Reactive Astrocytosis in a Mouse Model of Chronic Polyamine Catabolism Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of Purified Nerve Terminals and Astrocyte Processes
2.3. Western Blot
2.4. Polyamine Content
2.5. Catalase Activity
2.6. [Ca 2+]i Assay
2.7. Statistical Analysis
2.8. Chemicals
3. Results
3.1. Analysis of Ezrin and Vimentin Levels in the Cerebral Cortex of Dach-SMOX and Control Mice
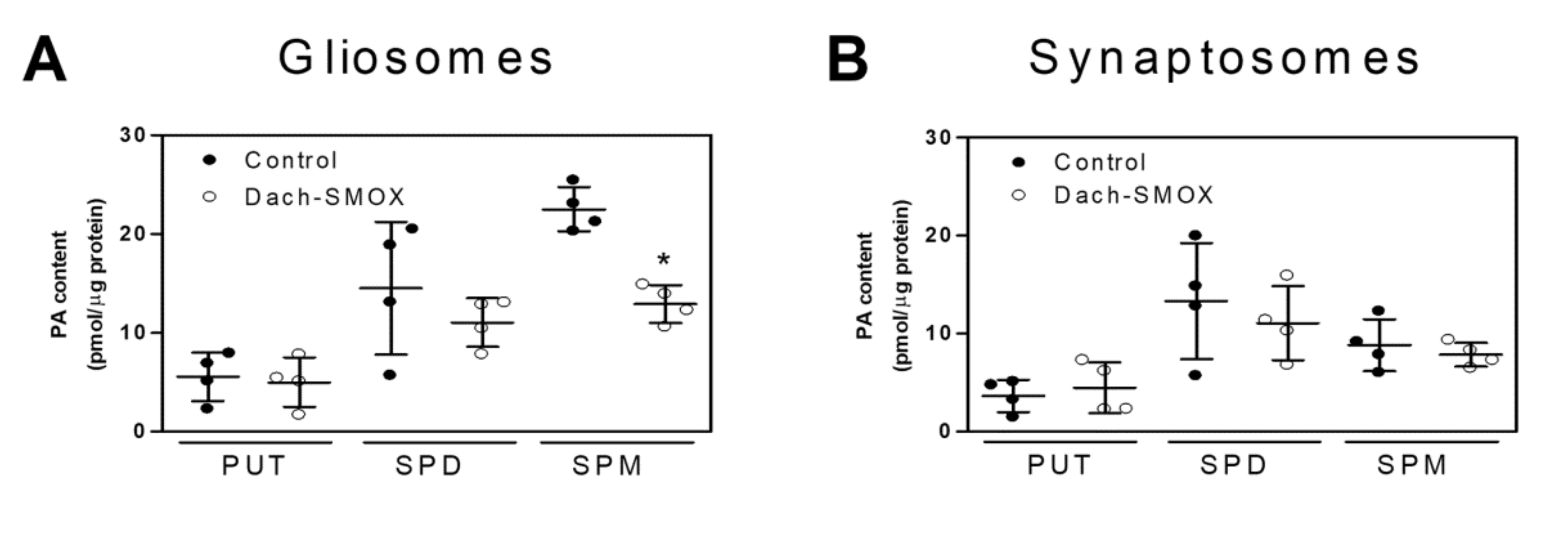
3.2. Polyamine Content in Purified Nerve Terminals and Astrocyte Processes
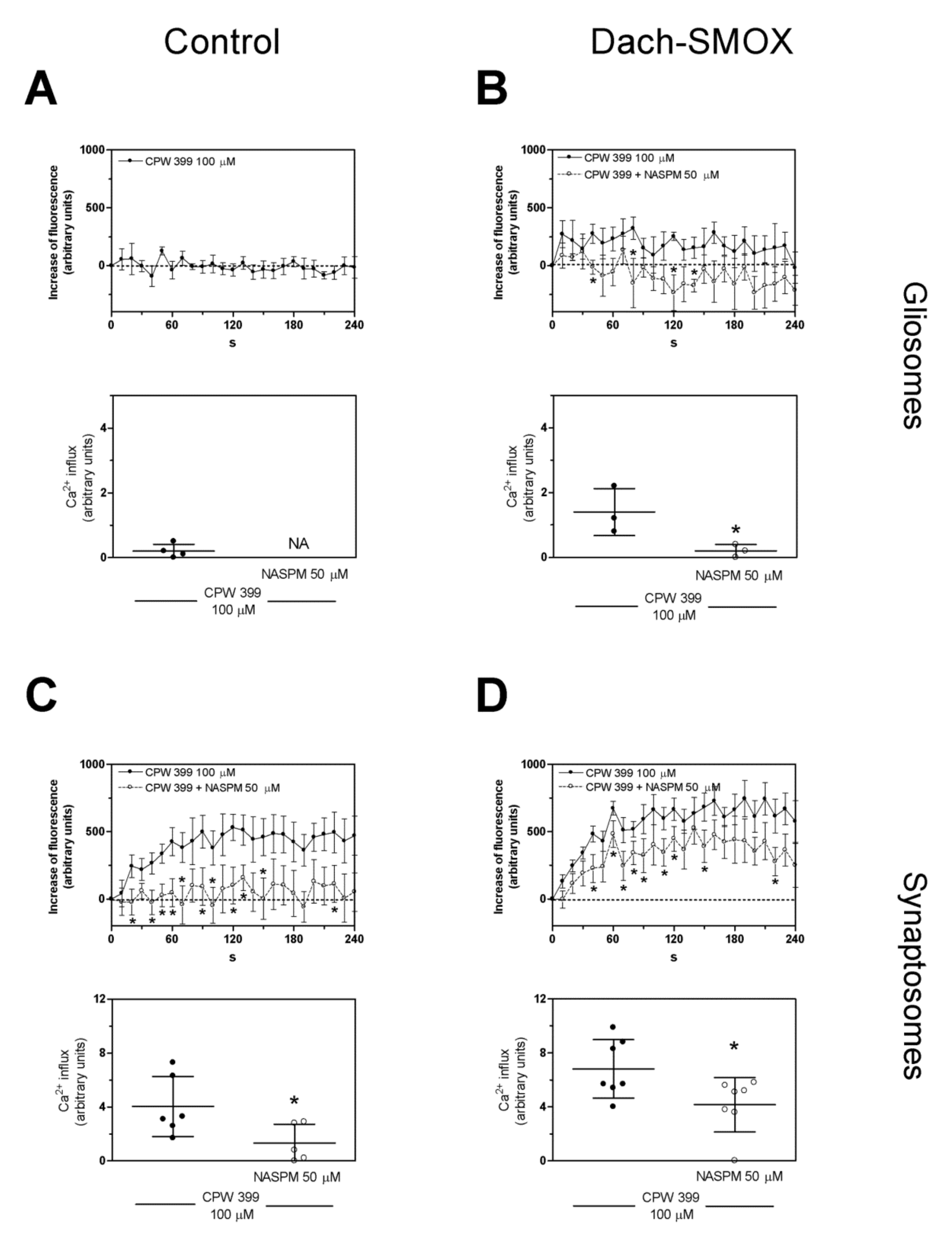
3.3. [Ca2+]i Evaluation in Purified Nerve Terminals and Astrocyte Processes
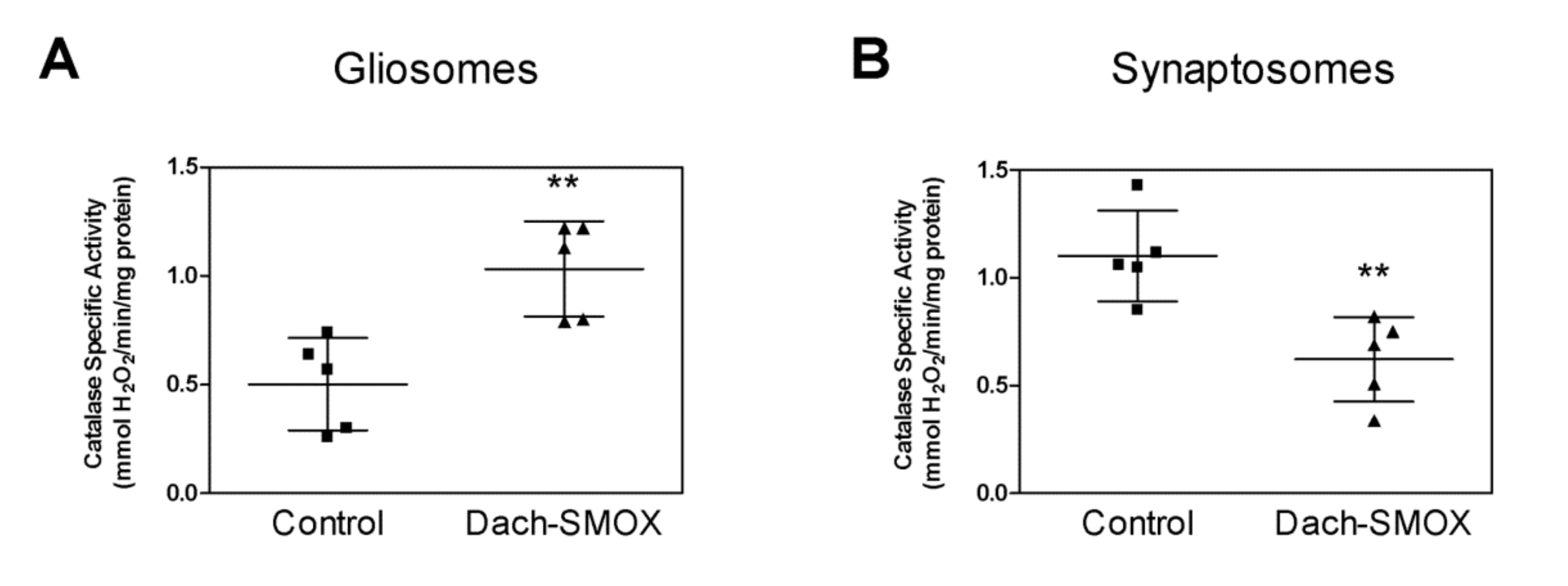
3.4. Oxidative Stress in Nerve Terminals and Astrocyte Processes from Dach-SMOX Cortex
4. Discussion
4.1. Increased Levels of the Astroglial Markers Ezrin and Vimentin
4.2. Altered Calcium Responses in Astrocyte Processes and Nerve Terminals
4.3. Reduction of Spermine in Astrocyte Processes, but Not in Nerve Terminals
4.4. Stimulation of Antioxidant Defense in Astrocyte Processes, and Impairment in Nerve Terminals
4.5. Insights on Polyamines and Neuron/Astrocytes Crosstalk in Dach-SMOX Mice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michael, A.J. Polyamines in Eukaryotes, Bacteria, and Archaea. J. Biol. Chem. 2016, 291, 14896–14903. [Google Scholar] [CrossRef] [Green Version]
- Cervelli, M.; Fratini, E.; Amendola, R.; Bianchi, M.; Signori, E.; Ferraro, E.; Lisi, A.; Federico, R.; Marcocci, L.; Mariottini, P. Increased Spermine Oxidase (SMO) Activity as a Novel Differentiation Marker of Myogenic C2C12 Cells. Int. J. Biochem. Cell Biol. 2009, 41, 934–944. [Google Scholar] [CrossRef]
- Fage, D.; Voltz, C.; Scatton, B.; Carter, C. Selective Release of Spermine and Spermidine from the Rat Striatum by N-Methyl-d-Aspartate Receptor Activation In Vivo. J. Neurochem. 1992, 58, 2170–2175. [Google Scholar] [CrossRef]
- Masuko, T.; Kusama-Eguchi, K.; Sakata, K.; Kusama, T.; Chaki, S.; Okuyama, S.; Williams, K.; Kashiwagi, K.; Igarashi, K. Polyamine Transport, Accumulation, and Release in Brain. J. Neurochem. 2003, 84, 610–617. [Google Scholar] [CrossRef]
