Cholinesterase Deficiency Syndrome—A Pitfall in the Use of Butyrylcholinesterase as a Biomarker for Wilson’s Disease
Abstract
:1. Introduction
2. Case Reports
- (1)
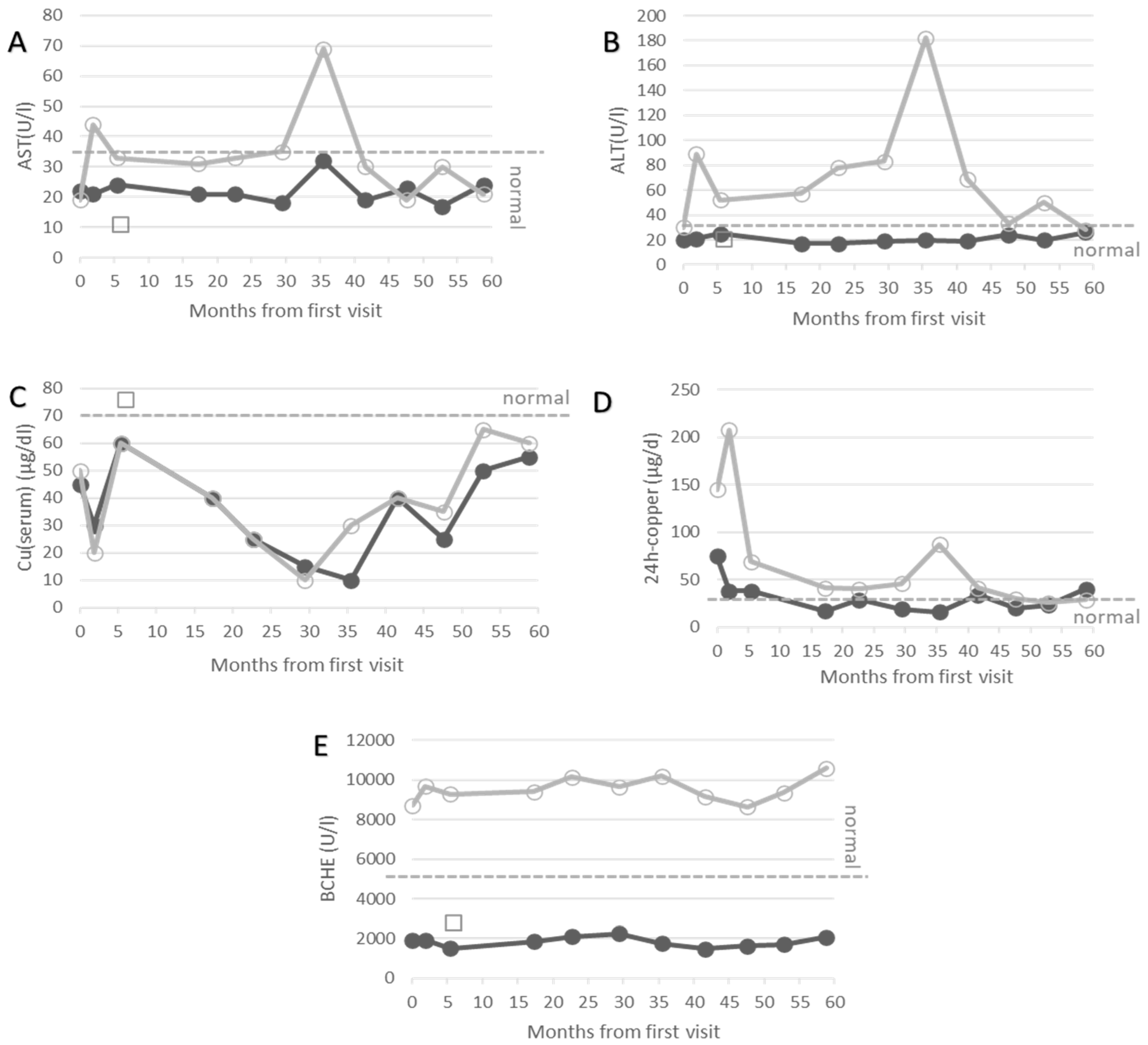
- Key Case 1 (male, age at recruitment: 18 years, BCHE-deficiency and WD)
- (2)
- Case 2 (male, age at recruitment: 20 years, WD)
- (3)
- Case 3 (female, age at recruitment: 14 years, BCHE-deficiency)
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santarpia, L.; Grandome, I.; Contaldo, F.; Pasanisi, F. Butyrylcholinesterase as a prognostic marker: A review of the literature. J. Cach. Sarco. Muscle 2013, 4, 31–39. [Google Scholar] [CrossRef]
- Ramachandran, J.; Sajith, K.G.; Priya, S.; Dutta, A.K.; Balasubramanian, K. Serum cholinesterase is an excellent biomarker of cirrhosis. Trop. Gastroenterol. 2014, 35, 15–20. [Google Scholar] [CrossRef]
- Seo, M.; Yamada, T.; Tamaki, S.; Hikoso, S.; Yasumura, Y.; Higuchi, Y.; Nakagawa, Y.; Uematsu, M.; Abe, H.; Fuji, H.; et al. Prognostic significance of serum cholinesterase level in patients with acute decompensated heart failure with preserved ejection fraction: Insights from the PURSUIT-HFpEF Registry. J. Am. Heart Assoc. 2020, 9, e014100. [Google Scholar] [CrossRef]
- Sato, T.; Yamauchi, H.; Suzuki, S.; Yoshihisa, A.; Yamaki, T.; Sugimoto, K.; Kunii, H.; Nakazato, K.; Suzuki, H.; Saitoh, S.-I.; et al. Serum cholinesterase is an important prognostic factor in chronic heart failure. Heart Vessel. 2015, 30, 204–210. [Google Scholar] [CrossRef]
- Sine, H.; El Grafel, K.; Alkhammal, S.; Achbani, A.; Filali, K. Serum cholinesterase biomarker study in farmers–Sous Massa regio-Morocco: Case-control study. Biomarkers 2019, 24, 771–775. [Google Scholar] [CrossRef]
- Nakajima, K.; Abe, T.; Saji, R.; Ogawa, F.; Taniguchi, H.; Yamaguchi, K.; Sakai, K.; Nakagawa, T.; Matsumura, R.; Oi, Y.; et al. Serum cholinesterase associated with COVID-19 pneumonia severity and mortality. J. Infect. 2021, 82, 282–327. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, H.; Gang Wan, Z.; Wang, J. Prolonged neuromuscular block associated with cholinesterase deficiency. Medicine 2018, 97, e13714. [Google Scholar] [CrossRef]
- Al-Eman, A. Butyryl-cholinesterase deficiency: A case report of delayed recovery after general anesthesia. Toxicol. Rep. 2021, 8, 1226–1228. [Google Scholar] [CrossRef]
