Adropin Serum Levels in Patients with Primary Sjögren’s Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Ethical Considerations
2.3. Clinical and Laboratory Evaluation
2.4. Definition of CV Risk
2.5. Evaluation of the Activity and Accumulated Irreversible Damage in Primary SS
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Laboratory Parameters of the Study Population
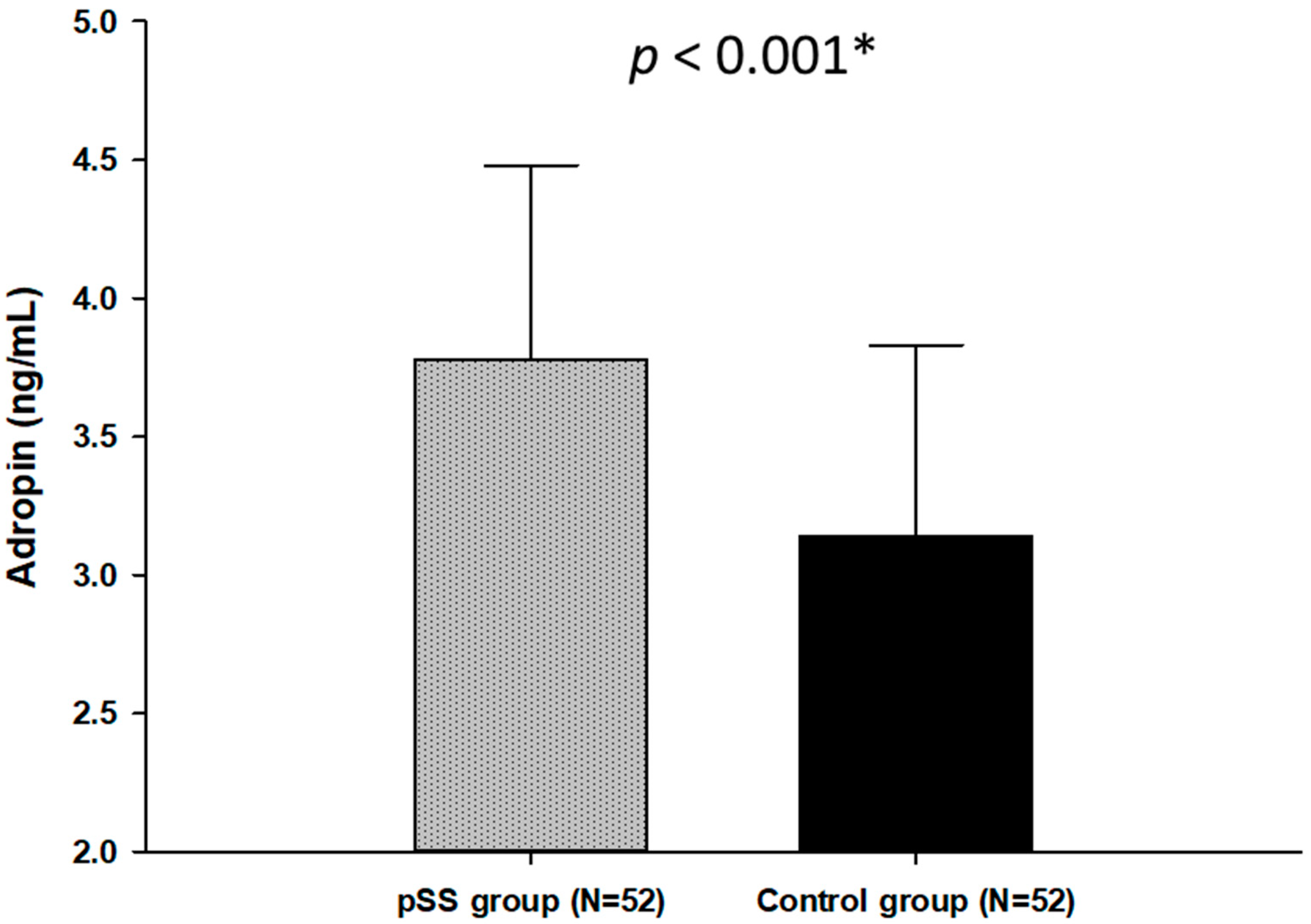
3.3. Serum Adropin Levels in Patients with pSS and Control Subjects
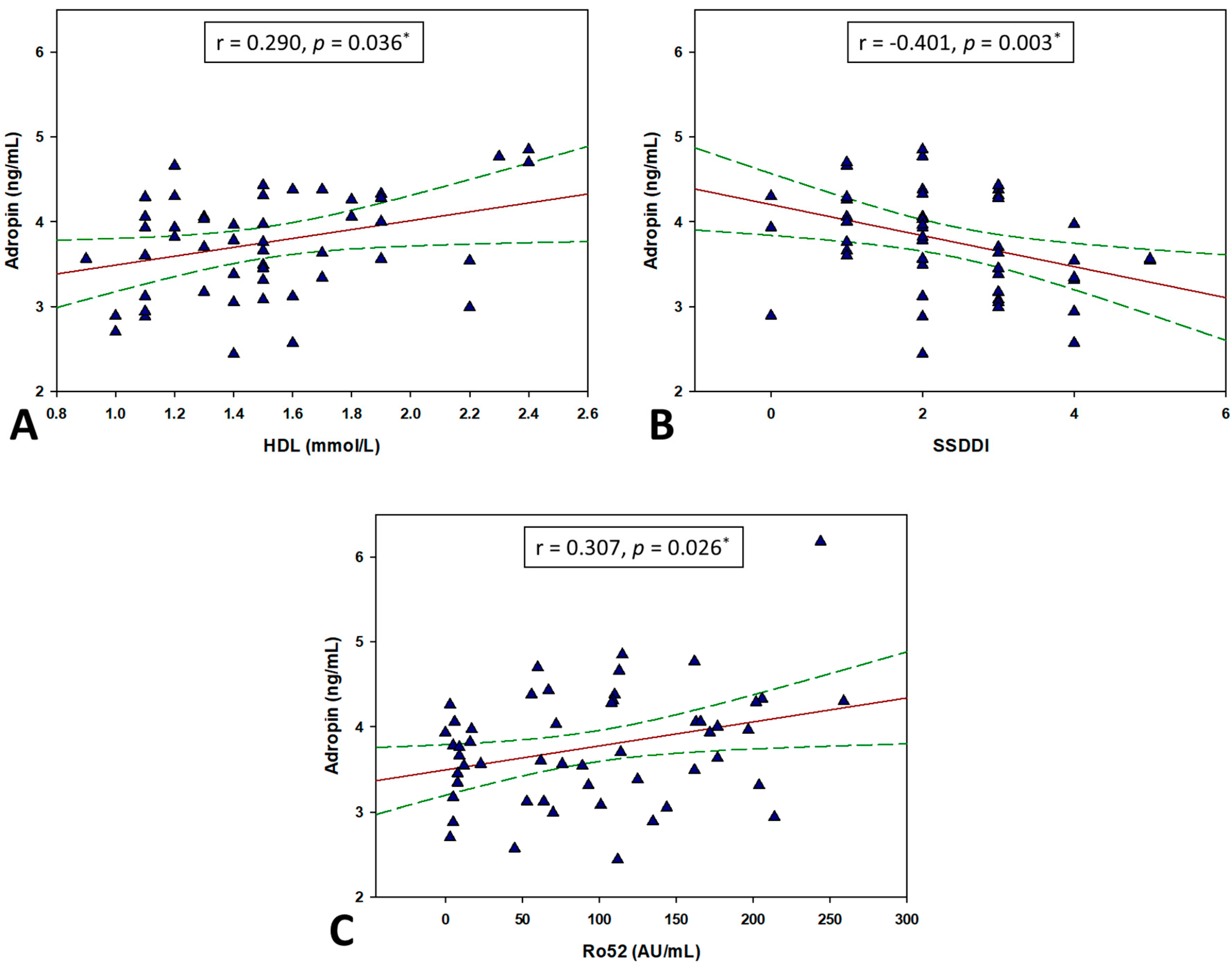
3.4. Correlation between Adropin and Other Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, R.; Shahane, A. The epidemiology of Sjögren’s syndrome. Clin. Epidemiol. 2014, 6, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Pérez-De-Lis, M.; Akasbi, M.; Sisó, A.; Diez-Cascon, P.; Brito-Zerón, P.; Diaz-Lagares, C.; Ortiz, J.; Perez-Alvarez, R.; Ramos-Casals, M.; Coca, A. Cardiovascular risk factors in primary Sjögren’s syndrome: A case-control study in 624 patients. Lupus 2010, 19, 941–948. [Google Scholar] [CrossRef]
- Horvath, I.F.; Szanto, A.; Papp, G.; Zeher, M. Clinical course, prognosis, and cause of death in primary Sjögren’s syndrome. J. Immunol. Res. 2014, 2014, 647507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vaudo, G.; Bocci, E.B.; Shoenfeld, Y.; Schillaci, G.; Wu, R. Precocious intima-media thickening in patients with primary Sjogren’s syndrome. Arthritis Rheum. 2005, 52, 3890–3897. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, F.; Sarzi-Puttini, P.; Signorello, M.C.; Gianturco, L.; Stella, D. New parameters for identifying subclinical atherosclerosis in patients with primary Sjogren’s syndrome: A pilot study. Clin. Exp. Rheumatol. 2014, 32, 361–368. [Google Scholar] [CrossRef]
- Akyel, A.; Tavil, Y.; Yayla, C.; Tufan, A.; Kaya, A. Endothelial dysfunction in primary Sjogren syndrome. West Indian Med. J. 2012, 61, 870–872. [Google Scholar] [CrossRef][Green Version]
- Melissaropoulos, K.; Bogdanos, D.; Dimitroulas, T.; Sakkas, L.I.; Kitas, G.D.; Daoussis, D. Primary Sjögren’s Syndrome and Cardiovascular Disease. Curr. Vasc. Pharmacol. 2020, 18, 447–454. [Google Scholar] [CrossRef]
- Li, L.; Xie, W.; Zheng, X.L.; Yin, W.D.; Tang, C.K. A novel peptide adropin in cardiovascular diseases. Clin. Chim. Acta 2016, 453, 107–113. [Google Scholar] [CrossRef]
