Integrated Multi-Omics for Novel Aging Biomarkers and Antiaging Targets
Abstract
1. Introduction
2. The Necessity of Distinguishing Chronological Age and Biological Age
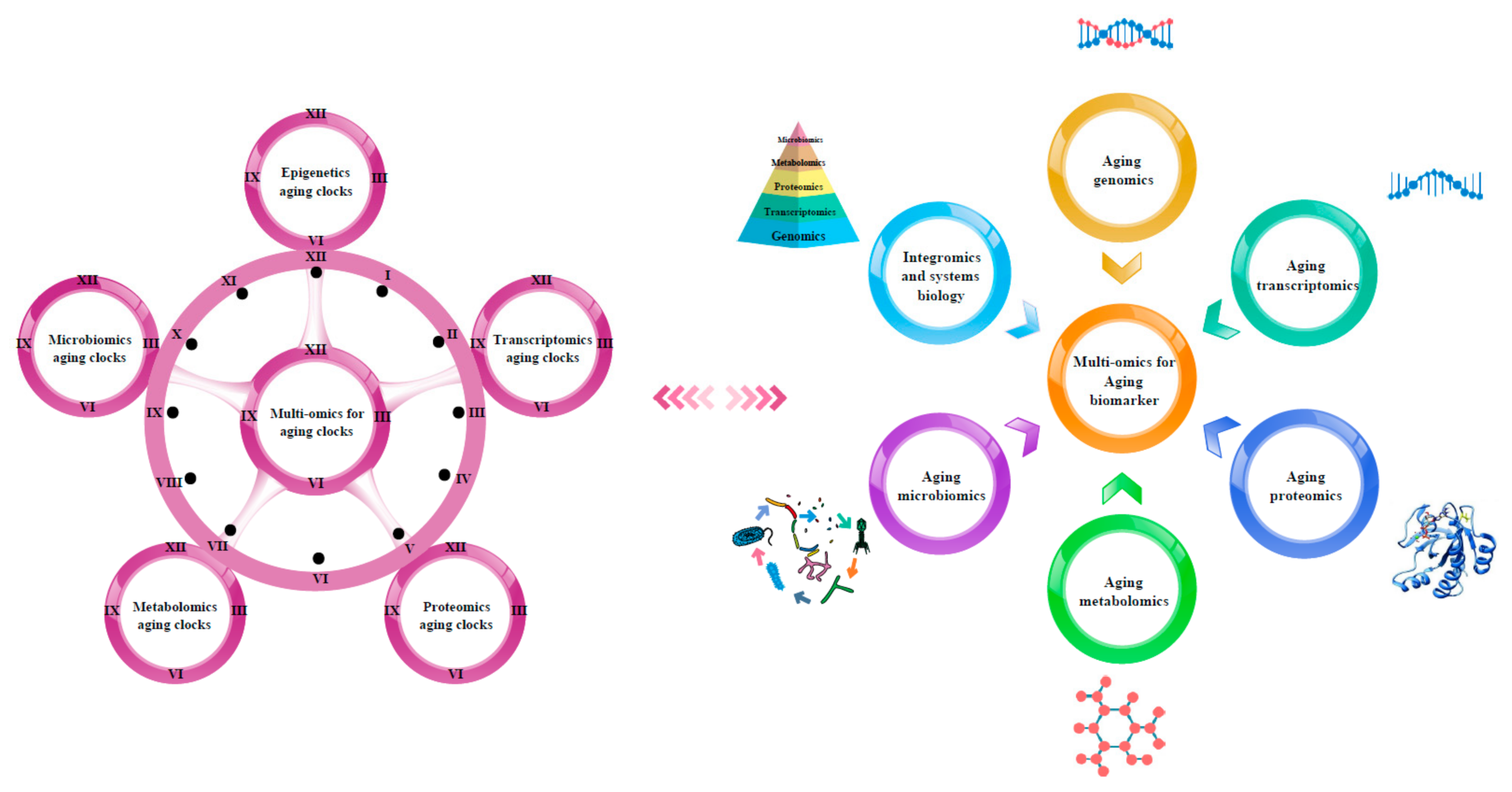
3. Multi-Omics for Aging Clocks
3.1. Epigenetics Aging Clocks
3.2. Transcriptomics Aging Clocks
3.3. Proteomics Aging Clocks
3.4. Metabolomics Aging Clocks
3.5. Microbiomics Aging Clocks
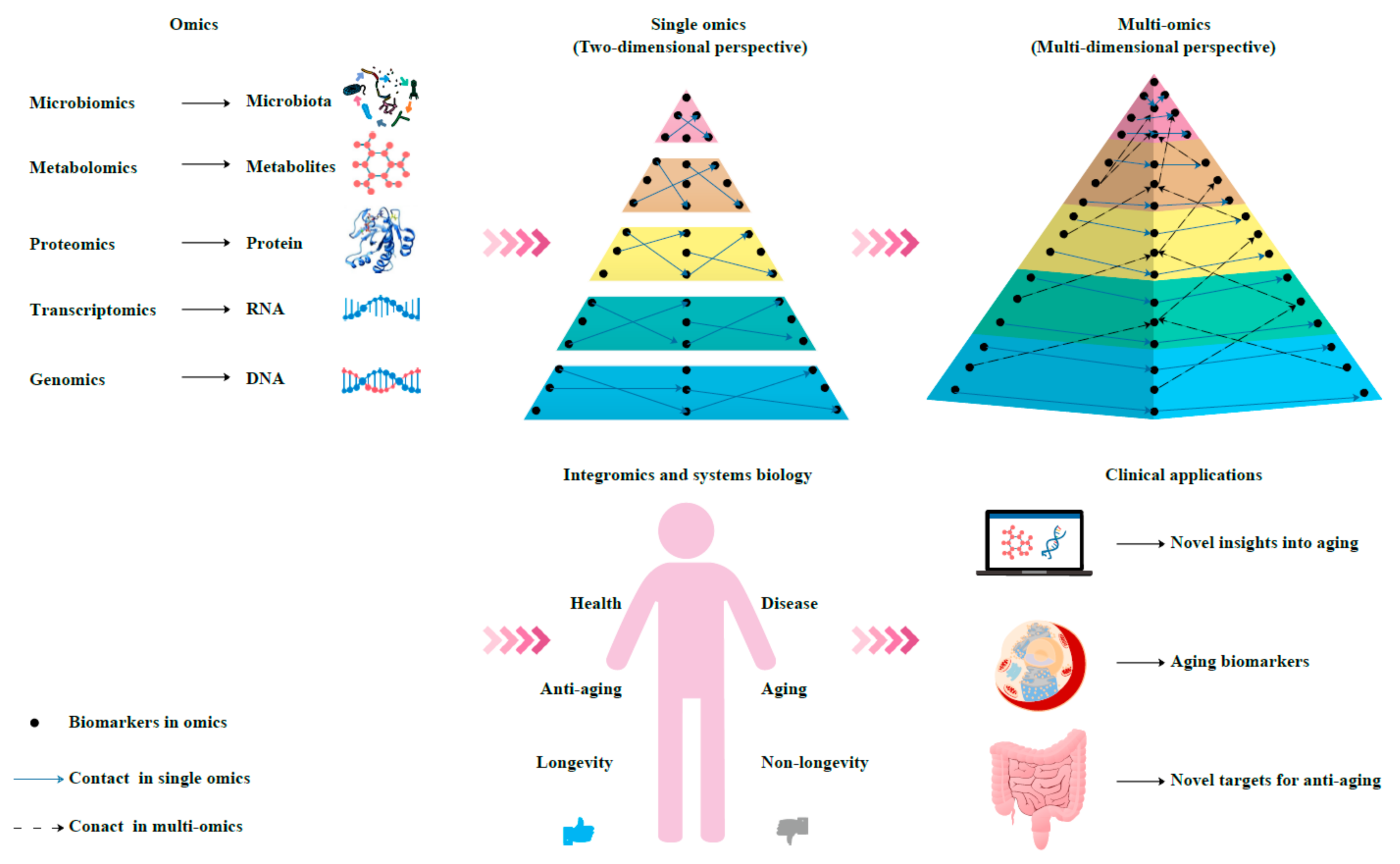
4. Multi-Omics Approach for the Discovery of Aging Biomarkers
4.1. Aging Genomics
4.1.1. Aging Epigenomics
4.1.2. Aging Gene Expression
4.1.3. Telomere-Based Biomarkers
4.2. Aging Transcriptomics
4.2.1. Transcriptomics-Based Biomarkers
4.2.2. MiRNAs, lncRNAs, and circRNAs-Based Biomarkers
4.3. Aging Proteomics
4.3.1. Proteomics-Based Biomarkers
4.3.2. Senescence-Associated Secretory Phenotype-Based Biomarkers
4.4. Aging Metabolomics
4.5. Aging Microbiomics
4.6. Early Biomarkers of Aging
5. Integromics and Systems Biology
6. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019: Highlights; ST/ESA/SER.A/423; United Nation: New York, NY, USA, 2019; Available online: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf (accessed on 21 January 2021).