- Skatchkov, S.N.; Woodbury-Fariña, M.A.; Eaton, M. The Role of Glia in Stress: Polyamines and Brain Disorders. Psychiatr. Clin. North. Am. 2014, 37, 653–678. [Google Scholar] [CrossRef] [Green Version]
- Skatchkov, S.N.; Antonov, S.M.; Eaton, M.J. Glia and Glial Polyamines. Role in Brain Function in Health and Disease. Biochem. Suppl. Ser. A Membr. Cell Biol. 2016, 10, 73–98. [Google Scholar] [CrossRef]
- Cervelli, M.; Bellavia, G.; D’Amelio, M.; Cavallucci, V.; Moreno, S.; Berger, J.; Nardacci, R.; Marcoli, M.; Maura, G.; Piacentini, M.; et al. A New Transgenic Mouse Model for Studying the Neurotoxicity of Spermine Oxidase Dosage in the Response to Excitotoxic Injury. PLoS ONE 2013, 8, e64810. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, M.; Angelucci, E.; Germani, F.; Amendola, R.; Mariottini, P. Inflammation, Carcinogenesis and Neurodegeneration Studies in Transgenic Animal Models for Polyamine Research. Amino Acids 2014, 46, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Vergani, L.; Passalacqua, M.; Ragazzoni, M.; Venturini, A.; Cecconi, F.; Berretta, N.; Mercuri, N.; D’Amelio, M.; Maura, G.; et al. Astrocyte-Dependent Vulnerability to Excitotoxicity in Spermine Oxidase-Overexpressing Mouse. NeuroMolecular Med. 2016, 18, 50–68. [Google Scholar] [CrossRef] [PubMed]
- Pietropaoli, S.; Leonetti, A.; Cervetto, C.; Venturini, A.; Mastrantonio, R.; Baroli, G.; Persichini, T.; Colasanti, M.; Maura, G.; Marcoli, M.; et al. Glutamate Excitotoxicity Linked to Spermine Oxidase Overexpression. Mol. Neurobiol. 2018, 55, 7259–7270. [Google Scholar] [CrossRef]
- Leonetti, A.; Baroli, G.; Fratini, E.; Pietropaoli, S.; Marcoli, M.; Mariottini, P.; Cervelli, M. Epileptic Seizures and Oxidative Stress in a Mouse Model Over-Expressing Spermine Oxidase. Amino Acids 2020, 52, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite Synapses: Glia, the Unacknowledged Partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Volterra, A.; Meldolesi, J. Astrocytes, from Brain Glue to Communication Elements: The Revolution Continues. Nat. Rev. Neurosci. 