- Yosaee, S.; Soltani, S.; Sekhavati, E.; Jazayeri, S. Adropin- A Novel Biomarker of Heart Disease: A Systematic Review Article. Iran. J. Public Health 2016, 45, 1568–1576. [Google Scholar]
- Kumar, K.G.; Trevaskis, J.L.; Lam, D.D.; Sutton, G.M.; Koza, R.A.; Chouljenko, V.N.; Kousoulas, K.G.; Rogers, P.M.; Kesterson, R.A.; Thearle, M.; et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008, 8, 468–481. [Google Scholar] [CrossRef]
- Lovren, F.; Pan, Y.; Quan, A.; Singh, K.K.; Shukla, P.C.; Gupta, M.; Al-Omran, M.; Teoh, H.; Verma, S. Adropin is a novel regulator of endothelial function. Circulation 2010, 122, S185–S192. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Niepolski, L.; Grzegorzewska, A.E. Salusins and adropin: New peptides potentially involved in lipid metabolism and atherosclerosis. Adv. Med. Sci. 2016, 61, 282–287. [Google Scholar] [CrossRef]
- Zhao, L.P.; You, T.; Chan, S.P.; Chen, J.C.; Xu, W.T. Adropin is associated with hyperhomocysteine and coronary atherosclerosis. Exp. Ther. Med. 2016, 11, 1065–1070. [Google Scholar] [CrossRef]
- Wu, L.; Fang, J.; Chen, L.; Zhao, Z.; Luo, Y.; Lin, C.; Fan, L. Low serum adropin is associated with coronary atherosclerosis in type 2 diabetic and non-diabetic patients. Clin. Chem. Lab Med. 2014, 52, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. European Study Group on Classification Criteria for Sjögren’s Syndrome. Classification criteria for Sjögren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Seror, R.; Ravaud, P.; Bowman, S.J.; Baron, G.; Tzioufas, A.; Theander, E. EULAR Sjögren’s syndrome disease activity index: Development of a consensus systemic disease activity index for primary Sjögren’s syndrome. Ann. Rheum. Dis. 2010, 69, 1103–1109. [Google Scholar] [CrossRef]
- Vitali, C.; Palombi, G.; Baldini, C.; Benucci, M.; Bombardieri, S.; Covelli, M.; Del Papa, N.; De Vita, S.; Epis, O.; Franceschini, F.; et al. Sjögren’s Syndrome Disease Damage Index and disease activity index: Scoring systems for the assessment of disease damage and disease activity in Sjögren’s syndrome, derived from an analysis of a cohort of Italian patients. Arthritis Rheum. 2007, 56, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Buchanan, M.R.; Anderson, T.J. Endothelial function testing as a biomarker of vascular disease. Circulation 2003, 108, 2054–2059. [Google Scholar] [CrossRef]
