Fibrillin-1 Regulates Arteriole Integrity in the Retina
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Whole Retina Immunohistochemistry
2.3. Antibodies for Immunolabeling
2.4. Analysis of the Retinal Vasculature
2.5. Statistics
3. Results
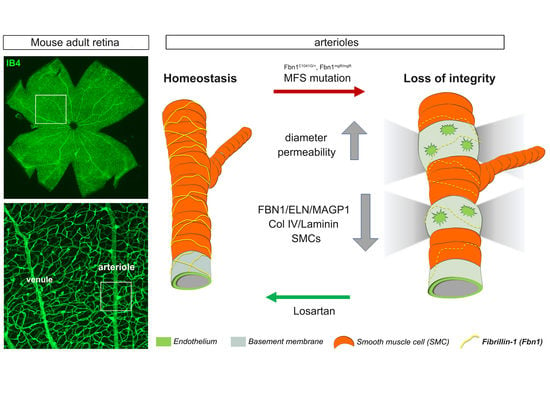
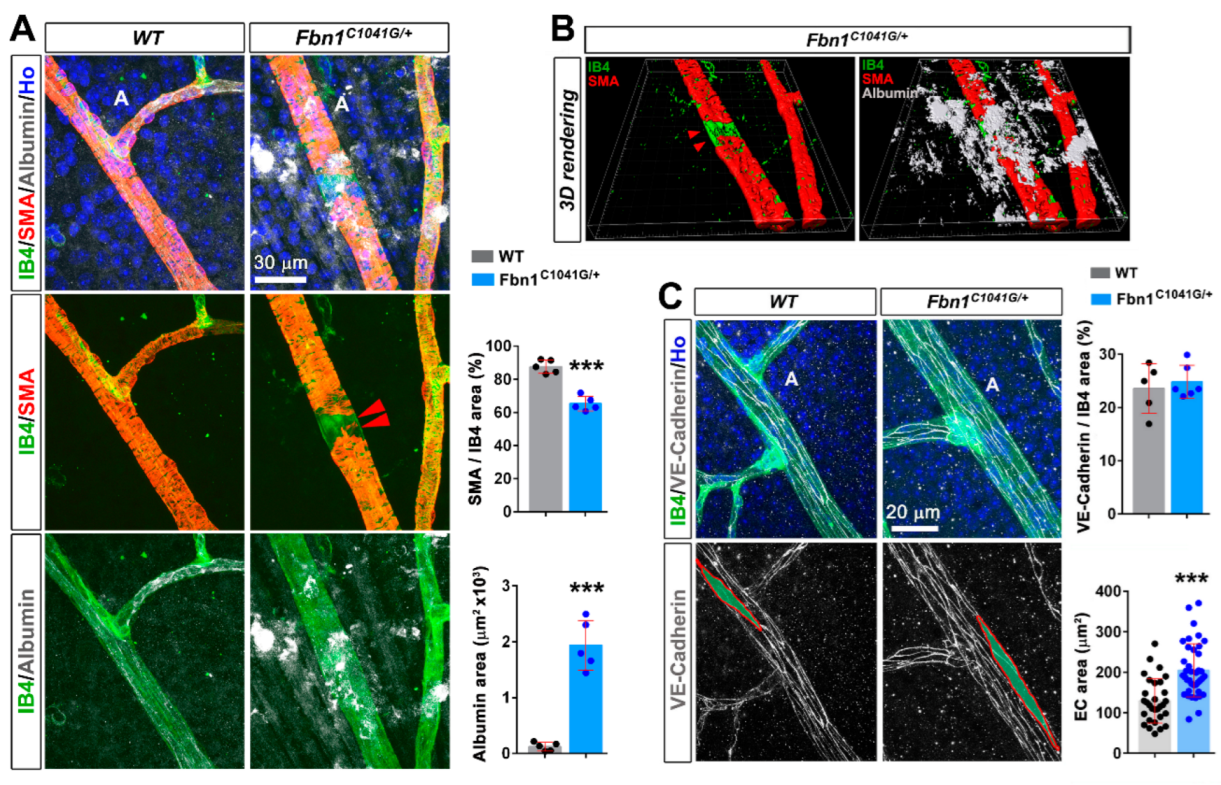
3.1. Fibrillin-1 Is Detected in the Arteriolar Wall and Its Deficiency Affects the Integrity of Arterioles
3.2. Fibrillin-1 Deficiency Also Affects the Integrity during Development and Aging of the Retinal Vasculature
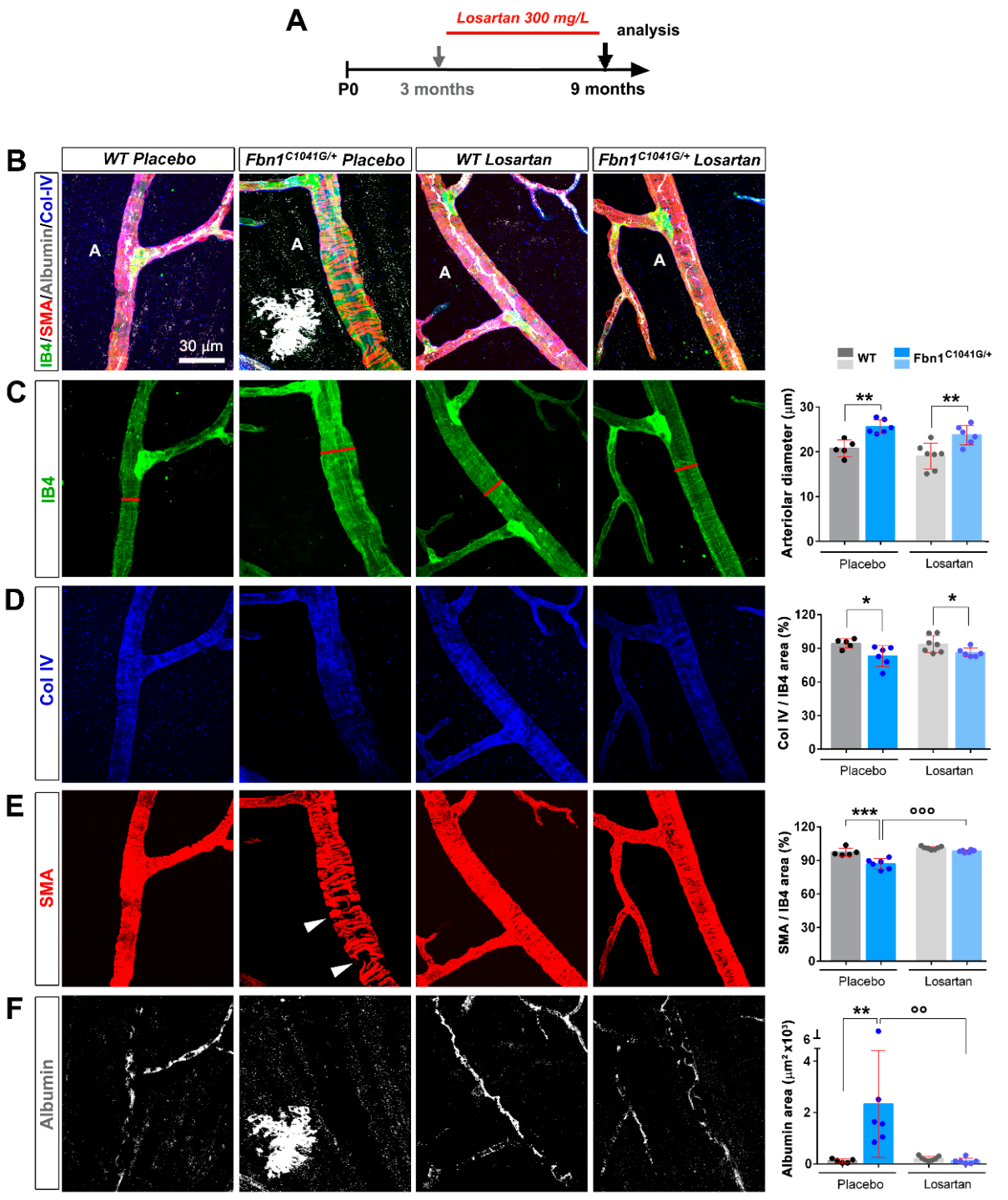
3.3. Focal Loss of VSMC Coverage, Poor BM Protein Coverage, and Vessel Leakiness Are Prevented by Losartan Treatment
3.4. Focal Loss of VSMC Coverage, Poor BM Protein Coverage, and Vessel Leakiness Are Reverted by Losartan Treatment
4. Discussion
4.1. The Retinal Arterioles Are Sheathed by Fibrillin-1 and MAGP1 during Their Formation and at Maturity
4.2. Mutant Fibrillin-1 Alters Arteriolar Integrity
4.3. Losartan Rescues Mutant Fibrillin-1 Induced Defects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelleher, C.M.; McLean, S.E.; Mecham, R.P. Vascular extracellular matrix and aortic development. Curr. Top. Dev. Biol. 2004, 62, 153–188. [Google Scholar] [CrossRef]
- Sakai, L.Y.; Keene, D.R.; Engvall, E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J. Cell Biol. 1986, 103, 2499–2509. [Google Scholar] [CrossRef]
- Schmelzer, C.E.H.; Duca, L. Elastic fibers: Formation, function, and fate during aging and disease. FEBS J. 2022, 289, 3704–3730. [Google Scholar] [CrossRef]
- Shi, Y.; Jones, W.; Beatty, W.; Tan, Q.; Mecham, R.P.; Kumra, H.; Reinhardt, D.P.; Gibson, M.A.; Reilly, M.A.; Rodriguez, J.; et al. Latent-transforming growth factor beta-binding protein-2 (LTBP-2) is required for longevity but not for development of zonular fibers. Matrix Biol. 2021, 95, 15–31. [Google Scholar] [CrossRef]
- Davis, E.C. Immunolocalization of microfibril and microfibril-associated proteins in the subendothelial matrix of the developing mouse aorta. J. Cell Sci. 1994, 107 Pt 3, 727–736. [Google Scholar] [CrossRef]
