and cytotoxic effects of bioactive compounds isolated from Onosma bourgaei. Med. Oncol. 2022, 39, 116. [Google Scholar] [CrossRef]
- Hsu, K.C.; Sung, T.Y.; Lin, C.T.; Chiu, Y.Y.; Hsu, J.T.; Hung, H.C.; Sun, C.M.; Barve, I.; Chen, W.L.; Huang, W.C.; et al. Anchor-based classification and type-C inhibitors for tyrosine kinases. Sci. Rep. 2015, 5, 10938. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Shamsi, A.; Shahbaaz, M.; Queen, A.; Khan, P.; Hasan, G.M.; Islam, A.; Alajmi, M.F.; Hussain, A.; Ahmad, F.; et al. Rosmarinic Acid Exhibits Anticancer Effects via MARK4 Inhibition. Sci. Rep. 2020, 10, 10300. [Google Scholar] [CrossRef] [PubMed]
- Parvizpour, S.; Masoudi-Sobhanzadeh, Y.; Pourseif, M.M.; Barzegari, A.; Razmara, J.; Omidi, Y. Pharmacoinformatics-based phytochemical screening for anticancer impacts of yellow sweet clover, Melilotus officinalis (Linn.) Pall. Comput. Biol. Med. 2021, 138, 104921. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Liu, J.; Gao, D.; Xu, Y.; He, L.; Zang, Y.; Li, N.; Lin, D. Detailed studies on the anticancer action of rosmarinic acid in human Hep-G2 liver carcinoma cells: Evaluating its effects on cellular apoptosis, caspase activation and suppression of cell migration and invasion. J. BU ON. Off. J. Balk. Union Oncol. 2020, 25, 1383–1389. [Google Scholar]
- Han, Y.H.; Kee, J.Y.; Hong, S.H. Rosmarinic Acid Activates AMPK to Inhibit Metastasis of Colorectal Cancer. Front Pharm. 2018, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, J.; Yang, Y.; Wang, X.; Chen, G.; Shi, A.; Lu, Y.; Jia, S.; Kang, X.; Lu, L. Rosmarinic acid exerts an anticancer effect on osteosarcoma cells by inhibiting DJ-1 via regulation of the PTEN-PI3K-Akt signaling pathway. Phytomedicine 2020, 68, 153186. [Google Scholar] [CrossRef]
- An, Y.; Zhao, J.; Zhang, Y.; Wu, W.; Hu, J.; Hao, H.; Qiao, Y.; Tao, Y.; An, L. Rosmarinic Acid Induces Proliferation Suppression of Hepatoma Cells Associated with NF-κB Signaling Pathway. Asian Pac. J. Cancer Prev. APJCP 2021, 22, 1623–1632. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Wang, C.; Shi, X.; Li, K. Rosmarinic acid inhibits proliferation and invasion of hepatocellular carcinoma cells SMMC 7721 via PI3K/AKT/mTOR signal pathway. Biomed Pharm. 2019, 120, 109443. [Google Scholar] [CrossRef]
- Şengelen, A.; Önay-Uçar, E. Rosmarinic acid and siRNA combined therapy represses Hsp27 (HSPB1) expression and induces apoptosis in human glioma cells. Cell Stress Chaperones 2018, 23, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Choi, S.; Park, Y.; Jin, H.S. Mucin1 and Mucin16: Therapeutic Targets for Cancer Therapy. Pharmaceuticals 2021, 14, 1053. [Google Scholar] [CrossRef] [PubMed]
- Radziejewska, I.; Supruniuk, K.; Bielawska, A. Anti-cancer effect of combined action of anti-MUC1 and rosmarinic acid in AGS gastric cancer cells. Eur. J. Pharm. 2021, 902, 174119. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Śliwiński, T.; Zajdel, K.; Malinowska, K.; Zielińska-Bliźniewska, H.; Kucharska, E.; Zajdel, R. In Vitro and In Silico Studies on Leonotis nepetifolia (L.) R. Br. Root Extract against Cancer Cells. Curr. Pharm. Biotechnol. 2022, 23, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.D.; Handley, M.D.; Gottesman, M.M. Is resistance useless? Multidrug resistance and collateral sensitivity. Trends Pharmacol. Sci. 2009, 30, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Yehya, A.H.S.; Asif, M.; Abdul Majid, A.M.S.; Oon, C.E. Complementary effects of Orthosiphon stamineus standardized ethanolic extract and rosmarinic acid in combination with gemcitabine on pancreatic cancer. Biomed. J. 2021, 44, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Juskowiak, B.; Bogacz, A.; Wolek, M.; Kamiński, A.; Uzar, I.; Seremak-Mrozikiewicz, A.; Czerny, B. Expression profiling of genes modulated by rosmarinic acid (RA) in MCF-7 breast cancer cells. Ginekol. Pol. 2018, 89, 541–545. [Google Scholar] [CrossRef]
