Function of Dental Follicle Progenitor/Stem Cells and Their Potential in Regenerative Medicine: From Mechanisms to Applications
Abstract
1. Introduction
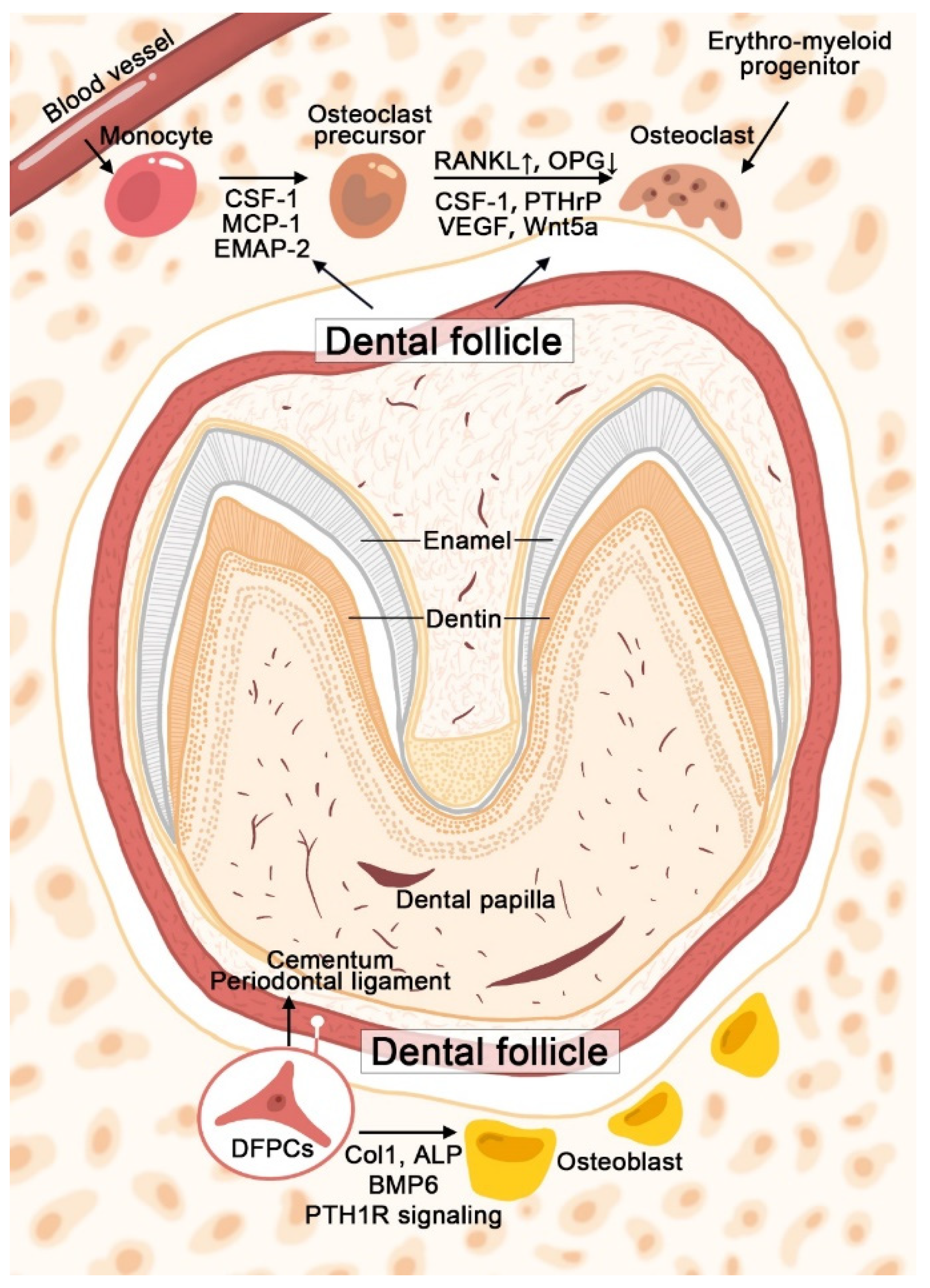
2. The Role of DFPCs during Tooth Eruption
2.1. Regulation of Osteoclastogenesis and Bone Resorption Process
2.2. DFPCs Contribute to Osteoblast Differentiation and Bone Formation
2.2.1. DFPCs Are Involved in Apically Alveolar Bone Formation In Vivo
2.2.2. DFPCs Differentiate into Osteogenic Lineage In Vitro
2.3. DFPCs Give Rise to Periodontal Attachment Apparatus
3. DFPCs in Tissue Engineering
3.1. The Potential of DFPCs in Dental Tissue Engineering
3.1.1. Craniofacial Bone Regeneration
3.1.2. Periodontal Tissue Regeneration
3.1.3. Root-Like Tissue Regeneration
3.1.4. Providing a Favorable Microenvironment
3.1.5. DFPCs and Scaffolds
3.2. Application in Other Diseases
3.2.1. Nervous Tissue Regeneration
3.2.2. Therapy of Autoimmune and Inflammatory Diseases
4. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chai, Y.; Jiang, X.; Ito, Y.; Bringas, P.; Han, J.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 2000, 127, 1671–1679. [Google Scholar] [CrossRef]
- Ten Cate, A.R. The development of the periodontium—A largely ectomesenchymally derived unit. Periodontology 2000 1997, 13, 9–19. [Google Scholar] [CrossRef]
- Cahill, D.R.; Marks, S.C., Jr. Tooth eruption: Evidence for the central role of the dental follicle. J. Oral Pathol. Med. 1980, 9, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Wise, G.E.; Yao, S. Regional differences of expression of bone morphogenetic protein-2 and RANKL in the rat dental follicle. Eur. J. Oral Sci. 2006, 114, 512–516. [Google Scholar] [CrossRef]
- Wise, G.E.; Lin, F.; Fan, W. Culture and characterization of dental follicle cells from rat molars. Cell Tissue Res. 1992, 267, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Morsczeck, C.; Götz, W.; Schierholz, J.; Zeilhofer, F.; Kühn, U.; Möhl, C.; Sippel, C.; Hoffmann, K.H. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. J. Int. Soc. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef]
- Tan, J.; Xu, X.; Fan, L.; Zheng, Y.; Kuang, W. Dental stem cell in tooth development and advances of adult dental stem cell in regenerative therapies. Curr. Stem Cell Res. Ther. 2015, 10, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Karamzadeh, R.; Eslaminejad, M.B.; Sharifi-Zarchi, A. Comparative in vitro evaluation of human dental pulp and follicle stem cell commitment. Cell J. 2017, 18, 609. [Google Scholar]
- Yagyuu, T.; Ikeda, E.; Ohgushi, H.; Tadokoro, M.; Hirose, M.; Maeda, M.; Inagake, K.; Kirita, T. Hard tissue-forming potential of stem/progenitor cells in human dental follicle and dental papilla. Arch. Oral Biol. 2010, 55, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kémoun, P.; Laurencin-Dalicieux, S.; Rue, J.; Farges, J.C.; Gennero, I.; Conte-Auriol, F.; Briand-Mesange, F.; Gadelorge, M.; Arzate, H.; Narayanan, A.S.; et al. Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res. 2007, 329, 283–294. [Google Scholar] [CrossRef]
- Pan, G.; Thomson, J.A. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007, 17, 42–49. [Google Scholar] [CrossRef]
- Gopinathan, G.; Kolokythas, A.; Luan, X.; Diekwisch, T.G. Epigenetic marks define the lineage and differentiation potential of two distinct neural crest-derived intermediate odontogenic progenitor populations. Stem Cells Dev. 2013, 22, 1763–1778. [Google Scholar] [CrossRef]
- Luan, X.; Ito, Y.; Dangaria, S.; Diekwisch, T.G. Dental follicle progenitor cell heterogeneity in the developing mouse periodontium. Stem Cells Dev. 2006, 15, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Pan, F.; Prpic, V.; Wise, G.E. Differentiation of stem cells in the dental follicle. J. Dent. Res. 2008, 87, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Pan, J.; Wu, P.; Huang, R.; Du, W.; Zhou, Y.; Wan, M.; Fan, Y.; Xu, X.; Zhou, X.; et al. Dental Follicle Cells: Roles in Development and Beyond. Stem Cells Int. 2019, 2019, 9159605. [Google Scholar] [CrossRef]
- Shoi, K.; Aoki, K.; Ohya, K.; Takagi, Y.; Shimokawa, H. Characterization of pulp and follicle stem cells from impacted supernumerary maxillary incisors. Pediatric Dent. 2014, 36, 79E–84E. [Google Scholar]