- Dzamba, B.J.; Keene, D.R.; Isogai, Z.; Charbonneau, N.L.; Karaman-Jurukovska, N.; Simon, M.; Sakai, L.Y. Assembly of epithelial cell fibrillins. J. Investig. Dermatol. 2001, 117, 1612–1620. [Google Scholar] [CrossRef]
- Tiedemann, K.; Sasaki, T.; Gustafsson, E.; Gohring, W.; Batge, B.; Notbohm, H.; Timpl, R.; Wedel, T.; Schlotzer-Schrehardt, U.; Reinhardt, D.P. Microfibrils at basement membrane zones interact with perlecan via fibrillin-1. J. Biol. Chem. 2005, 280, 11404–11412. [Google Scholar] [CrossRef]
- Hubmacher, D.; Reinhardt, D.P. Microfibrils and fibrillin. In The Extracellular Matrix: An Overview, Mecham RP, ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 233–265. [Google Scholar]
- Godwin, A.R.F.; Singh, M.; Lockhart-Cairns, M.P.; Alanazi, Y.F.; Cain, S.A.; Baldock, C. The role of fibrillin and microfibril binding proteins in elastin and elastic fibre assembly. Matrix Biol. 2019, 84, 17–30. [Google Scholar] [CrossRef]
- Sengle, G.; Sakai, L.Y. The fibrillin microfibril scaffold: A niche for growth factors and mechanosensation? Matrix Biol. 2015, 47, 3–12. [Google Scholar] [CrossRef]
- Zeyer, K.A.; Reinhardt, D.P. Fibrillin-containing microfibrils are key signal relay stations for cell function. J. Cell Commun. Signal. 2015, 9, 309–325. [Google Scholar] [CrossRef]
- Dietz, H.C.; Cutting, G.R.; Pyeritz, R.E.; Maslen, C.L.; Sakai, L.Y.; Corson, G.M.; Puffenberger, E.G.; Hamosh, A.; Nanthakumar, E.J.; Curristin, S.M.; et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 1991, 352, 337–339. [Google Scholar] [CrossRef]
- Collod-Beroud, G.; Le Bourdelles, S.; Ades, L.; Ala-Kokko, L.; Booms, P.; Boxer, M.; Child, A.; Comeglio, P.; De Paepe, A.; Hyland, J.C.; et al. Update of the UMD-FBN1 mutation database and creation of an FBN1 polymorphism database. Hum. Mutat. 2003, 22, 199–208. [Google Scholar] [CrossRef]
- Robinson, P.N.; Arteaga-Solis, E.; Baldock, C.; Collod-Beroud, G.; Booms, P.; De Paepe, A.; Dietz, H.C.; Guo, G.; Handford, P.A.; Judge, D.P.; et al. The molecular genetics of Marfan syndrome and related disorders. J. Med. Genet. 2006, 43, 769–787. [Google Scholar] [CrossRef]
