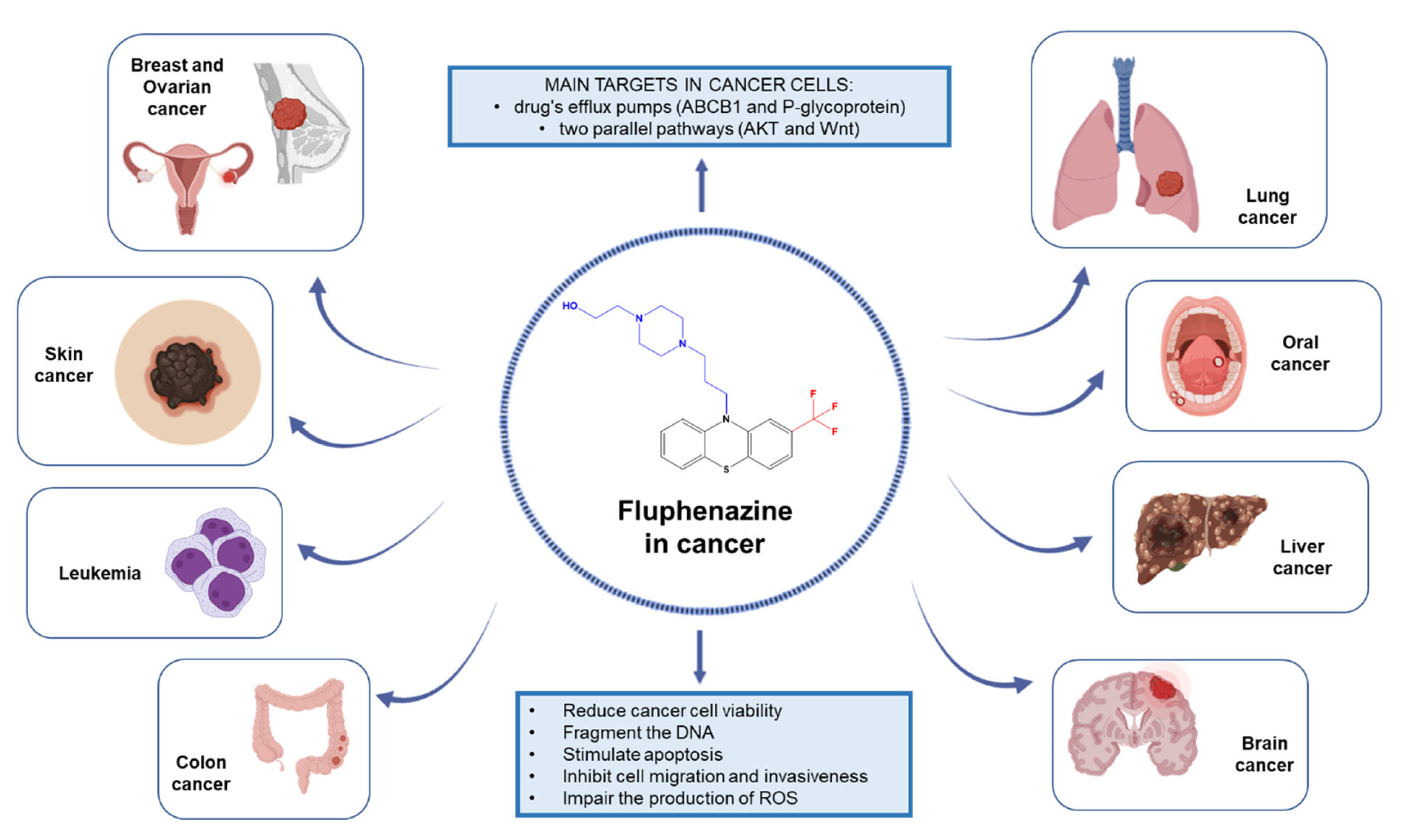
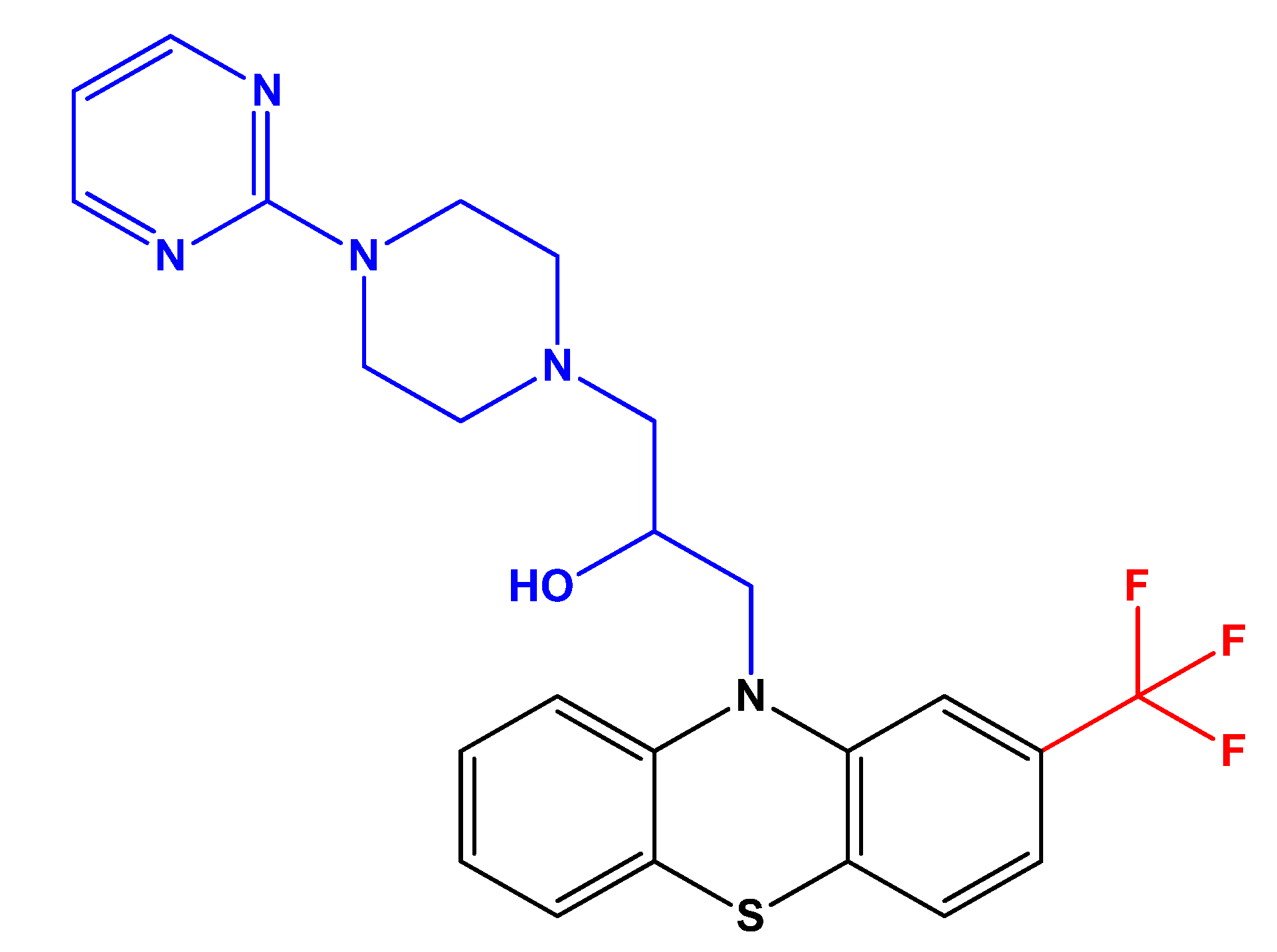
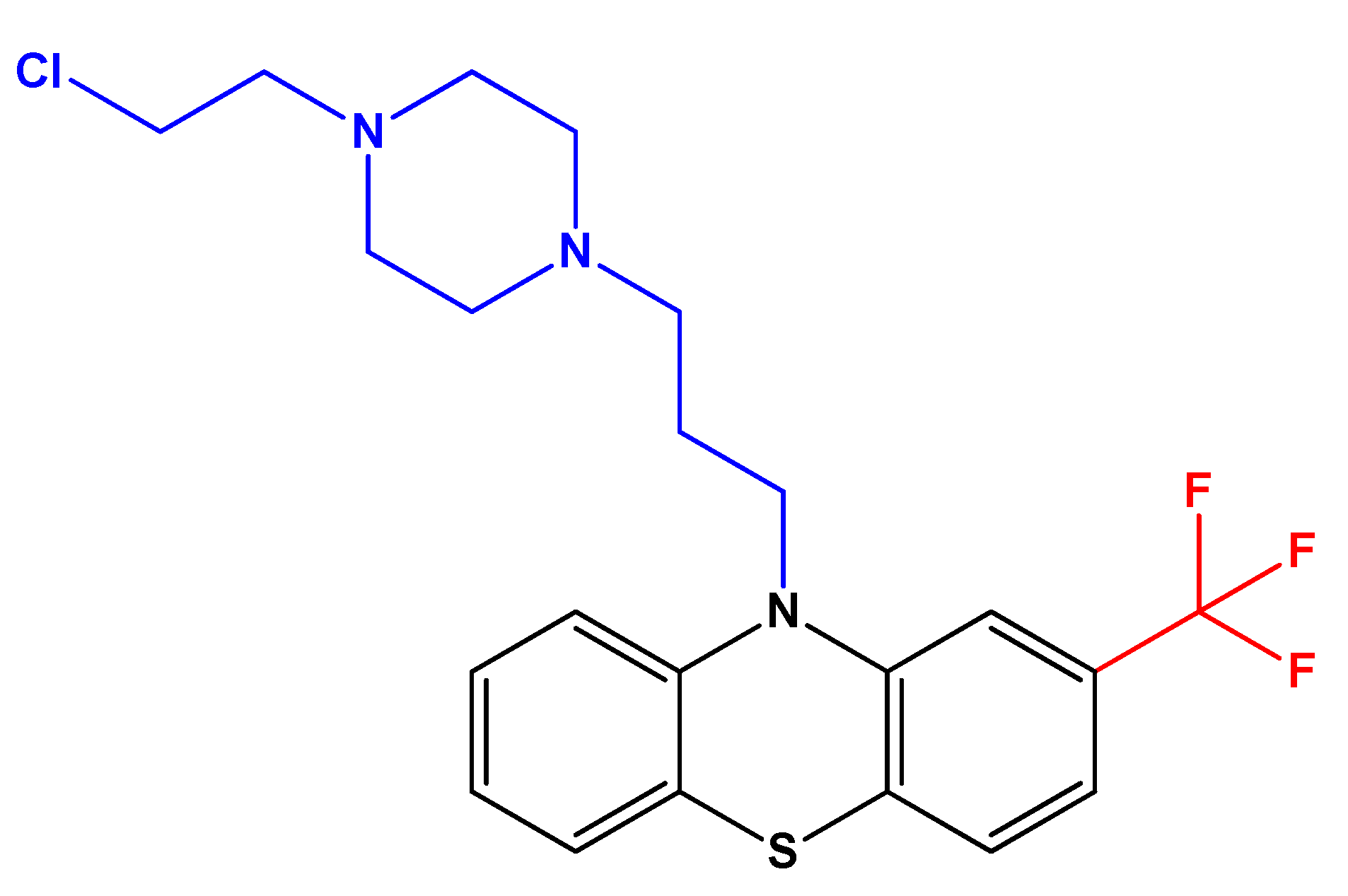
Antipsychotic Drug Fluphenazine against Human Cancer Cells
Abstract
1. Introduction
2. Evidence of Anticancer Effects of Fluphenazine against Different Human Cancer Cells
2.1. Lung Cancer
2.2. Breast Cancer
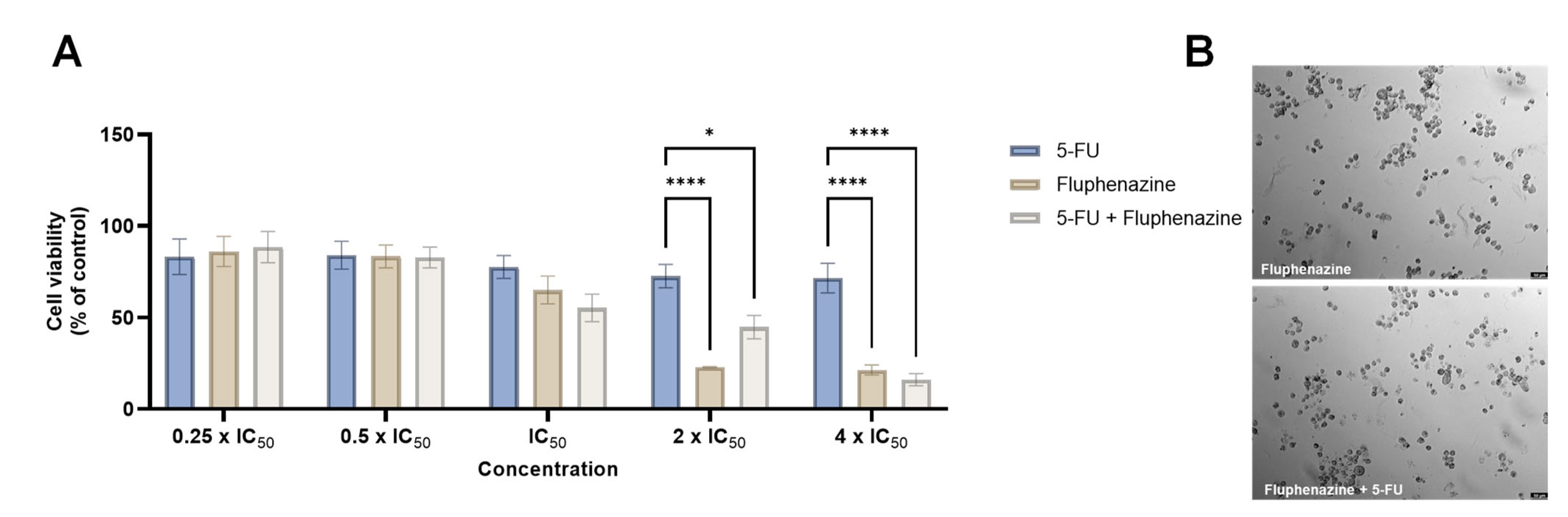
2.3. Colon Cancer
2.4. Liver Cancer
2.5. Brain Cancer
2.6. Leukemia
2.7. Oral Cancer
2.8. Ovarian Cancer
2.9. Melanoma
3. Discussion on the Future Directions Regarding Fluphenazine Use in Cancer Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Yabroff, K.R.; Wu, X.-C.; Negoita, S.; Stevens, J.; Coyle, L.; Zhao, J.; Mumphrey, B.J.; Jemal, A.; Ward, K.C. Association of the COVID-19 Pandemic with Patterns of Statewide Cancer Services. J. Natl. Cancer Inst. 2022, 114, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Antoszczak, M.; Markowska, A.; Markowska, J.; Huczyński, A. Old Wine in New Bottles: Drug Repurposing in Oncology. Eur. J. Pharmacol. 