Afamin Levels and Their Correlation with Oxidative and Lipid Parameters in Non-diabetic, Obese Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Sample Collection and Laboratory Measurements
2.3. Determination of Serum Afamin Level
2.4. Measurement of Serum Oxidized LDL Concentration
2.5. Measurement of Serum α- and γ-Tocopherol Levels by Gas Chromatography-Mass Spectrometry
2.6. HDL Subfraction Analysis
2.7. LDL Subfraction Analysis
2.8. Statistical Analysis
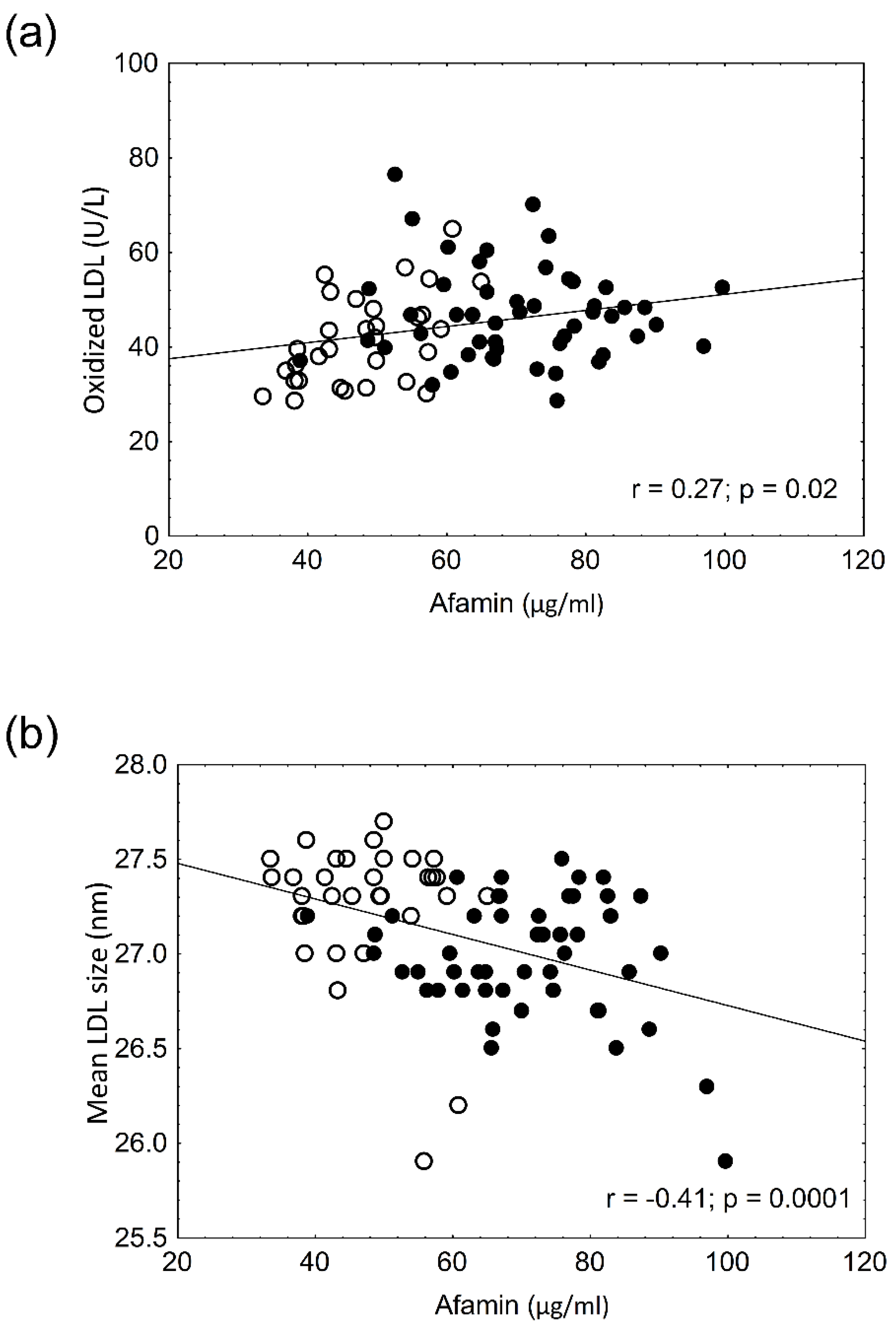
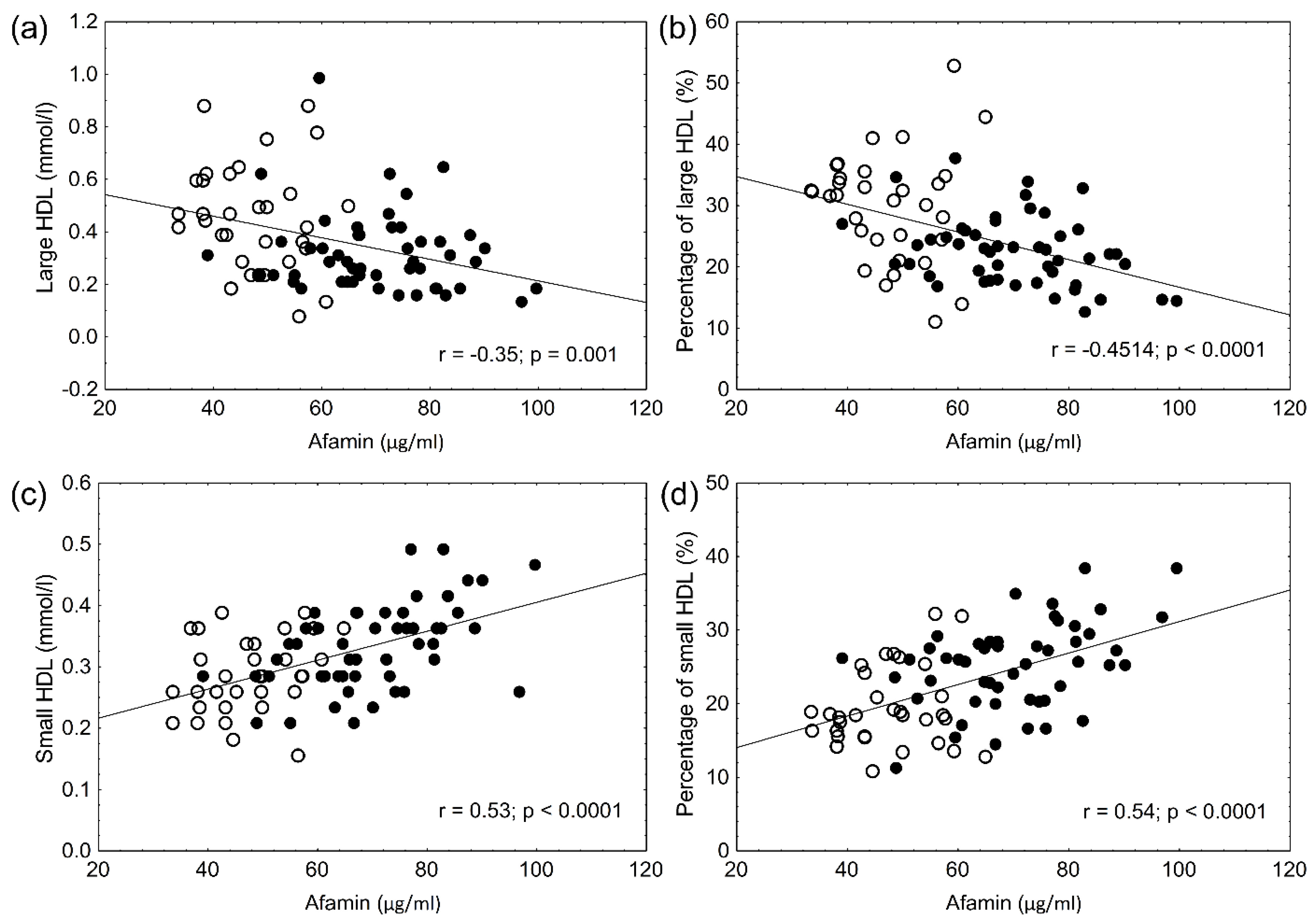
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahmoud, I.; Al-Wandi, A.; Gharaibeh, S.; Mohamed, S. Concordances and correlations between anthropometric indices of obesity: A systematic review. Public Health 2021, 198, 301–306. [Google Scholar] [CrossRef]
- Csige, I.; Ujvárosy, D.; Szabó, Z.; Lőrincz, I.; Paragh, G.; Harangi, M.; Somodi, S. The Impact of Obesity on the Cardiovascular System. J. Diabetes Res. 2018, 2018, 3407306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, A.R.D.O.D.; Zanuso, B.D.O.; Miola, V.F.B.; Barbalho, S.M.; Bueno, P.C.S.; Flato, U.A.P.; Detregiachi, C.R.P.; Buchaim, D.V.; Buchaim, R.L.; Tofano, R.J.; et al. Adipokines, Myokines, and Hepatokines: Crosstalk and Metabolic Repercussions. Int. J. Mol. Sci. 2021, 22, 2639. [Google Scholar] [CrossRef] [PubMed]
- Lichenstein, H.S.; Lyons, D.E.; Wurfel, M.M.; Johnson, D.A.; McGinley, M.D.; Leidli, J.C.; Trollinger, D.B.; Mayer, J.P.; Wright, S.D.; Zukowski, M.M. Afamin is a new member of the albumin, alpha-fetoprotein, and vitamin D-binding protein gene family. J. Biol. Chem. 1994, 269, 18149–18154. [Google Scholar] [CrossRef]
- Jackson, D.; Craven, R.A.; Hutson, R.C.; Graze, I.; Lueth, P.; Tonge, R.P.; Hartley, J.L.; Nickson, J.A.; Rayner, S.J.; Johnston, C.; et al. Proteomic Profiling Identifies Afamin as a Potential Biomarker for Ovarian Cancer. Clin. Cancer Res. 2007, 13, 7370–7379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jerkovic, L.; Voegele, A.F.; Chwatal, S.; Kronenberg, F.; Radcliffe, C.M.; Wormald, M.R.; Lobentanz, E.M.; Ezeh, B.; Eller, P.; Dejori, N.; et al. Afamin Is a Novel Human Vitamin E-Binding Glycoprotein Characterization and In Vitro Expression. J. Proteome Res. 2005, 4, 889–899. [Google Scholar] [CrossRef]