- Celik, A.; Balin, M.; Kobat, M.A.; Erdem, K.; Baydas, A.; Bulut, M.; Altas, Y.; Aydin, S.; Aydin, S. Deficiency of a new protein associated with cardiac syndrome X; called adropin. Cardiovasc. Ther. 2013, 31, 174–178. [Google Scholar] [CrossRef]
- Sato, K.; Yamashita, T.; Shirai, R.; Shibata, K.; Okano, T.; Yamaguchi, M.; Mori, Y.; Hirano, T.; Watanabe, T. Adropin Contributes to Anti-Atherosclerosis by Suppressing Monocyte-Endothelial Cell Adhesion and Smooth Muscle Cell Proliferation. Int. J. Mol. Sci. 2018, 19, 1293. [Google Scholar] [CrossRef]
- Wanchu, A.; Khullar, M.; Sud, A.; Bambery, P. Elevated nitric oxide production in patients with primary Sjögren’s syndrome. Clin. Rheumatol. 2000, 19, 360–364. [Google Scholar] [CrossRef]
- Couffinhal, T.; Duplàa, C.; Moreau, C.; Lamazière, J.M.; Bonnet, J. Regulation of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 in human vascular smooth muscle cells. Circ. Res. 1994, 74, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Kim, H.J.; Won, K.J.; Choi, W.S.; Park, S.H.; Song, H.; Park, P.J.; Park, T.K.; Lee, C.K.; Kim, B. Soluble form of vascular cell adhesion molecule 1 induces migration and proliferation of vascular smooth muscle cells. J. Vasc. Res. 2008, 45, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Konttinen, Y.T.; Platts, L.A.; Tuominen, S.; Eklund, K.K.; Santavirta, N.; Törnwall, J.; Sorsa, T.; Hukkanen, M.; Polak, J.M. Role of nitric oxide in Sjögren’s syndrome. Arthritis Rheum. 1997, 40, 875–883. [Google Scholar] [CrossRef]
- Yu, H.Y.; Zhao, P.; Wu, M.C.; Liu, J.; Yin, W. Serum adropin levels are decreased in patients with acute myocardial infarction. Regul. Pept. 2014, 190–191, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J.; Cirino, G.; Casini, A.; Napoli, C. Nitric oxide as a signaling molecule in the vascular system: An overview. J Cardiovasc. Pharmacol. 1999, 34, 879–886. [Google Scholar] [CrossRef]
- Akcilar, R.; Kocak, F.E.; Simsek, H.; Akcilar, A.; Bayat, Z.; Ece, E.; Kokdasgil, H. Antidiabetic and hypolipidemic effects of adropinin streoptozotocin-induced type 2 diabetic rats. Bratisl. Lek. Listy 2016, 117, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Akcılar, R.; Emel Koçak, F.; Şimşek, H. The effect of adropin on lipid and glucose metabolism in rats with hyperlipidemia. Iran. J. Basic Med. Sci. 2016, 19, 245–251. [Google Scholar]