- Carmona, J.J.; Michan, S. Biology of healthy aging and longevity. Rev. Investig. Clin. 2016, 68, 7–16. [Google Scholar]
- Tuttle, C.S.L.; Waaijer, M.E.C.; Slee-Valentijn, M.S.; Stijnen, T.; Westendorp, R.; Maier, A.B. Cellular senescence and chronological age in various human tissues: A systematic review and meta-analysis. Aging Cell 2020, 19, e13083. [Google Scholar] [CrossRef] [PubMed]
- Denic, A.; Glassock, R.J.; Rule, A.D. Structural and functional changes with the aging kidney. Adv. Chronic Kidney Dis. 2016, 23, 19–28. [Google Scholar] [CrossRef]
- Kim, I.H.; Kisseleva, T.; Brenner, D.A. Aging and liver disease. Curr. Opin. Gastroenterol. 2015, 31, 184–191. [Google Scholar] [CrossRef]
- Bernardes de Jesus, B.; Blasco, M.A. Potential of telomerase activation in extending health span and longevity. Curr. Opin. Cell Biol. 2012, 24, 739–743. [Google Scholar] [CrossRef]
- Gross, A.L.; Carlson, M.C.; Chu, N.M.; McAdams-DeMarco, M.A.; Mungas, D.; Simonsick, E.M. Derivation of a measure of physiological multisystem dysregulation: Results from WHAS and health ABC. Mech. Ageing Dev. 2020, 188, 111258. [Google Scholar] [CrossRef] [PubMed]
- Belloni, G.; Cesari, M. Frailty and intrinsic capacity: Two distinct but related constructs. Front. Med. 2019, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Zhavoronkov, A.; Mamoshina, P.; Vanhaelen, Q.; Scheibye-Knudsen, M.; Moskalev, A.; Aliper, A. Artificial intelligence for aging and longevity research: Recent advances and perspectives. Ageing Res. Rev. 2019, 49, 49–66. [Google Scholar] [CrossRef]
- Buerkle, A.; Moreno-Villanueva, M.; Bernhard, J.; Blasco, M.; Zondag, G.; Hoeijmakers, J.H.J.; Toussaint, O.; Grubeck-Loebenstein, B.; Mocchegiani, E.; Collino, S.; et al. MARK-AGE biomarkers of ageing. Mech. Ageing Dev. 2015, 151, 2–12. [Google Scholar] [CrossRef]
- Pilling, L.C.; Atkins, J.L.; Bowman, K.; Jones, S.E.; Tyrrell, J.; Beaumont, R.N.; Ruth, K.S.; Tuke, M.A.; Yaghootkar, H.; Wood, A.R.; et al. Human longevity is influenced by many genetic variants: Evidence from 75,000 UK Biobank participants. Aging 2016, 8, 547–563. [Google Scholar] [CrossRef]
- Zhavoronkov, A.; Buzdin, A.A.; Garazha, A.V.; Borisov, N.M.; Moskalev, A.A. Signaling pathway cloud regulation for in silico screening and ranking of the potential geroprotective drugs. Front. Genet. 2014, 5, 49. [Google Scholar] [CrossRef]
- Gott, A.; Andrews, C.; Hormigos, M.L.; Spencer, K.; Bateson, M.; Nettle, D. Chronological age, biological age, and individual variation in the stress response in the European starling: A follow-up study. PeerJ 2018, 6, e5842. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.J.; Wall, M.M.; Chen, C.; Levine, M.E.; Yaffe, K.; Roose, S.P.; Rutherford, B.R. Biological age, not chronological age, is associated with late-life depression. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, B.J.; Kang, H. Measurement of biological age may help to assess the risk of colorectal adenoma in screening colonoscopy. World J. Gastroenterol. 2017, 23, 6877–6883. [Google Scholar] [CrossRef]
- Cho, I.H.; Park, K.S.; Lim, C.J. An empirical comparative study on biological age estimation algorithms with an application of Work Ability Index (WAI). Mech. Ageing Dev. 2010, 131, 69–78. [Google Scholar] [CrossRef]
- Klemera, P.; Doubal, S. A new approach to the concept and computation of biological age. Mech. Ageing Dev. 2006, 127, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.J.; Han, L.L.; Liu, Q.; Shan, H.Y.; Lin, H.L.; Sun, X.F.; Chen, X.M. Evaluation of biological aging process—A population-based study of healthy people in china. Gerontology 2010, 56, 129–140. [Google Scholar] [CrossRef]
- Zhang, W.G.; Bai, X.J.; Sun, X.F.; Cai, G.Y.; Bai, X.Y.; Zhu, S.Y.; Zhang, M.; Chen, X.M. Construction of an integral formula of biological age for a healthy Chinese population using principle component analysis. J. Nutr. Health Aging 2014, 18, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Belsky, D.W.; Caspi, A.; Houts, R.; Cohen, H.J.; Corcoran, D.L.; Danese, A.; Harrington, H.; Israel, S.; Levine, M.E.; Schaefer, J.D. Quantification of biological aging in young adults. Proc. Natl. Acad. Sci. USA 2015, 112, E4104–E4110. [Google Scholar] [CrossRef] [PubMed]
- Mitnitski, A.; Howlett, S.E.; Rockwood, K. Heterogeneity of human aging and its assessment. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 877–884. [Google Scholar] [CrossRef]
- Horvath, S.; Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.B.; Gao, Y.; et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Leung, D.; Levine, M. Comparative analysis of epigenetic aging clocks from CpG characteristics to functional associations. bioRxiv 2019, 51, 512483. [Google Scholar] [CrossRef]
- Lin, Q.; Weidner, C.I.; Costa, I.G.; Marioni, R.E.; Ferreira, M.R.P.; Deary, I.J.; Wagner, W. DNA methylation levels at individual age-associated CpG sites can be indicative for life expectancy. Aging 2016, 8, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wong, A.; Kuh, D.; Paul, D.S.; Rakyan, V.K.; Leslie, R.D.; Zheng, S.J.C.; Widschwendter, M.; Beck, S.; Teschendorff, A. Correlation of an epigenetic mitotic clock with cancer risk. Genome Biol. 2016, 17, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Wilson, R.; Heiss, J.; Breitling, L.P.; Saum, K.U.; Schoettker, B.; Holleczek, B.; Waldenberger, M.; Peters, A.; Brenner, H. DNA methylation signatures in peripheral blood strongly predict all-cause mortality. Nat. Commun. 2017, 8, 14617. [Google Scholar] [CrossRef]
- Weidner, C.I.; Lin, Q.; Koch, C.M.; Eisele, L.; Beier, F.; Ziegler, P.; Bauerschlag, D.O.; Joeckel, K.H.; Erbel, R.; Muehleisen, T.W.; et al. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol. 2014, 15, R24. [Google Scholar] [CrossRef] [PubMed]
- Garagnani, P.; Bacalini, M.G.; Pirazzini, C.; Gori, D.; Giuliani, C.; Mari, D.; Di Blasio, A.M.; Gentilini, D.; Vitale, G.; Collino, S.; et al. Methylation of ELOVL2 gene as a new epigenetic marker of age. Aging Cell 2012, 11, 1132–1134. [Google Scholar] [CrossRef]
- Horvath, S.; Oshima, J.; Martin, G.M.; Lu, A.T.; Quach, A.; Cohen, H.; Felton, S.; Matsuyama, M.; Lowe, D.; Kabacik, S.; et al. Epigenetic clock for skin and blood cells applied to Hutchinson Gilford Progeria Syndrome and ex vivo studies. Aging 2018, 10, 1758–1775. [Google Scholar] [CrossRef] [PubMed]
- Bocklandt, S.; Lin, W.; Sehl, M.E.; Sanchez, F.J.; Sinsheimer, J.S.; Horvath, S.; Vilain, E. Epigenetic Predictor of Age. PLoS ONE 2011, 6, e14821. [Google Scholar] [CrossRef]
- Lu, A.T.; Quach, A.; Wilson, J.G.; Reiner, A.P.; Aviv, A.; Raj, K.; Hou, L.F.; Baccarelli, A.A.; Li, Y.; Stewart, J.D.; et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 2019, 11, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lemos, B. Ribosomal DNA harbors an evolutionarily conserved clock of biological aging. Genome Res. 2019, 29, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.J.; Joehanes, R.; Pilling, L.C.; Schurmann, C.; Conneely, K.N.; Powell, J.; Reinmaa, E.; Sutphin, G.L.; Zhernakova, A.; Schramm, K.; et al. The transcriptional landscape of age in human peripheral blood. Nat. Commun. 2015, 6, 9570. [Google Scholar] [CrossRef]
- Fleischer, J.G.; Schulte, R.; Tsai, H.H.; Tyagi, S.; Ibarra, A.; Shokhirev, M.N.; Huang, L.; Hetzer, M.; Navlakha, S. Predicting age from the transcriptome of human dermal fibroblasts. Genome Biol. 2018, 19, 221. [Google Scholar] [CrossRef] [PubMed]
- Putin, E.; Mamoshina, P.; Aliper, A.; Korzinkin, M.; Moskalev, A.; Kolosov, A.; Ostrovskiy, A.; Cantor, C.; Vijg, J.; Zhavoronkov, A. Deep biomarkers of human aging: Application of deep neural networks to biomarker development. Aging 2016, 8, 1021–1033. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, 1168. [Google Scholar] [CrossRef]
- Mamoshina, P.; Kochetov, K.; Putin, E.; Aliper, A.; Zhavoronkov, A. Testing for batch effect through age predictors. bioRxiv 2019, 531863. [Google Scholar] [CrossRef]
- Bathke, J.; Konzer, A.; Remes, B.; McIntosh, M.; Klug, G. Comparative analyses of the variation of the transcriptome and proteome of Rhodobacter sphaeroides throughout growth. BMC Genom. 2019, 20, 358. [Google Scholar] [CrossRef]
- Haider, S.; Pal, R. Integrated analysis of transcriptomic and proteomic data. Curr. Genom. 2013, 14, 91–110. [Google Scholar] [CrossRef]