- Li, F.R.; Fu, Y.Y.; Jiang, D.H.; Wu, Z.; Zhou, Y.J.; Guo, L.; Dong, Z.M.; Wang, Z.Z. Reversal effect of rosmarinic acid on multidrug resistance in SGC7901/Adr cell. J. Asian Nat. Prod. Res. 2013, 15, 276–285. [Google Scholar] [CrossRef]
- Jin, W.; Liao, X.; Lv, Y.; Pang, Z.; Wang, Y.; Li, Q.; Liao, Y.; Ye, Q.; Chen, G.; Zhao, K.; et al. MUC1 induces acquired chemoresistance by upregulating ABCB1 in EGFR-dependent manner. Cell Death Dis. 2017, 8, e2980. [Google Scholar] [CrossRef]
- Nath, S.; Daneshvar, K.; Roy, L.D.; Grover, P.; Kidiyoor, A.; Mosley, L.; Sahraei, M.; Mukherjee, P. MUC1 induces drug resistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis 2013, 2, e51. [Google Scholar] [CrossRef] [PubMed]
- Radziejewska, I.; Supruniuk, K.; Nazaruk, J.; Karna, E.; Popławska, B.; Bielawska, A.; Galicka, A. Rosmarinic acid influences collagen, MMPs, TIMPs, glycosylation and MUC1 in CRL-1739 gastric cancer cell line. Biomed. Pharm. 2018, 107, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.J.; Yan, H.; Wang, Y.J.; Yang, Y.; Li, X.B.; Shi, A.C.; Jing-Wen, X.; Yu-Bao, L.; Li, L.; Wang, X.X. Proteomics analysis demonstrating rosmarinic acid suppresses cell growth by blocking the glycolytic pathway in human HepG2 cells. Biomed. Pharm. 2018, 105, 334–349. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.; O’Neill, L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yang, S.; Cai, Z.; Pan, D.; Li, Z.; Huang, Z.; Zhang, P.; Zhu, H.; Lei, L.; Wang, W. Anti-Warburg effect of rosmarinic acid via miR-155 in gastric cancer cells. Drug Des. Dev. Ther. 2015, 9, 2695–2703. [Google Scholar] [CrossRef]
- Xu, Y.; Han, S.; Lei, K.; Chang, X.; Wang, K.; Li, Z.; Liu, J. Anti-Warburg effect of rosmarinic acid via miR-155 in colorectal carcinoma cells. Eur. J. Cancer Prev. 2016, 25, 481–489. [Google Scholar] [CrossRef]
- Aiello, N.M.; Kang, Y. Context-dependent EMT programs in cancer metastasis. J. Exp. Med. 2019, 216, 1016–1026. [Google Scholar] [CrossRef]
- Yang, K.; Shen, Z.; Zou, Y.; Gao, K. Rosmarinic acid inhibits migration, invasion, and p38/AP-1 signaling via miR-1225-5p in colorectal cancer cells. J. Recept. Signal Transduct. 2021, 41, 284–293. [Google Scholar] [CrossRef]
- Han, Y.; Ma, L.; Zhao, L.; Feng, W.; Zheng, X. Rosmarinic inhibits cell proliferation, invasion and migration via up-regulating miR-506 and suppressing MMP2/16 expression in pancreatic cancer. Biomed. Pharm. 2019, 115, 108878. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Bergers, G.; Brekken, R.; McMahon, G.; Vu, T.H.; Itoh, T.; Tamaki, K.; Tanzawa, K.; Thorpe, P.; Itohara, S.; Werb, Z.; et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000, 2, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, Z.; Ji, G.; Liu, J. Inhibition of bone metastasis from breast carcinoma by rosmarinic acid. Planta Med. 2010, 76, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.B.; Aisha, A.F.; Nassar, Z.D.; Siddiqui, J.M.; Ismail, Z.; Omari, S.M.; Parish, C.R.; Majid, A.M. Cat’s whiskers tea (Orthosiphon stamineus) extract inhibits growth of colon tumor in nude mice and angiogenesis in endothelial cells via suppressing VEGFR phosphorylation. Nutr. Cancer 2012, 64, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Zheng, R.L. Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett. 2006, 239, 271–280. [Google Scholar] [CrossRef]