- Chen, G.; Sun, Q.; Xie, L.; Jiang, Z.; Feng, L.; Yu, M.; Guo, W.; Tian, W. Comparison of the Odontogenic Differentiation Potential of Dental Follicle, Dental Papilla, and Cranial Neural Crest Cells. J. Endod. 2015, 41, 1091–1099. [Google Scholar] [CrossRef]
- Park, B.W.; Jang, S.J.; Byun, J.H.; Kang, Y.H.; Choi, M.J.; Park, W.U.; Lee, W.J.; Rho, G.J. Cryopreservation of human dental follicle tissue for use as a resource of autologous mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2017, 11, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.M. Shedding new light on the mysteries of tooth eruption. Proc. Natl. Acad. Sci. USA 2019, 116, 353–355. [Google Scholar] [CrossRef]
- Morsczeck, C. Chapter 20—Dental Follicle Stem Cells. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Vishwakarma, A., Sharpe, P., Shi, S., Ramalingam, M., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 271–277. [Google Scholar]
- Marks, S.C., Jr.; Cahill, D. Regional control by the dental follicle of alterations in alveolar bone metabolism during tooth eruption. J. Oral Pathol. Med. 1987, 16, 164–169. [Google Scholar]
- Nagata, M.; Ono, N.; Ono, W. Mesenchymal Progenitor Regulation of Tooth Eruption: A View from PTHrP. J. Dent. Res. 2020, 99, 133–142. [Google Scholar] [CrossRef]
- Honda, M.J.; Imaizumi, M.; Tsuchiya, S.; Morsczeck, C. Dental follicle stem cells and tissue engineering. J. Oral Sci. 2010, 52, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Chen, M.; He, L.; Cai, B.; Du, Y.; Zhang, X.; Zhou, C.; Wang, C.; Mao, J.J.; Ling, J. Wnt5a regulates dental follicle stem/progenitor cells of the periodontium. Stem Cell Res. Ther. 2014, 5, 135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uribe, P.; Plakwicz, P.; Larsson, L.; Czochrowska, E.; Westerlund, A.; Ransjö, M. Study on site-specific expression of bone formation and resorption factors in human dental follicles. Eur. J. Oral Sci. 2018, 126, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Wise, G. Cellular and molecular basis of tooth eruption. Orthod. Craniofacial Res. 2009, 12, 67–73. [Google Scholar] [CrossRef]
- Wise, G.E.; King, G.J. Mechanisms of tooth eruption and orthodontic tooth movement. J. Dent. Res. 2008, 87, 414–434. [Google Scholar] [CrossRef]
- Wise, G.E.; Fan, W. Changes in the tartrate-resistant acid phosphatase cell population in dental follicles and bony crypts of rat molars during tooth eruption. J. Dent. Res. 1989, 68, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Volejnikova, S.; Laskari, M.; Marks, S.C., Jr.; Graves, D.T. Monocyte recruitment and expression of monocyte chemoattractant protein-1 are developmentally regulated in remodeling bone in the mouse. Am. J. Pathol. 1997, 150, 1711–1721. [Google Scholar]
- Walker, D.G. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science 1975, 190, 784–785. [Google Scholar] [CrossRef]
- Gomez Perdiguero, E.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; de Bruijn, M.F.; Geissmann, F.; et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015, 518, 547–551. [Google Scholar] [CrossRef]
- Udagawa, N.; Takahashi, N.; Akatsu, T.; Tanaka, H.; Sasaki, T.; Nishihara, T.; Koga, T.; Martin, T.J.; Suda, T. Origin of osteoclasts: Mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc. Natl. Acad. Sci. USA 1990, 87, 7260–7264. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.E.; MacDougall, M.; Horn, D.; Woodruff, K.; Zimmer, S.N.; Rebel, V.I.; Fajardo, R.; Feng, J.Q.; Gluhak-Heinrich, J.; Harris, M.A. Meox2Cre-mediated disruption of CSF-1 leads to osteopetrosis and osteocyte defects. Bone 2012, 50, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yao, S.; Wise, G.E. Regulation of SFRP-1 Expression in the Rat Dental Follicle. Connect. Tissue Res. 2012, 53, 366–372. [Google Scholar] [CrossRef]
- Fox, S.W.; Lovibond, A.C. Current insights into the role of transforming growth factor-β in bone resorption. Mol. Cell. Endocrinol. 2005, 243, 19–26. [Google Scholar] [CrossRef]
- Wise, G.E. In vivo effect of interleukin-1α on colony-stimulating factor-1 gene expression in the dental follicle of the rat molar. Arch. Oral Biol. 1998, 43, 163–165. [Google Scholar] [CrossRef]