2005, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, P.; Bergersen, L.H.; Bhaukaurally, K.; Bezzi, P.; Santello, M.; Domercq, M.; Matute, C.; Tonello, F.; Gundersen, V.; Volterra, A. Glutamate Exocytosis from Astrocytes Controls Synaptic Strength. Nat. Neurosci. 2007, 10, 331–339. [Google Scholar] [CrossRef]
- Bernardinelli, Y.; Muller, D.; Nikonenko, I. Astrocyte-Synapse Structural Plasticity. Neural Plast. 2014, 2014, 232105. [Google Scholar] [CrossRef] [Green Version]
- Sofroniew, M. V Molecular Dissection of Reactive Astrogliosis and Glial Scar Formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef] [Green Version]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Cervetto, C.; Maura, G.; Marcoli, M. Inhibition of Presynaptic Release-Facilitatory Kainate Autoreceptors by Extracellular Cyclic GMP. J. Pharmacol. Exp. Ther. 2010, 332, 210–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervetto, C.; Venturini, A.; Guidolin, D.; Maura, G.; Passalacqua, M.; Tacchetti, C.; Cortelli, P.; Genedani, S.; Candiani, S.; Ramoino, P.; et al. Homocysteine and A2A-D2 Receptor-Receptor Interaction at Striatal Astrocyte Processes. J. Mol. Neurosci. 2018, 65, 456–466. [Google Scholar] [CrossRef]
- Amaroli, A.; Marcoli, M.; Venturini, A.; Passalacqua, M.; Agnati, L.F.; Signore, A.; Raffetto, M.; Maura, G.; Benedicenti, S.; Cervetto, C. Near-Infrared Laser Photons Induce Glutamate Release from Cerebrocortical Nerve Terminals. J. Biophotonics 2018, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Frattaroli, D.; Venturini, A.; Passalacqua, M.; Nobile, M.; Alloisio, S.; Tacchetti, C.; Maura, G.; Agnati, L.; Marcoli, M. Calcium-Permeable AMPA Receptors Trigger Vesicular Glutamate Release from Bergmann Gliosomes. Neuropharmacology 2015, 99. [Google Scholar] [CrossRef]
- Venturini, A.; Passalacqua, M.; Pelassa, S.; Pastorino, F.; Tedesco, M.; Cortese, K.; Gagliani, M.C.; Leo, G.; Maura, G.; Guidolin, D.; et al. Exosomes from Astrocyte Processes: Signaling to Neurons. Front. Pharmacol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Iga, K.; Shibata, T.; Shudo, M.; Kataoka, K. Glial Plasmalemmal Vesicles: A Subcellular Fraction from Rat Hippocampal Homogenate Distinct from Synaptosomes. Glia 1993, 9, 48–56. [Google Scholar] [CrossRef]