- De Robertis, M.; Massi, E.; Poeta, M.L.; Carotti, S.; Morini, S.; Cecchetelli, L.; Signori, E.; Fazio, V.M. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J. Carcinog. 2011, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Karmokar, A.; Marczylo, T.H.; Cai, H.; Steward, W.P.; Gescher, A.J.; Brown, K. Dietary intake of rosmarinic acid by Apc(Min) mice, a model of colorectal carcinogenesis: Levels of parent agent in the target tissue and effect on adenoma development. Mol. Nutr. Food Res. 2012, 56, 775–783. [Google Scholar] [CrossRef]
- Lubov, J.E.; Cvammen, W.; Kemp, M.G. The Impact of the Circadian Clock on Skin Physiology and Cancer Development. Int. J. Mol. Sci. 2021, 22, 6112. [Google Scholar] [CrossRef]
- Rudolf, J.; Raad, H.; Taieb, A.; Rezvani, H.R. NADPH Oxidases and Their Roles in Skin Homeostasis and Carcinogenesis. Antioxid. Redox Signal. 2018, 28, 1238–1261. [Google Scholar] [CrossRef]
- Gupta, D.; Archoo, S.; Naikoo, S.H.; Abdullah, S.T. Rosmarinic Acid: A Naturally Occurring Plant Based Agent Prevents Impaired Mitochondrial Dynamics and Apoptosis in Ultraviolet-B-Irradiated Human Skin Cells. Photochem. Photobiol. 2022, 98, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Baldasquin-Caceres, B.; Gomez-Garcia, F.J.; López-Jornet, P.; Castillo-Sanchez, J.; Vicente-Ortega, V. Chemopreventive potential of phenolic compounds in oral carcinogenesis. Arch. Oral Biol. 2014, 59, 1101–1107. [Google Scholar] [CrossRef]
- Paluszczak, J.; Krajka-Kuźniak, V.; Baer-Dubowska, W. The effect of dietary polyphenols on the epigenetic regulation of gene expression in MCF7 breast cancer cells. Toxicol. Lett. 2010, 192, 119–125. [Google Scholar] [CrossRef]
- Li, W.; Li, Q.; Wei, L.; Pan, X.; Huang, D.; Gan, J.; Tang, S. Rosmarinic Acid Analogue-11 Induces Apoptosis of Human Gastric Cancer SGC-7901 Cells via the Epidermal Growth Factor Receptor (EGFR)/Akt/Nuclear Factor kappa B (NF-κB) Pathway. Med. Sci. Monit. Basic Res. 2019, 25, 63–75. [Google Scholar] [CrossRef]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Reviews. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Nam, K.H.; Kim, K.; Yi, S.A.; Lee, J.; Han, J.W. Rosmarinic Acid Methyl Ester Regulates Ovarian Cancer Cell Migration and Reverses Cisplatin Resistance by Inhibiting the Expression of Forkhead Box M1. Pharmaceuticals 2020, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H.; Yi, S.A.; Nam, G.; Noh, J.S.; Park, J.W.; Lee, M.G.; Park, J.H.; Oh, H.; Lee, J.; Lee, K.R.; et al. Identification of a novel S6K1 inhibitor, rosmarinic acid methyl ester, for treating cisplatin-resistant cervical cancer. BMC Cancer 2019, 19, 773. [Google Scholar] [CrossRef] [PubMed]
- Canturk, Z.; Dikmen, M.; Artagan, O.; Ozarda, M.G.; Ozturk, N. Cytotoxic Effects of Resveratrol, Rutin and Rosmarinic Acid on ARH-77 Human (Multiple Myeloma) Cell Line. Nat. Prod. Commun. 2016, 11, 1441–1444. [Google Scholar] [CrossRef]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef]
- Citron, M.L.; Berry, D.A.; Cirrincione, C.; Hudis, C.; Winer, E.P.; Gradishar, W.J.; Davidson, N.E.; Martino, S.; Livingston, R.; Ingle, J.N.; et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 1431–1439. [Google Scholar] [CrossRef]