2020, 866, 172784. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, J.P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug Repositioning: A Brief Overview. J. Pharm. Pharmacol. 2020, 72, 1145–1151. [Google Scholar] [CrossRef]
- Pantziarka, P.; Bouche, G.; Meheus, L.; Sukhatme, V.; Sukhatme, V.P.; Vikas, P. The Repurposing Drugs in Oncology (ReDO) Project. Ecancermedicalscience 2014, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Roney, M.S.I.; Park, S.-K. Antipsychotic Dopamine Receptor Antagonists, Cancer, and Cancer Stem Cells. Arch. Pharm. Res. 2018, 41, 384–408. [Google Scholar] [CrossRef]
- Fond, G.; Macgregor, A.; Attal, J.; Larue, A.; Brittner, M.; Ducasse, D.; Capdevielle, D. Antipsychotic Drugs: Pro-Cancer or Anti-Cancer? A Systematic Review. Med. Hypotheses 2012, 79, 38–42. [Google Scholar] [CrossRef]
- Zong, D.; Zielinska-Chomej, K.; Juntti, T.; Mörk, B.; Lewensohn, R.; Hååg, P.; Viktorsson, K. Harnessing the Lysosome-Dependent Antitumor Activity of Phenothiazines in Human Small Cell Lung Cancer. Cell Death Dis. 2014, 5, e1111. [Google Scholar] [CrossRef] [PubMed]
- Munson, J.M.; Fried, L.; Rowson, S.A.; Bonner, M.Y.; Karumbaiah, L.; Diaz, B.; Courtneidge, S.A.; Knaus, U.G.; Brat, D.J.; Arbiser, J.L.; et al. Anti-Invasive Adjuvant Therapy with Imipramine Blue Enhances Chemotherapeutic Efficacy Against Glioma. Sci. Transl. Med. 2012, 4, 127ra36. [Google Scholar] [CrossRef]
- Abdelaleem, M.; Ezzat, H.; Osama, M.; Megahed, A.; Alaa, W.; Gaber, A.; Shafei, A.; Refaat, A. Prospects for Repurposing CNS Drugs for Cancer Treatment. Oncol. Rev. 2019, 13, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Varga, B.; Csonka, Á.; Csonka, A.; Molnár, J.; Amaral, L.; Spengler, G. Possible Biological and Clinical Applications of Phenothiazines. Anticancer Res. 2017, 37, 5983–5993. [Google Scholar] [CrossRef] [PubMed]
- Spengler, G.; Csonka, Á.; Molnár, J.; Amaral, L. The Anticancer Activity of the Old Neuroleptic Phenothiazine-Type Drug Thioridazine. Anticancer Res. 2016, 36, 5701–5706. [Google Scholar] [CrossRef] [PubMed]
- Hendouei, N.; Saghafi, F.; Shadfar, F.; Hosseinimehr, S.J. Molecular Mechanisms of Anti-Psychotic Drugs for Improvement of Cancer Treatment. Eur. J. Pharmacol. 2019, 856, 172402. [Google Scholar] [CrossRef] [PubMed]
- Otręba, M.; Kośmider, L. In Vitro Anticancer Activity of Fluphenazine, Perphenazine and Prochlorperazine. A Review. J. Appl. Toxicol. 2021, 41, 82–94. [Google Scholar] [CrossRef]
- World Health Organization. WHO Model List of Essential Medicines; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Fluphenazine: Uses, Interactions, Mechanism of Action | DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00623 (accessed on 17 August 2022).