- Polini, B.; Cervetto, C.; Carpi, S.; Pelassa, S.; Gado, F.; Ferrisi, R.; Bertini, S.; Nieri, P.; Marcoli, M.; Manera, C. Positive Allosteric Modulation of CB1 and CB2 Cannabinoid Receptors Enhances the Neuroprotective Activity of a Dual CB1R/CB2R Orthosteric Agonist. Life 2020, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.; Gado, F.; Di Cesare Mannelli, L.; Cervetto, C.; Carpi, S.; Reynoso-Moreno, I.; Polini, B.; Vallini, E.; Chicca, S.; Lucarini, E.; et al. The Endocannabinoid System Dual-Target Ligand N-Cycloheptyl-1,2-Dihydro-5-Bromo-1-(4-Fluorobenzyl)-6-Methyl-2-Oxo-Pyridine-3-Carboxamide Improves Disease Severity in a Mouse Model of Multiple Sclerosis. Eur. J. Med. Chem. 2020, 208, 112858. [Google Scholar] [CrossRef]
- Ceci, R.; Duranti, G.; Leonetti, A.; Pietropaoli, S.; Spinozzi, F.; Marcocci, L.; Amendola, R.; Cecconi, F.; Sabatini, S.; Mariottini, P.; et al. Adaptive Responses of Heart and Skeletal Muscle to Spermine Oxidase Overexpression: Evaluation of a New Transgenic Mouse Model. Free Radic. Biol. Med. 2017, 103, 216–225. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Wiechelman, K.J.; Braun, R.D.; Fitzpatrick, J.D. Investigation of the Bicinchoninic Acid Protein Assay: Identification of the Groups Responsible for Color Formation. Anal. Biochem. 1988, 175, 231–237. [Google Scholar] [CrossRef]
- Franchi, A.; Pedrazzi, M.; Casazza, A.A.; Millo, E.; Damonte, G.; Salis, A.; Liessi, N.; Onofri, F.; Marte, A.; Casagrande, S.; et al. A Bioactive Olive Pomace Extract Prevents the Death of Murine Cortical Neurons Triggered by NMDAR Over-Activation. Molecules 2020, 25, 4385. [Google Scholar] [CrossRef]
- Pedrazzi, M.; Patrone, M.; Passalacqua, M.; Ranzato, E.; Colamassaro, D.; Sparatore, B.; Pontremoli, S.; Melloni, E. Selective Proinflammatory Activation of Astrocytes by High-Mobility Group Box 1 Protein Signaling. J. Immunol. 2007, 179, 8525–8532. [Google Scholar] [CrossRef] [Green Version]