- Yu, C.; Chen, D.Q.; Liu, H.X.; Li, W.B.; Lu, J.W.; Feng, J.F. Rosmarinic acid reduces the resistance of gastric carcinoma cells to 5-fluorouracil by downregulating FOXO4-targeting miR-6785-5p. Biomed. Pharm. 2019, 109, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Ozgun, G.S.; Ozgun, E. The cytotoxic concentration of rosmarinic acid increases MG132-induced cytotoxicity, proteasome inhibition, autophagy, cellular stresses, and apoptosis in HepG2 cells. Hum. Exp. Toxicol. 2020, 39, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Hu, G.; Cai, X. The success and the challenge of all-trans retinoic acid in the treatment of cancer. Crit. Rev. Food Sci. Nutr. 2019, 59, S71–S80. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Tallman, M.S. Acute promyelocytic leukemia (APL): Remaining challenges towards a cure for all. Leuk. Lymphoma 2019, 60, 3107–3115. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.K.; Noh, E.K.; Yoon, D.J.; Jo, J.C.; Koh, S.; Baek, J.H.; Park, J.H.; Min, Y.J.; Kim, H. Rosmarinic acid potentiates ATRA-induced macrophage differentiation in acute promyelocytic leukemia NB4 cells. Eur. J. Pharm. 2015, 747, 36–44. [Google Scholar] [CrossRef]
- Alcaraz, M.; Alcaraz-Saura, M.; Achel, D.G.; Olivares, A.; López-Morata, J.A.; Castillo, J. Radiosensitizing effect of rosmarinic acid in metastatic melanoma B16F10 cells. Anticancer Res. 2014, 34, 1913–1921. [Google Scholar]
- Ferreira-Gonçalves, T.; Gaspar, M.M.; Coelho, J.M.P.; Marques, V.; Viana, A.S.; Ascensão, L.; Carvalho, L.; Rodrigues, C.M.P.; Ferreira, H.A.; Ferreira, D.; et al. The Role of Rosmarinic Acid on the Bioproduction of Gold Nanoparticles as Part of a Photothermal Approach for Breast Cancer Treatment. Biomolecules 2022, 12, 71. [Google Scholar] [CrossRef]
| Disease | Model | Treatment | Outcome | Ref |
|---|---|---|---|---|
| Colorectal carcinogenesis | Wistar male rats given DMH orally 20 mg/kg, once a day | RA 10mg/kg, once a day | Inhibited the carcinogenic effect through circulatory antioxidant enzymes (SOD↑, CAT↑, GSH↑, and GPx↑) | [75] |
| Colorectal carcinogenesis | Male albino wistar rat given DMH 20 mg/kg subcutaneously for 4 weeks | RA 2.5, 5, and 10 mg/kg | Reduced the polyp incidence through CYP450↓, lipid peroxidation↓, SOD↑, CAT↑, GPx↑, and GSH↑. | [74] |
| Colorectal carcinogenesis | Wistar rats with subcutaneous injection of 40 mg/kg DMH for 2 weeks | RA 4, 8 and 16 mg/kg body weight | Reduced DNA damage and frequency of the formation of ACF | [92] |
| Colorectal carcinogenesis | Male Wistar rats with subcutaneous injection of DMH 20 mg/kg. | Oral RA 5mg/kg body weight 30 weeks in total | Inhibited the tumor formation and reduced expressions of TNF-α, IL-6, and COX-2, and increased SOD, CAT, GPx, and TBARS | [76] |
| Colorectal carcinogenesis | Male Wistar rats with subcutaneous injection of DMH 20 mg/kg for 15 weeks | Daily RA 5mg/kg orally | Protected the activity of antioxidant enzymes (CYP450↓ and CYP4502E1↓) and reduced the formation of ACF | [77] |
| Colorectal carcinogenesis | Male Sprague-Dawley rats intraperitoneally injected with 15 mg/kg AOM once a week for 4 weeks | RA 5 mg/kg orally per day | Increased the total antioxidant status, and decreased the expression of IL-6 and total oxidant status | [79] |