- Hait, W.N.; Gesmonde, J.F.; Lazo, J.S. Effect of Anti-Calmodulin Drugs on the Growth and Sensitivity of C6 Rat Glioma Cells to Bleomycin. Anticancer Res. 1994, 14, 1711–1721. [Google Scholar]
- Berchtold, M.W.; Villalobo, A. The Many Faces of Calmodulin in Cell Proliferation, Programmed Cell Death, Autophagy, and Cancer. Biochim. Biophys. Acta—Mol. Cell Res. 2014, 1843, 398–435. [Google Scholar] [CrossRef]
- Study of Fluphenazine in Relapsed or Relapsed-and-Refractory Multiple Myeloma—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00821301?term=fluphenazine&draw=2&rank=1 (accessed on 18 August 2022).
- Fluphenazine in Treating Patients with Refractory Advanced Multiple Myeloma—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00335647?term=fluphenazine&draw=2&rank=4 (accessed on 18 August 2022).
- Hilf, R.; Bell, C.; Goldenberg, H.; Michel, I. Effect of Fluphenazine HCl on R3230AC Mammary Carcinoma and Mammary Glands of the Rat. Cancer Res. 1971, 31, 1111–1117. [Google Scholar]
- Laing, L.P.; Boegman, R.J.; Roder, J.C. The Inhibitory Effect of Phenothiazines on NK-Mediated Cytolysis of Tumor Cells. Immunopharmacology 1984, 8, 1–12. [Google Scholar] [CrossRef]
- Cieślik-Boczula, K.; Szwed, J.; Jaszczyszyn, A.; Gasiorowski, K.; Koll, A. Interactions of Dihydrochloride Fluphenazine with DPPC Liposomes: ATR-IR and 31P NMR Studies. J. Phys. Chem. B 2009, 113, 15495–15502. [Google Scholar] [CrossRef]
- Cieślik-Boczula, K.; Świątek, P.; Jaszczyszyn, A.; Zawilska, P.; Gąsiorowski, K.; Malinka, W.; Köhler, G. Phase Separation in Phosphatidylcholine Membrane Caused by the Presence of a Pyrimidine Analogue of Fluphenazine with High Anti-Multidrug-Resistance Activity. J. Phys. Chem. B 2014, 118, 3605–3615. [Google Scholar] [CrossRef]
- Zhu, J.; Smith, K.; Hsieh, P.N.; Mburu, Y.K.; Chattopadhyay, S.; Sen, G.C.; Sarkar, S.N. High-Throughput Screening for TLR3-IFN Regulatory Factor 3 Signaling Pathway Modulators Identifies Several Antipsychotic Drugs as TLR Inhibitors. J. Immunol. 2010, 184, 5768–5776. [Google Scholar] [CrossRef] [PubMed]
- Jaszczyszyn, A.; Gąsiorowski, K.; Swiątek, P.; Malinka, W.; Cieślik-Boczula, K.; Petrus, J.; Czarnik-Matusewicz, B. New Fluphenazine Analogues as Inhibitors of P-Glycoprotein in Human Lymphocyte Cultures. Contemp. Oncol. 2012, 16, 332–337. [Google Scholar] [CrossRef]
- Li, Y.; McGreal, S.; Zhao, J.; Huang, R.; Zhou, Y.; Zhong, H.; Xia, M.; Ding, W.-X. A Cell-Based Quantitative High-Throughput Image Screening Identified Novel Autophagy Modulators. Pharmacol. Res. 2016, 110, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Dubey, A.; Kamboj, N.K.; Sahoo, A.K.; Kang, S.G.; Yadava, U. Drug Repurposing for Ligand-Induced Rearrangement of Sirt2 Active Site-Based Inhibitors via Molecular Modeling and Quantum Mechanics Calculations. Sci. Rep. 2021, 11, 10169. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, G.B.; Okutachi, S.; Abankwa, D. Potential of Phenothiazines to Synergistically Block Calmodulin and Reactivate PP2A in Cancer Cells. PLoS ONE 2022, 17, e0268635. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Min, Y.K.; Kim, S.H. Calmodulin Inhibition Contributes to Sensitize TRAIL-Induced Apoptosis in Human Lung Cancer H1299 Cells. Biochem. Cell Biol. 2009, 87, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Zong, D.; Hååg, P.; Yakymovych, I.; Lewensohn, R.; Viktorsson, K. Chemosensitization by Phenothiazines in Human Lung Cancer Cells: Impaired Resolution of ΓH2AX and Increased Oxidative Stress Elicit Apoptosis Associated with Lysosomal Expansion and Intense Vacuolation. Cell Death Dis. 2011, 2, e181. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, W.; Shen, X.; Wang, Q.; Lv, J.; Liu, M.; Cheng, F.; Zhao, Z.; Pang, X. Repurposing Sertraline Sensitizes Non-Small Cell Lung Cancer Cells to Erlotinib by Inducing Autophagy. JCI Insight 2018, 3, e98921. [Google Scholar] [CrossRef]
- Ford, J.M.; Prozialeck, W.C.; Hait, W.N. Structural Features Determining Activity of Phenothiazines and Related Drugs for Inhibition of Cell Growth and Reversal of Multidrug Resistance. Mol. Pharmacol. 1989, 35, 105–115. [Google Scholar]
- Goyette, M.-A.A.; Cusseddu, R.; Elkholi, I.; Abu-Thuraia, A.; El-Hachem, N.; Haibe-Kains, B.; Gratton, J.-P.P.; Côté, J.-F.F. AXL Knockdown Gene Signature Reveals a Drug Repurposing Opportunity for a Class of Antipsychotics to Reduce Growth and Metastasis of Triple-Negative Breast Cancer. Oncotarget 2019, 10, 2055–2067. [Google Scholar] [CrossRef]
- Xu, F.; Xia, Y.; Feng, Z.; Lin, W.; Xue, Q.; Jiang, J.; Yu, X.; Peng, C.; Luo, M.; Yang, Y.; et al. Repositioning Antipsychotic Fluphenazine Hydrochloride for Treating Triple Negative Breast Cancer with Brain Metastases and Lung Metastases. Am. J. Cancer Res. 2019, 9, 459–478. [Google Scholar] [PubMed]
- Duarte, D.; Cardoso, A.; Vale, N. Synergistic Growth Inhibition of HT-29 Colon and MCF-7 Breast Cancer Cells with Simultaneous and Sequential Combinations of Antineoplastics and CNS Drugs. Int. J. Mol. Sci. 2021, 22, 7408. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.; Rêma, A.; Amorim, I.; Vale, N. Drug Combinations: A New Strategy to Extend Drug Repurposing and Epithelial-Mesenchymal Transition in Breast and Colon Cancer Cells. Biomolecules 2022, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.; Falcão, S.I.; El Mehdi, I.; Vilas-Boas, M.; Vale, N.; El Mehdi, I.; Vilas-Boas, M.; Vale, N. Honeybee Venom Synergistically Enhances the Cytotoxic Effect of CNS Drugs in HT-29 Colon and MCF-7 Breast Cancer Cell Lines. Pharmaceutics 2022, 14, 511. [Google Scholar] [CrossRef] [PubMed]
- Klutzny, S.; Lesche, R.; Keck, M.; Kaulfuss, S.; Schlicker, A.; Christian, S.; Sperl, C.; Neuhaus, R.; Mowat, J.; Steckel, M.; et al. Functional Inhibition of Acid Sphingomyelinase by Fluphenazine Triggers Hypoxia-Specific Tumor Cell Death. Cell Death Dis. 2017, 8, e2709. [Google Scholar] [CrossRef]
- Środa-Pomianek, K.; Michalak, K.; Świątek, P.; Poła, A.; Palko-Łabuz, A.; Wesołowska, O. Increased Lipid Peroxidation, Apoptosis and Selective Cytotoxicity in Colon Cancer Cell Line LoVo and Its Doxorubicin-Resistant Subline LoVo/Dx in the Presence of Newly Synthesized Phenothiazine Derivatives. Biomed. Pharmacother. 2018, 106, 624–636. [Google Scholar] [CrossRef]
- Środa-Pomianek, K.; Michalak, K.; Palko-Łabuz, A.; Uryga, A.; Świątek, P.; Majkowski, M.; Wesołowska, O. The Combined Use of Phenothiazines and Statins Strongly Affects Doxorubicin-Resistance, Apoptosis, and Cox-2 Activity in Colon Cancer Cells. Int. J. Mol. Sci. 2019, 20, 955. [Google Scholar] [CrossRef]