- Tin, A.; Yu, B.; Ma, J.; Masushita, K.; Daya, N.; Hoogeveen, R.C.; Ballantyne, C.M.; Couper, D.; Rebholz, C.M.; Grams, M.E.; et al. Reproducibility and variability of protein analytes measured using a multiplexed modified aptamer assay. J. Appl. Lab. Med. 2019, 4, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Biancotto, A.; Moaddel, R.; Moore, A.Z.; Gonzalez-Freire, M.; Aon, M.A.; Candia, J.; Zhang, P.B.; Cheung, F.; Fantoni, G.; et al. Plasma proteomic signature of age in healthy humans. Aging Cell 2018, 17, e12799. [Google Scholar] [CrossRef]
- Johnson, A.A.; Shokhirev, M.N.; Wyss-Coray, T.; Lehallier, B. Systematic review and analysis of human proteomics aging studies unveils a novel proteomic aging clock and identifies key processes that change with age. Ageing Res. Rev. 2020, 60, 101070. [Google Scholar] [CrossRef]
- Lehallier, B.; Gate, D.; Schaum, N.; Nanasi, T.; Lee, S.E.; Yousef, H.; Losada, P.M.; Berdnik, D.; Keller, A.; Verghese, J.; et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 2019, 25, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J.; Friedrich, N.; Wittfeld, K.; Pietzner, M.; Budde, K.; Van der Auwera, S.; Lohmann, T.; Teumer, A.; Voelzke, H.; Nauck, M.; et al. Measuring biological age via metabonomics: The metabolic age score. J. Proteome Res. 2016, 15, 400–410. [Google Scholar] [CrossRef] [PubMed]
- van den Akker, E.B.; Trompet, S.; Wolf, J.J.H.B.; Beekman, M.; Suchiman, H.E.D.; Deelen, J. Predicting biological age based on the BBMRI-NL 1H-NMR metabolomics repository. bioRxiv 2019, 632919. [Google Scholar] [CrossRef]
- Choi, J.; Hur, T.Y.; Hong, Y. Influence of altered gut microbiota composition on aging and aging-related diseases. J. Lifestyle Med. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Galkin, F.; Mamoshina, P.; Aliper, A.; Putin, E.; Moskalev, V.; Gladyshev, V.N.; Zhavoronkov, A. Human gut microbiome aging clock based on taxonomic profiling and deep learning. iScience 2020, 23, 101199. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Woodmansey, E.J.; McMurdo, M.E.T.; Macfarlane, G.T.; Macfarlane, S. Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl. Environ. Microbiol. 2004, 70, 6113–6122. [Google Scholar] [CrossRef]
- Gonzalez-Bautista, E.; Andrieu, S.; Gutierrez-Robledo, L.M.; Garcia-Chanes, R.E.; De Souto Barreto, P. In the quest of a standard index of intrinsic capacity. A critical literature review. J. Nutr. Health Aging 2020, 24, 959–965. [Google Scholar] [CrossRef]
- Buck Louis, G.M.; Smarr, M.M.; Patel, C.J. The exposome research paradigm: An opportunity to understand the environmental basis for human health and disease. Curr. Environ. Health Rep. 2017, 4, 89–98. [Google Scholar] [CrossRef]
- Horvath, S.; Gurven, M.; Levine, M.E.; Trumble, B.C.; Kaplan, H.; Allayee, H.; Ritz, B.R.; Chen, B.; Lu, A.T.; Rickabaugh, T.M.; et al. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. 2016, 17, 171. [Google Scholar] [CrossRef] [PubMed]
- Day, K.; Waite, L.L.; Thalacker-Mercer, A.; West, A.; Bamman, M.M.; Brooks, J.D.; Myers, R.M.; Absher, D. Differential DNA methylation with age displays both common and dynamic features across human tissues that are influenced by CpG landscape. Genome Biol. 2013, 14, R102. [Google Scholar] [CrossRef]
- Horvath, S.; Zhang, Y.; Langfelder, P.; Kahn, R.S.; Boks, M.P.M.; van Eijk, K.; van den Berg, L.H.; Ophoff, R. Aging effects on DNA methylation modules in human brain and blood tissue. Genome Biol. 2012, 13, R97. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Ritz, B.R. Increased epigenetic age and granulocyte counts in the blood of Parkinson’s disease patients. Aging 2015, 7, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Hosgood, H.D.; Chen, B.; Absher, D.; Assimes, T.; Horvath, S. DNA methylation age of blood predicts future onset of lung cancer in the women’s health initiative. Aging 2015, 7, 690–700. [Google Scholar] [CrossRef]
- Vidal-Bralo, L.; Lopez-Golan, Y.; Gonzalez, A. Simplified assay for epigenetic age estimation in whole blood of adults. Front. Genet. 2016, 7, 126. [Google Scholar] [CrossRef]
- Seaborne, R.A.; Strauss, J.; Cocks, M.; Shepherd, S.; O’Brien, T.D.; van Someren, K.A.; Bell, P.G.; Murgatroyd, C.; Morton, J.P.; Stewart, C.E.; et al. Human skeletal muscle possesses an epigenetic memory of hypertrophy. Sci. Rep. 2018, 8, 1898. [Google Scholar] [CrossRef]
- Quach, A.; Levine, M.E.; Tanaka, T.; Lu, A.T.; Chen, B.H.; Ferrucci, L.; Ritz, B.; Bandinelli, S.; Neuhouser, M.; Beasley, J.; et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging 2017, 9, 419–446. [Google Scholar] [CrossRef]
- Giannakou, M.E.; Goss, M.; Junger, M.A.; Hafen, E.; Leevers, S.J.; Partridge, L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science 2004, 305, 361. [Google Scholar] [CrossRef]
- Bluher, M.; Kahn, B.B.; Kahn, C.R. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 2003, 299, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Soerensen, M.; Kruse, T.A.; Christensen, K.; Christiansen, L. A novel permutation test for case-only analysis identifies epistatic effects on human longevity in the FOXO gene family. Aging Cell 2013, 12, 690–694. [Google Scholar] [CrossRef]
- Mahley, R.W.; Huang, Y. Apolipoprotein E sets the stage: Response to injury triggers neuropathology. Neuron 2012, 76, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Tyner, S.D.; Venkatachalam, S.; Choi, J.; Jones, S.; Ghebranious, N.; Igelmann, H.; Lu, X.B.; Soron, G.; Cooper, B.; Brayton, C.; et al. p53 mutant mice that display early ageing-associated phenotypes. Nature 2002, 415, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H. Telomeres and telomerase: Their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005, 579, 859–862. [Google Scholar] [CrossRef]
- Honig, L.S.; Kang, M.S.; Cheng, R.; Eckfeldt, J.H.; Thyagarajan, B.; Leiendecker-Foster, C.; Province, M.A.; Sanders, J.L.; Perls, T.; Christensen, K.; et al. Heritability of telomere length in a study of long-lived families. Neurobiol. Aging 2015, 36, 2785–2790. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef]
- Boccardi, V.; Paolisso, G. Telomerase activation: A potential key modulator for human healths pan and longevity. Ageing Res. Rev. 2014, 15, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Strong, M.A.; Vidal-Cardenas, S.L.; Karim, B.; Yu, H.; Guo, N.; Greider, C.W. Phenotypes in mTERT(+/−) and mTERT(−/−) mice are due to short telomeres, not telomere-independent functions of telomerase reverse transcriptase. Mol. Cell. Biol. 2011, 31, 2369–2379. [Google Scholar] [CrossRef]
- Jaskelioff, M.; Muller, F.L.; Paik, J.H.; Thomas, E.; Jiang, S.; Adams, A.C.; Sahin, E.; Kost-Alimova, M.; Protopopov, A.; Cadinanos, J.; et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 2011, 469, 102–106. [Google Scholar] [CrossRef]
- Wang, Q.; Zhan, Y.; Pedersen, N.L.; Fang, F.; Hagg, S. Telomere length and all-cause mortality: A meta-analysis. Ageing Res. Rev. 2018, 48, 11–20. [Google Scholar] [CrossRef]
- Lapham, K.; Kvale, M.N.; Lin, J.; Connell, S.; Croen, L.A.; Dispensa, B.P.; Fang, L.; Hesselson, S.; Hoffmann, T.J.; Iribarren, C.; et al. Automated assay of telomere length measurement and informatics for 100,000 subjects in the genetic epidemiology research on adult health and aging (GERA) cohort. Genetics 2015, 200, 1061–1072. [Google Scholar] [CrossRef]
- Simoncini, T.; Hafezl-Moghadam, A.; Brazil, D.P.; Ley, K.; Chin, W.W.; Liao, J.K. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 2000, 407, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Austad, S.N. Why women live longer than men: Sex differences in longevity. Gend. Med. 2006, 3, 79–92. [Google Scholar] [CrossRef]
- Needham, B.L.; Rehkopf, D.; Adler, N.; Gregorich, S.; Lin, J.; Blackburn, E.H.; Epel, E. Leukocyte telomere length and mortality in the national health and nutrition examination survey, 1999-2002. Epidemiology 2015, 26, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Deelen, J.; Beekman, M.; Codd, V.; Trompet, S.; Broer, L.; Hagg, S.; Fischer, K.; Thijssen, P.E.; Suchiman, H.E.D.; Postmus, I.; et al. Leukocyte telomere length associates with prospective mortality independent of immune-related parameters and known genetic markers. Int. J. Epidemiol. 2014, 43, 878–886. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.; Franceschi, C. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or Foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef]