| Colorectal carcinogenesis | Male BALB/c mice with oral administration of AOM5-ASA 75 mg/kg/day intraperitoneally for 7 days, then supplied drinking water containing 1–2% DSS for 49 days | RA 30 mg/kg/day orally | Inhibited TLR4 mediated the activation of NF-κB and STAT3 and eliminated the progression of colitis-associated colon cancer | [9] |
| Colorectal carcinogenesis | APC10.1 cells; C57BL/6J-ApcMin/+ mouse model | RA 100 µM; 0.3% RA in the diet, 360 mg/kg per day | Decreased numbers of large adenomas (>3 mm) | [136] |
| Skin carcinogenesis | DMBA/TPA induced skin papilloma mouse model | Topical application RA 1.35 mg/mouse | Inhibited MDA, chemokines and arachidonic acid and prevented DNA from oxidative damage | [20] |
| Skin carcinogenesis | HaCaT cells exposed to UVA | RA 2.7–18 mg/mL | Attenuated ROS generation and DNA damage in UVB-irradiated keratinocytes by LBE | [24] |
| Skin carcinogenesis | HaCaT cells exposed to UVB | RA 2.5 or 5 µM | Downregulated the inflammasome components (NLRP3 and IL-1β production) via Nrf2/HO-1 antioxidant system and prevented skin changes caused by UVB | [78] |
| Skin carcinogenesis | B16 melanoma cells; Female albino Swiss mouses exposed to UVA light 3 times a week, total 100 times | 2% RA in the diet to rats; Cell administration RA at 1 mg/mL | RA increased the Tyr activity in vitro. Oral RA inhibited skin changes caused by UVA exposure (skin photocarcinogenesis) | [70] |
| Oral carcinogenesis | 0.5% DMBA liquid paraffin treated on left buccal pouches of golden Syrian hamster model for 14 weeks | RA orally 100 mg/kg | Suppressed oral carcinogenesis through upregulation of SOD, CAT, GSH, GPx and downregulation of TBARS and BCL-2 | [73] |
| Oral carcinogenesis | Male Syrian hamster intravenous injection of 0.5% DMBA | RA 1.3 mg/15mL | Reduced the intensity and invasiveness of the tumor | [140] |
| Tumor angiogenesis | Human umbilical vein endothelial cells (HUVECs) | RA 50, 100 and 200 mM | Suppression of ROS generation and downregulation the release of VEGF and IL-8 | [134] |
| Disease | Model (IC50) | Treatment | Outcome | Ref |
|---|---|---|---|---|
| Glioma | U251 and U343 glioma cells | RA 100, 200, and 400 µM | Inhibited BCL-2 and promoted the expression of BAX and cleaved caspase-3 protein, and downregulated PI3K/AKT/NF-κB signaling pathway through targeting Fyn. | [10] |
| Glioma | U-87 MG cells (IC50 for 48 h:373.48 μM) | RA 80 and 215 µM | Inhibited the expression of HSP27 and enhanced the activity of caspase-3 | [111] |
| Oral cancer | SCC-15 cells | RA 10, 20, and 40 µM | Increased the expression of cleaved caspase-3 and BAX/BCL-2 ratio, induced G2/M cell cycle arrest, and inhibited migration through downregulation of MMP-2 and MMP-9 | [100] |
| Breast cancer | MDA-MB-231 (IC50 for 48 h: 321.75 ± 9.75 uM) and MDA-MB-468 cells (IC50 for 48 h: 340.45 ± 7.57 uM) | RA 125 and 250 µM | Induced G0/G1 cell cycle arrest and apoptosis through regulation of apoptosis-related genes (HRK↑, TNFRSF25↑, BNIP3↑, TNF↑, GADD45A↑, BNIP3↑, TNFSF10↓, BIRC5↓ and TNFRSF11B↓) | [97] |
| Breast cancer | MCF7 cell line | RA 20 and 40 µM | Regulated the methylation pattern via DNMT1 for chemoprevention of cancer | [141] |
| Breast-derived bone metastases | MDA-MB-231BO human bone-homing breast cancer cells (IC50: 118.04 µg/mL) | RA 7.5, 15, 30, and 60 µg/mL | Inhibited the metastasis of breast cancer by suppression of IL-8 through NF-κB ligand/ TNF receptor superfamily member 11a /osteoprotegerin pathway | [132] |
