Gelatin-Graphene Oxide Nanocomposite Hydrogels for Kluyveromyces lactis Encapsulation: Potential Applications in Probiotics and Bioreactor Packings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Media
2.2. Synthesis and Characterization of Graphene Oxide (GO)
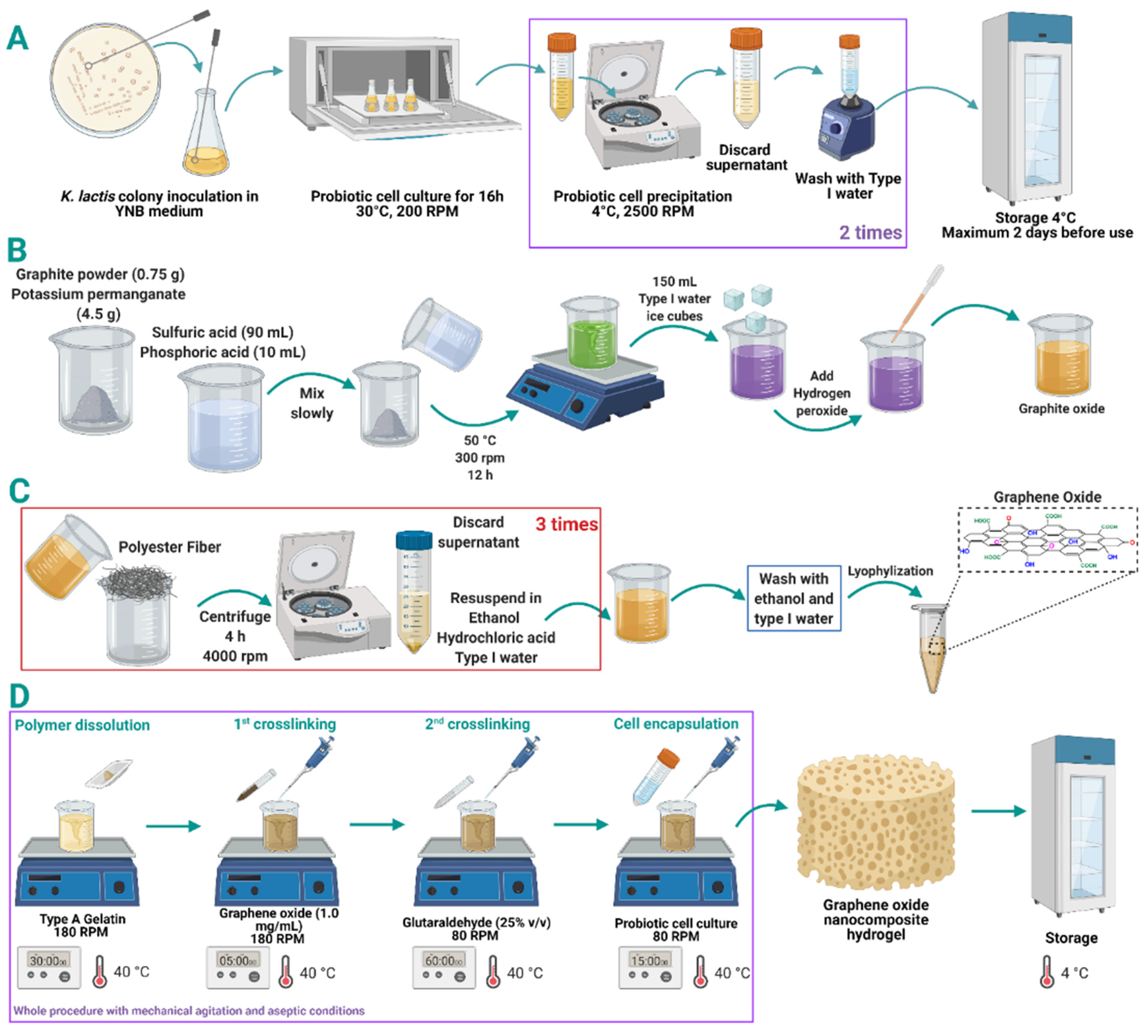
2.3. Preparation of Gelatin-GO Hydrogels
2.4. Preparation of GO Hydrogel Enriched Probiotic Hydrogel
2.5. Survival Rate of Encapsulated Probiotics
2.6. Morphological Structure and Beads Conformation
2.7. Swelling Percentage Determination
2.8. Rheological Response
2.9. Performance of Encapsulates in a Milliliter Scale Bioreactor
2.10. Thermal Stability Analyses
2.11. Mechanical Resistance Evaluation
2.12. Performance of Encapsulates in the Simulated Gastrointestinal Tract (GIT) Media
3. Results
3.1. Graphene Oxide Synthesis and Characterization
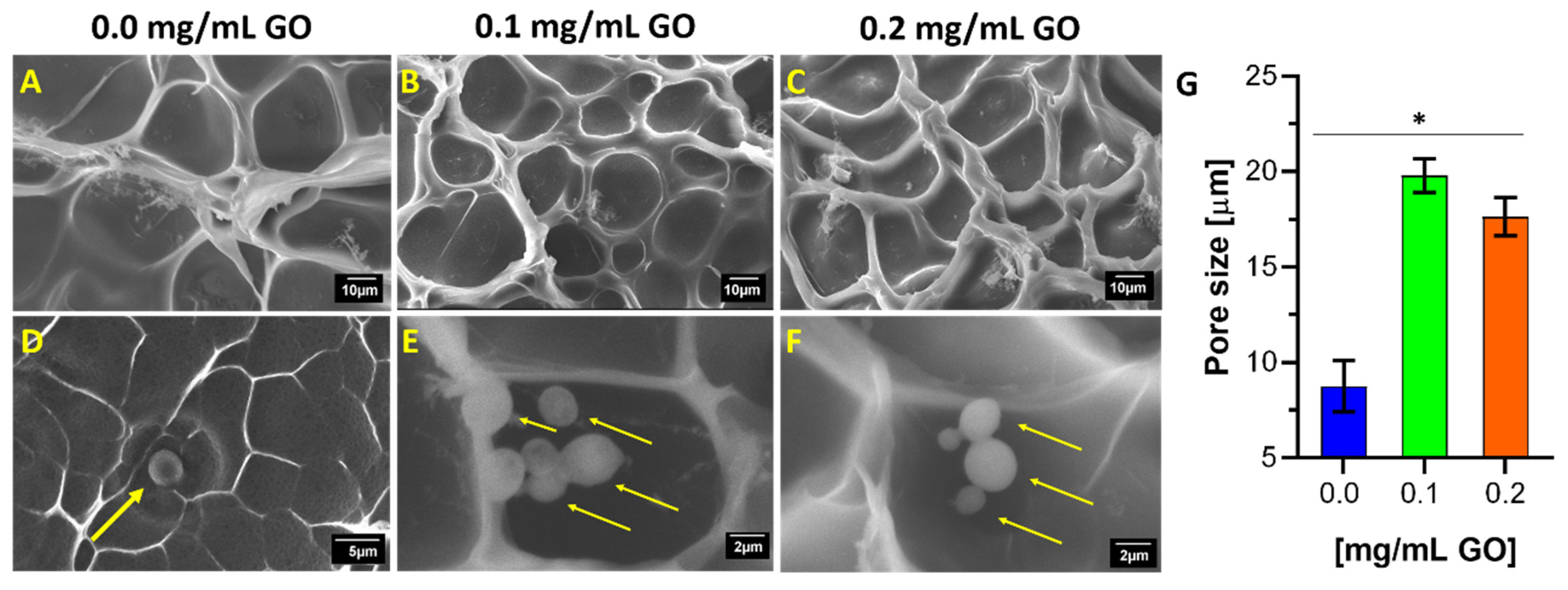
3.2. Morphological Structure of Hydrogels and Encapsulated Cells
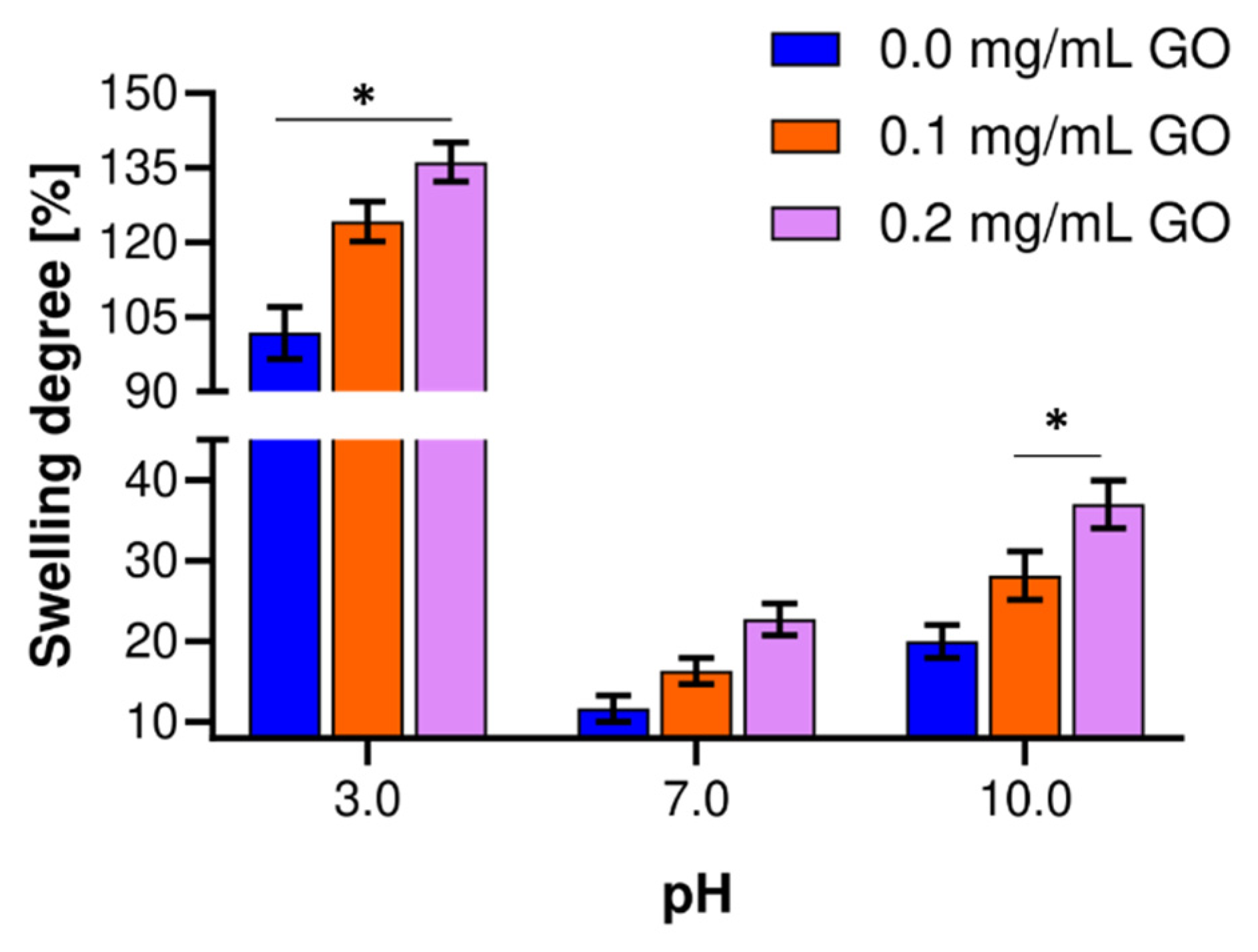
3.3. Swelling Degree of Nanocomposite Hydrogels
3.4. Rheological Response of Hydrogels Nanocomposites
3.5. Mechanical Resistance Evaluation
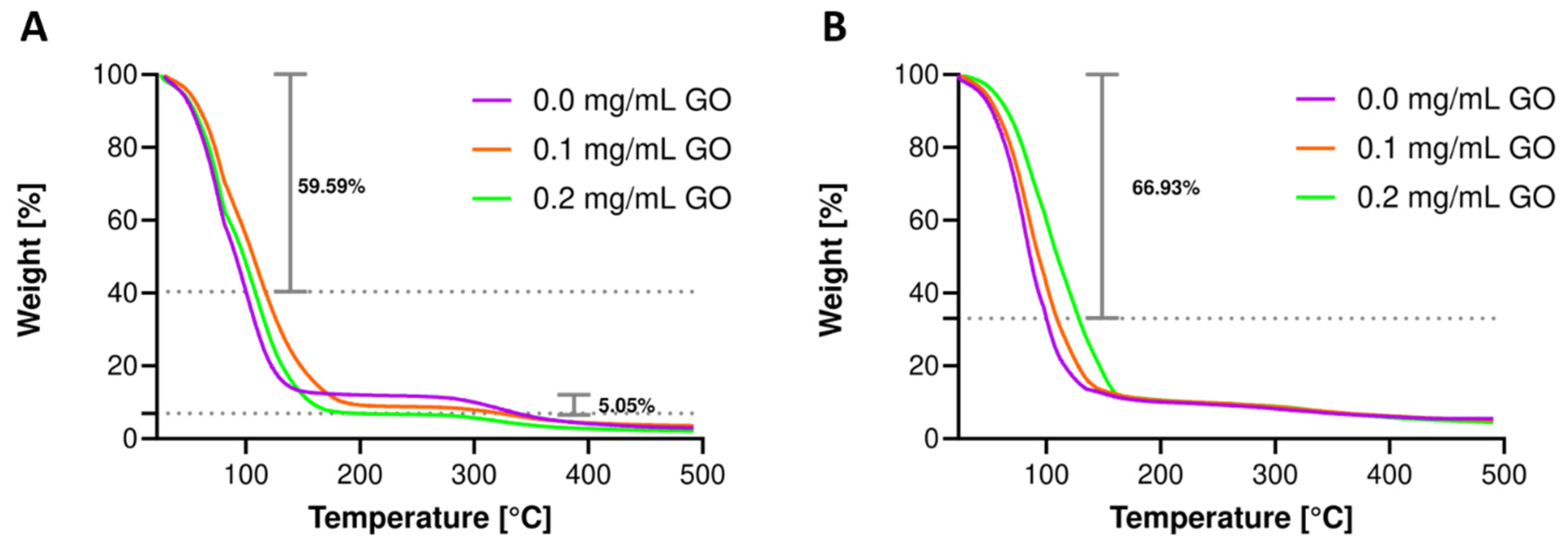
3.6. Thermal Resistance Evaluation
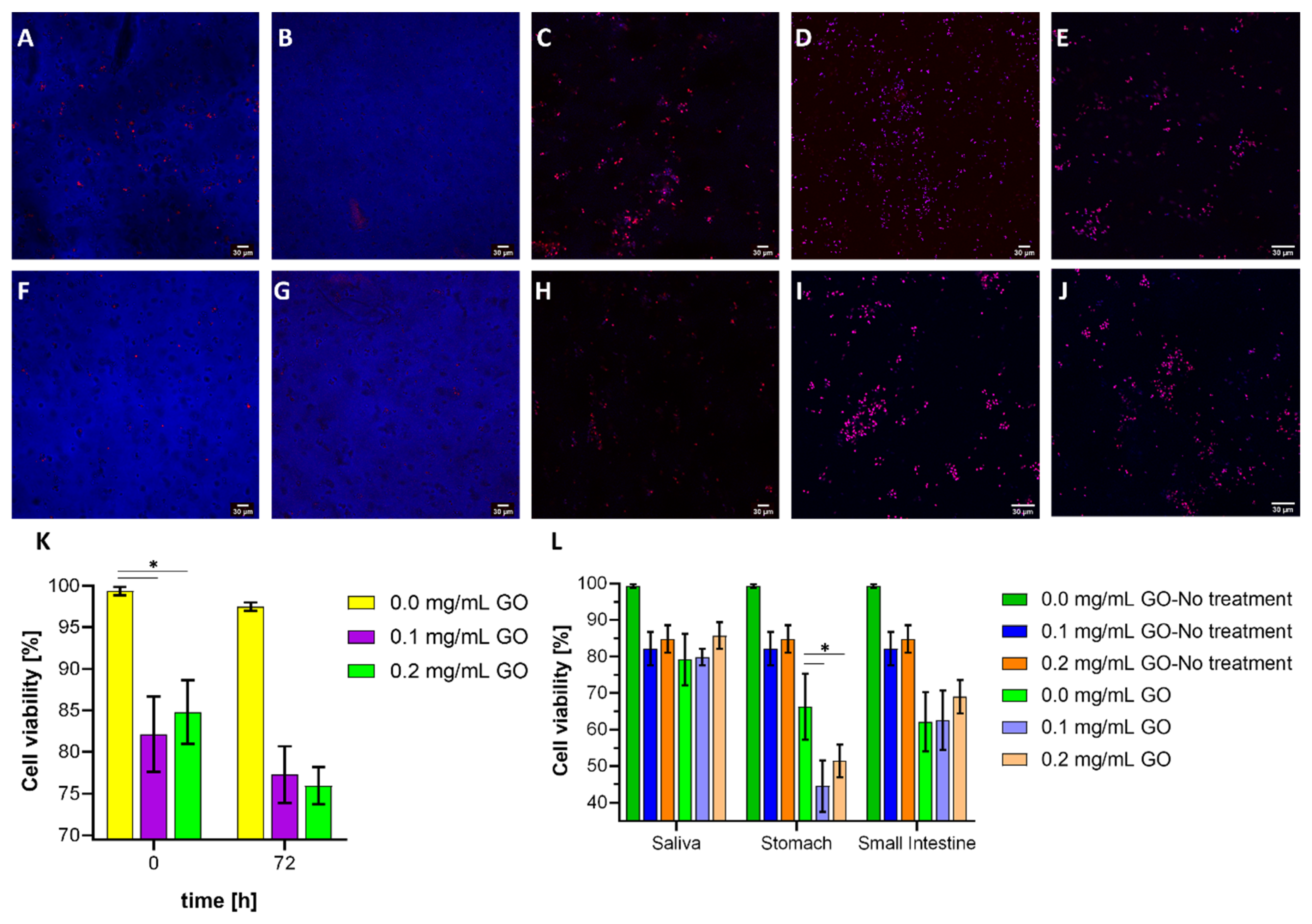
3.7. Cell Viability Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shimoda, A.; Yamamoto, Y.; Sawada, S.I.; Akiyoshi, K. Biodegradable nanogel-integrated hydrogels for sustained protein delivery. Macromol. Res. 2012, 20, 266–270. [Google Scholar] [CrossRef]
- Martínez-Martínez, M.; Rodríguez-Berna, G.; Bermejo, M.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M.; Merino, V. Covalently crosslinked organophosphorous derivatives-chitosan hydrogel as a drug delivery system for oral administration of camptothecin. Eur. J. Pharm. Biopharm. 2019, 136, 174–183. [Google Scholar] [CrossRef]