- Yolbas, S.; Kara, M.; Kalayci, M.; Yildirim, A.; Gundogdu, B.; Aydin, S.; Koca, S.S. ENHO gene expression and serum adropin level in rheumatoid arthritis and systemic lupus erythematosus. Adv. Clin. Exp. Med. 2018, 27, 1637–1641. [Google Scholar] [CrossRef]
- Yolbas, S.; Kara, M.; Yilmaz, M.; Aydin, S.; Koca, S.S. Serum adropin level and ENHO gene expression in systemic sclerosis. Clin. Rheumatol. 2016, 35, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Bozic, J.; Borovac, J.A.; Galic, T.; Kurir, T.T.; Supe-Domic, D.; Dogas, Z. Adropin and Inflammation Biomarker Levels in Male Patients with Obstructive Sleep Apnea: A Link With Glucose Metabolism and Sleep Parameters. J. Clin. Sleep Med. 2018, 14, 1109–1118. [Google Scholar] [CrossRef]
- Brnić, D.; Martinovic, D.; Zivkovic, P.M.; Tokic, D.; Tadin Hadjina, I.; Rusic, D.; Vilovic, M.; Supe-Domic, D.; Tonkic, A.; Bozic, J. Serum adropin levels are reduced in patients with inflammatory bowel diseases. Sci. Rep. 2020, 10, 9264. [Google Scholar] [CrossRef]
- Kuliczkowska-Płaksej, J.; Mierzwicka, A.; Jończyk, M.; Stachowska, B.; Urbanovych, A.; Bolanowski, M. Adropin in women with polycystic ovary syndrome. Endokrynol. Pol. 2019, 70, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Tičinović Kurir, T.; Miličević, T.; Novak, A.; Vilović, M.; Božić, J. Adropin—Potential Link in Cardiovascular Protection for Obese Male Type 2 Diabetes Mellitus Patients Treated with Liraglutide. Acta Clin. Croat. 2020, 59, 344–350. [Google Scholar] [CrossRef]
- Retamozo, S.; Akasbi, M.; Brito-Zerón, P.; Bosch, X.; Bove, A.; Perez-de-Lis, M.; Jimenez, I.; Soto-Cardenas, M.J.; Gandia, M.; Diaz-Lagares, C.; et al. Anti-Ro52 antibody testing influences the classification and clinical characterisation of primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2012, 30, 686–692. [Google Scholar] [PubMed]
- Bartoloni, E.; Baldini, C.; Schillaci, G.; Quartuccio, L.; Priori, R.; Carubbi, F.; Bini, V.; Alunno, A.; Bombardieri, S.; De Vita, S.; et al. Cardiovascular disease risk burden in primary Sjögren’s syndrome: Results of a population-based multicentre cohort study. J. Intern. Med. 2015, 278, 185–192. [Google Scholar] [CrossRef]