- Voegele, A.F.; Jerković, L.; Wellenzohn, B.; Eller, P.; Kronenberg, F.; Liedl, K.R.; Dieplinger, H. Characterization of the vitamin E-binding properties of human plasma afamin. Biochemistry 2002, 41, 14532–14538. [Google Scholar] [CrossRef]
- Kronenberg, F.; Kollerits, B.; Kiechl, S.; Lamina, C.; Kedenko, L.; Meisinger, C.; Willeit, J.; Huth, C.; Wietzorrek, G.; Altmann, M.E.; et al. Plasma Concentrations of Afamin Are Associated with the Prevalence and Development of Metabolic Syndrome. Circ. Cardiovasc. Genet. 2014, 7, 822–829. [Google Scholar] [CrossRef] [Green Version]
- Köninger, A.; Edimiris, P.; Koch, L.; Enekwe, A.; Lamina, C.; Kasimir-Bauer, S.; Kimmig, R.; Dieplinger, H. Serum concentrations of afamin are elevated in patients with polycystic ovary syndrome. Endocr. Connect. 2014, 3, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Kollerits, B.; Lamina, C.; Huth, C.; Marques-Vidal, P.; Kiechl, S.; Seppälä, I.; Cooper, J.; Hunt, S.C.; Meisinger, C.; Herder, C.; et al. Plasma Concentrations of Afamin Are Associated with Prevalent and Incident Type 2 Diabetes: A Pooled Analysis in More Than 20,000 Individuals. Diabetes Care 2017, 40, 1386–1393. [Google Scholar] [CrossRef] [Green Version]
- Kurdiova, T.; Balaz, M.; Kovanicova, Z.; Zemkova, E.; Kuzma, M.; Belan, V.; Payer, J.; Gasperikova, D.; Dieplinger, H.; Ukropcova, B.; et al. Serum Afamin a Novel Marker of Increased Hepatic Lipid Content. Front. Endocrinol. 2021, 12, 670425. [Google Scholar] [CrossRef]
- Yu, Y.H.; Ginsberg, H.N. Adipocyte signaling and lipid homeostasis: Sequelae of insulin-resistant adipose tissue. Circ. Res. 2005, 96, 1042–1052. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [Green Version]
- Björnson, E.; Adiels, M.; Taskinen, M.-R.; Borén, J. Kinetics of plasma triglycerides in abdominal obesity. Curr. Opin. Lipidol. 2017, 28, 11–18. [Google Scholar] [CrossRef]
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R.; et al. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieczorek, E.; Ćwiklińska, A.; Kuchta, A.; Kortas-Stempak, B.; Gliwińska, A.; Jankowski, M. The Differential Effects of HDL Subpopulations on Lipoprotein Lipase (LPL)-Mediated VLDL Catabolism. Biomedicines 2021, 9, 1839. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef] [PubMed]