- Schiweck, J.; Eickholt, B.J.; Murk, K. Important shapeshifter: Mechanisms allowing astrocytes to respond to the changing nervous system during development, injury and disease. Front. Cell. Neurosci. 2018, 12, 261. [Google Scholar] [CrossRef] [Green Version]
- Derouiche, A.; Geiger, K.D. Perspectives for Ezrin and Radixin in Astrocytes: Kinases, Functions and Pathology. Int. J. Mol. Sci. 2019, 20, 3776. [Google Scholar] [CrossRef] [Green Version]
- Lavialle, M.; Aumann, G.; Anlauf, E.; Proöls, F.; Arpin, M.; Derouiche, A. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 12915–12919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazargani, N.; Attwell, D. Astrocyte calcium signaling: The third wave. Nat. Neurosci. 2016, 19, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Lia, A.; Henriques, V.J.; Zonta, M.; Chiavegato, A.; Carmignoto, G.; Gómez-Gonzalo, M.; Losi, G. Calcium signals in astrocyte microdomains, a decade of great advances. Front. Cell. Neurosci. 2021, 15, 673433. [Google Scholar] [CrossRef] [PubMed]
- Cason, A.L.; Ikeguchi, Y.; Skinner, C.; Wood, T.C.; Holden, K.R.; Lubs, H.A.; Martinez, F.; Simensen, R.J.; Stevenson, R.E.; Pegg, A.E.; et al. X-linked spermine synthase gene (SMS) defect: The first polyamine deficiency syndrome. Eur. J. Hum. Genet. 2003, 11, 937–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeguchi, Y.; Bewley, M.C.; Pegg, A.E. Aminopropyltransferases: Function, structure and genetics. J. Biochem. 2006, 139, 1–9. [Google Scholar] [CrossRef]
- Sequeira, A.; Gwadry, F.G.; French-Mullen, J.M.; Canetti, L.; Gingras, Y.; Casero, R.A., Jr.; Rouleau, G.; Benkelfa, G.; Turecki, G. Implication of SSAT by gene expression and genetic variation in suicide and major depression. Arch. Gen. Psychiatry 2006, 63, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Virgili, M.; Crochemore, C.; Pena-Altamira, E.; Contestabile, A. Regional and temporal alterations of ODC/polyamine system during ALS-like neurodegenerative motor syndrome in G93A transgenic mice. Neurochem. Int. 2006, 48, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Izumi, M.; Osano, Y.; Miura, N.; Takatsu, S.; Terao, S.; Mitsuma, T. Polyamine concentrations in the brain of vitamin B12-deficient rats. Exp. Biol. Med. 2003, 228, 1069–1071. [Google Scholar] [CrossRef] [PubMed]