- Gulen, B.; Demircivi, P. Synthesis and characterization of montmorillonite/ciprofloxacin/TiO2 porous structure for controlled drug release of ciprofloxacin tablet with oral administration. Appl. Clay Sci. 2020, 197. [Google Scholar] [CrossRef]
- Shah, A.V.; Desai, H.H.; Thool, P.; Dalrymple, D.; Serajuddin, A.T.M. Development of self-microemulsifying drug delivery system for oral delivery of poorly water-soluble nutraceuticals. Drug Dev. Ind. Pharm. 2018, 44, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Bin, W.; Tu, B.; Li, X.; Wang, W.; Liao, S.; Sun, C. A Delivery System for Oral Administration of Proteins/Peptides Through Bile Acid Transport Channels. J. Pharm. Sci. 2019, 108, 2143–2152. [Google Scholar] [CrossRef]
- Horigome, A.; Hisata, K.; Odamaki, T.; Iwabuchi, N. Colonization of Supplemented Bifidobacterium breve M-16V in Low Birth Weight Infants and Its Effects on Their Gut Microbiota Weeks. Front. Microbiol. 2021, 12, 785. [Google Scholar] [CrossRef]
- Cazorla, S.I.; Maldonado-Galdeano, C.; Weill, R.; De Paula, J.; Perdigón, G.D.V. Oral administration of probiotics increases Paneth cells and intestinal antimicrobial activity. Front. Microbiol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Secher, T.; Kassem, S.; Benamar, M.; Bernard, I.; Boury, M.; Barreau, F.; Oswald, E.; Saoudi, A. Oral administration of the probiotic strain Escherichia coli Nissle 1917 reduces susceptibility to neuroinflammation and repairs experimental autoimmune encephalomyelitis-induced intestinal barrier dysfunction. Front. Immunol. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Aindelis, G.; Tiptiri-kourpeti, A.; Lampri, E.; Spyridopoulou, K.; Lamprianidou, E.; Kotsianidis, I.; Ypsilantis, P.; Pappa, A.; Chlichlia, K. Immune Responses Raised in an Experimental Colon. Cancers 2020, 12, 368. [Google Scholar] [CrossRef] [Green Version]