- Wise, G.; Zhao, L. Immunostaining and transcriptional enhancement of interleukin-1 receptor type I in the rat dental follicle. Arch. Oral Biol. 1997, 42, 339–344. [Google Scholar] [CrossRef]
- Que, B.G.; Lumpkin, S.J.; Wise, G.E. Implications for tooth eruption of the effect of interleukin-1α on nuclear factor-κB gene expression in the rat dental follicle. Arch. Oral Biol. 1999, 44, 961–967. [Google Scholar] [CrossRef]
- Wise, G.E.; Yao, S.; Odgren, P.R.; Pan, F. CSF-1 regulation of osteoclastogenesis for tooth eruption. J. Dent. Res. 2005, 84, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xie, N.; Ling, J.; Du, Y.; Gu, H. Proteomic analysis of the effects of CSF-1 and IL-1α on dental follicle cells. Mol. Med. Rep. 2016, 14, 2405–2414. [Google Scholar] [CrossRef]
- Bradaschia-Correa, V.; Moreira, M.M.; Arana-Chavez, V.E. Reduced RANKL expression impedes osteoclast activation and tooth eruption in alendronate-treated rats. Cell Tissue Res. 2013, 353, 79–86. [Google Scholar] [CrossRef]
- Alfaqeeh, S.A.; Gaete, M.; Tucker, A.S. Interactions of the tooth and bone during development. J. Dent. Res. 2013, 92, 1129–1135. [Google Scholar] [CrossRef]
- Yao, S.; Liu, D.; Pan, F.; Wise, G.E. Effect of vascular endothelial growth factor on RANK gene expression in osteoclast precursors and on osteoclastogenesis. Arch. Oral Biol. 2006, 51, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Sun, X.; Wang, X.; Zhang, C.; Zheng, S. Abnormal bone remodelling activity of dental follicle cells from a cleidocranial dysplasia patient. Oral Dis. 2018, 24, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, X.; Zhang, C.; Liu, Y.; Yang, X.; Yan, W.; Liu, Z.; Wang, Y.; Zheng, S. RUNX2 mutation impairs bone remodelling of dental follicle cells and periodontal ligament cells in patients with cleidocranial dysplasia. Mutagenesis 2016, 31, 677–685. [Google Scholar] [CrossRef]
- Ge, J.; Guo, S.; Fu, Y.; Zhou, P.; Zhang, P.; Du, Y.; Li, M.; Cheng, J.; Jiang, H. Dental Follicle Cells Participate in Tooth Eruption via the RUNX2-MiR-31-SATB2 Loop. J. Dent. Res. 2015, 94, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Philbrick, W.M.; Dreyer, B.E.; Nakchbandi, I.A.; Karaplis, A.C. Parathyroid hormone-related protein is required for tooth eruption. Proc. Natl. Acad. Sci. USA 1998, 95, 11846–11851. [Google Scholar] [CrossRef]
- Nakchbandi, I.A.; Weir, E.E.; Insogna, K.L.; Philbrick, W.M.; Broadus, A.E. Parathyroid hormone-related protein induces spontaneous osteoclast formation via a paracrine cascade. Proc. Natl. Acad. Sci. USA 2000, 97, 7296–7300. [Google Scholar] [CrossRef]
- Sun, H.; Li, Q.; Zhang, Y.; Bi, Y.; Li, X.; Shu, Y.; Chen, X.; Jin, Z.; Ge, C. Regulation of OPG and RANKL expressed by human dental follicle cells in osteoclastogenesis. Cell Tissue Res. 2015, 362, 399–405. [Google Scholar] [CrossRef]
- Wise, G.E.; Ding, D.; Yao, S. Regulation of secretion of osteoprotegerin in rat dental follicle cells. Eur. J. Oral Sci. 2004, 112, 439–444. [Google Scholar] [CrossRef]
- Zappitelli, T.; Aubin, J.E. The “connexin” between bone cells and skeletal functions. J. Cell. Biochem. 2014, 115, 1646–1658. [Google Scholar] [CrossRef]
- Ransjö, M.; Sahli, J.; Lie, A. Expression of connexin 43 mRNA in microisolated murine osteoclasts and regulation of bone resorption in vitro by gap junction inhibitors. Biochem. Biophys. Res. Commun. 2003, 303, 1179–1185. [Google Scholar] [CrossRef]
- Matemba, S.F.; Lie, A.; Ransjö, M. Regulation of osteoclastogenesis by gap junction communication. J. Cell. Biochem. 2006, 99, 528–537. [Google Scholar] [CrossRef]
- Civitelli, R. Cell-cell communication in the osteoblast/osteocyte lineage. Arch. Biochem. Biophys. 2008, 473, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Wise, G.E.; Yao, S.; Henk, W.G. Bone formation as a potential motive force of tooth eruption in the rat molar. Clin. Anat. Off. J. Am. Assoc. Clin. Anat. Br. Assoc. Clin. Anat. 2007, 20, 632–639. [Google Scholar] [CrossRef]