- Vergès, B. Pathophysiology of diabetic dyslipidaemia: Where are we? Diabetologia 2015, 58, 886–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perugini, C.; Bagnati, M.; Cau, C.; Bordone, R.; Paffoni, P.; Re, R.; Zoppis, E.; Albano, E.; Bellomo, G. Distribution of lipid-soluble antioxidants in lipoproteins from healthy subjects. ii. effects of in vivo supplementation with α-tocopherol. Pharmacol. Res. 2000, 41, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Kayden, H.J.; Traber, M. Absorption, lipoprotein transport, and regulation of plasma concentrations of vitamin E in humans. J. Lipid Res. 1993, 34, 343–358. [Google Scholar] [CrossRef]
- Brockes, C.; Buchli, C.; Locher, R.; Koch, J.; Vetter, W. Vitamin E prevents extensive lipid peroxidation in patients with hypertension. Br. J. Biomed. Sci. 2003, 60, 5–8. [Google Scholar] [CrossRef]
- Eroğlu, H.; Örgül, G.; Tonyalı, N.V.; Biriken, D.; Polat, N.; Yücel, A.; Yazihan, N.; Şahin, D. The Role of Afamin and Other Trace Elements in the Prediction of GDM: A Tertiary Center Experience. Biol. Trace Element Res. 2021, 199, 4418–4422. [Google Scholar] [CrossRef] [PubMed]
- Varga, V.E.; Lőrincz, H.; Szentpéteri, A.; Juhász, L.; Seres, I.; Paragh, G.; Balla, J.; Harangi, M. Changes in serum afamin and vitamin E levels after selective LDL apheresis. J. Clin. Apher. 2018, 33, 569–575. [Google Scholar] [CrossRef]
- Zerbinati, C.; Galli, F.; Regolanti, R.; Poli, G.; Iuliano, L. Gas chromatography–mass spectrometry microanalysis of alpha- and gamma-tocopherol in plasma and whole blood. Clin. Chim. Acta 2015, 446, 156–162. [Google Scholar] [CrossRef]
- Barakat, H.A.; McLendon, V.D.; Marks, R.; Pories, W.; Heath, J.; Carpenter, J.W. Influence of morbid obesity and non-insulin-dependent diabetes mellitus on high-density lipoprotein composition and subpopulation distribution. Metabolism 1992, 41, 37–41. [Google Scholar] [CrossRef]
- Davidson, M.H. Apolipoprotein measurements: Is more widespread use clinically indicated? Clin. Cardiol. 2009, 32, 482–486. [Google Scholar] [CrossRef]