| Gastric cancer | MKN45 cells (IC50 for 24 h: 240.2 μM); MKN45 cells injected into BALB/c-nude mice | RA 60, 120.1, and 240.2 µM; RA 2 mg/kg injected intraperitoneally for 14 days | Inhibited Warburg effect (glucose consumption, lactate generation, and HIF-1α) through downregulation of IL-6/STAT3 pathway | [125] |
| Gastric cancer | CRL-1739 cells (IC50 for 24 h: 240 μM) | RA 100 and 200 μM | Inhibited the expression of MMP-9, TIMP-1, MUC1, Tn antigens and T antigens, increased the expression of collagen I | [122] |
| Gastric cancer | GES-1 (IC50 for 24 h: 289.425 ± 0.854 μmol/L) and SGC-7901 cells (IC50 for 24 h: 73.299 ± 2.011 μmol/L) | RA analogue-11 10, 20, and 40μmol/L | Promoted apoptosis via the EGFR/AKT/NF-κB pathway in gastric cancer cells. | [142] |
| HCC | HepG2 cells | RA 5 and 10 µg/mL | Induced apoptosis through increasing the mRNA levels of Jun, Jun-B, Fos-B, BAX and caspase-8, and decreased BCL-2 mRNA expression | [19] |
| HCC | H22 tumor-bearing mice | Intraperitoneal injection of RA 75, 150, and 300 mg/kg | Inhibited inflammatory cytokines (IL-1β, IL-6, TNF-α, TGF-β), angiogenic factors (VEGF) and phosphorylation of p65. The tumor inhibition rates in different concentrations of RA (39.03%, 42.98%, and 48.24%) | [88] |
| HCC | HepG2 cells (IC50 for 48 h: 33 ± 0.74 μg/mL) | RA 6.25, 12.5, 25, 50, and 100 µg/mL | Inhibited the expression of GLUT-1 and HK-2 to suppress the glycolytic pathway. | [123] |
| HCC | HepG2 cells | RA 7, 14, and 28 µM | Induced apoptosis (caspase-3↑, caspase-9↑ and BAX/BCL-2 ratio↑), inhibited migration, and invasion | [106] |
| HCC | HepG2 cells | RA 100, 200, and 400 µM | Reduced the expression of MMP-2, MMP-9, and BCL-2, promoted the expression of BAX and Caspase-3, and downregulated PI3K/AKT/NF-κB signaling pathway through targeting Fyn. | [109] |
| HCC | SMMC 7721 cells; Tumor bearing model of nude mice | RA 20, 50, and 100 µmol/L; RA 5, 10, and 20 mg/kg for 5 days | Downregulated PI3K/AKT/mTOR signaling pathway to induce apoptosis, inhibited EMT in vitro and tumor growth in vivo | [110] |
| Pancreatic cancer | PANC-1, PATU-8988, MIA PaCa-2 and BxPC-3 cells; Tumor bearing model of nude mice (MIA PaCa-2 cells) | RA 100, 200, 300, 400, and 500 μM; Orally 50 mg/kg RA 50 mg/kg orally for 30 days | Enhanced proteasome-mediated degradation of Gli1 and inhibited the expression of downstream VEGF, Cyclin D1 and snail1. Induced apoptosis and inhibited invasion and proliferation in vitro; Suppressed tumor growth in vivo | [98] |
| Pancreatic cancer | Panc-1 (IC50 for 24 h: 104.2 ± 4.5 μM) and SW1990 cells (IC50 for 24 h: 118.9 ± 6.7 μM); Nude mice injected subcutaneously into Panc-1 cells | RA 100 µM; 10 and 50 mg/kg orally for 30 days | Inhibited mRNA expression of MMP2 and MMP16 via miR-506; Inhibited tumor growth in the xenograft mice model. | [129] |
| CRC | HCT15 and CO115 cells | RA 10, 50, and 100 µM | Inhibited cell proliferation through inhibitory of phospho-ERK in HCT15 | [35] |
| CRC | HCT8 (IC50: 298.1 μM), HCT116 (IC50: 319.8 μM), Ls174-T (IC50: 539.4 μM), and Lovo (IC50: 576.3 μM) cells | RA 0, 75, and 150 µM | Inhibited IL-1β, TNFα, IL-6, and STAT3 against Warburg effect | [126] |
| CRC | CT26 and HCT116; BALB/c mice inoculated with CT26 via the lateral tail vein | RA 50, 100, and 200 µM; oral injection of RA (100 mg/kg/day) for 14 days | Induced G0/G1 cell cycle arrest and apoptosis (caspases↑, Bcl-XL↓, and BCL-2↓), inhibited EMT and invasion via AMPK phosphorylation; Reduced lung metastasis of CRC cells | [107] |