- Carta, L.; Pereira, L.; Arteaga-Solis, E.; Lee-Arteaga, S.Y.; Lenart, B.; Starcher, B.; Merkel, C.A.; Sukoyan, M.; Kerkis, A.; Hazeki, N.; et al. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J. Biol. Chem. 2006, 281, 8016–8023. [Google Scholar] [CrossRef]
- Habashi, J.P.; Judge, D.P.; Holm, T.M.; Cohn, R.D.; Loeys, B.L.; Cooper, T.K.; Myers, L.; Klein, E.C.; Liu, G.; Calvi, C.; et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006, 312, 117–121. [Google Scholar] [CrossRef]
- Judge, D.P.; Biery, N.J.; Keene, D.R.; Geubtner, J.; Myers, L.; Huso, D.L.; Sakai, L.Y.; Dietz, H.C. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J. Clin. Investig. 2004, 114, 172–181. [Google Scholar] [CrossRef]
- Neptune, E.R.; Frischmeyer, P.A.; Arking, D.E.; Myers, L.; Bunton, T.E.; Gayraud, B.; Ramirez, F.; Sakai, L.Y.; Dietz, H.C. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003, 33, 407–411. [Google Scholar] [CrossRef]
- Pereira, L.; Lee, S.Y.; Gayraud, B.; Andrikopoulos, K.; Shapiro, S.D.; Bunton, T.; Biery, N.J.; Dietz, H.C.; Sakai, L.Y.; Ramirez, F. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc. Natl. Acad. Sci. USA 1999, 96, 3819–3823. [Google Scholar] [CrossRef]
- Stahl, A.; Connor, K.M.; Sapieha, P.; Chen, J.; Dennison, R.J.; Krah, N.M.; Seaward, M.R.; Willett, K.L.; Aderman, C.M.; Guerin, K.I.; et al. The mouse retina as an angiogenesis model. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2813–2826. [Google Scholar] [CrossRef]
- Pozzi, A.; Yurchenco, P.D.; Iozzo, R.V. The nature and biology of basement membranes. Matrix Biol. 2017, 57–58, 1–11. [Google Scholar] [CrossRef]
- Lindsay, M.E. Medical management of aortic disease in children with Marfan syndrome. Curr. Opin. Pediatrics 2018, 30, 639–644. [Google Scholar] [CrossRef]
- Milewicz, D.M.; Prakash, S.K.; Ramirez, F. Therapeutics Targeting Drivers of Thoracic Aortic Aneurysms and Acute Aortic Dissections: Insights from Predisposing Genes and Mouse Models. Annu. Rev. Med. 2017, 68, 51–67. [Google Scholar] [CrossRef]
- Holm, T.M.; Habashi, J.P.; Doyle, J.J.; Bedja, D.; Chen, Y.; van Erp, C.; Lindsay, M.E.; Kim, D.; Schoenhoff, F.; Cohn, R.D.; et al. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 2011, 332, 358–361. [Google Scholar] [CrossRef]