- Camps, J.; Castañé, H.; Rodríguez-Tomàs, E.; Baiges-Gaya, G.; Hernández-Aguilera, A.; Arenas, M.; Iftimie, S.; Joven, J. On the Role of Paraoxonase-1 and Chemokine Ligand 2 (C-C motif) in Metabolic Alterations Linked to Inflammation and Disease. A 2021 Update. Biomolecules 2021, 11, 971. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Bunyan, J. Vitamin E and the biological antioxidant theory. Nutr. Abstr. Rev. 1969, 39, 321–345. [Google Scholar] [CrossRef]
- Castañé, H.; Baiges-Gaya, G.; Hernández-Aguilera, A.; Rodríguez-Tomàs, E.; Fernández-Arroyo, S.; Herrero, P.; Delpino-Rius, A.; Canela, N.; Menendez, J.; Camps, J.; et al. Coupling Machine Learning and Lipidomics as a Tool to Investigate Metabolic Dysfunction-Associated Fatty Liver Disease. A General Overview. Biomolecules 2021, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Liebler, D.C.; Kling, D.S.; Reed, D.J. Antioxidant protection of phospholipid bilayers by alpha-tocopherol. Control of alpha-tocopherol status and lipid peroxidation by ascorbic acid and glutathione. J. Biol. Chem. 1986, 261, 12114–12119. [Google Scholar] [CrossRef]
- Newaz, M.; Nawal, N. Effect of α-tocopherol on lipid peroxidation and total antioxidant status in spontaneously hypertensive rats. Am. J. Hypertens. 1998, 11, 1480–1485. [Google Scholar] [CrossRef] [Green Version]
- Devaraj, S.; Tang, R.; Adams-Huet, B.; Harris, A.; Seenivasan, T.; de Lemos, J.A.; Jialal, I. Effect of high-dose alpha-tocopherol supplementation on biomarkers of oxidative stress and inflammation and carotid atherosclerosis in patients with coronary artery disease. Am. J. Clin. Nutr. 2007, 86, 1392–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodis, H.N.; Mack, W.J.; LaBree, L.; Mahrer, P.R.; Sevanian, A.; Liu, C.R.; Liu, C.H.; Hwang, J.; Selzer, R.H.; Azen, S.P. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: The Vitamin E Atherosclerosis Prevention Study (VEAPS). Circulation 2002, 106, 1453–1459. [Google Scholar] [CrossRef] [Green Version]
- Jha, P. Alpha-tocopherol may reduce the risk of non-fatal myocardial infarction among patients with angiographically proven coronary atherosclerosis. Evid.-Based Cardiovasc. Med. 1997, 1, 12–14. [Google Scholar]