- Nikam, P.S.; Kingston, J.J.; Belagal Motatis, A.K. Oral co-administration of bivalent protein r-BL with U-Omp19 elicits mucosal immune responses and reduces S. Typhimurium shedding in BALB/c mice. Immunol. Lett. 2021, 231, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chang, A.K.; Li, Y.; Tao, X.; Liu, W.; Su, W.; Li, Z.; Liang, X. Screening and tissue distribution of protein tyrosine phosphatase 1B inhibitors in mice following oral administration of Garcinia mangostana L. ethanolic extract. Food Chem. 2021, 357, 129759. [Google Scholar]
- Han, S.; Lu, Y.; Xie, J.; Fei, Y.; Zheng, G.; Wang, Z.; Liu, J.; Lv, L.; Ling, Z.; Berglund, B.; et al. Probiotic Gastrointestinal Transit and Colonization After Oral Administration: A Long Journey. Front. Cell. Infect. Microbiol. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Gleeson, J.P. Diet, food components and the intestinal barrier. Nutr. Bull. 2017, 42, 123–131. [Google Scholar] [CrossRef]
- Wright, L.; Barnes, T.J.; Prestidge, C.A. Oral delivery of protein-based therapeutics: Gastroprotective strategies, physiological barriers and in vitro permeability prediction. Int. J. Pharm. 2020, 585. [Google Scholar] [CrossRef]
- Raddatz, G.C.; de Menezes, C.R. Microencapsulation and co-encapsulation of bioactive compounds for application in food: Challenges and perspectives. Cienc. Rural 2021, 51, 1–8. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Mishra, H.N. Novel approaches for co-encapsulation of probiotic bacteria with bioactive compounds, their health benefits and functional food product development: A review. Trends Food Sci. Technol. 2021, 109, 340–351. [Google Scholar] [CrossRef]
- Šeregelj, V.; Ćetković, G.; Čanadanović-Brunet, J.; Tumbas Šaponjac, V.; Vulić, J.; Stajčić, S. Encapsulation and degradation kinetics of bioactive compounds from sweet potato peel during storage. Food Technol. Biotechnol. 2020, 58, 1–27. [Google Scholar] [CrossRef]