- Salmon, J.E.; Roman, M.J. Subclinical atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. Am. J. Med. 2008, 121 (Suppl. 1), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Shoenfeld, Y.; Gerli, R.; Doria, A.; Matsuura, E.; Cerinic, M.M.; Ronda, N.; Jara, L.J.; Abu-Shakra, M.; Meroni, P.L.; Sherer, Y. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation 2005, 112, 3337–3347. [Google Scholar] [CrossRef] [PubMed]
- Sabio, J.M.; Sánchez-Berná, I.; Martinez-Bordonado, J.; Vargas-Hitos, J.A.; Navarrete-Navarrete, N.; Expósito Ruíz, M.; Jiménez-Alonso, J. Prevalence of and factors associated with increased arterial stiffness in patients with primary Sjögren’s syndrome. Arthritis Care Res. 2015, 67, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Gravani, F.; Papadaki, I.; Antypa, E.; Nezos, A.; Masselou, K.; Ioakeimidis, D.; Koutsilieris, M.; Moutsopoulos, H.M.; Mavragani, C.P. Subclinical atherosclerosis and impaired bone health in patients with primary Sjogren’s syndrome: Prevalence, clinical and laboratory associations. Arthritis Res. Ther. 2015, 17, 99. [Google Scholar] [CrossRef]
- Gu, X.; Li, H.; Zhu, X.; Gu, H.; Chen, J.; Wang, L.; Harding, P.; Xu, W. Inverse Correlation Between Plasma Adropin and ET-1 Levels in Essential Hypertension: A Cross-Sectional Study. Medicine 2015, 94, e1712. [Google Scholar] [CrossRef]
- Gulen, B.; Eken, C.; Kucukdagli, O.T.; Serinken, M.; Kocyigit, A.; Kılıc, E.; Uyarel, H. Adropin levels and target organ damage secondary to high blood pressure in the ED. Am. J. Emerg. Med. 2016, 34, 2061–2064. [Google Scholar] [CrossRef] [PubMed]
- Çelik, H.T.; Akkaya, N.; Erdamar, H.; Gok, S.; Kazanci, F.; Demircelik, B.; Cakmak, M.; Yigitoglu, R. The Effects of Valsartan and Amlodipine on the Levels of Irisin, Adropin, and Perilipin. Clin. Lab 2015, 61, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Boric-Skaro, D.; Mizdrak, M.; Luketin, M.; Martinovic, D.; Tokic, D.; Vilovic, M.; Supe-Domic, D.; Kurir, T.T.; Bozic, J. Serum Adropin Levels in Patients on Hemodialysis. Life 2021, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S. Three new players in energy regulation: Preptin, adropin and irisin. Peptides 2014, 56, 94–110. [Google Scholar] [CrossRef]
- Ganesh Kumar, K.; Zhang, J.; Gao, S.; Rossi, J.; McGuinness, O.P.; Halem, H.H.; Culler, M.D.; Mynatt, R.L.; Butler, A.A. Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity 2012, 20, 1394–1402. [Google Scholar] [CrossRef]
| Parameter | pSS Group (N = 52) | Control Group (N = 52) | p * |
|---|---|---|---|
