The Prognostic Value of Locoregional Interventions for BRAF V600E Metastatic Colorectal Cancer: A Retrospective Cohort Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Treatment Features and Definitions
2.3. Statistical Analysis
3. Results
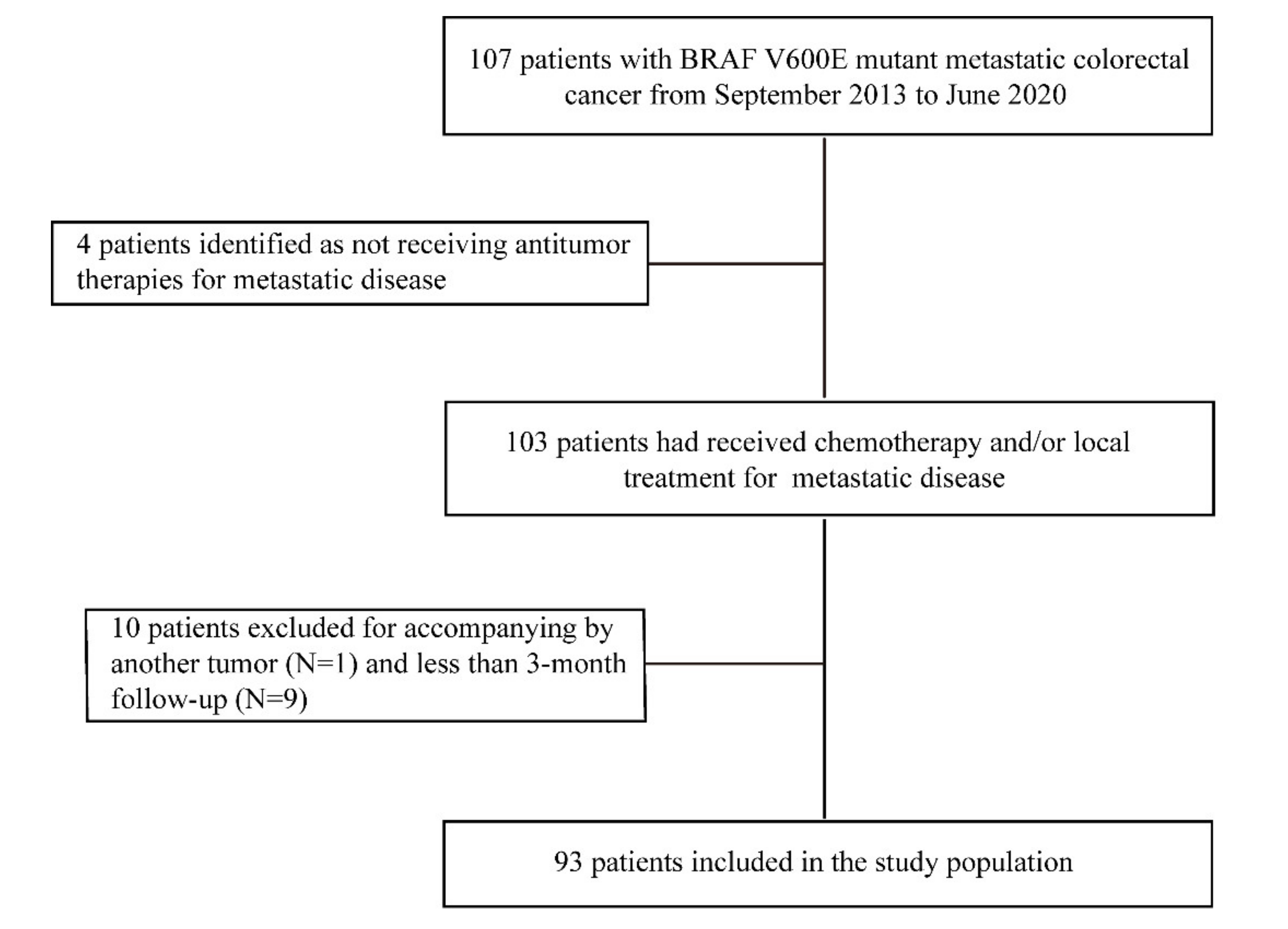
3.1. Study Population
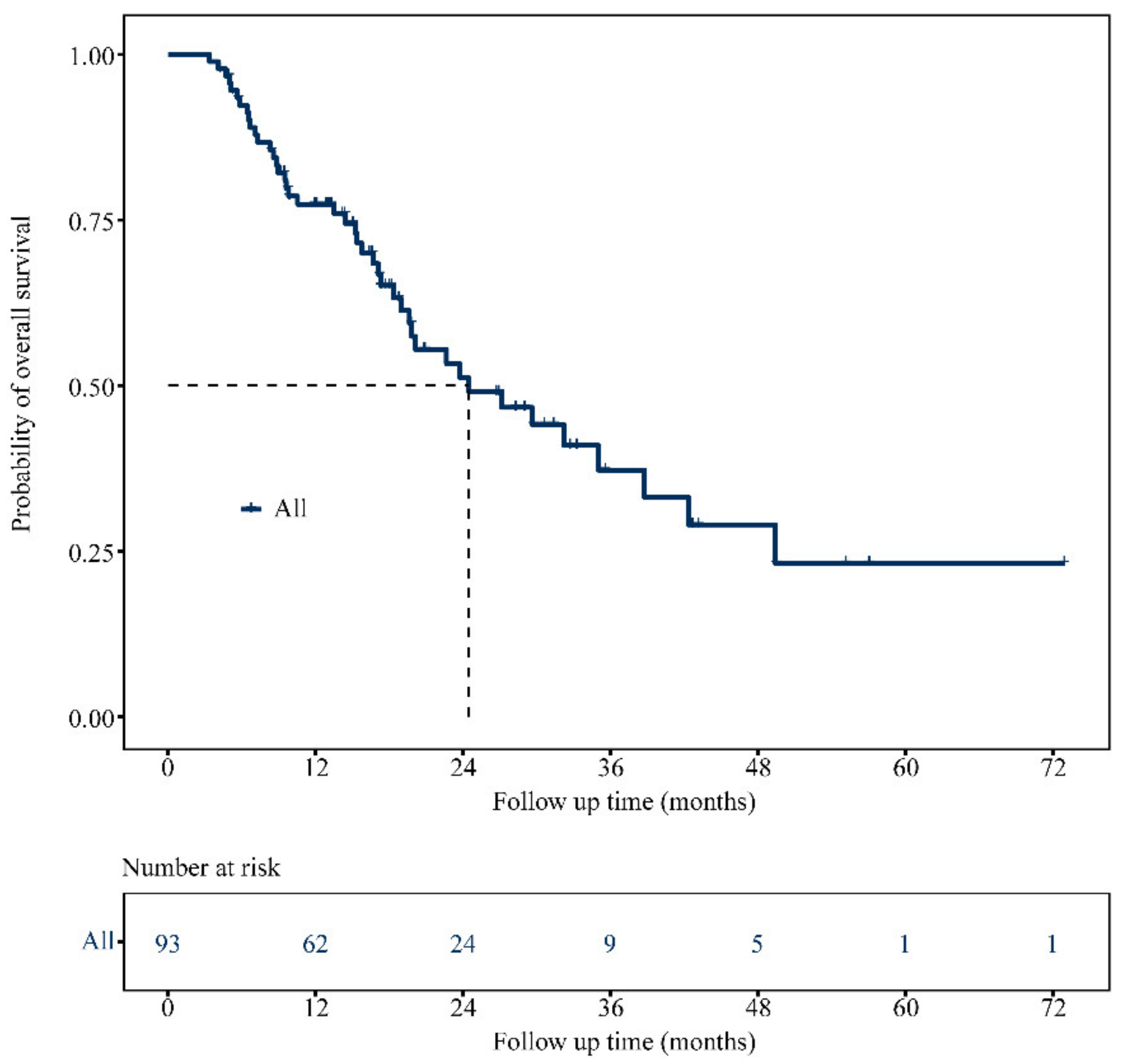
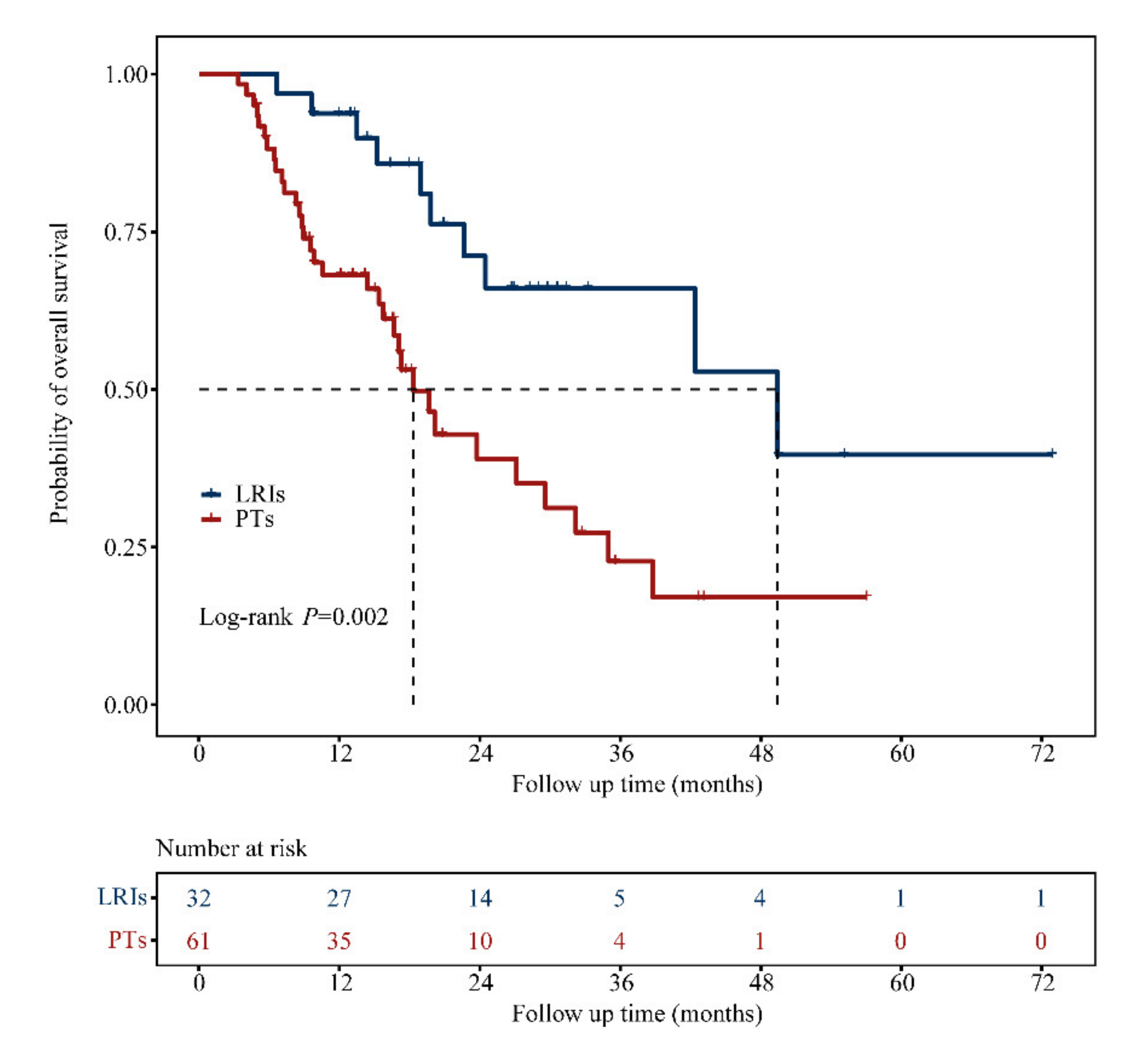
3.2. OS Analysis
3.3. Clinical Characteristics between Treatment Groups
3.4. Focus on the LRIs Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Richman, S.D.; Seymour, M.T.; Chambers, P.; Elliott, F.; Daly, C.L.; Meade, A.M.; Taylor, G.; Barrett, J.H.; Quirke, P. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: Results from the MRC FOCUS trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 5931–5937. [Google Scholar] [CrossRef] [PubMed]
- Seligmann, J.F.; Fisher, D.; Smith, C.G.; Richman, S.D.; Elliott, F.; Brown, S.; Adams, R.; Maughan, T.; Quirke, P.; Cheadle, J.; et al. Investigating the poor outcomes of BRAF-mutant advanced colorectal cancer: Analysis from 2530 patients in randomised clinical trials. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, T.; Shimokawa, M.; Kotaka, M.; Matsumoto, T.; Nagai, H.; Boku, S.; Shibata, N.; Yasui, H.; Satake, H. Clinical and prognostic features of patients with detailed RAS/BRAF-mutant colorectal cancer in Japan. BMC Cancer 2021, 21, 518. [Google Scholar] [CrossRef]
- Maughan, T.S.; Adams, R.A.; Smith, C.G.; Meade, A.M.; Seymour, M.T.; Wilson, R.H.; Idziaszczyk, S.; Harris, R.; Fisher, D.; Kenny, S.L.; et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial. Lancet 2011, 377, 2103–2114. [Google Scholar] [CrossRef]
- Tie, J.; Gibbs, P.; Lipton, L.; Christie, M.; Jorissen, R.N.; Burgess, A.W.; Croxford, M.; Jones, I.; Langland, R.; Kosmider, S.; et al. Optimizing targeted therapeutic development: Analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int. J. Cancer 2011, 128, 2075–2084. [Google Scholar] [CrossRef]
- Tran, B.; Kopetz, S.; Tie, J.; Gibbs, P.; Jiang, Z.-Q.; Lieu, C.H.; Agarwal, A.; Maru, D.M.; Sieber, O.; Desai, J. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011, 117, 4623–4632. [Google Scholar] [CrossRef]