- De Faria, P.A.; Bettanin, F.; Cunha, R.L.O.R.; Paredes-Gamero, E.J.; Homem-de-Mello, P.; Nantes, I.L.; Rodrigues, T. Cytotoxicity of Phenothiazine Derivatives Associated with Mitochondrial Dysfunction: A Structure-Activity Investigation. Toxicology 2015, 330, 44–54. [Google Scholar] [CrossRef]
- Cheng, J.S.; Wang, J.L.; Lo, Y.K.; Chou, K.J.; Lee, K.C.; Liu, C.P.; Chang, H.T.; Jan, C.R. Effects of the Antianginal Drug Fendiline on Ca2+ Movement in Hepatoma Cells. Hum. Exp. Toxicol. 2001, 20, 359–364. [Google Scholar] [CrossRef]
- Hamid, R.; Rotshteyn, Y.; Rabadi, L.; Parikh, R.; Bullock, P. Comparison of Alamar Blue and MTT Assays for High Through-Put Screening. Toxicol. In Vitro 2004, 18, 703–710. [Google Scholar] [CrossRef]
- Silver, M.A.; Yang, Z.W.; Ganguli, R.; Nimgaonkar, V.L.; Andrew Silver, M.; Wei Yang, Z.; Ganguli, R.; Nimgaonkar, V.L. An Inhibitory Effect of Psychoactive Drugs on a Human Neuroblastoma Cell Line. Biol. Psychiatry 1994, 35, 824–826. [Google Scholar] [CrossRef]
- Gil-Ad, I.; Offen, D.; Shtaif, B.; Galili-Mosberg, R.; Weizman, A. Haloperidol Induces Neurotoxicity in Mouse Embryo Brain Tissue. In Progress in Alzheimer’s and Parkinson’s Diseases; Springer: Boston, MA, USA, 1998; pp. 163–169. [Google Scholar] [CrossRef]
- Gil-Ad, I.; Shtaif, B.; Levkovitz, Y.; Dayag, M.; Zeldich, E.; Weizman, A. Characterization of Phenothiazine-Incluced Apoptosis in Neuroblastoma and Glioma Cell Lines: Clinical Relevance and Possible Application for Brain-Derived Tumors. J. Mol. Neurosci. 2004, 22, 189–198. [Google Scholar] [CrossRef]
- De Preter, K.; De Brouwer, S.; Van Maerken, T.; Pattyn, F.; Schramm, A.; Eggert, A.; Vandesompele, J.; Speleman, F. Meta-Mining of Neuroblastoma and Neuroblast Gene Expression Profiles Reveals Candidate Therapeutic Compounds. Clin. Cancer Res. 2009, 15, 3690–3696. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.W.; Liang, Y.H.; Kuo, Y.L.; Chuu, C.P.; Lin, C.Y.; Lee, M.H.; Wu, A.T.H.; Yeh, C.T.; Chen, E.T.; Peng, J.W.; et al. Identification of Thioridazine, an Antipsychotic Drug, as an Antiglioblastoma and Anticancer Stem Cell Agent Using Public Gene Expression Data. Cell Death Dis. 2015, 6, e1753. [Google Scholar] [CrossRef]
- Jacobs, K.A.; André-Grégoire, G.; Maghe, C.; Thys, A.; Li, Y.; Harford-Wright, E.; Trillet, K.; Douanne, T.; Alves Nicolau, C.; Frénel, J.; et al. Paracaspase MALT1 Regulates Glioma Cell Survival by Controlling Endo-lysosome Homeostasis. EMBO J. 2020, 39. [Google Scholar] [CrossRef]
- Hait, W.N.; Lee, G.L. Characteristics of the Cytotoxic Effects of the Phenothiazine Class of Calmodulin Antagonists. Biochem. Pharmacol. 1985, 34, 3973–3978. [Google Scholar] [CrossRef]
- Seredenina, T.; Chiriano, G.; Filippova, A.; Nayernia, Z.; Mahiout, Z.; Fioraso-Cartier, L.; Plastre, O.; Scapozza, L.; Krause, K.H.; Jaquet, V. A Subset of N-Substituted Phenothiazines Inhibits NADPH Oxidases. Free Radic. Biol. Med. 2015, 86, 239–249. [Google Scholar] [CrossRef][Green Version]
- Cheon, J.H.; Lee, B.M.; Kim, H.S.; Yoon, S. Highly Halaven-Resistant KBV20C Cancer Cells Can Be Sensitized by Co-Treatment with Fluphenazine. Anticancer Res. 2016, 36, 5867–5874. [Google Scholar] [CrossRef][Green Version]