- Habashi, J.P.; Doyle, J.J.; Holm, T.M.; Aziz, H.; Schoenhoff, F.; Bedja, D.; Chen, Y.; Modiri, A.N.; Judge, D.P.; Dietz, H.C. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science 2011, 332, 361–365. [Google Scholar] [CrossRef]
- Singh, M.N.; Lacro, R.V. Recent Clinical Drug Trials Evidence in Marfan Syndrome and Clinical Implications. Can. J. Cardiol. 2016, 32, 66–77. [Google Scholar] [CrossRef]
- Muthu, M.L.; Tiedemann, K.; Fradette, J.; Komarova, S.; Reinhardt, D.P. Fibrillin-1 regulates white adipose tissue development, homeostasis, and function. Matrix Biol. 2022, 110, 106–128. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Combs, M.D.; Knutsen, R.H.; Broekelmann, T.J.; Toennies, H.M.; Brett, T.J.; Miller, C.A.; Kober, D.L.; Craft, C.S.; Atkinson, J.J.; Shipley, J.M.; et al. Microfibril-associated glycoprotein 2 (MAGP2) loss of function has pleiotropic effects in vivo. J. Biol. Chem. 2013, 288, 28869–28880. [Google Scholar] [CrossRef]
- Mecham, R.P.; Gibson, M.A. The microfibril-associated glycoproteins (MAGPs) and the microfibrillar niche. Matrix Biol. 2015, 47, 13–33. [Google Scholar] [CrossRef]
- Hazlewood, R.J.; Chen, Q.; Clark, F.K.; Kuchtey, J.; Kuchtey, R.W. Differential effects of angiotensin II type I receptor blockers on reducing intraocular pressure and TGFbeta signaling in the mouse retina. PLoS ONE 2018, 13, e0201719. [Google Scholar] [CrossRef]
- Jensen, S.A.; Reinhardt, D.P.; Gibson, M.A.; Weiss, A.S. Protein interaction studies of MAGP-1 with tropoelastin and fibrillin-1. J Biol Chem 2001, 276, 39661–39666. [Google Scholar] [CrossRef] [PubMed]
- Penner, A.S.; Rock, M.J.; Kielty, C.M.; Shipley, J.M. Microfibril-associated glycoprotein-2 interacts with fibrillin-1 and fibrillin-2 suggesting a role for MAGP-2 in elastic fiber assembly. J. Biol. Chem. 2002, 277, 35044–35049. [Google Scholar] [CrossRef]
- Rock, M.J.; Cain, S.A.; Freeman, L.J.; Morgan, A.; Mellody, M.; Marson, A.; Shuttlrworth, C.A.; Weiss, A.S.; Kielty, C.M. Molecular basis of elastic fiber formation. Critical interactions and a tropoelastin-fibrillin-1 cross-link. J. Biol. Chem. 2004, 279, 23748–23758. [Google Scholar] [CrossRef]