- Wise, G.E.; He, H.; Gutierrez, D.L.; Ring, S.; Yao, S. Requirement of alveolar bone formation for eruption of rat molars. Eur. J. Oral Sci. 2011, 119, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Oralová, V.; Chlastáková, I.; Radlanski, R.J.; Matalová, E. Distribution of BMP6 in the alveolar bone during mouse mandibular molar eruption. Connect. Tissue Res. 2014, 55, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; He, H.; Gutierrez, D.L.; Rad, M.R.; Liu, D.; Li, C.; Flanagan, M.; Wise, G.E. Expression of bone morphogenetic protein-6 in dental follicle stem cells and its effect on osteogenic differentiation. Cells Tissues Organs 2013, 198, 438–447. [Google Scholar] [CrossRef]
- Ono, W.; Sakagami, N.; Nishimori, S.; Ono, N.; Kronenberg, H.M. Parathyroid hormone receptor signalling in osterix-expressing mesenchymal progenitors is essential for tooth root formation. Nat. Commun. 2016, 7, 11277. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Nagata, M.; Gupta, A.; Matsushita, Y.; Yamaguchi, T.; Mizuhashi, K.; Maki, K.; Ruellas, A.C.; Cevidanes, L.S.; Kronenberg, H.M.; et al. Autocrine regulation of mesenchymal progenitor cell fates orchestrates tooth eruption. Proc. Natl. Acad. Sci. USA 2019, 116, 575–580. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, J.; Li, J.; Zhao, H.; Ho, T.V.; Chai, Y. An Nfic-hedgehog signaling cascade regulates tooth root development. Dev. Camb. Engl. 2015, 142, 3374–3382. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Bi, R.; Liu, W.; Guan, S.; Li, P.; Song, D.; Xu, R.; Zheng, L.; Yuan, Q.; Zhou, X.; et al. Role of PTH1R Signaling in Prx1+ Mesenchymal Progenitors during Eruption. J. Dent. Res. 2020, 99, 1296–1305. [Google Scholar] [CrossRef]
- Klingelhoffer, C.; Reck, A.; Ettl, T.; Morsczeck, C. The parathyroid hormone-related protein is secreted during the osteogenic differentiation of human dental follicle cells and inhibits the alkaline phosphatase activity and the expression of DLX3. Tissue Cell 2016, 48, 334–339. [Google Scholar] [CrossRef]
- Morsczeck, C. Gene expression of runx2, Osterix, c-fos, DLX-3, DLX-5, and MSX-2 in dental follicle cells during osteogenic differentiation in vitro. Calcif. Tissue Int. 2006, 78, 98–102. [Google Scholar] [CrossRef]
- Viale-Bouroncle, S.; Klingelhöffer, C.; Ettl, T.; Reichert, T.E.; Morsczeck, C. A protein kinase A (PKA)/β-catenin pathway sustains the BMP2/DLX3-induced osteogenic differentiation in dental follicle cells (DFCs). Cell. Signal. 2015, 27, 598–605. [Google Scholar] [CrossRef]
- Morsczeck, C.; Reck, A.; Beck, H.C. The hedgehog-signaling pathway is repressed during the osteogenic differentiation of dental follicle cells. Mol. Cell. Biochem. 2017, 428, 79–86. [Google Scholar] [CrossRef]
- Li, C.; Yang, X.; He, Y.; Ye, G.; Li, X.; Zhang, X.; Zhou, L.; Deng, F. Bone morphogenetic protein-9 induces osteogenic differentiation of rat dental follicle stem cells in P38 and ERK1/2 MAPK dependent manner. Int. J. Med Sci. 2012, 9, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, D.; Jing, X.; Li, C. DKK1 and TNF-alpha influence osteogenic differentiation of adBMP9-infected-rDFCs. Oral Dis. 2020, 26, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Pieles, O.; Reichert, T.E.; Morsczeck, C. Classical isoforms of protein kinase C (PKC) and Akt regulate the osteogenic differentiation of human dental follicle cells via both β-catenin and NF-κB. Stem Cell Res. Ther. 2021, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, J.; Ling, J.; Du, Y.; Hou, Y. Nkd2 promotes the differentiation of dental follicle stem/progenitor cells into osteoblasts. Int. J. Mol. Med. 2018, 42, 2403–2414. [Google Scholar] [CrossRef]
- Viale-Bouroncle, S.; Klingelhöffer, C.; Ettl, T.; Morsczeck, C. The WNT inhibitor APCDD1 sustains the expression of β-catenin during the osteogenic differentiation of human dental follicle cells. Biochem. Biophys. Res. Commun. 2015, 457, 314–317. [Google Scholar] [CrossRef]
- Deng, L.; Hong, H.; Zhang, X.; Chen, D.; Chen, Z.; Ling, J.; Wu, L. Down-regulated lncRNA MEG3 promotes osteogenic differentiation of human dental follicle stem cells by epigenetically regulating Wnt pathway. Biochem. Biophys. Res. Commun. 2018, 503, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Um, S.; Lee, J.H.; Seo, B.M. TGF-β2 downregulates osteogenesis under inflammatory conditions in dental follicle stem cells. Int. J. Oral Sci. 2018, 10, 29. [Google Scholar] [CrossRef]