- Lockridge, O.; Norgren, R.B., Jr.; Johnson, R.C.; Blake, T.A. Naturally occurring genetic variants of human acetylcholinesterase and butyrylcholinesterase and their potential impact on the risk of toxicity from cholinesterase inhibitors. Chem. Res. Toxicol. 2016, 29, 1381–1392. [Google Scholar] [CrossRef]
- Alexander, D.R. Pseudocholinesterase deficiency. Medscape 2017, 14, 1–6. [Google Scholar]
- Zhang, Q.-L.; Xu, M.-J.; Wang, T.-L.; Zhu, Z.-Q.; Lai, F.; Zheng, X.-C. Newly discovered COLQ gene mutation and its clinical features in patients with acetyl cholinesterase deficiency. J. Integr. Neurosc. 2018, 17, 439–446. [Google Scholar] [CrossRef]
- Wilson, K.S.A. Progressive lenticular degeneration: A familial nervous disease associated with cirrhosis of the liver. Brain 1912, 34, 295–507. [Google Scholar] [CrossRef]
- Hefter, H.; Arslan, M.; Kruschel, T.S.; Novak, M.; Rosenthal, D.; Meuth, S.G.; Albrecht, P.; Hartmann, C.; Samadzadeh, S. Pseudocholinesterase as a biomarker for Wilson’s disease. J. Hep. 2022; submitted. [Google Scholar]
- Pantuk, E. All variants of plasma cholinesterase are determined by mutations of one gene on chromosome 3q26. Anesth. Analg. 1993, 77, 380–386. [Google Scholar]
- Johnson, G.; Moore, S.W. Why has butyrylcholinesterase been retained? Structural and functional diversification in a duplicated gene. Neurochem. Int. 2012, 61, 783–797. [Google Scholar] [CrossRef]
- Rosenman, K.D.; Guss, P.S. Prevalence of congenital deficiency in serum cholinesterase. Arch. Environ. Health 1997, 52, 42–44. [Google Scholar] [CrossRef]
- Shab, P.; Jacobs, D. Plasma cholinesterase deficiency in Turkish patients. Anaesthesia 2016, 71, 982. [Google Scholar] [CrossRef]
- Yildizhan, E.; Tomruk, N.; Dereli, M.; Özdemir, A.; Yıldırım, H.; Canbek, Ö. Modified electroconvulsive therapy in pseudocholinesterase deficiency: A case report. In 25th European Congress Psychiatry; Cambridge University Press: Cambridge, UK, 2017; Volume 415, pp. S710–S771. [Google Scholar]
- Wallace, D.F.; Dooley, J.S. ATP7B variant penetrance explains differences between genetic and clinical prevalence estimates for Wilson disease. Hum. Genet. 2020, 139, 1065–1075. [Google Scholar] [CrossRef]