| Colon carcinoma Lung cancer | Ls174-T human colon carcinoma cells. Lewis lung carcinoma (LLC) cells injected into C57BL/6 mice | RA 37.5, 75, 150, and 300 µg/mL in vitro; RA 1, 2, and 4 mg/kg intraperitoneal injection for 20 days | Inhibited the activities of EGFR and VEGFR, and then suppressed the nuclear translocation of NF-κB and activity of p-AKT and p-ERK resulting in downregulation of the mRNA and protein expression of MMP-2, MMP-9, and VEGF in vitro. Inhibited the formation of metastasis nodules. | [60] |
| CRC | HT-29 cells | RA 50, 100, and 200 µM | Inhibited EMT (E-cadherin↑, N-cadherin↓, MMP-1, -3, and -9↓) via the p38/AP-1 signaling | [128] |
| Ovarian cancer | OVCAR-3 cells | RA 10, 40, and 160 µM | Regulated the expression of lncRNA MALAT-1, inhibited cell migration and induced apoptosis. | [94] |
| Ovarian cancer | SKOV-3, TOV-21G and DDP resistant daughter line TOV/CisR | RA methyl ester 40 µM; DDP 5µM; combination therapy | Accelerated apoptosis in DDP resistant ovarian cancer cell line through inhibitory of FOXM1 | [146] |
| Cervical cancer | HeLa and SiHa cells | RA methyl ester 80 µM; DDP 5µM; combination therapy | Exerted apoptosis effects against cervical cancer by inhibiting mTOR/S6K1 pathway | [147] |
| Prostate cancer | PC-3, DU145 cells | RA 200 µM | Induced G0/G1 cell cycle arrest (Cyclin D1↓, Cyclin E↓, CyclinB1↓ and p21↑) and apoptosis, enhanced transcription of p53 by inhibition of HDAC2. | [96] |
| Osteosarcoma | U2OS (IC50 for 48h: 28 ± 1.14 μg/mL) and MG63 (IC50 for 48h: 25 ± 1.37 μg/mL) osteosarcoma cells. | RA 12.5, 25, and 50 µg/mL | Induced apoptosis (caspase-3, -8, and -9↑ and BAX/BCL-2 ratio↑), inhibited EMT and invasion (MMP-2↓, MMP-9↓) through DJ-1 mediated upregulation of PTEN and downregulation of PI3K/AKT | [108] |
| MM | ARH-77 cells | RA 50, 100, and 200 µM | Exerted cytotoxic effects and decreased the mitochondrial activity | [148] |
| Leukemia | U937 cells using TNF- α 10 ng/mL induced oxidative stress | RA 60 µM | Reduced NF- κB and ROS production, promoted apoptosis | [87] |
| Acute lymphoblastic leukemia | CCRF-CEM (IC50 for 48h: 14.6 ± 1.58 μM) and CEM/ADR5000 (IC50 for 48h: 44.5 ± 5.3 μM) cells | RA 15, 30, and 60 µM | Targeted IKK-β to inhibit NF-κB signaling pathway, caused disruption of MMP and cell adhesion and promoted caspase-independent cell death | [90] |
| Disease | Model | Treatment | Outcome | Ref |
|---|---|---|---|---|
| Lung cancer | A549 and A549/DDP (DDP resistance) cells | RA 10, 15, 20, and 40 µg/mL; DDP 1 µg/mL; combination therapy | Inhibited proliferation and invasion, and enhanced chemosensitivity to DDP based on downregulation of MDR1 mRNA expression | [11] |
| Renal cancer | 786-O cells | RA 25, 50, and 100 µM; DDP 5µM; combination therapy | Induced G2/M phase arrest and apoptosis in renal cancer cells. | [99] |
| Ovarian cancer | A2780 and DDP resistant daughter line A2780CP70 | RA 2.5, 5, and 10 g/mL | Showed synergistic anti-proliferation effect with DDP on A2780 cells | [18] |
| Melanoma | A375 cells | RA 50, 100, and 200 µg/mL; DDP 8 µM; combination therapy | Inhibited cell proliferation, invasion, and melanin synthesis, and increased apoptosis and DDP sensitivity via inhibitory of ADAM17/EGFR/AKT/GSK3β axis | [101] |
| Breast cancer | Female Swiss albino mice with intradermal injection of 0.1 mL Ehrlich ascites carcinoma | Oral RA 50 mg/kg; Paclitaxel 10 mg/kg/three times weekly intraperitoneally; combination therapy | Exerted chemo-preventive in combination with paclitaxel, suppressed NF-κB, TNF-α, and VEGF, increased in apoptotic markers p53, caspase-3, and BAX/BCL-2 ratio | [89] |