- Li, Z.Z.; Wang, H.T.; Lee, G.Y.; Yang, Y.; Zou, Y.P.; Wang, B.; Gong, C.J.; Cai, Y.; Ren, J.G.; Zhao, J.H. Bleomycin: A novel osteogenesis inhibitor of dental follicle cells via a TGF-β1/SMAD7/RUNX2 pathway. Br. J. Pharmacol. 2021, 178, 312–327. [Google Scholar] [CrossRef]
- Zhang, F.; Liang, M.; Zhao, C.; Fu, Y.; Yu, S. NFIC promotes the vitality and osteogenic differentiation of rat dental follicle cells. J. Mol. Histol. 2019, 50, 471–482. [Google Scholar] [CrossRef]
- Pieles, O.; Hartmann, M.; Morsczeck, C. AMP-activated protein kinase and the down-stream activated process of autophagy regulate the osteogenic differentiation of human dental follicle cells. Arch. Oral Biol. 2021, 122, 104951. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.L.; Barrias, C.C.; Monteiro, F.J.M. Clarifying the Tooth-Derived Stem Cells Behavior in a 3D Biomimetic Scaffold for Bone Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2020, 8, 724. [Google Scholar] [CrossRef]
- Saygin, N.E.; Giannobile, W.V.; Somerman, M.J. Molecular and cell biology of cementum. Periodontology 2000 2000, 24, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Diekwisch, T.G. The developmental biology of cementum. Int. J. Dev. Biol. 2001, 45, 695–706. [Google Scholar]
- Zeichner-David, M.; Oishi, K.; Su, Z.; Zakartchenko, V.; Chen, L.S.; Arzate, H.; Bringas, P., Jr. Role of Hertwig’s epithelial root sheath cells in tooth root development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2003, 228, 651–663. [Google Scholar] [CrossRef]
- Nemoto, E.; Sakisaka, Y.; Tsuchiya, M.; Tamura, M.; Nakamura, T.; Kanaya, S.; Shimonishi, M.; Shimauchi, H. Wnt3a signaling induces murine dental follicle cells to differentiate into cementoblastic/osteoblastic cells via an osterix-dependent pathway. J. Periodontal. Res. 2016, 51, 164–174. [Google Scholar] [CrossRef]
- Mohamed, F.F.; Ge, C.; Binrayes, A.; Franceschi, R.T. The Role of Discoidin Domain Receptor 2 in Tooth Development. J. Dent. Res. 2020, 99, 214–222. [Google Scholar] [CrossRef]
- Sowmya, S.; Chennazhi, K.P.; Arzate, H.; Jayachandran, P.; Nair, S.V.; Jayakumar, R. Periodontal Specific Differentiation of Dental Follicle Stem Cells into Osteoblast, Fibroblast, and Cementoblast. Tissue Eng. Part C Methods 2015, 21, 1044–1058. [Google Scholar] [CrossRef]
- Orimoto, A.; Kurokawa, M.; Handa, K.; Ishikawa, M.; Nishida, E.; Aino, M.; Mitani, A.; Ogawa, M.; Tsuji, T.; Saito, M. F-spondin negatively regulates dental follicle differentiation through the inhibition of TGF-β activity. Arch. Oral Biol. 2017, 79, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, S.; Ohshima, S.; Yamakoshi, Y.; Simmer, J.P.; Honda, M.J. Osteogenic differentiation capacity of porcine dental follicle progenitor cells. Connect. Tissue Res. 2010, 51, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Rad, M.; Bova, J.F.; Orooji, M.; Pepping, J.; Qureshi, A.; Del Piero, F.; Hayes, D.; Yao, S. Evaluation of bone regeneration potential of dental follicle stem cells for treatment of craniofacial defects. Cytotherapy 2015, 17, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Oshima, M.; Inoue, K.; Nakajima, K.; Tachikawa, T.; Yamazaki, H.; Isobe, T.; Sugawara, A.; Ogawa, M.; Tanaka, C.; Saito, M.; et al. Functional tooth restoration by next-generation bio-hybrid implant as a bio-hybrid artificial organ replacement therapy. Sci. Rep. 2014, 4, 6044. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yang, Z.; Zhou, W.; Jin, F.; Song, Y.; Wang, Y.; Huo, N.; Chen, L.; Qian, H.; Hou, R.; et al. Periapical follicle stem cell: A promising candidate for cementum/periodontal ligament regeneration and bio-root engineering. Stem Cells Dev. 2010, 19, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Wu, J.; Jin, F.; Tang, L.; Yu, J.; Xu, L.; Yang, Z.; Wu, G.; Duan, Y.; Jin, Y. Dentin non-collagenous proteins (dNCPs) can stimulate dental follicle cells to differentiate into cementoblast lineages. Biol. Cell 2008, 100, 291–302. [Google Scholar] [CrossRef]
- Guo, S.; Guo, W.; Ding, Y.; Gong, J.; Zou, Q.; Xie, D.; Chen, Y.; Wu, Y.; Tian, W. Comparative study of human dental follicle cell sheets and periodontal ligament cell sheets for periodontal tissue regeneration. Cell Transplant. 2013, 22, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Kang, J.; Ji, B.; Guo, W.; Ding, Y.; Wu, Y.; Tian, W. Periodontal-Derived Mesenchymal Cell Sheets Promote Periodontal Regeneration in Inflammatory Microenvironment. Tissue Eng. Part A 2017, 23, 585–596. [Google Scholar] [CrossRef]