- Jones, J.C.; Renfro, L.A.; Al-Shamsi, H.O.; Schrock, A.B.; Rankin, A.; Zhang, B.Y.; Kasi, P.M.; Voss, J.S.; Leal, A.D.; Sun, J.; et al. BRAF Mutations Define a Clinically Distinct Molecular Subtype of Metastatic Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, G.N.; Dal Pozzo, C.A.; Depetris, I.; Schirripa, M.; Brignola, S.; Biason, P.; Balistreri, M.; Dal Santo, L.; Lonardi, S.; Munari, G.; et al. The heterogeneous clinical and pathological landscapes of metastatic-mutated colorectal cancer. Cancer Cell Int. 2020, 20, 30. [Google Scholar] [CrossRef]
- Tol, J.; Nagtegaal, I.D.; Punt, C.J. BRAF mutation in metastatic colorectal cancer. N. Engl. J. Med. 2009, 361, 99. [Google Scholar]
- Sveen, A.; Kopetz, S.; Lothe, R.A. Biomarker-guided therapy for colorectal cancer: Strength in complexity. Nat. Rev. Clin. Oncol. 2020, 17, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology—Colon Cancer, Version 2.2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 4 April 2021).
- Kobayashi, S.; Takahashi, S.; Takahashi, N.; Masuishi, T.; Shoji, H.; Shinozaki, E.; Yamaguchi, T.; Kojima, M.; Gotohda, N.; Nomura, S.; et al. Survival Outcomes of Resected BRAF V600E Mutant Colorectal Liver Metastases: A Multicenter Retrospective Cohort Study in Japan. Ann. Surg. Oncol. 2020, 27, 3307–3315. [Google Scholar] [CrossRef]
- Gagniere, J.; Dupre, A.; Gholami, S.S.; Pezet, D.; Boerner, T.; Gonen, M.; Kingham, T.P.; Allen, P.J.; Balachandran, V.P.; De Matteo, R.P.; et al. Is Hepatectomy Justified for BRAF Mutant Colorectal Liver Metastases?: A Multi-institutional Analysis of 1497 Patients. Ann. Surg. 2020, 271, 147–154. [Google Scholar] [CrossRef]
- Prasanna, T.; Wong, R.; Price, T.; Shapiro, J.; Tie, J.; Wong, H.-L.; Nott, L.; Roder, D.; Lee, M.; Kosmider, S.; et al. Metastasectomy and BRAF mutation; an analysis of survival outcome in metastatic colorectal cancer. Curr. Probl. Cancer 2021, 45, 100637. [Google Scholar] [CrossRef]
- Johnson, B.; Jin, Z.; Truty, M.J.; Smoot, R.L.; Nagorney, D.M.; Kendrick, M.L.; Kipp, B.R.; Grothey, A. Impact of Metastasectomy in the Multimodality Approach for BRAF V600E Metastatic Colorectal Cancer: The Mayo Clinic Experience. Oncologist 2018, 23, 128–134. [Google Scholar] [CrossRef]