- Janne, J.; Alhonen, L.; Pietila, M.; Keinanen, T.A.; Uimari, A.; Hyvonen, M.T.; Pirinen, E.; Jarvinen, A. Genetic manipulation of polyamine catabolism in rodents. J. Biochem. 2006, 139, 155–160. [Google Scholar] [CrossRef]
- Antony, T.; Hoyer, W.; Cherny, D.; Heim, G.; Jovin, T.M.; Subramaniam, V. Cellular polyamines promote the aggregation of α-synuclein. J. Biol. Chem. 2003, 278, 3235–3240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colton, C.A.; Xu, Q.; Burke, J.R.; Bae, S.Y.; Wakefield, J.K.; Nair, A.; Strittmatter, W.J.; Vitek, M.P. Disrupted spermine homeostasis: A novel mechanism in polyglutamine-mediated aggregation and cell death. J. Neurosci. 2004, 24, 7118–7127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collingridge, G.L.; Bliss, T.V.P. NMDA Receptors—Their Role in Long-Term Potentiation. Trends Neurosci. 1987, 10, 288–293. [Google Scholar] [CrossRef]
- Collingridge, G.L.; Singer, W. Excitatory Amino Acid Receptors and Synaptic Plasticity. Trends Pharmacol. Sci. 1990, 11, 290–296. [Google Scholar] [CrossRef]
- Bashir, Z.I.; Alford, S.; Davies, S.N.; Randall, A.D.; Collingridge, G.L. Long-Term Potentiation of NMDA Receptor-Mediated Synaptic Transmission in the Hippocampus. Nature 1991, 349, 156–158. [Google Scholar] [CrossRef]
- Williams, K.; Zappia, A.M.; Pritchett, D.B.; Shen, Y.M.; Molinoff, P.B. Sensitivity of the N-Methyl-D-Aspartate Receptor to Polyamines Is Controlled by NR2 Subunits. Mol. Pharmacol. 1994, 45, 803–809. [Google Scholar]
- Zhang, L.; Zheng, X.; Paupard, M.C.; Wang, A.P.; Santchi, L.; Friedman, L.K.; Zukin, R.S.; Bennett, M. V Spermine Potentiation of Recombinant N-Methyl-D-Aspartate Receptors Is Affected by Subunit Composition. Proc. Natl. Acad. Sci. USA 1994, 91, 10883–10887. [Google Scholar] [CrossRef] [Green Version]