- Abdul Mudalip, S.K.; Khatiman, M.N.; Hashim, N.A.; Che Man, R.; Arshad, Z.I.M. A short review on encapsulation of bioactive compounds using different drying techniques. Mater. Today Proc. 2021, 42, 288–296. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Y.; Yue, W.; Qin, W.; Dong, H.; Vasanthan, T. Nanostructures of protein-polysaccharide complexes or conjugates for encapsulation of bioactive compounds. Trends Food Sci. Technol. 2021, 109, 169–196. [Google Scholar] [CrossRef]
- Khayambashi, P.; Iyer, J.; Pillai, S.; Upadhyay, A.; Zhang, Y.; Tran, S.D. Hydrogel encapsulation of mesenchymal stem cells and their derived exosomes for tissue engineering. Int. J. Mol. Sci. 2021, 22, 684. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; Fabra, M.J.; López-Rubio, A. Development of gelatin-coated ι-carrageenan hydrogel capsules by electric field-aided extrusion. Impact of phenolic compounds on their performance. Food Hydrocoll. 2019, 90, 523–533. [Google Scholar] [CrossRef]
- Demisli, S.; Mitsou, E.; Pletsa, V.; Xenakis, A.; Papadimitriou, V. Development and study of nanoemulsions and nanoemulsion-based hydrogels for the encapsulation of lipophilic compounds. Nanomaterials 2020, 10, 2464. [Google Scholar] [CrossRef]
- Wang, B.; Wang, S.; Zhang, Q.; Deng, Y.; Li, X.; Peng, L.; Zuo, X.; Piao, M.; Kuang, X.; Sheng, S.; et al. Recent advances in polymer-based drug delivery systems for local anesthetics. Acta Biomater. 2019, 96, 55–67. [Google Scholar] [CrossRef]
- Bai, S.; Jia, D.; Ma, X.; Liang, M.; Xue, P.; Kang, Y.; Xu, Z. Cylindrical polymer brushes-anisotropic unimolecular micelle drug delivery system for enhancing the effectiveness of chemotherapy. Bioact. Mater. 2021, 6, 2894–2904. [Google Scholar] [CrossRef]