| Male sex (N, %) | 4 (7.7) | 3 (5.8) | 0.718 |
| Age (years) | 59.3 ± 11.1 | 57.8 ± 10.5 | 0.477 |
| Body weight (kg) | 70.9 ± 11.6 | 71.9 ± 10.1 | 0.654 |
| Body height (cm) | 168.8 ± 7.1 | 168.3 ± 6.8 | 0.715 |
| Body mass index (kg/m2) | 24.8 ± 3.4 | 25.4 ± 3.1 | 0.418 |
| SBP (mmHg) | 126.2 ± 16.8 | 118.9 ± 13.2 | 0.015 |
| DBP (mmHg) | 81.4 ± 7.6 | 77.6 ± 8.1 | 0.014 |
| FRS (%) | 3.4 (1.3–5.5) | 2.1 (1.0–3.3) | 0.042 |
| Smoking (N, %) | 9 (17.3) | 8 (15.4) | 0.792 |
| Disease duration (years) † | 6 (3.5–10) | - | - |
| ESSDAI | 2 (1–3) | - | - |
| SSDDI | 2 (2–3) | - | - |
| Parameter | pSS Group (N = 52) | Control Group (N = 52) | p * |
|---|---|---|---|
| Erythrocytes (×1012/L) | 4.37 ± 0.42 | 4.52 ± 0.32 | 0.047 |
| Hemoglobin (g/L) | 130.5 ± 11.0 | 138.4 ± 11.5 | <0.001 |
| MCV (fL) | 87.1 ± 12.8 | 93.1 ± 16.1 | 0.034 |
| Leukocytes (×1012/L) | 4.88 ± 1.38 | 5.94 ± 1.81 | 0.001 |
| Platelets (×109/L) | 230.8 ± 52.1 | 244.1 ± 47.9 | 0.179 |
| Urea (mmoL/L) | 5.51 ± 1.65 | 5.47 ± 1.44 | 0.915 |
| Creatinine (mmoL/L) | 65.9 ± 18.5 | 63.1 ± 14.6 | 0.378 |
| ESR (mm/h) | 16.5 (8.0–27.5) | 10.0 (6.0–16.0) | 0.006 |
| hsCRP (mg/L) | 1.30 (0.8–3.8) | 1.15 (0.7–3.3) | 0.767 |
| TC (mmoL/L) | 5.60 ± 1.12 | 6.13 ± 0.93 | 0.010 |
| LDL (mmoL/L) | 3.56 ± 0.96 | 3.91 ± 1.15 | 0.093 |
| TG (mmoL/L) | 1.18 ± 0.42 | 1.42 ± 0.85 | 0.072 |
| HDL (mmoL/L) | 1.52 ± 0.37 | 1.57 ± 0.32 | 0.436 |
| C3 (g/L) | 1.21 ± 0.22 | 1.19 ± 0.17 | 0.567 |
| C4 (g/L) | 0.26 ± 0.19 | 0.31 ± 0.09 | 0.197 |
| Anti SSB/La (AU/mL) | 33.5 (2.5–81.5) | - | - |
| Anti SSA/Ro52 (AU/mL) | 91.0 (16.5–162.0) | - | - |
| Anti SSA/Ro60 (AU/mL) | 103.0 (56.5–117.0) | - | - |
| Parameter | Lower Adropin (<3.73 ng/mL) (N = 26) | Higher Adropin (>3.73 ng/mL) (N = 26) | p * |
|---|---|---|---|
| Age (years) | 51.0 ± 9.83 | 57.6 ± 12.3 | 0.284 |
| Body mass index (kg/m2) | 24.8 ± 4.1 | 24.83 ± 2.5 | 0.978 |
| SBP (mmHg) | 123.6 ± 18.46 | 128.8 ± 14.9 | 0.270 |
| DBP (mmHg) | 79.6 ± 8.8 | 80.8 ± 5.9 | 0.086 |
| Total cholesterol (mmoL/L) | 5.56 ± 1.08 | 5.76 ± 1.21 | 0.535 |
| HDL (mmoL/L) | 1.46 ± 0.36 | 1.56 ± 0.38 | 0.362 |
| LDL (mmoL/L) | 3.35 ± 0.89 | 3.76 ± 0.99 | 0.119 |
| TG (μmol/L) | 1.15 ± 0.43 | 1.20 ± 0.40 | 0.694 |
| hsCRP (mg/L) | 1.35 (0.9–2.7) | 1.15 (0.80–5.6) | 0.869 |
| Anti SSB/La (AU/mL) | 36.0 (7.0–79.0) | 9.5 (2.0–88.0) | 0.734 |