- Bai, Y.; Bai, Y.; Matsuzaka, K.; Hashimoto, S.; Fukuyama, T.; Wu, L.; Miwa, T.; Liu, X.; Wang, X.; Inoue, T. Cementum- and periodontal ligament-like tissue formation by dental follicle cell sheets co-cultured with Hertwig’s epithelial root sheath cells. Bone 2011, 48, 1417–1426. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, W.; Chen, J.; Chen, G.; Tian, W.; Bai, D. Are Hertwig’s epithelial root sheath cells necessary for periodontal formation by dental follicle cells? Arch. Oral Biol. 2018, 94, 1–9. [Google Scholar] [CrossRef]
- Tian, Y.; Bai, D.; Guo, W.; Li, J.; Zeng, J.; Yang, L.; Jiang, Z.; Feng, L.; Yu, M.; Tian, W. Comparison of human dental follicle cells and human periodontal ligament cells for dentin tissue regeneration. Regen. Med. 2015, 10, 461–479. [Google Scholar] [CrossRef]
- Liaw, K.; Delfini, R.H.; Abrahams, J.J. Dental Implant Complications. Semin. Ultrasound CT MR 2015, 36, 427–433. [Google Scholar] [CrossRef]
- Yang, B.; Chen, G.; Li, J.; Zou, Q.; Xie, D.; Chen, Y.; Wang, H.; Zheng, X.; Long, J.; Tang, W.; et al. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix—Based scaffold. Biomaterials 2012, 33, 2449–2461. [Google Scholar] [CrossRef]
- Guo, W.; Gong, K.; Shi, H.; Zhu, G.; He, Y.; Ding, B.; Wen, L.; Jin, Y. Dental follicle cells and treated dentin matrix scaffold for tissue engineering the tooth root. Biomaterials 2012, 33, 1291–1302. [Google Scholar] [CrossRef]
- Luo, X.; Yang, B.; Sheng, L.; Chen, J.; Li, H.; Xie, L.; Chen, G.; Yu, M.; Guo, W.; Tian, W. CAD based design sensitivity analysis and shape optimization of scaffolds for bio-root regeneration in swine. Biomaterials 2015, 57, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Soleymaninejadian, E.; Pramanik, K.; Samadian, E. Immunomodulatory properties of mesenchymal stem cells: Cytokines and factors. Am. J. Reprod. Immunol. 2012, 67, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, B.; Tian, J.; Hong, H.; Du, Y.; Li, K.; Li, X.; Wang, N.; Yu, X.; Wei, X. Dental Follicle Stem Cells Ameliorate Lipopolysaccharide-Induced Inflammation by Secreting TGF-β3 and TSP-1 to Elicit Macrophage M2 Polarization. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 51, 2290–2308. [Google Scholar] [CrossRef] [PubMed]
- Asami, T.; Ishii, M.; Namkoong, H.; Yagi, K.; Tasaka, S.; Asakura, T.; Suzuki, S.; Kamo, T.; Okamori, S.; Kamata, H.; et al. Anti-inflammatory roles of mesenchymal stromal cells during acute Streptococcus pneumoniae pulmonary infection in mice. Cytotherapy 2018, 20, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Chen, X.; Li, K.; Wang, N.; Li, M.; Yang, B.; Yu, X.; Wei, X. Dental follicle stem cells rescue the regenerative capacity of inflamed rat dental pulp through a paracrine pathway. Stem Cell Res. Ther. 2020, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, L.; Liu, W.; Li, Q.; Jin, Z.; Jin, Y. Dental follicle cells rescue the regenerative capacity of periodontal ligament stem cells in an inflammatory microenvironment. PLoS ONE 2014, 9, e108752. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Liu, L.; Deng, M.; Liu, R.; Zhang, L.; Nie, X. In vitro cementoblast-like differentiation of postmigratory neural crest-derived p75+ stem cells with dental follicle cell conditioned medium. Exp. Cell Res. 2015, 337, 76–86. [Google Scholar] [CrossRef]
- Wagers, A.J. The stem cell niche in regenerative medicine. Cell Stem Cell 2012, 10, 362–369. [Google Scholar] [CrossRef]
- Handa, K.; Saito, M.; Yamauchi, M.; Kiyono, T.; Sato, S.; Teranaka, T.; Sampath Narayanan, A. Cementum matrix formation in vivo by cultured dental follicle cells. Bone 2002, 31, 606–611. [Google Scholar] [CrossRef]
- Shinagawa-Ohama, R.; Mochizuki, M.; Tamaki, Y.; Suda, N.; Nakahara, T. Heterogeneous Human Periodontal Ligament-Committed Progenitor and Stem Cell Populations Exhibit a Unique Cementogenic Property Under In Vitro and In Vivo Conditions. Stem Cells Dev. 2017, 26, 632–645. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Hu, Y.; Sun, J.; Guo, W.; Li, H.; Chen, J.; Huo, F.; Tian, W.; Li, S. Treated dentin matrix particles combined with dental follicle cell sheet stimulate periodontal regeneration. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2019, 35, 1238–1253. [Google Scholar] [CrossRef]