- Loupakis, F.; Intini, R.; Cremolini, C.; Orlandi, A.; Sartore-Bianchi, A.; Pietrantonio, F.; Pella, N.; Spallanzani, A.; Dell’Aquila, E.; Scartozzi, M.; et al. A validated prognostic classifier for BRAF-mutated metastatic colorectal cancer: The ‘BRAF BeCool’ study. Eur. J. Cancer 2019, 118, 121–130. [Google Scholar] [CrossRef]
- Moretto, R.; Rossini, D.; Zucchelli, G.; Lonardi, S.; Bergamo, F.; Santini, D.; Cupini, S.; Tomasello, G.; Caponnetto, S.; Zaniboni, A.; et al. Oligometastatic colorectal cancer: Prognosis, role of locoregional treatments and impact of first-line chemotherapy-a pooled analysis of TRIBE and TRIBE2 studies by Gruppo Oncologico del Nord Ovest. Eur. J. Cancer 2020, 139, 81–89. [Google Scholar] [CrossRef]
- Sánchez-Hidalgo, J.M.; Rodríguez-Ortiz, L.; Arjona-Sánchez, Á.; Rufián-Peña, S.; Casado-Adam, Á.; Cosano-Álvarez, A.; Briceño-Delgado, J. Colorectal peritoneal metastases: Optimal management review. World J. Gastroenterol. 2019, 25, 3484–3502. [Google Scholar] [CrossRef]
- Cremolini, C.; Antoniotti, C.; Stein, A.; Bendell, J.; Gruenberger, T.; Rossini, D.; Masi, G.; Ongaro, E.; Hurwitz, H.; Falcone, A.; et al. Individual Patient Data Meta-Analysis of FOLFOXIRI Plus Bevacizumab Versus Doublets Plus Bevacizumab as Initial Therapy of Unresectable Metastatic Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 3314–3324. [Google Scholar] [CrossRef]
- Fouchardière, C.; Cohen, R.; Malka, D.; Guimbaud, R.; Bourien, H.; Lièvre, A.; Cacheux, W.; Artru, P.; François, E.; Gilabert, M.; et al. Characteristics of BRAF V600E Mutant, Deficient Mismatch Repair/Proficient Mismatch Repair, Metastatic Colorectal Cancer: A Multicenter Series of 287 Patients. Oncologist 2019, 24, e1331. [Google Scholar] [CrossRef]
- Morris, V.K.; Kee, B.K.; Overman, M.J.; Fogelman, D.R.; Dasari, A.; Raghav, K.P.S.; Shureiqi, I.; Johnson, B.; Parseghian, C.M.; Wolff, R.A. Clinical and Pathologic Factors Associated with Survival in Brafv600e Colorectal Cancers; American Society of Clinical Oncology: Alexandria, VA, USA, 2020. [Google Scholar]
- Passiglia, F.; Bronte, G.; Bazan, V.; Galvano, A.; Vincenzi, B.; Russo, A. Can KRAS and BRAF mutations limit the benefit of liver resection in metastatic colorectal cancer patients? A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2016, 99, 150–157. [Google Scholar] [CrossRef]