- Niki, E. Do free radicals play causal role in atherosclerosis? Low density lipoprotein oxidation and vitamin E revisited. J. Clin. Biochem. Nutr. 2011, 48, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Maes, M.; Weeckx, S.; Wauters, A.; Neels, H.; Scharpé, S.; Verkerk, R.; Demedts, P.; DeSnyder, R. Biological variability in serum vitamin E concentrations: Relation to serum lipids. Clin. Chem. 1996, 42, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A. Reflections on a century of vitamin E research: Looking at the past with an eye on the future. Free Radic. Biol. Med. 2021, 175, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Vitamin E as a Potential Interventional Treatment for Metabolic Syndrome: Evidence from Animal and Human Studies. Front. Pharmacol. 2017, 8, 444. [Google Scholar] [CrossRef] [Green Version]
| Obese Patients | Controls | p-Value | |
|---|---|---|---|
| n = 50 | n = 32 | ||
| Gender (F/M) | 43/7 | 27/5 | n.s. |
| Age (yrs) | 44.2 ± 13.5 | 41.8 ± 6.0 | n.s. |
| BMI (kg/m2) | 42.0 ± 8.6 | 24.2 ± 2.5 | <0.001 |
| Waist circumference (cm) | 119.8 ± 16.9 | 83.6 ± 9.3 | <0.001 |
| hsCRP (mg/L) | 8.2 (3.2–13.1) | 1.4 (0.5–2.5) | <0.001 |
| sTSH (mU/L) | 1.98 ± 0.98 | 2.06 ± 1.22 | n.s. |
| AST (U/L) | 23.5 ± 9.0 | 18.7 ± 3.9 | <0.01 |
| ALT (U/L) | 29.4 ± 15.3 | 18.1 ± 7.9 | <0.001 |
| γ-GTP (U/L) | 33.6 ± 21.4 | 24.3 ± 15.4 | <0.05 |
| LDH (U/L) | 355.6 ± 84 | 222.2± 73.2 | <0.001 |
| Uric acid (µmol/L) | 315.2 ± 91.6 | 254.5 ± 63.7 | <0.001 |
| Cholesterol (mmol/L) | 5.0 ± 0.8 | 5.0 ± 0.8 | n.s. |
| Triglyceride (mmol/L) | 1.4 (1.1–2.0) | 1.0 (0.75–1.39) | <0.01 |
| HDL-C (mmol/L) | 1.4 ± 0.3 | 1.6 ± 0.5 | <0.001 |
| LDL-C (mmol/L) | 3.2 ± 0.7 | 2.9 ± 0.6 | <0.05 |
| ApoA-I (g/L) | 1.48 ± 0.24 | 1.71 ± 0.31 | <0.001 |
| ApoB (g/L) | 0.86 ± 0.20 | 0.88 ± 0.23 | n.s. |
| Glucose (mmol/L) | 5.4 ± 0.7 | 4.8 ± 0.5 | <0.001 |
| OGTT 0′ | 4.9 ± 0.8 | ||
| OGTT 120′ | 7 ± 2 | ||
| HbA1C (%) | 5.8 ± 0.5 | 5.1 ± 0.3 | <0.001 |
| Insulin (mU/L) | 21 ± 15.9 | ||
| HOMA-IR | 3.75 (2.4–6.52) | ||
| C-peptide (pmol/L) | 1325 (1055–1619) |
| Obese | Control | p-Value | ||
|---|---|---|---|---|
| HDL | Large HDL% | 22.5 ± 5.7 | 30.9 ± 9.4 | <0.05 |
| Intermediate HDL% | 52.3 ± 3.4 | 50.2 ± 4.7 | n.s. | |
| Small HDL% | 25.2 ± 5.9 | 18.9 ± 5.7 | <0.05 | |