- Sharma, T.; Gopal, L.; Shanmugam, M.P.; Bhende, P.S.; Agrawal, R.; Shetty, N.S.; Gopalakrishna, M.; Rao, M.K.; Balusamy, S. Retinal detachment in Marfan syndrome: Clinical characteristics and surgical outcome. Retina 2002, 22, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Maumenee, I.H. The eye in the Marfan syndrome. Trans. Am. Ophthalmol. Soc. 1981, 79, 684–733. [Google Scholar] [PubMed]
- Evain, B.; Langlois, M.; Francois, P. Retinal detachment and Marfan’s syndrome. Bull. Soc. D’ophtalmologie Fr. 1986, 86, 875–882. [Google Scholar]
- Nakamura, M.; Itoh, S.; Makita, S.; Ohira, A.; Arakawa, N.; Hiramori, K. Peripheral resistance vessel dysfunction in Marfan syndrome. Am. Heart J. 2000, 139, 661–666. [Google Scholar] [CrossRef]
- Syyong, H.T.; Chung, A.W.; Yang, H.H.; van Breemen, C. Dysfunction of endothelial and smooth muscle cells in small arteries of a mouse model of Marfan syndrome. Br. J. Pharmacol. 2009, 158, 1597–1608. [Google Scholar] [CrossRef]
- Sellers, S.L.; Milad, N.; Chan, R.; Mielnik, M.; Jermilova, U.; Huang, P.L.; de Crom, R.; Hirota, J.A.; Hogg, J.C.; Sandor, G.G.; et al. Inhibition of Marfan Syndrome Aortic Root Dilation by Losartan: Role of Angiotensin II Receptor Type 1-Independent Activation of Endothelial Function. Am. J. Pathol. 2018, 188, 574–585. [Google Scholar] [CrossRef]
- Vassequi-Silva, T.; Pereira, D.S.; Nery Diez, A.C.C.; Braga, G.G.; Godoy, J.A.; Mendes, C.B.; Dos Santos, L.; Krieger, J.E.; Antunes, E.; Costa, F.T.M.; et al. Losartan and captopril treatment rescue normal thrombus formation in microfibril associated glycoprotein-1 (MAGP1) deficient mice. Thromb. Res. 2016, 138, 7–15. [Google Scholar] [CrossRef]
- Watanabe, T.; Suzuki, J.; Yamawaki, H.; Sharma, V.K.; Sheu, S.S.; Berk, B.C. Losartan metabolite EXP3179 activates Akt and endothelial nitric oxide synthase via vascular endothelial growth factor receptor-2 in endothelial cells: Angiotensin II type 1 receptor-independent effects of EXP3179. Circulation 2005, 112, 1798. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Larson, J.D.; Ekker, S.C. Functional analysis of zebrafish microfibril-associated glycoprotein-1 (Magp1) in vivo reveals roles for microfibrils in vascular development and function. Blood 2006, 107, 4364–4374. [Google Scholar] [CrossRef] [PubMed]