- Tosi, F.; Magni, E.; Amatu, A.; Mauri, G.; Bencardino, K.; Truini, M.; Veronese, S.; De Carlis, L.; Ferrari, G.; Nichelatti, M.; et al. Effect of KRAS and BRAF Mutations on Survival of Metastatic Colorectal Cancer After Liver Resection: A Systematic Review and Meta-Analysis. Clin. Colorectal Cancer 2017, 16, e153–e163. [Google Scholar] [CrossRef]
- Cloyd, J.; Tzeng, C.; Mizuno, T.; Conrad, C.; Omichi, K.; Aloia, T.; Vauthey, J.; Chun, Y. Braf mutation is not a contraindication to resection of colorectal liver metastases. Health Promot. Board 2017, 19, S55–S56. [Google Scholar] [CrossRef][Green Version]
- Pitroda, S.P.; Khodarev, N.N.; Huang, L.; Uppal, A.; Wightman, S.C.; Ganai, S.; Joseph, N.; Pitt, J.; Brown, M.; Forde, M.; et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat. Commun. 2018, 9, 1793. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Lefevre, J.H.; Chevalier, J.; Brouquet, A.; Marchal, F.; Classe, J.-M.; Ferron, G.; Guilloit, J.-M.; Meeus, P.; Goéré, D.; et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Cashin, P.H.; Mahteme, H.; Spång, N.; Syk, I.; Frödin, J.E.; Torkzad, M.; Glimelius, B.; Graf, W. Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: A randomised trial. Eur. J. Cancer 2016, 53, 155–162. [Google Scholar] [CrossRef]
- Quenet, F.; Goéré, D.; Mehta, S.S.; Roca, L.; Dumont, F.; Hessissen, M.; Saint-Aubert, B.; Elias, D. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann. Surg. 2011, 254, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, G.; Petrelli, F.; Ghidini, M.; Russo, A.; Passalacqua, R.; Barni, S. FOLFOXIRI Plus Bevacizumab as Conversion Therapy for Patients with Initially Unresectable Metastatic Colorectal Cancer: A Systematic Review and Pooled Analysis. JAMA Oncol. 2017, 3, e170278. [Google Scholar] [CrossRef]
- Margonis, G.A.; Sergentanis, T.N.; Ntanasis-Stathopoulos, I.; Andreatos, N.; Tzanninis, I.-G.; Sasaki, K.; Psaltopoulou, T.; Wang, J.; Buettner, S.; He, J. Impact of surgical margin width on recurrence and overall survival following R0 hepatic resection of colorectal metastases: A systematic review and meta-analysis. Ann. Surg. 2018, 267, 1047–1055. [Google Scholar] [CrossRef]