- van der Harst, P.; van der Steege, G.; de Boer, R.A.; Voors, A.A.; Hall, A.S.; Mulder, M.J.; van Gilst, W.; van Veldhuisen, D. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007, 49, 1459–1464. [Google Scholar] [CrossRef]
- Forero, D.A.; Gonzalez-Giraldo, Y.; Lopez-Quintero, C.; Castro-Vega, L.J.; Barreto, G.E.; Perry, G. Meta-analysis of telomere length in Alzheimer’s disease. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2016, 71, 1069–1073. [Google Scholar] [CrossRef]
- Forero, D.A.; Gonzalez-Giraldo, Y.; Lopez-Quintero, C.; Castro-Vega, L.J.; Barreto, G.E.; Perry, G. Telomere length in Parkinson’s disease: A meta-analysis. Exp. Gerontol. 2016, 75, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Panossian, L.A.; Porter, V.R.; Valenzuela, H.F.; Zhu, X.; Reback, E.; Masterman, D.; Cummings, J.L.; Effros, R.B. Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol. Aging 2003, 24, 77–84. [Google Scholar] [CrossRef]
- D’Mello, M.J.J.; Ross, S.A.; Briel, M.; Anand, S.S.; Gerstein, H.; Pare, G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: Systematic review and meta-analysis. Circ. Cardiovasc. Genet. 2015, 8, 82–90. [Google Scholar] [CrossRef]
- Dai, D.F.; Chiao, Y.A.; Marcinek, D.J.; Szeto, H.H.; Rabinovitch, P.S. Mitochondrial oxidative stress in aging and healthspan. Longev. Healthspan 2014, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Serra, V.; von Zglinicki, T.; Lorenz, M.; Saretzki, G. Extracellular superoxide dismutase is a major antioxidant in human fibroblasts and slows telomere shortening. J. Biol. Chem. 2003, 278, 6824–6830. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Trimarchi, J.R.; Smith, P.J.S.; Keefe, D.L. Mitochondrial dysfunction leads to telomere attrition and genomic instability. Aging Cell 2002, 1, 40–46. [Google Scholar] [CrossRef]
- Shay, J.W. Role of telomeres and telomerase in aging and cancer. Cancer Discov. 2016, 6, 584–593. [Google Scholar] [CrossRef]
- Savage, S.A.; Gadalla, S.M.; Chanock, S.J. The long and short of telomeres and cancer association studies. JNCI J. Natl. Cancer Inst. 2013, 105, 448–449. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mensa, E.; Latini, S.; Ramini, D.; Storci, G.; Bonafe, M.; Olivieri, F. The telomere world and aging: Analytical challenges and future perspectives. Ageing Res. Rev. 2019, 50, 27–42. [Google Scholar] [CrossRef]
- He, J.; Tu, C.; Liu, Y. Role of lncRNAs in aging and age-related diseases. Aging Med. 2018, 1, 158–175. [Google Scholar] [CrossRef]
- Huan, T.; Chen, G.; Liu, C.; Bhattacharya, A.; Rong, J.; Chen, B.H.; Seshadri, S.; Tanriverdi, K.; Freedman, J.E.; Larson, M.G.; et al. Age-associated microRNA expression in human peripheral blood is associated with all-cause mortality and age-related traits. Aging Cell 2018, 17, e12687. [Google Scholar] [CrossRef]
- Gupta, S.C.; Tripathi, Y.N. Potential of long non-coding RNAs in cancer patients: From biomarkers to therapeutic targets. Int. J. Cancer 2017, 140, 1955–1967. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, K.; Panda, A.; Kang, M.J.; Xu, J.; Selimyan, R.; Yoon, J.H.; Martindale, J.L.; De, S.; Wood, W.H.; Becker, K.G.; et al. Senescence-associated lncRNAs: Senescence-associated long noncoding RNAs. Aging Cell 2013, 12, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Frenk, S.; Houseley, J. Gene expression hallmarks of cellular ageing. Biogerontology 2018, 19, 547–566. [Google Scholar] [CrossRef]
- Westerterp, M.; Tsuchiya, K.; Tattersall, I.W.; Fotakis, P.; Bochem, A.E.; Molusky, M.M.; Ntonga, V.; Abramowicz, S.; Parks, J.S.; Welch, C.L.; et al. Deficiency of ATP-binding cassette transporters A1 and G1 in endothelial cells accelerates atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1328–1337. [Google Scholar] [CrossRef]
- D’Aquila, P.; Crocco, P.; De Rango, F.; Indiveri, C.; Bellizzi, D.; Rose, G.; Passarino, G. A genetic variant of ASCT2 hampers in vitro RNA splicing and correlates with human longevity. Rejuvenation Res. 2018, 21, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Klucken, J.; Buchler, C.; Orso, E.; Kaminski, W.E.; Porsch-Ozcurumez, M.; Liebisch, C.; Kapinsky, M.; Diederich, W.; Drobnik, W.; Dean, M.; et al. ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc. Natl. Acad. Sci. USA 2000, 97, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Balliu, B.; Durrant, M.; de Goede, O.; Abell, N.; Li, X.; Liu, B. Genetic dysregulation of gene expression and splicing during a ten-year period of human aging. bioRxiv 2019, 519520. [Google Scholar] [CrossRef]
- Nakamura, S.; Kawai, K.; Takeshita, Y.; Honda, M.; Takamura, T.; Kaneko, S.; Matoba, R.; Matsubara, K. Identification of blood biomarkers of aging by transcript profiling of whole blood. Biochem. Biophys. Res. Commun. 2012, 418, 313–318. [Google Scholar] [CrossRef]
- Harries, L.W.; Hernandez, D.; Henley, W.; Wood, A.R.; Holly, A.C.; Bradley-Smith, R.M.; Yaghootkar, H.; Dutta, A.; Murray, A.; Frayling, T.M.; et al. Human aging is characterized by focused changes in gene expression and deregulation of alternative splicing. Aging Cell 2011, 10, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Holly, A.C.; Melzer, D.; Pilling, L.C.; Henley, W.; Hernandez, D.G.; Singleton, A.B.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L.; Harries, L.W. Towards a gene expression biomarker set for human biological age. Aging Cell 2013, 12, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Dhahbi, J.M. Circulating small noncoding RNAs as biomarkers of aging. Ageing Res. Rev. 2014, 17, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, O.; Hinault, C.; Van Obberghen, E. MicroRNAs and metabolism crosstalk in energy homeostasis. Cell Metab. 2013, 18, 312–324. [Google Scholar] [CrossRef]
- Hayes, J.; Peruzzi, P.P.; Lawler, S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol. Med. 2014, 20, 460–469. [Google Scholar] [CrossRef]
- Jung, M.; Pfeifer, G.P. Aging and DNA methylation. BMC Biol. 2015, 13, 7. [Google Scholar] [CrossRef]
- Huan, T.; Rong, J.; Tanriverdi, K.; Meng, Q.; Bhattacharya, A.; McManus, D.D.; Joehanes, R.; Assimes, T.L.; McPherson, R.; Samani, N.J.; et al. Dissecting the roles of microRNAs in coronary heart disease via integrative genomic analyses. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Huan, T.; Rong, J.; Liu, C.; Zhang, X.; Tanriverdi, K.; Joehanes, R.; Chen, B.H.; Murabito, J.M.; Yao, C.; Courchesne, P.; et al. Genome-wide identification of microRNA expression quantitative trait loci. Nat. Commun. 2015, 6, 7601. [Google Scholar] [CrossRef]
- Shi, L.; Liao, J.; Liu, B.; Zeng, F.; Zhang, L. Mechanisms and therapeutic potential of microRNAs in hypertension. Drug Discov. Today 2015, 20, 1188–1204. [Google Scholar] [CrossRef]
- Iacomino, G.; Siani, A. Role of microRNAs in obesity and obesity-related diseases. Genes Nutr. 2017, 12, 23. [Google Scholar] [CrossRef]
- Feng, J.; Xing, W.; Xie, L. Regulatory roles of microRNAs in diabetes. Int. J. Mol. Sci. 2016, 17, 1729. [Google Scholar] [CrossRef] [PubMed]
- Noren Hooten, N.; Fitzpatrick, M.; Wood, W.H., III; De, S.; Ejiogu, N.; Zhang, Y.; Mattison, J.A.; Becker, K.G.; Zonderman, A.B.; Evans, M.K.; et al. Age-related changes in microRNA levels in serum. Aging 2013, 5, 725–740. [Google Scholar] [CrossRef] [PubMed]
- ElSharawy, A.; Keller, A.; Flachsbart, F.; Wendschlag, A.; Jacobs, G.; Kefer, N.; Brefort, T.; Leidinger, P.; Backes, C.; Meese, E.; et al. Genome-wide miRNA signatures of human longevity. Aging Cell 2012, 11, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Noren Hooten, N.; Abdelmohsen, K.; Gorospe, M.; Ejiogu, N.; Zonderman, A.B.; Evans, M.K. MicroRNA expression patterns reveal differential expression of target genes with age. PLoS ONE 2010, 5, e10724. [Google Scholar] [CrossRef]
- Kinser, H.E.; Pincus, Z. MicroRNAs as modulators of longevity and the aging process. Hum. Genet. 2020, 139, 291–308. [Google Scholar] [CrossRef]
- Eyileten, C.; Wicik, Z.; De Rosa, S.; Mirowska-Guzel, D.; Soplinska, A.; Indolfi, C.; Jastrzebska-Kurkowska, I.; Czlonkowska, A.; Postula, M. MicroRNAs as diagnostic and prognostic biomarkers in ischemic stroke-A comprehensive review and bioinformatic analysis. Cells 2018, 7, 249. [Google Scholar] [CrossRef]