- Williams, K. Interactions of Polyamines with Ion Channels. Biochem. J. 1997, 325, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The Glutamate Receptor Ion Channels. Pharmacol. Rev. 1999, 51, 7–61. [Google Scholar] [PubMed]
- Gandhi, S.; Abramov, A.Y. Mechanism of Oxidative Stress in Neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, C.; Kong, J. Oxidative Stress in Neurodegenerative Diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [Green Version]


| Mechanism | Type of Change | Consequence | Ref |
|---|---|---|---|
| Ca2+-permeable AMPA receptor | expression of functional | glutamate release | [9] |
| Xc- transporter | increased function | glutamate release (in-outside transport) | [10] |
| EAAT1, EAAT2 | reduced expression | impaired glutamate clearance | [10] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervetto, C.; Averna, M.; Vergani, L.; Pedrazzi, M.; Amato, S.; Pelassa, S.; Giuliani, S.; Baldini, F.; Maura, G.; Mariottini, P.; et al. Reactive Astrocytosis in a Mouse Model of Chronic Polyamine Catabolism Activation. Biomolecules 2021, 11, 1274. https://doi.org/10.3390/biom11091274
Cervetto C, Averna M, Vergani L, Pedrazzi M, Amato S, Pelassa S, Giuliani S, Baldini F, Maura G, Mariottini P, et al. Reactive Astrocytosis in a Mouse Model of Chronic Polyamine Catabolism Activation. Biomolecules. 2021; 11(9):1274. https://doi.org/10.3390/biom11091274
Chicago/Turabian StyleCervetto, Chiara, Monica Averna, Laura Vergani, Marco Pedrazzi, Sarah Amato, Simone Pelassa, Stefano Giuliani, Francesca Baldini, Guido Maura, Paolo Mariottini, and et al. 2021. "Reactive Astrocytosis in a Mouse Model of Chronic Polyamine Catabolism Activation" Biomolecules 11, no. 9: 1274. https://doi.org/10.3390/biom11091274
APA StyleCervetto, C., Averna, M., Vergani, L., Pedrazzi, M., Amato, S., Pelassa, S., Giuliani, S., Baldini, F., Maura, G., Mariottini, P., Marcoli, M., & Cervelli, M. (2021). Reactive Astrocytosis in a Mouse Model of Chronic Polyamine Catabolism Activation. Biomolecules, 11(9), 1274. https://doi.org/10.3390/biom11091274