- Taieb, J.; Kourie, H.R.; Emile, J.-F.; Le Malicot, K.; Balogoun, R.; Tabernero, J.; Mini, E.; Folprecht, G.; Van Laethem, J.-L.; Mulot, C.; et al. Association of Prognostic Value of Primary Tumor Location in Stage III Colon Cancer with RAS and BRAF Mutational Status. JAMA Oncol. 2018, 4, e173695. [Google Scholar] [CrossRef] [PubMed]
- Bachet, J.B.; Moreno-Lopez, N.; Vigano, L.; Marchese, U.; Gelli, M.; Raoux, L.; Truant, S.; Laurent, C.; Herrero, A.; Le Roy, B.; et al. BRAF mutation is not associated with an increased risk of recurrence in patients undergoing resection of colorectal liver metastases. Br. J. Surg. 2019, 106, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Bruzzi, M.; Auclin, E.; Lo Dico, R.; Voron, T.; Karoui, M.; Espin, E.; Cianchi, F.; Weitz, J.; Buggenhout, A.; Malafosse, R.; et al. Influence of Molecular Status on Recurrence Site in Patients Treated for a Stage III Colon Cancer: A Post Hoc Analysis of the PETACC-8 Trial. Ann. Surg. Oncol. 2019, 26, 3561–3567. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A.; Shi, Q.; Allegra, C.J.; Smyrk, T.C.; Thibodeau, S.N.; Goldberg, R.M.; Meyers, J.P.; Pogue-Geile, K.L.; Yothers, G.; Sargent, D.J.; et al. Association of DNA Mismatch Repair and Mutations in BRAF and KRAS with Survival after Recurrence in Stage III Colon Cancers: A Secondary Analysis of 2 Randomized Clinical Trials. JAMA Oncol. 2017, 3, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.E.; Johnson, B.; Kugathasan, L.; Morris, V.K.; Raghav, K.; Swanson, L.; Lim, H.J.; Renouf, D.J.; Gill, S.; Wolber, R.; et al. Population-based Screening for in Metastatic Colorectal Cancer Reveals Increased Prevalence and Poor Prognosis. Clin. Cancer Res. 2020, 26, 4599–4605. [Google Scholar] [CrossRef] [PubMed]
- Venderbosch, S.; Nagtegaal, I.D.; Maughan, T.S.; Smith, C.G.; Cheadle, J.P.; Fisher, D.; Kaplan, R.; Quirke, P.; Seymour, M.T.; Richman, S.D.; et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: A pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin. Cancer Res. 2014, 20, 5322–5330. [Google Scholar] [CrossRef]
- Loupakis, F.; Biason, P.; Prete, A.A.; Cremolini, C.; Pietrantonio, F.; Pella, N.; Dell’Aquila, E.; Sperti, E.; Zichi, C.; Intini, R.; et al. CK7 and consensus molecular subtypes as major prognosticators in BRAF mutated metastatic colorectal cancer. Br. J. Cancer 2019, 121, 593–599. [Google Scholar] [CrossRef]