| Large HDL (mmol/L) | 0.32 ± 0.16 | 0.53 ± 0.31 | <0.05 | |
| Intermediate HDL (mmol/L) | 0.71 ± 0.17 | 0.78 ± 0.17 | n.s. | |
| Small HDL (mmol/L) | 0.33 ± 0.07 | 0.28 ± 0.06 | <0.01 | |
| LDL | Large LDL % | 25.8 ± 4.1 | 21.4 ± 5.9 | <0.05 |
| Small-dense LDL % | 2.0 ± 1.6 | 1.0 ± 2.1 | <0.001 | |
| Large LDL (mmol/L) | 1.32 ± 0.36 | 1.08 ± 0.35 | <0.01 | |
| Small-dense LDL (mmol/L) | 0.11 ± 0.12 | 0.05 ± 0.11 | <0.05 | |
| Mean LDL size (nm) (minimum-maximum) | 26.98 ± 0.3 (25.9–27.5) | 27.25 ± 0.3 (25.9–27.7) | <0.001 |
| Obese | Control | p | |
|---|---|---|---|
| Afamin (µg/mL) | 70.4 ± 12.9 | 47.6 ± 8.5 | <0.001 |
| α-tocopherol (µg/mL) | 9.4 (7.9–13.2) | 8.2 (7.2–9.7) | <0.05 |
| γ-tocopherol (µg/mL) | 0.2 (0.16–0.31) | 0.12 (0.1–0.17) | <0.001 |
| α-Tocopherol/cholesterol | 1.95 (1.62–2.51) | 1.64 (1.49–1.946) | <0.05 |
| γ-Tocopherol/cholesterol | 0.04 (0.03–0.06) | 0.03 (0.02–0.033) | <0.001 |
| oxLDL (U/L) | 46.8 ± 10 | 40.2 ± 10.1 | <0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhász, I.; Ujfalusi, S.; Seres, I.; Lőrincz, H.; Varga, V.E.; Paragh, G., Jr.; Somodi, S.; Harangi, M.; Paragh, G. Afamin Levels and Their Correlation with Oxidative and Lipid Parameters in Non-diabetic, Obese Patients. Biomolecules 2022, 12, 116. https://doi.org/10.3390/biom12010116
Juhász I, Ujfalusi S, Seres I, Lőrincz H, Varga VE, Paragh G Jr., Somodi S, Harangi M, Paragh G. Afamin Levels and Their Correlation with Oxidative and Lipid Parameters in Non-diabetic, Obese Patients. Biomolecules. 2022; 12(1):116. https://doi.org/10.3390/biom12010116
Chicago/Turabian StyleJuhász, Imre, Szilvia Ujfalusi, Ildikó Seres, Hajnalka Lőrincz, Viktória Evelin Varga, György Paragh, Jr., Sándor Somodi, Mariann Harangi, and György Paragh. 2022. "Afamin Levels and Their Correlation with Oxidative and Lipid Parameters in Non-diabetic, Obese Patients" Biomolecules 12, no. 1: 116. https://doi.org/10.3390/biom12010116
APA StyleJuhász, I., Ujfalusi, S., Seres, I., Lőrincz, H., Varga, V. E., Paragh, G., Jr., Somodi, S., Harangi, M., & Paragh, G. (2022). Afamin Levels and Their Correlation with Oxidative and Lipid Parameters in Non-diabetic, Obese Patients. Biomolecules, 12(1), 116. https://doi.org/10.3390/biom12010116