| Breast cancer | MCF-7 cells | RA 1.5, 15, or 50 µM; DOX 0.2 µM; combination therapy | Decreased the MDM2 gene expression and potentiated the effect of DOX | [118] |
| Gastric cancer | AGS cells | RA 100 and 200 µM; Anti-MUC1 antibody 5 µg/mL combination therapy | Inhibited the expression of MUC1, BCL-2, Tn antigens and T antigens, increased the expression of caspase-9, BAX, and BAD | [113] |
| Gastric cancer | SGC7901/Adr cells (DOX resistance) | RA 2.4 and 12 µM | Reversed the MDR of SGC7901/Adr cells, increased sensitivity to DOX and Rh123 through downregulating the expression of MDR1 transcript levels | [119] |
| Gastric cancer | SGC7901 and SGC7901/5-Fu (5-Fu resistance) cells | RA 15 µg/mL; 5-Fu 50 µg/mL; combination therapy | Enhanced chemosensitivity to 5-Fu, increased FOXO4 by downregulating miR-6785-5p and miR-642a-3p | [151] |
| HCC | HepG2 and Bel-7402 Cells | RA 25, 50, and 100 µg/mL; DOX 0.4 µg/mL; combination therapy | Enhanced DNA damage and apoptosis (BAX/BCL-2 ratio↑) | [93] |
| HCC | HepG2 cells | RA 10, 100, and 1000 mM; MG132 1 µM; combination therapy | Synergistically increased cytotoxicity, proteasome inhibition, autophagy, and apoptosis | [152] |
| Pancreatic cancer | Panc-1 cells | RA 10 and 20 µM; Gemcitabine 12.5 nM; combination therapy | Exerted anti-migration, pro-apoptosis effects and enhanced the efficacy of gemcitabine through downregulation of MRP-4, MRP-5, and Notch1 intracellular domain | [117] |
| APL | NB4 cells | RA 40 mM; ATRA 10 nM; combination therapy | RA potentiated ATRA-induced macrophage differentiation in APL cells and increased CCR-1, CCR-2, and ICAM-1 expression through activation of ERK and NF-κB | [155] |
| APL | HL-60 cells | RA 100, 125, and 150 µM; Ara-C 5, 10, and 20 nM; combination therapy | Synergistically inhibited DNA synthesis to potentiated the anti-proliferative effect of Ara-C | [81] |
| HNSCC | UM-SCC-1, UM-SCC-6, and OSC-2 cells | RA 80 µg/mL; Blue light 400–500 nm; 60 J/cm2, 2 min; combination therapy | Reduced EGFR activation and H2O2 production. | [80] |
| Metastatic melanoma | B16F10 cells | RA 20 and 40μM; RA combination with X-rays | Specifically sensitized radiation induces apoptosis of tumor cells | [156] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Xu, L.; Jin, D.; Xin, Y.; Tian, L.; Wang, T.; Zhao, D.; Wang, Z.; Wang, J. Rosmarinic Acid and Related Dietary Supplements: Potential Applications in the Prevention and Treatment of Cancer. Biomolecules 2022, 12, 1410. https://doi.org/10.3390/biom12101410
Zhao J, Xu L, Jin D, Xin Y, Tian L, Wang T, Zhao D, Wang Z, Wang J. Rosmarinic Acid and Related Dietary Supplements: Potential Applications in the Prevention and Treatment of Cancer. Biomolecules. 2022; 12(10):1410. https://doi.org/10.3390/biom12101410
Chicago/Turabian StyleZhao, Jiachao, Liwei Xu, Di Jin, Yu Xin, Lin Tian, Tan Wang, Daqing Zhao, Zeyu Wang, and Jing Wang. 2022. "Rosmarinic Acid and Related Dietary Supplements: Potential Applications in the Prevention and Treatment of Cancer" Biomolecules 12, no. 10: 1410. https://doi.org/10.3390/biom12101410
APA StyleZhao, J., Xu, L., Jin, D., Xin, Y., Tian, L., Wang, T., Zhao, D., Wang, Z., & Wang, J. (2022). Rosmarinic Acid and Related Dietary Supplements: Potential Applications in the Prevention and Treatment of Cancer. Biomolecules, 12(10), 1410. https://doi.org/10.3390/biom12101410