| Characteristics | No. of Patients (%) | |
|---|---|---|
| Overall (N = 93) | ||
| Age at metastases | Median, range | 52.0 (14.0–79.0) |
| Gender | Male | 62 (66.7) |
| Female | 31 (33.3) | |
| Primary tumor site | Left | 52 (55.9) |
| Right | 39 (41.9) | |
| Multisite | 2 (2.2) | |
| Differentiation | Well or moderate | 66 (71.0) |
| Poor | 26 (27.9) | |
| Unknown | 1 (1.1) | |
| Primary tumor resected | Yes | 68 (73.1) |
| No | 25 (26.9) | |
| Primary T stage | T3 | 31 (33.3) |
| T4 | 47 (50.6) | |
| Tx | 15 (16.1) | |
| Primary lymph node status | Positive | 77 (82.8) |
| Negative | 16 (17.2) | |
| Previous adjuvant chemotherapy | Yes | 22 (23.7) |
| No | 71 (76.3) | |
| Metastatic disease | Synchronous | 71 (76.3) |
| Metachronous | 22 (23.7) | |
| Number of organs involved | <2 | 46 (49.5) |
| ≥2 | 47 (50.5) | |
| Oligometastatic state a | OMD | 11 (12.0) |
| Non-OMD | 81 (88.0) | |
| Liver or lung metastasis only | Yes | 20 (21.5) |
| No | 73 (78.5) | |
| Peritoneal metastasis only | Yes | 22 (23.7) |
| No | 71 (76.3) | |
| Multiple non-peritoneal metastases b | Yes | 26 (28.0) |
| No | 67 (72.0) | |
| First-line chemotherapy | Tri-chemo | 32 (34.4) |
| Bi-chemo | 54 (58.1) | |
| Others | 3 (3.2) | |
| Unknown | 4 (4.3) | |
| Bev in first-line chemotherapy | Yes | 48 (51.6) |
| No | 41 (44.1) | |
| Unknown | 4 (4.3) | |
| Treatment of metastases | LRIs | 32 (34.4) |
| PTs | 61 (65.6) | |
| RAS status | Mutant | 3 (3.2) |
| Wide-type | 85 (91.4) | |
| Unknown | 5 (5.4) | |
| MMR/MSI status | dMMR/MSI-H | 1 (1.1) |
| pMMR/MSS | 86 (92.5) | |
| Unknown | 6 (6.4) |
| Variables | N | Univariate Cox Analysis | Multivariate Cox Analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | ||
| Age at metastases (<65 vs. ≥65) | 76/17 | 1.19 | 0.55–2.59 | 0.666 | |||
| Gender (Male vs. female) | 62/31 | 0.79 | 0.40–1.54 | 0.485 | |||
| Primary tumor site (Left vs. right) | 52/39 | 0.87 | 0.46–1.65 | 0.667 | |||
| Differentiation (Well or moderate vs. poor) | 66/26 | 1.14 | 0.58–2.23 | 0.705 | |||
| Primary tumor resected (Yes vs. no) | 68/25 | 1.93 | 0.94–3.98 | 0.075 | |||