- Roy-O’Reilly, M.A.; Ahnstedt, H.; Spychala, M.S.; Munshi, Y.; Aronowski, J.; Sansing, L.H.; McCullough, L.D. Aging exacerbates neutrophil pathogenicity in ischemic stroke. Aging 2020, 12, 436–461. [Google Scholar] [CrossRef]
- Li, X.; Khanna, A.; Li, N.; Wang, E. Circulatory miR-34a as an RNA-based, noninvasive biomarker for brain aging. Aging 2011, 3, 985–1002. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, F.; Spazzafumo, L.; Santini, G.; Lazzarini, R.; Albertini, M.C.; Rippo, M.R.; Galeazzi, R.; Abbatecola, A.M.; Marcheselli, F.; Monti, D.; et al. Age-related differences in the expression of circulating microRNAs: miR-21 as a new circulating marker of inflammaging. Mech. Ageing Dev. 2012, 133, 675–685. [Google Scholar] [CrossRef]
- Kumar, S.; Vijayan, M.; Reddy, P.H. MicroRNA-455-3p as a potential peripheral biomarker for Alzheimer’s disease. Hum. Mol. Genet. 2017, 26, 3808–3822. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, A.P.; Yin, X.; Reddy, P.H. Novel MicroRNA-455-3p and its protective effects against abnormal APP processing and amyloid beta toxicity in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2428–2440. [Google Scholar] [CrossRef] [PubMed]
- Fatica, A.; Bozzoni, I. Long non-coding RNAs: New players in cell differentiation and development. Nat. Rev. Genet. 2014, 15, 7–21. [Google Scholar] [CrossRef]
- Jin, L.; Song, Q.; Zhang, W.; Geng, B.; Cai, J. Roles of long noncoding RNAs in aging and aging complications. Biochim. Biophys Acta Mol. Basis Dis. 2019, 1865, 1763–1771. [Google Scholar] [CrossRef]
- Pereira Fernandes, D.; Bitar, M.; Jacobs, F.M.J.; Barry, G. Long non-coding RNAs in neuronal aging. Non-Coding RNA 2018, 4, 12. [Google Scholar] [CrossRef]
- Boon, R.A.; Hofmann, P.; Michalik, K.M.; Lozano-Vidal, N.; Berghaeuser, D.; Fischer, A.; Knau, A.; Jae, N.; Schuermann, C.; Dimmeler, S. Long noncoding RNA Meg3 controls endothelial cell aging and function implications for regenerative angiogenesis. J. Am. Coll. Cardiol. 2016, 68, 2589–2591. [Google Scholar] [CrossRef] [PubMed]
- Knupp, D.; Miura, P. CircRNA accumulation: A new hallmark of aging? Mech. Ageing Dev. 2018, 173, 71–79. [Google Scholar] [CrossRef]
- Alhasan, A.A.; Izuogu, O.G.; Al-Balool, H.H.; Steyn, J.S.; Evans, A.; Colzani, M.; Ghevaert, C.; Mountford, J.C.; Marenah, L.; Elliott, D.J.; et al. Circular RNA enrichment in platelets is a signature of transcriptome degradation. Blood 2016, 127, E1–E11. [Google Scholar] [CrossRef]
- Memczak, S.; Papavasileiou, P.; Peters, O.; Rajewsky, N. Identification and characterization of circular RNAs as a new class of putative biomarkers in human blood. PLoS ONE 2015, 10, e141214. [Google Scholar] [CrossRef]
- Koh, W.; Pan, W.; Gawad, C.; Fan, H.C.; Kerchner, G.A.; Wyss-Coray, T.; Blumenfeld, Y.J.; El-Sayed, Y.Y.; Quake, S.R. Noninvasive in vivo monitoring of tissue-specific global gene expression in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 7361–7366. [Google Scholar] [CrossRef] [PubMed]
- Bahn, J.H.; Zhang, Q.; Li, F.; Chan, T.M.; Lin, X.; Kim, Y.; Wong, D.T.W.; Xiao, X. The landscape of microRNA, piwi-interacting RNA, and circular RNA in human saliva. Clin. Chem. 2015, 61, 221–230. [Google Scholar] [CrossRef]
- Pan, T.; Sun, X.Q.; Liu, Y.X.; Li, H.; Deng, G.B.; Lin, H.H.; Wang, S. Heat stress alters genome-wide profiles of circular RNAs in Arabidopsis. Plant Mol. Biol. 2018, 96, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Rybak-Wolf, A.; Stottmeister, C.; Glazar, P.; Jens, M.; Pino, N.; Giusti, S.; Hanan, M.; Behm, M.; Bartok, O.; Ashwal-Fluss, R.; et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol. Cell 2015, 58, 870–885. [Google Scholar] [CrossRef]
- Fanciulli, A.; Wenning, G.K. Multiple-system atrophy. N. Engl. J. Med. 2015, 372, 249–263. [Google Scholar] [CrossRef]
- Pastushkova, L.K.; Kononikhin, A.S.; Tiys, E.S.; Dobrokhotov, I.V.; Ivanisenko, V.A.; Nikolaev, E.N.; Larina, I.M.; Popov, I.A. Characteristics of age-dependent changes in urine proteome in healthy men. Adv. Gerontol. 2016, 6, 123–128. [Google Scholar] [CrossRef]
- Diz, A.P.; Martinez-Fernandez, M.; Rolan-Alvarez, E. Proteomics in evolutionary ecology: Linking the genotype with the phenotype. Mol. Ecol. 2012, 21, 1060–1080. [Google Scholar] [CrossRef]
- Semba, R.D.; Zhang, P.; Zhu, M.; Fabbri, E.; Gonzalez-Freire, M.; Moaddel, R.; Geng-Spyropoulos, M.; Ferrucci, L. A targeted proteomic assay for the measurement of plasma proteoforms related to human aging phenotypes. Proteomics 2017, 17, 1600232. [Google Scholar] [CrossRef] [PubMed]
- Mehra, V.C.; Ramgolam, V.S.; Bender, J.R. Cytokines and cardiovascular disease. J. Leukoc. Biol. 2005, 78, 805–818. [Google Scholar] [CrossRef]
- Swardfager, W.; Lanctot, K.; Rothenburg, L.; Wong, A.; Cappell, J.; Herrmann, N. A meta-analysis of cytokines in Alzheimer’s disease. Biol. Psychiatry 2010, 68, 930–941. [Google Scholar] [CrossRef]
- Menni, C.; Kiddle, S.J.; Mangino, M.; Vinuela, A.; Psatha, M.; Steves, C.; Sattlecker, M.; Buil, A.; Newhouse, S.; Nelson, S.; et al. Circulating proteomic signatures of chronological age. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Santos-Lozano, A.; Valenzuela, P.L.; Llavero, F.; Lista, S.; Carrera-Bastos, P.; Hampel, H.; Pareja-Galeano, H.; Galvez, B.G.; Lopez, J.A.; Vazquez, J.; et al. Successful aging: Insights from proteome analyses of healthy centenarians. Aging 2020, 12, 3502–3515. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, P.; Federico, A.; Morris, M.; Gurinovich, A.; Tanaka, T.; Chandler, K.B.; Andersen, S.L.; Denis, G.; Costello, K.; Ferrucci, L.; et al. Protein signatures of centenarians and their offspring suggest centenarians age slower than other humans. Aging Cell 2021, 20, e13290. [Google Scholar] [CrossRef] [PubMed]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Martucci, M.; Chiariello, A.; Franceschi, C.; Salvioli, S. Mitochondria, immunosenescence and inflammaging: A role for mitokines? Semin. Immunopathol. 2020, 42, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Coppe, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Munoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Cellular senescence: Putting the paradoxes in perspective. Curr. Opin. Genet. Dev. 2011, 21, 107–112. [Google Scholar] [CrossRef]
- Rodier, F.; Coppe, J.P.; Patil, C.K.; Hoeijmakers, W.A.M.; Munoz, D.P.; Raza, S.R.; Freund, A.; Campeau, E.; Davalos, A.; Campisi, J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009, 11, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Orjalo, A.V.; Bhaumik, D.; Gengler, B.K.; Scott, G.K.; Campisi, J. Cell surface-bound IL-1 alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl. Acad. Sci. USA 2009, 106, 17031–17036. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Patil, C.K.; Campisi, J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. Embo J. 2011, 30, 1536–1548. [Google Scholar] [CrossRef]
- Laberge, R.M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.; et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015, 1548, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Watroba, M.; Szukiewicz, D. The role of sirtuins in aging and age-related diseases. Adv. Med. Sci. 2016, 61, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Watroba, M.; Dudek, I.; Skoda, M.; Stangret, A.; Rzodkiewicz, P.; Szukiewicz, D. Sirtuins, epigenetics and longevity. Ageing Res. Rev. 2017, 40, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res. Rev. 2012, 11, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef]
- Dang, W.; Steffen, K.K.; Perry, R.; Dorsey, J.A.; Johnson, F.B.; Shilatifard, A.; Kaeberlein, M.; Kennedy, B.K.; Berger, S.L. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature 2009, 459, 802–807. [Google Scholar] [CrossRef]
- Blackburn, E.H. Switching and signaling at the telomere. Cell 2001, 106, 661–673. [Google Scholar] [CrossRef]
- Jazwinski, S.M.; Yashin, A.I. Aging and health—A systems biology perspective. Introduction. Interdiscip. Top. Gerontol. 2015, 40, 7–12. [Google Scholar] [CrossRef][Green Version]