- Guo, W.; Chen, L.; Gong, K.; Ding, B.; Duan, Y.; Jin, Y. Heterogeneous dental follicle cells and the regeneration of complex periodontal tissues. Tissue Eng. Part A 2012, 18, 459–470. [Google Scholar] [CrossRef]
- Lucaciu, O.; Soriţău, O.; Gheban, D.; Ciuca, D.R.; Virtic, O.; Vulpoi, A.; Dirzu, N.; Câmpian, R.; Băciuţ, G.; Popa, C.; et al. Dental follicle stem cells in bone regeneration on titanium implants. BMC Biotechnol. 2015, 15, 114. [Google Scholar] [CrossRef]
- Veernala, I.; Giri, J.; Pradhan, A.; Polley, P.; Singh, R.; Yadava, S.K. Effect of Fluoride Doping in Laponite Nanoplatelets on Osteogenic Differentiation of Human Dental Follicle Stem Cells (hDFSCs). Sci. Rep. 2019, 9, 915. [Google Scholar] [CrossRef]
- Olteanu, D.; Filip, A.; Socaci, C.; Biris, A.R.; Filip, X.; Coros, M.; Rosu, M.C.; Pogacean, F.; Alb, C.; Baldea, I.; et al. Cytotoxicity assessment of graphene-based nanomaterials on human dental follicle stem cells. Colloids Surf. B Biointerfaces 2015, 136, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Mohanty, N.; Suk, J.W.; Nagaraja, A.; An, J.; Piner, R.D.; Cai, W.; Dreyer, D.R.; Berry, V.; Ruoff, R.S. Biocompatible, robust free-standing paper composed of a TWEEN/graphene composite. Adv. Mater. 2010, 22, 1736–1740. [Google Scholar] [CrossRef]
- Schwab, J.M.; Brechtel, K.; Mueller, C.A.; Failli, V.; Kaps, H.P.; Tuli, S.K.; Schluesener, H.J. Experimental strategies to promote spinal cord regeneration--an integrative perspective. Prog. Neurobiol. 2006, 78, 91–116. [Google Scholar] [CrossRef] [PubMed]
- Rowland, J.W.; Hawryluk, G.W.; Kwon, B.; Fehlings, M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg. Focus 2008, 25, E2. [Google Scholar] [CrossRef]
- Norenberg, M.D.; Smith, J.; Marcillo, A. The pathology of human spinal cord injury: Defining the problems. J. Neurotrauma 2004, 21, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Völlner, F.; Ernst, W.; Driemel, O.; Morsczeck, C. A two-step strategy for neuronal differentiation in vitro of human dental follicle cells. Differ. Res. Biol. Divers. 2009, 77, 433–441. [Google Scholar] [CrossRef]
- Li, X.; Yang, C.; Li, L.; Xiong, J.; Xie, L.; Yang, B.; Yu, M.; Feng, L.; Jiang, Z.; Guo, W.; et al. A therapeutic strategy for spinal cord defect: Human dental follicle cells combined with aligned PCL/PLGA electrospun material. BioMed Res. Int. 2015, 2015, 197183. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.; Sun, L.; Guo, W.; Tian, W. Potential of human dental stem cells in repairing the complete transection of rat spinal cord. J. Neural Eng. 2017, 14, 026005. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Su, J.; Roberts, A.I.; Shou, P.; Rabson, A.B.; Ren, G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012, 33, 136–143. [Google Scholar] [CrossRef]
- Saparov, A.; Ogay, V.; Nurgozhin, T.; Jumabay, M.; Chen, W.C. Preconditioning of Human Mesenchymal Stem Cells to Enhance Their Regulation of the Immune Response. Stem Cells Int. 2016, 2016, 3924858. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Wang, Y.; Jin, Y.; Zhang, Q.; Zhang, Y.; Wang, L.; Shen, B.; Yin, S.; Liu, W.; Cui, L.; et al. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008, 18, 846–857. [Google Scholar] [CrossRef]
- Ulusoy, C.; Zibandeh, N.; Yıldırım, S.; Trakas, N.; Zisimopoulou, P.; Küçükerden, M.; Tașlı, H.; Tzartos, S.; Göker, K.; Tüzün, E.; et al. Dental follicle mesenchymal stem cell administration ameliorates muscle weakness in MuSK-immunized mice. J. Neuroinflammation 2015, 12, 231. [Google Scholar] [CrossRef]
- Jayawant, S.; Parr, J.; Vincent, A. Autoimmune myasthenia gravis. Handb. Clin. Neurol. 2013, 113, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Borish, L. The immunology of asthma: Asthma phenotypes and their implications for personalized treatment. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2016, 117, 108–114. [Google Scholar] [CrossRef]