| Primary lymph node status (Negative vs. positive) | 16/77 | 0.90 | 0.40–2.06 | 0.809 | |||
| Previous adjuvant chemotherapy (Yes vs. no) | 22/71 | 1.41 | 0.67–2.94 | 0.366 | |||
| Metastatic disease (Metachronous vs. synchronous) | 22/71 | 1.80 | 0.80–4.05 | 0.155 | |||
| Number of organs involved (<2 vs. ≥2) | 46/47 | 2.05 | 1.11–3.79 | 0.023 | |||
| Oligometastatic state (Non-OMD vs. OMD) | 81/11 | 0.13 | 0.02–0.97 | 0.046 | |||
| Liver or lung metastasis only (Yes vs. no) | 20/73 | 2.36 | 0.92–6.02 | 0.073 | |||
| Peritoneal metastasis only (Yes vs. no) | 22/71 | 1.79 | 0.85–3.76 | 0.126 | |||
| Multiple non-peritoneal metastases (Yes vs. no) | 26/67 | 0.70 | 0.36–1.37 | 0.296 | |||
| First-line chemotherapy (Bi-chemo or others vs. tri-chemo) | 57/32 | 0.97 | 0.48–1.97 | 0.931 | |||
| Bev in first-line treatment (Yes vs. no) | 48/41 | 1.37 | 0.73–2.58 | 0.333 | |||
| Treatment of metastases (PTs vs. LRIs) | 61/32 | 0.34 | 0.17–0.70 | 0.003 | 0.46 | 0.22–0.98 | 0.044 |
| Characteristics | No. of Patients (%) | |||
|---|---|---|---|---|
| PTs (N = 61) | LRIs (N = 32) | p Value | ||
| Age at metastases | <65 | 50 (82.0) | 26 (81.2) | 0.932 |
| ≥65 | 11 (18.0) | 6 (18.8) | ||
| Gender | Male | 39 (63.9) | 23 (71.9) | 0.440 |
| Female | 22 (36.1) | 9 (28.1) | ||
| Primary tumor site | Left | 32 (52.5) | 20 (62.5) | 0.448 |
| Right | 28 (45.9) | 11 (34.4) | ||
| Multisite | 1 (1.6) | 1 (3.1) | ||
| Differentiation | Well or moderate | 42 (68.9) | 24 (75.0) | 0.388 |
| Poor | 19 (31.1) | 7 (21.9) | ||
| Unknown | -- | 1 (3.1) | ||
| Primary tumor resected | Yes | 36 (59.0) | 32 (100.0) | <0.001 |
| No | 25 (41.0) | -- | ||
| Primary T stage | T3 | 17 (27.9) | 14 (43.8) | 0.103 |
| T4 | 31 (50.8) | 16 (50.0) | ||
| Tx | 13 (21.3) | 2 (6.2) | ||
| Primary lymph node status | Positive | 54 (88.5) | 23 (71.9) | 0.043 |
| Negative | 7 (11.5) | 9 (28.1) | ||
| Previous adjuvant chemotherapy | Yes | 14 (23.0) | 8 (25.0) | 0.825 |
| No | 47 (77.0) | 24 (75.0) | ||
| Metastatic disease | Synchronous | 47 (77.0) | 24 (75.0) | 0.825 |
| Metachronous | 14 (23.0) | 8 (25.0) | ||
| Number of organs involved | <2 | 19 (31.1) | 27 (84.4) | <0.001 |
| ≥2 | 42 (68.9) | 5 (15.6) | ||
| Oligometastatic state a | OMD | 1 (1.7) | 10 (31.3) | <0.001 |