- Bjedov, I.; Partridge, L. A longer and healthier life with TOR down-regulation: Genetics and drugs. Biochem. Soc. Trans. 2011, 39, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Vanhooren, V.; Santos, A.N.; Voutetakis, K.; Petropoulos, I.; Libert, C.; Simm, A.; Gonos, E.S.; Friguet, B. Protein modification and maintenance systems as biomarkers of ageing. Mech. Ageing Dev. 2015, 151, 71–84. [Google Scholar] [CrossRef]
- Oien, D.B.; Moskovitz, J. Substrates of the methionine sulfoxide reductase system and their physiological relevance. Curr. Top. Dev. Biol. 2008, 80, 93–133. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S. Emerging insights into the metabolic alterations in aging using metabolomics. Metabolites 2019, 9, 301. [Google Scholar] [CrossRef]
- Wang, Z.; Bian, L.; Mo, C.; Shen, H.; Zhao, L.J.; Su, K.J.; Kukula, M.; Lee, J.T.; Armstrong, D.W.; Recker, R.; et al. Quantification of aminobutyric acids and their clinical applications as biomarkers for osteoporosis. Commun. Biol. 2020, 3, 39. [Google Scholar] [CrossRef]
- Jylhava, J.; Pedersen, N.L.; Hagg, S. Biological age predictors. EBioMedicine 2017, 21, 29–36. [Google Scholar] [CrossRef]
- Yaneske, E.; Angione, C. The poly-omics of ageing through individual-based metabolic modelling. BMC Bioinform. 2018, 19, 415–496. [Google Scholar] [CrossRef]
- Rivero-Segura, N.A.; Bello-Chavolla, O.Y.; Barrera-Vazquez, O.S.; Gutierrez-Robledo, L.M.; Gomez-Verjan, J.C. Promising biomarkers of human aging: In search of a multi-omics panel to understand the aging process from a multidimensional perspective. Ageing Res. Rev. 2020, 64, 101164. [Google Scholar] [CrossRef] [PubMed]
- Zierer, J.; Menni, C.; Kastenmuler, G.; Spector, T.D. Integration of ‘omics’ data in aging research: From biomarkers to systems biology. Aging Cell 2015, 14, 933–944. [Google Scholar] [CrossRef]
- Robinson, O.; Chadeau Hyam, M.; Karaman, I.; Climaco Pinto, R.; Ala-Korpela, M.; Handakas, E.; Fiorito, G.; Gao, H.; Heard, A.; Jarvelin, M.R.; et al. Determinants of accelerated metabolomic and epigenetic aging in a UK cohort. Aging Cell 2020, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Jove, M.; Mate, I.; Naudi, A.; Mota-Martorell, N.; Portero-Otin, M.; De la Fuente, M.; Pamplona, R. Human aging is a metabolome-related matter of gender. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C. The plasticity of aging: Insights from long-lived mutants. Cell 2005, 120, 449–460. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending Healthy Life Span-From Yeast to Humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Junnila, R.K.; List, E.O.; Berryman, D.E.; Murrey, J.W.; Kopchick, J.J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 2013, 9, 366–376. [Google Scholar] [CrossRef]
- Massudi, H.; Grant, R.; Braidy, N.; Guest, J.; Farnsworth, B.; Guillemin, G.J. Age-associated changes In oxidative stress and NAD(+) metabolism in human tissue. PLoS ONE 2012, 7, e42357. [Google Scholar] [CrossRef]
- Verdin, E. NAD(+) in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef]
- Mendelsohn, A.R.; Larrick, J.W. The NAD+/PARP1/SIRT1 axis in aging. Rejuvenation Res. 2017, 20, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; D’Amico, D.; Ropelle, E.R.; Lutolf, M.P.; Aebersold, R.; et al. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 2016, 352, 1436–1443. [Google Scholar] [CrossRef]
- Igarashi, M.; Miura, M.; Williams, E.; Jaksch, F.; Kadowaki, T.; Yamauchi, T.; Guarente, L. NAD(+) supplementation rejuvenates aged gut adult stem cells. Aging Cell 2019, 18, e12935. [Google Scholar] [CrossRef]
- Nacarelli, T.; Lau, L.; Fukumoto, T.; Zundell, J.; Fatkhutdinov, N.; Wu, S.; Aird, K.M.; Iwasaki, O.; Kossenkov, A.V.; Schultz, D.; et al. NAD(+) metabolism governs the proinflammatory senescence-associated secretome. Nat. Cell Biol. 2019, 21, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16(Ink4a)-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, J.; Purkayastha, S.; Tang, Y.; Zhang, H.; Yin, Y.; Li, B.; Liu, G.; Cai, D.S. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappa B and GnRH. Nature 2013, 497, 211–216. [Google Scholar] [CrossRef]
- Melendez, A.; Talloczy, Z.; Seaman, M.; Eskelinen, E.L.; Hall, D.H.; Levine, B. Autophagy genes are essential for dauer development and life-span extension in C-elegans. Science 2003, 301, 1387–1391. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, A.; Cumming, R.C.; Brech, A.; Isakson, P.; Schubert, D.R.; Finley, K.D. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy 2008, 4, 176–184. [Google Scholar] [CrossRef]
- Pyo, J.O.; Yoo, S.M.; Ahn, H.H.; Nah, J.; Hong, S.H.; Kam, T.I.; Jung, S.; Jung, Y.K. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat. Commun. 2013, 4, 2300. [Google Scholar] [CrossRef]
- Almajwal, A.M.; Alam, I.; Abulmeaty, M.; Razak, S.; Pawelec, G.; Alam, W. Intake of dietary advanced glycation end products influences inflammatory markers, immune phenotypes, and antiradical capacity of healthy elderly in a little-studied population. Food Sci. Nutr. 2020, 8, 1046–1057. [Google Scholar] [CrossRef]
- Grosskopf, A.; Simm, A. Carbohydrates in nutrition: Friend or foe? Z. Gerontol. Geriatr. 2020, 53, 290–294. [Google Scholar] [CrossRef]
- Chak, C.M.; Lacruz, M.E.; Adam, J.; Brandmaier, S.; Covic, M.; Huang, J.; Meisinger, C.; Tiller, D.; Prehn, C.; Adamski, J.; et al. Ageing investigation using two-time-point metabolomics data from KORA and CARLA studies. Metabolites 2019, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Deelen, J.; Kettunen, J.; Fischer, K.; van der Spek, A.; Trompet, S.; Kastenmueller, G.; Boyd, A.; Zierer, J.; van den Akker, E.B.; Ala-Korpela, M.; et al. A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nat. Commun. 2019, 10, 3346. [Google Scholar] [CrossRef] [PubMed]
- Kohler, I.; Verhoeven, A.; Derks, R.J.E.; Giera, M. Analytical pitfalls and challenges in clinical metabolomics. Bioanalysis 2016, 8, 1509–1532. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Morate, E.; Gimeno-Mallench, L.; Stromsnes, K.; Sanz-Ros, J.; Roman-Dominguez, A.; Parejo-Pedrajas, S.; Ingles, M.; Olaso, G.; Gambini, J.; Mas-Bargues, C. Relationship between diet, microbiota, and healthy aging. Biomedicines 2020, 8, 287. [Google Scholar] [CrossRef]
- Salvador Barrera-Vazquez, O.; Carlos Gomez-Verjan, J. The unexplored world of human virome, mycobiome, and archaeome in aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, 1834–1837. [Google Scholar] [CrossRef]
- Aleman, F.D.D.; Valenzano, D.R. Microbiome evolution during host aging. PLoS Pathog. 2019, 15, e1007727. [Google Scholar] [CrossRef]
- Finlay, B.B.; Pettersson, S.; Melby, M.K.; Bosch, T.C.G. The microbiome mediates environmental effects on aging. BioEssays 2019, 41, e1800257. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The gut microbiome, aging, and longevity: A systematic review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108, 4586–4591. [Google Scholar] [CrossRef]
- Thomas, V.; Clark, J.; Dore, J. Fecal microbiota analysis: An overview of sample collection methods and sequencing strategies. Future Microbiol. 2015, 10, 1485–1504. [Google Scholar] [CrossRef]
- Kumar, M.; Babaei, P.; Ji, B.; Nielsen, J. Human gut microbiota and healthy aging: Recent developments and future prospective. Nutr. Healthy Aging 2016, 4, 3–16. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef]
- Hopkins, M.J.; Sharp, R.; Macfarlane, G.T. Variation in human intestinal microbiota with age. Dig. Liver Dis. 2002, 34, S12–S18. [Google Scholar] [CrossRef]
- Vaiserman, A.M.; Koliada, A.K.; Marotta, F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017, 35, 36–45. [Google Scholar] [CrossRef]
- O’Hagan, C.; Li, J.V.; Marchesi, J.R.; Plummer, S.; Garaiova, I.; Good, M.A. Long-term multi-species Lactobacillus and Bifidobacterium dietary supplement enhances memory and changes regional brain metabolites in middle-aged rats. Neurobiol. Learn. Mem. 2017, 144, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Giacosa, A.; Faliva, M.A.; Perna, S.; Allieri, F.; Castellazzi, A.M. Review on microbiota and effectiveness of probiotics use in older. World J. Clin. Cases 2015, 3, 156–162. [Google Scholar] [CrossRef]