- Froidure, A.; Mouthuy, J.; Durham, S.R.; Chanez, P.; Sibille, Y.; Pilette, C. Asthma phenotypes and IgE responses. Eur. Respir. J. 2016, 47, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Genç, D.; Zibandeh, N.; Nain, E.; Gökalp, M.; Özen, A.O.; Göker, M.K.; Akkoç, T. Dental follicle mesenchymal stem cells down-regulate Th2-mediated immune response in asthmatic patients mononuclear cells. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2018, 48, 663–678. [Google Scholar] [CrossRef]
- Genç, D.; Zibandeh, N.; Nain, E.; Arığ, Ü.; Göker, K.; Aydıner, E.K.; Akkoç, T. IFN-γ stimulation of dental follicle mesenchymal stem cells modulates immune response of CD4+ T lymphocytes in Der p1+ asthmatic patients in vitro. Allergol. Immunopathol. 2019, 47, 467–476. [Google Scholar] [CrossRef]
| Scaffolds | Tissue Regeneration | Origin | Transplantation Model | Reference |
|---|---|---|---|---|
| hydroxyapatite (HA) powder | Fibrous tissues and cementum-like matrix | Bovine | SCID mice subcutaneous pockets | Handa et al. 2002 [108] |
| HA-coated dental implant | Bone-like and PDL tissues | Murine | Mice tooth-loss model | Oshima et al. 2014 [88] |
| HA ceramic discs | Cement/woven bone-like tissues | Human | Immunocompromised rats subcutaneous pockets | Yagyuu et al. 2010 [9] |
| HA/collagen-gel | Acellular cementum-like tissues | Human | SCID mice subcutaneous pockets | Shinagawa-Ohama et al. 2016 [109] |
| HA/tricalcium phosphate particles | Bone-like tissues | Human | Immunodeficient mice subcutaneous pockets | Um et al. 2018 [74] |
| Collagen nanohydroxyapatite/phosphoserine biocomposite cryogel | Bone-like tissues | Human | Immunodeficient mice subcutaneous pockets | Salgado et al. 2020 [78] |
| Treated dentin matrix (TDM) | Periodontal-like tissues | Canine | Canine one-wall periodontal intrabony defects | Yang et al. 2019 [110] |
| TDM | Root-like tissues | Rat | Rats alveolar fossa | Guo et al. 2012 [99] |
| TDM | Dentin-like tissues | Human | Immunodeficient mice subcutaneous pockets | Tian et al. 2015 [96] |
| TDM | Periodontal-like tissues | Human | Nude mice subcutaneous pockets | Guo et al. 2013 [92] |
| TDM | Root-like tissues | Human | Immunodeficient mice subcutaneous pockets | Yang et al. 2012 [98] |
| Extracellular matrix | Bone-like tissues | Porcine | Immunocompromised rats critical size parietal defect | Tsuchiya et al. 2010 [86] |
| Dentin non-collagenous proteins (dNCPs) | Cementum-like tissues | Rat | Rats renal capsules | Wu et al. 2008 [91] |
| Ceramic bovine bone | Cementum-PDL complex | Human | Nude mice subcutaneous pockets | Guo et al. 2012 [111] |
| Titanium implants with hydroxyapatite (TiHA), with silicatitanate (TiSiO2) | Enhanced osteogenic differentiation capabilities | Human | In vitro | Lucaciu et al. 2015 [112] |
| Fuoride nanosilicate platelets (NS+F) | Enhanced osteogenic differentiation capabilities | Human | In vitro | Veernala et al. 2019 [113] |
| Graphene-oxide (GO), Thermally reduced graphene oxide (TRGO), Nitrogen-doped graphene (N-Gr) | low levels of cytotoxicity and mitochondria induced damage | Human | In vitro | Olteanu et al. 2015 [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, R.; Lyu, P.; Song, Y.; Li, P.; Song, D.; Cui, C.; Fan, Y. Function of Dental Follicle Progenitor/Stem Cells and Their Potential in Regenerative Medicine: From Mechanisms to Applications. Biomolecules 2021, 11, 997. https://doi.org/10.3390/biom11070997
Bi R, Lyu P, Song Y, Li P, Song D, Cui C, Fan Y. Function of Dental Follicle Progenitor/Stem Cells and Their Potential in Regenerative Medicine: From Mechanisms to Applications. Biomolecules. 2021; 11(7):997. https://doi.org/10.3390/biom11070997
Chicago/Turabian StyleBi, Ruiye, Ping Lyu, Yiming Song, Peiran Li, Dongzhe Song, Chen Cui, and Yi Fan. 2021. "Function of Dental Follicle Progenitor/Stem Cells and Their Potential in Regenerative Medicine: From Mechanisms to Applications" Biomolecules 11, no. 7: 997. https://doi.org/10.3390/biom11070997
APA StyleBi, R., Lyu, P., Song, Y., Li, P., Song, D., Cui, C., & Fan, Y. (2021). Function of Dental Follicle Progenitor/Stem Cells and Their Potential in Regenerative Medicine: From Mechanisms to Applications. Biomolecules, 11(7), 997. https://doi.org/10.3390/biom11070997