| Non-OMD | 59 (98.3) | 22 (68.7) | ||
| Metastatic location(s) | Liver or lung only | 6 (9.8) | 14 (43.8) | <0.001 |
| Peritoneal only | 9 (14.8) | 13 (40.6) | 0.005 | |
| Distant lymph nodes only | 4 (6.6) | -- | 0.295 | |
| Non-isolated peritoneal metastasis b | 17 (27.9) | 4 (12.5) | 0.092 | |
| Involved one other organ | 5 (29.4) | 4 (100.0) | 0.021 | |
| Involved > one other organ | 12 (70.6) | -- | -- | |
| Multiple non-peritoneal metastases b | 25 (41.0) | 1 (3.1) | <0.001 | |
| First-line chemotherapy | Tri-chemo | 19 (31.2) | 13 (40.6) | 0.180 |
| Bi-chemo | 39 (63.9) | 15 (46.9) | ||
| Others | 2 (3.3) | 1 (3.1) | ||
| Unknown | 1 (1.6) | 3 (9.4) | ||
| Bev in first-line chemotherapy | Yes | 30 (49.2) | 18 (56.2) | 0.113 |
| No | 30 (49.2) | 11 (34.4) | ||
| Unknown | 1 (1.6) | 3 (9.4) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, L.-F.; Ji, X.-M.; Ren, C.; Wang, Z.-Q.; Lin, C.-P.; Chen, D.-L.; Cai, Y.-Q.; Jin, Y.; Qiu, M.-Z.; Du, Z.-M.; et al. The Prognostic Value of Locoregional Interventions for BRAF V600E Metastatic Colorectal Cancer: A Retrospective Cohort Analysis. Biomolecules 2021, 11, 1268. https://doi.org/10.3390/biom11091268
Ye L-F, Ji X-M, Ren C, Wang Z-Q, Lin C-P, Chen D-L, Cai Y-Q, Jin Y, Qiu M-Z, Du Z-M, et al. The Prognostic Value of Locoregional Interventions for BRAF V600E Metastatic Colorectal Cancer: A Retrospective Cohort Analysis. Biomolecules. 2021; 11(9):1268. https://doi.org/10.3390/biom11091268
Chicago/Turabian StyleYe, Liu-Fang, Xiao-Meng Ji, Chao Ren, Zhi-Qiang Wang, Chun-Ping Lin, Dong-Liang Chen, Yan-Qing Cai, Ying Jin, Miao-Zhen Qiu, Zi-Ming Du, and et al. 2021. "The Prognostic Value of Locoregional Interventions for BRAF V600E Metastatic Colorectal Cancer: A Retrospective Cohort Analysis" Biomolecules 11, no. 9: 1268. https://doi.org/10.3390/biom11091268
APA StyleYe, L.-F., Ji, X.-M., Ren, C., Wang, Z.-Q., Lin, C.-P., Chen, D.-L., Cai, Y.-Q., Jin, Y., Qiu, M.-Z., Du, Z.-M., Xi, S.-Y., Zhang, D.-S., Wang, F., Wang, F.-H., Xu, R.-H., Li, Y.-H., & Wang, D.-S. (2021). The Prognostic Value of Locoregional Interventions for BRAF V600E Metastatic Colorectal Cancer: A Retrospective Cohort Analysis. Biomolecules, 11(9), 1268. https://doi.org/10.3390/biom11091268