- Kretzmann, J.A.; Luther, D.C.; Evans, C.W.; Jeon, T.; Jerome, W.; Gopalakrishnan, S.; Lee, Y.-W.; Norret, M.; Iyer, K.S.; Rotello, V.M. Regulation of Proteins to the Cytosol Using Delivery Systems with Engineered Polymer Architecture. J. Am. Chem. Soc. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnurch, A.; Malkawi, A.; Jalil, A.; Nazir, I.; Matuszczak, B.; Kennedy, R. Self-emulsifying drug delivery systems: Hydrophobic drug polymer complexes provide a sustained release in vitro. Mol. Pharm. 2020, 17, 3709–3719. [Google Scholar] [CrossRef]
- Mdlovu, N.V.; Lin, K.S.; Chen, Y.; Juang, R.S.; Chang, T.W.; Mdlovu, N.B. Formulation and characterization of multifunctional polymer modified-iron oxide magnetic nanocarrier for doxorubicin delivery. J. Taiwan Inst. Chem. Eng. 2019, 104, 260–272. [Google Scholar] [CrossRef]
- Pinteala, M.; Abadie, M.J.M.; Rusu, R.D. Smart supra- and macro-molecular tools for biomedical applications. Materials 2020, 13, 3343. [Google Scholar] [CrossRef] [PubMed]
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-responsive materials for tissue engineering and drug delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef] [PubMed]
- Reza Saboktakin, M.; Tabatabaei, R.M. Supramolecular hydrogels as drug delivery systems. Int. J. Biol. Macromol. 2015, 75, 426–436. [Google Scholar] [CrossRef]
- Ebara, M.; Kotsuchibashi, Y.; Narain, R. Smart Biomaterials; Springer: Berlin/Heidelberg, Germany, 2014; Volume 305, ISBN 9784431543992. [Google Scholar]
- Liarou, E.; Varlas, S.; Skoulas, D.; Tsimblouli, C.; Sereti, E.; Dimas, K.; Iatrou, H. Smart polymersomes and hydrogels from polypeptide-based polymer systems through α-amino acid N-carboxyanhydride ring-opening polymerization. From chemistry to biomedical applications. Prog. Polym. Sci. 2018, 83, 28–78. [Google Scholar] [CrossRef]