- Kim, J.Y.; Tae, I.H.; Lee, B.-M.M.; Kim, H.S.; Yoon, S. Low Doses of the Anti-Psychotic Drug Aripiprazole Have Strong P-Gp-Inhibitory Activity and Sensitize Anti-Mitotic Drug-Resistant Cancer Cells. Anticancer Res. 2018, 38, 5101–5108. [Google Scholar] [CrossRef]
- Kim, J.Y.; Son, J.Y.; Lee, B.-M.; Kim, H.S.; Yoon, S. Aging-Related Repositioned Drugs, Donepezil and Sildenafil Citrate, Increase Apoptosis of Anti-Mitotic Drug-Resistant KBV20C Cells Through Different Molecular Mechanisms. Anticancer Res. 2018, 38, 5149–5157. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, K.S.; Kim, I.S.; Yoon, S. Histamine Receptor Antagonists, Loratadine and Azelastine, Sensitize P-Gp-Overexpressing Antimitotic Drug-Resistant KBV20C Cells through Different Molecular Mechanisms. Anticancer Res. 2019, 39, 3767–3775. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Yang, Y.R.; Lee, S.K.; Kim, S.H.; Kim, Y.H.; Cha, J.Y.; Oh, S.W.; Ha, J.R.; Ryu, S.H.; Suh, P.G. Potential Inhibition of PDK1/Akt Signaling by Phenothiazines Suppresses Cancer Cell Proliferation and Survival. Ann. N. Y. Acad. Sci. 2008, 1138, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ad, I.; Shtaif, B.; Levkovitz, Y.; Nordenberg, J.; Taler, M.; Korov, I.; Weizman, A. Phenothiazines Induce Apoptosis in a B16 Mouse Melanoma Cell Line and Attenuate in Vivo Melanoma Tumor Growth. Oncol. Rep. 2006, 15, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, O.F.; Gowda, R.; Noory, M.A.; Robertson, G.P. Modulating Cancer Cell Survival by Targeting Intracellular Cholesterol Transport. Br. J. Cancer 2017, 117, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xu, F.; Xiong, M.; Yang, H.; Lin, W.; Xie, Y.; Xi, H.; Xue, Q.; Ye, T.; Yu, L. Repurposing of Antipsychotic Trifluoperazine for Treating Brain Metastasis, Lung Metastasis and Bone Metastasis of Melanoma by Disrupting Autophagy Flux. Pharmacol. Res. 2021, 163, 105295. [Google Scholar] [CrossRef]
- Menilli, L.; García-Argáez, A.N.; Dalla Via, L.; Miolo, G. The Neuroleptic Drug Fluphenazine Induces a Significant UVA-Mediated Cytotoxic Effect on Three Human Cancer Cell Lines through Apoptosis. Photochem. Photobiol. Sci. 2019, 18, 2232–2239. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-Catenin Signaling Pathway in Cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef]
- Nitulescu, G.M.; Van De Venter, M.; Nitulescu, G.; Ungurianu, A.; Juzenas, P.; Peng, Q.; Olaru, O.T.; Grǎdinaru, D.; Tsatsakis, A.; Tsoukalas, D.; et al. The Akt Pathway in Oncology Therapy and beyond (Review). Int. J. Oncol. 2018, 53, 2319–2331. [Google Scholar] [CrossRef]
- Davis, C. Fluphenazine. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–7. ISBN 9780080552323. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, D.; Vale, N. Antipsychotic Drug Fluphenazine against Human Cancer Cells. Biomolecules 2022, 12, 1360. https://doi.org/10.3390/biom12101360
Duarte D, Vale N. Antipsychotic Drug Fluphenazine against Human Cancer Cells. Biomolecules. 2022; 12(10):1360. https://doi.org/10.3390/biom12101360
Chicago/Turabian StyleDuarte, Diana, and Nuno Vale. 2022. "Antipsychotic Drug Fluphenazine against Human Cancer Cells" Biomolecules 12, no. 10: 1360. https://doi.org/10.3390/biom12101360
APA StyleDuarte, D., & Vale, N. (2022). Antipsychotic Drug Fluphenazine against Human Cancer Cells. Biomolecules, 12(10), 1360. https://doi.org/10.3390/biom12101360