- Bull, P.C.; Thomas, G.R.; Rommens, J.M.; Forbes, J.R.; Cox, D.W. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nature Genet. 1993, 5, 327–337. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; Xu, A.; Zhang, S.; Jiang, H.; Pei, P.; Li, H.; Lv, T.; Yang, Y.; Qian, N.; et al. Identification of mutations in the ATP7B gene in 14 Wilson disease children. Medicine 2021, 100, e25463. [Google Scholar] [CrossRef]
- Czlonkowska, A.; Gromadzka, G.; Chabik, G. Monozygotic female twins discordant for phenotype of Wilson’s disease. Mov. Disord. 2009, 24, 1066–1069. [Google Scholar] [CrossRef]
- Samadzadeh, S. Long-Term Follow-Up of 115 Patients with Wilson’s Disease. Dr. Med Dissertation, University of Düsseldorf, Düsseldorf, Germany, 2022. Available online: https://docserv.uni-duesseldorf.de/servlets/DocumentServlet?id=59305 (accessed on 6 July 2022).
- Samadzadeh, S.; Kruschel, T.; Novak, M.; Kallenbach, M.; Hefter, H. Different Response Behavior to Therapeutic Approaches in Homozygotic Wilson’s Disease Twins with Clinical Phenotypic Variability: Case Report and Literature Review. Genes 2022, 13, 1217. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arslan, M.; Novak, M.; Rosenthal, D.; Hartmann, C.J.; Albrecht, P.; Samadzadeh, S.; Hefter, H. Cholinesterase Deficiency Syndrome—A Pitfall in the Use of Butyrylcholinesterase as a Biomarker for Wilson’s Disease. Biomolecules 2022, 12, 1398. https://doi.org/10.3390/biom12101398
Arslan M, Novak M, Rosenthal D, Hartmann CJ, Albrecht P, Samadzadeh S, Hefter H. Cholinesterase Deficiency Syndrome—A Pitfall in the Use of Butyrylcholinesterase as a Biomarker for Wilson’s Disease. Biomolecules. 2022; 12(10):1398. https://doi.org/10.3390/biom12101398
Chicago/Turabian StyleArslan, Max, Max Novak, Dietmar Rosenthal, Christian J. Hartmann, Philipp Albrecht, Sara Samadzadeh, and Harald Hefter. 2022. "Cholinesterase Deficiency Syndrome—A Pitfall in the Use of Butyrylcholinesterase as a Biomarker for Wilson’s Disease" Biomolecules 12, no. 10: 1398. https://doi.org/10.3390/biom12101398
APA StyleArslan, M., Novak, M., Rosenthal, D., Hartmann, C. J., Albrecht, P., Samadzadeh, S., & Hefter, H. (2022). Cholinesterase Deficiency Syndrome—A Pitfall in the Use of Butyrylcholinesterase as a Biomarker for Wilson’s Disease. Biomolecules, 12(10), 1398. https://doi.org/10.3390/biom12101398