- Zhang, H.; Niu, C.; Zhang, Y.; Wang, X.; Yang, B. A mechanically strong polyvinyl alcohol/poly(2-(N,N′-dimethyl amino) ethyl methacrylate)-poly (acrylic acid) hydrogel with pH-responsiveness. Colloid Polym. Sci. 2020, 298, 619–628. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Benito-Garzón, L.; Fung, S.; Kohn, J.; Vázquez-Lasa, B.; San Román, J. Bioadhesive functional hydrogels: Controlled release of catechol species with antioxidant and antiinflammatory behavior. Mater. Sci. Eng. C 2019, 105. [Google Scholar] [CrossRef]
- Vafaei, A.; Rahbarghazi, R.; Kharaziha, M.; Avval, N.A.; Rezabakhsh, A.; Karimipour, M. Polycaprolactone fumarate acts as an artificial neural network to promote the biological behavior of neural stem cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 246–256. [Google Scholar] [CrossRef]
- Puentes, P.R.; Henao, M.C.; Torres, C.E.; Gómez, S.C.; Gómez, L.A.; Burgos, J.C.; Arbeláez, P.; Osma, J.F.; Muñoz-Camargo, C.; Reyes, L.H.; et al. Design, screening, and testing of non-rational peptide libraries with antimicrobial activity: In silico and experimental approaches. Antibiotics 2020, 9, 854. [Google Scholar] [CrossRef] [PubMed]
- Giubilini, A. Antibiotic resistance as a tragedy of the commons: An ethical argument for a tax on antibiotic use in humans. Bioethics 2019, 33, 776–784. [Google Scholar] [CrossRef]
- Vargason, A.M.; Anselmo, A.C. Evaluation of Surface Modified Live Biotherapeutic Products for Oral Delivery. ACS Biomater. Sci. Eng. 2020. [Google Scholar] [CrossRef]
- James, A.; Wang, Y. Characterization, health benefits and applications of fruits and vegetable probiotics. CYTA J. Food 2019, 17, 770–780. [Google Scholar] [CrossRef]
- Hammam, A.R.A.; Ahmed, M.S.I. Technological aspects, health benefits, and sensory properties of probiotic cheese. SN Appl. Sci. 2019, 1. [Google Scholar] [CrossRef] [Green Version]
- Bamgbose, T.; Anvikar, A.R.; Alberdi, P.; Abdullahi, I.O.; Inabo, H.I.; Bello, M.; Cabezas-Cruz, A.; de la Fuente, J. Functional Food for the Stimulation of the Immune System Against Malaria. Probiotics Antimicrob. Proteins 2021. [Google Scholar] [CrossRef] [PubMed]