- Han, B.; Sivaramakrishnan, P.; Lin, C.C.J.; Neve, I.A.A.; He, J.; Tay, L.W.R.; Sowa, J.N.; Sizovs, A.; Du, G.W.; Wang, J.; et al. Microbial genetic composition tunes host longevity. Cell 2017, 169, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Wilms, E.; Masclee, A.A.M.; Smidt, H.; Zoetendal, E.G.; Jonkers, D. Age-dependent changes in GI physiology and microbiota: Time to reconsider? Gut 2018, 67, 2213–2222. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Wu, G.D.; Lewis, J.D. Analysis of the human gut microbiome and association with disease. Clin. Gastroenterol. Hepatol. 2013, 11, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu. Rev. Med. 2011, 62, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Schneiderhan, J.; Master-Hunter, T.; Locke, A. Targeting gut flora to treat and prevent disease. J. Fam. Pract. 2016, 65, 33–38. [Google Scholar]
- Rodriguez-Castano, G.P.; Caro-Quintero, A.; Reyes, A.; Lizcano, F. Advances in gut microbiome research, opening new strategies to cope with a western lifestyle. Front. Genet. 2017, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Candela, M.; Biagi, E.; Brigidi, P.; O’Toole, P.W.; De Vos, W.M. Maintenance of a healthy trajectory of the intestinal microbiome during aging: A dietary approach. Mech. Ageing Dev. 2014, 136, 70–75. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Buford, T.W. (Dis)Trust your gut: The gut microbiome in age-related inflammation, health, and disease. Microbiome 2017, 5, 80. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.; et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 2017, 21, 455–466. [Google Scholar] [CrossRef]
- Rojas-Gutierrez, E.; Munoz-Arenas, G.; Trevino, S.; Espinosa, B.; Chavez, R.; Rojas, K.; Flores, G.; Diaz, A.; Guevara, J. Alzheimer’s disease and metabolic syndrome: A link from oxidative stress and inflammation to neurodegeneration. Synapse 2017, 71, e21990. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y.; Dore, J.; Schiffrina, E.J. The inflammatory status of old age can be nurtured from the intestinal environment. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pena, C.; Alvarez-Cisneros, T.; Quiroz-Baez, R.; Friedland, R.P. Microbiota and aging: A review and commentary. Arch. Med. Res. 2017, 48, 681–689. [Google Scholar] [CrossRef]
- Rojo, D.; Mendez-Garcia, C.; Raczkowska, B.A.; Bargiela, R.; Moya, A.; Ferrer, M.; Barbas, C. Exploring the human microbiome from multiple perspectives: Factors altering its composition and function. FEMS Microbiol. Rev. 2017, 41, 453–478. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Ventura, M.; Butto, L.F.; Duranti, S.; O’Toole, P.W.; Motherway, M.O.C.; van Sinderen, D. Molecular dialogue between the human gut microbiota and the host: A Lactobacillus and Bifidobacterium perspective. Cell. Mol. Life Sci. 2014, 71, 183–203. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Biagi, E.; Rampelli, S.; Turroni, S.; Quercia, S.; Candela, M.; Brigidi, P. The gut microbiota of centenarians: Signatures of longevity in the gut microbiota profile. Mech. Ageing Dev. 2017, 165, 180–184. [Google Scholar] [CrossRef]
- Kogut, M.H.; Lee, A.; Santin, E. Microbiome and pathogen interaction with the immune system. Poult. Sci. 2020, 99, 1906–1913. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, S.; Sanchez-Carrillo, S.; Ciordia, S.; Mena, M.C.; Mendez-Garcia, C.; Rojo, D.; Bargiela, R.; Zubeldia-Varela, E.; Martinez-Martinez, M.; Barbas, C.; et al. Functional microbiome deficits associated with ageing: Chronological age threshold. Aging Cell 2020, 19, e13063. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Chavez, L.A.; Roldan-Roldan, G.; Garcia-Juarez, B.; Gonzalez-Esquivel, D.; Perez de la Cruz, G.; Pineda, B.; Ramirez-Ortega, D.; Munoz, I.G.; Herrera, B.J.; Rios, C.; et al. Low serum tryptophan levels as an indicator of global cognitive performance in nondemented women over 50 years of age. Oxidative Med. Cell. Longev. 2018, 2018, 8604718. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.J.; Yin, Y.L. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell. Infect. Microbiol. 2018, 8, e56564. [Google Scholar] [CrossRef]
- Collino, S.; Montoliu, I.; Martin, F.P.J.; Scherer, M.; Mari, D.; Salvioli, S.; Bucci, L.; Ostan, R.; Monti, D.; Biagi, E.; et al. Metabolic signatures of extreme longevity in northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS ONE 2013, 8, e56564. [Google Scholar] [CrossRef]
- Mace, J.L.; Porter, R.J.; Dalrymple-Alford, J.C.; Wesnes, K.A.; Anderson, T.J. Effects of acute tryptophan depletion on neuropsychological and motor function in Parkinson’s disease. J. Psychopharmacol. 2010, 24, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Cisek, K.; Krochmal, M.; Klein, J.; Mischak, H. The application of multi-omics and systems biology to identify therapeutic targets in chronic kidney disease. Nephrol. Dial. Transplant. 2016, 31, 2003–2011. [Google Scholar] [CrossRef]
- Montaner, J.; Ramiro, L.; Simats, A.; Tiedt, S.; Makris, K.; Jickling, G.C.; Debette, S.; Sanchez, J.C.; Bustamante, A. Multilevel omics for the discovery of biomarkers and therapeutic targets for stroke. Nat. Rev. Neurol. 2020, 16, 247–264. [Google Scholar] [CrossRef]
- de Tayrac, M.; Le, S.; Aubry, M.; Mosser, J.; Husson, F. Simultaneous analysis of distinct Omics data sets with integration of biological knowledge: Multiple Factor Analysis approach. BMC Genom. 2009, 10, 32. [Google Scholar] [CrossRef]
- West, L.; Vidwans, S.J.; Campbell, N.P.; Shrager, J.; Simon, G.R.; Bueno, R.; Dennis, P.A.; Otterson, G.A.; Salgia, R. A novel classification of lung cancer into molecular subtypes. PLoS ONE 2012, 7, e31906. [Google Scholar] [CrossRef]
- Collisson, E.A.; Sadanandam, A.; Olson, P.; Gibb, W.J.; Truitt, M.; Gu, S.; Cooc, J.; Weinkle, J.; Kim, G.E.; Jakkula, L.; et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 2011, 17, 500–503. [Google Scholar] [CrossRef]
- Higdon, R.; Earl, R.K.; Stanberry, L.; Hudac, C.M.; Montague, E.; Stewart, E.; Janko, I.; Choiniere, J.; Broomall, W.; Kolker, N.; et al. The promise of multi-omics and clinical data integration to identify and target personalized healthcare approaches in autism spectrum disorders. Omics—J. Integr. Biol. 2015, 19, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Solovev, I.; Shaposhnikov, M.; Moskalev, A. Multi-omics approaches to human biological age estimation. Mech. Ageing Dev. 2020, 185, 111192. [Google Scholar] [CrossRef]
- Ahadi, S.; Zhou, W.; Schussler-Fiorenza Rose, S.M.; Sailani, M.R.; Contrepois, K.; Avina, M.; Ashland, M.; Brunet, A.; Snyder, M. Personal aging markers and ageotypes revealed by deep longitudinal profiling. Nat. Med. 2020, 26, 83–90. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Mitteldorf, J. A clinical trial using methylation age to evaluate current antiaging practices. Rejuvenation Res. 2019, 22, 201–209. [Google Scholar] [CrossRef]
- Soriano-Tarraga, C.; Giralt-Steinhauer, E.; Mola-Caminal, M.; Ois, A.; Rodriguez-Campello, A.; Cuadrado-Godia, E.; Fernandez-Cadenas, I.; Cullell, N.; Roquer, J.; Jimenez-Conde, J. Biological age is a predictor of mortality in ischemic stroke. Sci. Rep. 2018, 8, 4148. [Google Scholar] [CrossRef]
- Cole, J.H.; Ritchie, S.J.; Bastin, M.E.; Hernandez, M.C.V.; Maniega, S.M.; Royle, N.; Corley, J.; Pattie, A.; Harris, S.E.; Zhang, Q.; et al. Brain age predicts mortality. Mol. Psychiatry 2018, 23, 1385–1392. [Google Scholar] [CrossRef]
- Tat-Thang, V.; Vivot, A.; Porcher, R. Impact of biomarker-based design strategies on the risk of false-positive findings in targeted therapy evaluation. Clin. Cancer Res. 2018, 24, 6257–6264. [Google Scholar] [CrossRef]
- Yao, M.; Wang, L.; Yao, Y.; Gu, H.B.; Yao, D.F. Biomarker-based microRNA therapeutic strategies for hepatocellular carcinoma. J. Clin. Transl. Hepatol. 2014, 2, 253–258. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blennow, K. Biomarkers in Alzheimer’s disease drug development. Nat. Med. 2010, 16, 1218–1222. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Howell, P.R.; Anderson, R.M. Aging and caloric restriction research: A biological perspective with translational potential. EBioMedicine 2017, 21, 37–44. [Google Scholar] [CrossRef] [PubMed]
| Omics | Biomarkers | Function/Application | References |
|---|---|---|---|
| Genomics | DNA methylation aging clocks | Biological age estimation method | [38] |
| DNA methylation GrimAge | Been correlation with diseases and can predict mortality | [57] | |