| AntiSSA/Ro52 (AU/mL) | 73.0 (12.0–125.0) | 109.0 (17.0–172.0) | 0.272 |
| Anti SSA/Ro60 (AU/mL) | 101.0 (60.0–116.0) | 107.5 (52.0–118.0) | 0.963 |
| Disease duration (years) † | 6 (4.0–12.0) | 6 (3.0–10.0) | 0.607 |
| ESSDAI | 2.0 (1.0–3.0) | 2.5 (1.0–3.0) | 0.641 |
| SSDDI | 3.0 (2.0–4.0) | 2.0 (1.0–2.0) | <0.001 |
| FRS (%) | 4.25 (1.2–6.2) | 2.3 (1.4–5.2) | 0.374 |
| Parameter | r * | p |
|---|---|---|
| Age (years) | −0.133 | 0.345 |
| Body mass index (kg/m2) | −0.133 | 0.348 |
| hsCRP (mg/L) | 0.158 † | 0.262 |
| TC (mmoL/L) | 0.224 | 0.109 |
| TG (mmoL/L) | −0.083 | 0.557 |
| HDL (mmoL/L) | 0.290 | 0.036 |
| LDL (mmoL/L) | 0.178 | 0.206 |
| SBP (mmHg) | 0.118 | 0.404 |
| DBP (mmHg) | 0.155 | 0.271 |
| Anti SSA/Ro60 (AU/mL) | 0.123 † | 0.385 |
| Anti SSA/Ro52 (AU/mL) | 0.307 † | 0.026 |
| Anti SSB/La (AU/mL) | −0.009 † | 0.946 |
| ESSDAI | 0.051 † | 0.721 |
| SSDDI | −0.401 † | 0.003 |
| Disease duration (years) | −0.041 † | 0.770 |
| Variable | β 1 | SE 2 | t-Value | p |
|---|---|---|---|---|
| Age (years) | −0.013 | 0.011 | −1.134 | 0.263 |
| Sex | 0.220 | 0.367 | 0.602 | 0.550 |
| BMI (kg/m2) | −0.007 | 0.002 | −0.268 | 0.794 |
| Anti SSA/Ro52 | 0.001 | 0.001 | 1.551 | 0.128 |
| HDL | 0.903 | 0.283 | 3.191 | 0.002 |
| FRS | 0.025 | 0.032 | 0.768 | 0.447 |
| Disease duration † | 0.006 | 0.015 | 0.394 | 0.695 |
| ESSDAI | 0.044 | 0.061 | 0.718 | 0.476 |
| SSDDI | −0.202 | 0.073 | −2.754 | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danolić, M.J.; Perković, D.; Petrić, M.; Barišić, I.; Gugo, K.; Božić, J. Adropin Serum Levels in Patients with Primary Sjögren’s Syndrome. Biomolecules 2021, 11, 1296. https://doi.org/10.3390/biom11091296
Danolić MJ, Perković D, Petrić M, Barišić I, Gugo K, Božić J. Adropin Serum Levels in Patients with Primary Sjögren’s Syndrome. Biomolecules. 2021; 11(9):1296. https://doi.org/10.3390/biom11091296
Chicago/Turabian StyleDanolić, Marijana Janković, Dijana Perković, Marin Petrić, Igor Barišić, Katarina Gugo, and Joško Božić. 2021. "Adropin Serum Levels in Patients with Primary Sjögren’s Syndrome" Biomolecules 11, no. 9: 1296. https://doi.org/10.3390/biom11091296
APA StyleDanolić, M. J., Perković, D., Petrić, M., Barišić, I., Gugo, K., & Božić, J. (2021). Adropin Serum Levels in Patients with Primary Sjögren’s Syndrome. Biomolecules, 11(9), 1296. https://doi.org/10.3390/biom11091296