- Sabir, F.; Qindeel, M.; Zeeshan, M.; Ain, Q.U.; Rahdar, A. Onco-Receptors Targeting in Lung Cancer via Application of Surface-Modified and Hybrid Nanoparticles: A Cross-Disciplinary Review. Processes 2021, 9, 621. [Google Scholar] [CrossRef]
- Dash, B.S.; Jose, G.; Lu, Y.J.; Chen, J.P. Functionalized reduced graphene oxide as a versatile tool for cancer therapy. Int. J. Mol. Sci. 2021, 22, 2989. [Google Scholar] [CrossRef]
- Yu, H.; Park, J.Y.; Kwon, C.W.; Hong, S.C.; Park, K.M.; Chang, P.S. An overview of nanotechnology in food science: Preparative methods, practical applications, and safety. J. Chem. 2018, 2018. [Google Scholar] [CrossRef]
- Lattuada, E.; Leo, M.; Caprara, D.; Salvatori, L.; Stoppacciaro, A.; Sciortino, F.; Filetici, P. DNA-GEL, Novel Nanomaterial for Biomedical Applications and Delivery of Bioactive Molecules. Front. Pharmacol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Amini, S.M. Preparation of antimicrobial metallic nanoparticles with bioactive compounds. Mater. Sci. Eng. C 2019, 103. [Google Scholar] [CrossRef]
- Mladenovi, M.; Saoud, M.; Pergal, M.V. pH-Responsive Release of Ruthenium Metallotherapeutics from Mesoporous Silica-Based Nanocarriers. Pharmaceutics 2021, 13, 460. [Google Scholar] [CrossRef] [PubMed]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric Nanoparticles for Antimicrobial Therapies: An Up-to-Date Overview. Polymers 2021, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Ghawanmeh, A.A.; Ali, G.A.M.; Algarni, H.; Sarkar, S.M.; Chong, K.F. Graphene oxide-based hydrogels as a nanocarrier for anticancer drug delivery. Nano Res. 2019, 12, 973–990. [Google Scholar] [CrossRef]