| DNAm pattern of 353 CpG sites | Estimate physiological aging | [56] | |
| 73 CpG sites | Immune system | [23,24] | |
| 10 CpG sites | Predictor of cancer mortality and cardiovascular disease | [28] | |
| The increase in DNAmAge | Cancer, age-related cartilage degenerative diseases, and tumor tissues | [58,59] | |
| Forkhead box O3 gene (FOXO3) | Related to prolonged lifespan | [62,63,64] | |
| The apolipoprotein E gene (APOE) | Regulation of the cholesterol and lipid metabolism and cell repair | [65] |
| Omics | Biomarkers | Function/Application | References |
|---|---|---|---|
| Transcriptomics | Transcriptomics aging clocks | Predictors of age | [35] |
| Transcriptome aging of skin fibroblasts | Determining the biological age | [36] | |
| The number of ABCG1 | Determines human lifespan | [96,97] | |
| BIRC2 gene | An apoptosis regulator of inflammation, cell proliferation and mitotic kinase signal transduction | [99] | |
| The expression of 11 genes (AMZ1, MANEAL, PARP3, KIAA0408, ISM1, CRIP1, NEFL, PHLDA3, DDB2, CHN1, CAPN2) | Positively correlated with aging | [100] | |
| The expression of 4 genes (MXRA8, SLC4A10, CD248, and PLEKHA7) | Negatively correlated with aging | [100] | |
| miR-22-3p and miR-28-3p | Positively correlated with age | [92] | |
| miR-425-3p, miR-182-5p, miR-99b-5p, etc. | Negatively correlated with age | [92] | |
| miR-181a, miR-434-3p, miR-431, miR-29, and miR-126 | In sarcopenia | [115] | |
| miR-19a-3p | A biomarker for ischemic stroke | [116] | |
| the expression of miR-34a | Associated with human hearing loss | [118] | |
| miR-21 | A potential biomarker of inflammation | [119] | |
| miR455-3p | As early biomarkers of AD | [120,121] | |
| lncRNAs | Provide different regulatory layers in the cell aging process, which can be used to intervene in this process | [124] | |
| Downregulation of lncRNA | Lung adenocarcinoma transcript 1 associated with metastasis in proliferating cells induces decreased cell growth | [94] | |
| Telomere-lncRNA | Can regulate the telomerase activity and survival rate of neural stem cells during aging | [124] | |
| Age-related lncRNA expression disorders | May affect neurogenesis and synaptic plasticity processes | [124] | |
| Meg3 | Related to cardiovascular aging | [125] | |
| CircRNAs | May be valuable biomarkers in the aging brain | [126] | |
| Multiple circRNAs are upregulated | In multiple system atrophy (MSA), which is a sporadic neurodegenerative disease | [133] |
| Omics | Biomarkers | Function/Application | References |
|---|---|---|---|
| Proteomics | Proteomics aging clocks | Accurately predict the age of a person | [44] |
| GDF15, PTN, ADAMTS5, FSHB, SOST, CHRDL1, NPPB, EFEMP1, MMP12, and CTSV | Related to aging | [43] | |
| LGALS3BP, MASP2, DNASE1, ANPEP, IGFBP1, etc. | Assess the rate of aging | [134] | |
| Circulating peptides (GDF8 and GDF11 pro-peptides and GDF8 and GDF11 mature proteins) and proteins | Be related to the accelerated dominant aging phenotype, and they are all involved in the inflammatory process | [136] | |
| CLEC3B, CRISP3, IGFAS, TAS1R3, and TGFBI | Be related to healthy aging | [140] | |
| AOPEP, CD14, CDKL1, and CRTAC1 | Be related to nonhealthy aging | [140] | |
| Serine protease inhibitors, SCT1, and GDF15 | As biomarkers of aging | [142] | |
| GDF15 | A promising biomarker of aging | [143] | |
| Sirtuins | Affecting genome stability, inflammation alleviation, metabolic homeostasis, lifespan, and health maintenance | [150,151] | |
| The NF-κB signaling pathway | Regulating the expression of IL-6 and IL-8 | [147] | |
| AMP-activated protein kinase (AMPK) | Affecting animal and human lifespan and health | [152] | |
| Telomerase | Counteract telomere shortening associated with the cell cycle | [155] | |
| Methionine sulfoxide | A marker of biological aging | [158] | |
| Methionine sulfoxide reductase | Protect the cell from biological oxidative stress | [159] |
| Omics | Biomarkers | Function/Application | References |
|---|---|---|---|
| Metabolomics | CoA catabolism, vitamin E metabolism, lysine metabolism, tryptophan metabolism, tyrosine metabolism, etc. | Related to aging | [166] |
| Monoacylglycerides, diacylglycerols and phosphoserine, etc. | Show a decreasing trend with age | [167] | |
| The product of proteolysis and l-γ-glutamyl-l-leucine | Increases independently of gender during aging | [167] | |
| 25-hydroxy-hexanoic acid, eicosapentaenoic acid, phosphoserine, etc. | Show a negative trend in the elderly | [167] | |
| Nicotinamide adenine dinucleotide (NAD+) | Plays a vital role in mitochondrial electron transport. can help maintain health and extend the life of mice | [171,172,173] | |
| Higher advanced glycation end products (AGEs) levels | Suffered from oxidative damage, leading to immune aging | [182,183] | |
| Metabolic profile (polyunsaturated fatty acids/total fatty acids, histidine, leucine, etc.) | May be an indirect predictor of mortality related to clinical trials and medical decision-making | [185] | |
| Inhibiting the activity of NF-κB | Extends the life of fruit fly and mouse | [177,178] | |
| The autophagy–lysosomal signaling pathway | Maintain the normal cell functions and extend the lifespan | [179,180,181] |
| Omics | Biomarkers | Function/Application | References |
|---|---|---|---|
| Microbiomics | The abundance of Bifidobacterium, Bacteroides, Lactobacillus, Ruminococcus, and Bacillus decreased, while the number of Streptococcus, Enterobacter, Clostridium, and Escherichia increased | During the aging process | [49] |
| The ratio of Firmicutes to Bacteroidetes | Can be used as a criterion for metabolic health, and the ratio will decrease with age | [200] | |
| Bacteroides, Ruminococcus, Faecalibacterium, Coprococcus, Parabacteroides, Clostridium, Alistipes, etc. | Bacteria with anti-inflammatory and immunomodulatory effects | [216,217] | |
| Christensenellaceae, along with Akkermansia and Lactobacillus | Promote immune regulation, defend against inflammation, and promote healthy metabolic homeostasis | [218,219] | |
| Christensenellaceae, Akkermansia, Bifidobacterium | Associated with immunological and metabolic health | [220] | |
| Decrease in Blautia, Coprococcus, Roseburia, and Faecalibacterium and significant increase in Desulfovibrionaceae and Enterobacteriaceae | Linked to longevity | [220] | |
| Akkermansia, Lactobacillus, and Christensenellaceae | Longevity-related strains play an antioxidant role in humans, which helps achieve healthy aging and longevity | In our study |
| Omics | Biomarkers | Function/Application | References |
|---|---|---|---|
| Integromics and systems biology | The method of comprehensive analysis of different omics data | This method combines experimental data of multiple omics levels with computational models and analyzes them as a whole to identify valuable data | [227] |
| Multi-factor analysis or partial least square regression analysis | Can identify the main sources of data differences | [228,229] | |
| Multi-omics methods | Used for disease identification and personalized treatment in cancer | [230,231] | |
| Multi-omics and integration with clinical data | Used as a way to accelerate precision medicine and personalized medicine | [232] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Xie, X.; Liang, T.; Ma, J.; Yang, L.; Yang, J.; Li, L.; Xi, Y.; Li, H.; Zhang, J.; et al. Integrated Multi-Omics for Novel Aging Biomarkers and Antiaging Targets. Biomolecules 2022, 12, 39. https://doi.org/10.3390/biom12010039
Wu L, Xie X, Liang T, Ma J, Yang L, Yang J, Li L, Xi Y, Li H, Zhang J, et al. Integrated Multi-Omics for Novel Aging Biomarkers and Antiaging Targets. Biomolecules. 2022; 12(1):39. https://doi.org/10.3390/biom12010039
Chicago/Turabian StyleWu, Lei, Xinqiang Xie, Tingting Liang, Jun Ma, Lingshuang Yang, Juan Yang, Longyan Li, Yu Xi, Haixin Li, Jumei Zhang, and et al. 2022. "Integrated Multi-Omics for Novel Aging Biomarkers and Antiaging Targets" Biomolecules 12, no. 1: 39. https://doi.org/10.3390/biom12010039
APA StyleWu, L., Xie, X., Liang, T., Ma, J., Yang, L., Yang, J., Li, L., Xi, Y., Li, H., Zhang, J., Chen, X., Ding, Y., & Wu, Q. (2022). Integrated Multi-Omics for Novel Aging Biomarkers and Antiaging Targets. Biomolecules, 12(1), 39. https://doi.org/10.3390/biom12010039