- Craft, C.S.; Broekelmann, T.J.; Mecham, R.P. Microfibril-associated glycoproteins MAGP-1 and MAGP-2 in disease. Matrix Biol. 2018, 71–72, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Weinbaum, J.S.; Broekelmann, T.J.; Pierce, R.A.; Werneck, C.C.; Segade, F.; Craft, C.S.; Knutsen, R.H.; Mecham, R.P. Deficiency in microfibril-associated glycoprotein-1 leads to complex phenotypes in multiple organ systems. J. Biol. Chem. 2008, 283, 25533–25543. [Google Scholar] [CrossRef] [PubMed]
- Broekelmann, T.J.; Bodmer, N.K.; Mecham, R.P. Identification of the growth factor-binding sequence in the extracellular matrix protein MAGP-1. J. Biol. Chem. 2020, 295, 2687–2697. [Google Scholar] [CrossRef]
- Lee, J.J.; Galatioto, J.; Rao, S.; Ramirez, F.; Costa, K.D. Losartan Attenuates Degradation of Aorta and Lung Tissue Micromechanics in a Mouse Model of Severe Marfan Syndrome. Ann. Biomed Eng. 2016, 44, 2994–3006. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Martin, J.D.; Liu, H.; Lacorre, D.A.; Jain, S.R.; Kozin, S.V.; Stylianopoulos, T.; Mousa, A.S.; Han, X.; Adstamongkonkul, P.; et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 2013, 4, 2516. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso, F.; Li, L.; Fremaux, I.; Reinhardt, D.P.; Génot, E. Fibrillin-1 Regulates Arteriole Integrity in the Retina. Biomolecules 2022, 12, 1330. https://doi.org/10.3390/biom12101330
Alonso F, Li L, Fremaux I, Reinhardt DP, Génot E. Fibrillin-1 Regulates Arteriole Integrity in the Retina. Biomolecules. 2022; 12(10):1330. https://doi.org/10.3390/biom12101330
Chicago/Turabian StyleAlonso, Florian, Ling Li, Isabelle Fremaux, Dieter Peter Reinhardt, and Elisabeth Génot. 2022. "Fibrillin-1 Regulates Arteriole Integrity in the Retina" Biomolecules 12, no. 10: 1330. https://doi.org/10.3390/biom12101330
APA StyleAlonso, F., Li, L., Fremaux, I., Reinhardt, D. P., & Génot, E. (2022). Fibrillin-1 Regulates Arteriole Integrity in the Retina. Biomolecules, 12(10), 1330. https://doi.org/10.3390/biom12101330