- Shan, S.; Jia, S.; Lawson, T.; Yan, L.; Lin, M.; Liu, Y. The use of TAT peptide-functionalized graphene as a highly nuclear-targeting carrier system for suppression of choroidal melanoma. Int. J. Mol. Sci. 2019, 20, 4454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zheng, X.; Chu, Q. Bio-based nanomaterials for cancer therapy. Nano Today 2021, 38, 101134. [Google Scholar] [CrossRef]
- Andretto, V.; Rosso, A.; Briançon, S.; Lollo, G. Nanocomposite systems for precise oral delivery of drugs and biologics. Drug Deliv. Transl. Res. 2021, 445–470. [Google Scholar] [CrossRef]
- Rabiee, N.; Bagherzadeh, M.; Ghadiri, A.M.; Fatahi, Y. Bio-multifunctional noncovalent porphyrin functionalized carbon—based nanocomposite. Sci. Rep. 2021, 11, 6604. [Google Scholar] [CrossRef] [PubMed]
- Gholamali, I.; Yadollahi, M. Bio-nanocomposite Polymer Hydrogels Containing Nanoparticles for Drug Delivery: A Review. Regen. Eng. Transl. Med. 2021. [Google Scholar] [CrossRef]
- Ghibaudo, F.; Gerbino, E.; Copello, G.J.; Campo Dall’ Orto, V.; Gómez-Zavaglia, A. Pectin-decorated magnetite nanoparticles as both iron delivery systems and protective matrices for probiotic bacteria. Colloids Surf. B Biointerfaces 2019, 180, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; He, L.; Ramakrishana, S.; Luo, H. Graphene nanomaterials for regulating stem cell fate in neurogenesis and their biocompatibility. Curr. Opin. Biomed. Eng. 2019, 10, 69–78. [Google Scholar] [CrossRef]
- Shi, H.; Liu, W.; Xie, Y.; Yang, M.; Liu, C.; Zhang, F.; Wang, S.; Liang, L.; Pi, K. Synthesis of carboxymethyl chitosan-functionalized graphene nanomaterial for anticorrosive reinforcement of waterborne epoxy coating. Carbohydr. Polym. 2021, 252. [Google Scholar] [CrossRef]
- Talesara, V.; Garman, P.D.; Lee, J.L.; Lu, W. Thermal management of high-power switching transistors using thick CVD-Grown graphene nanomaterial. IEEE Trans. Power Electron. 2020, 35, 578–590. [Google Scholar] [CrossRef]
- Phan, L.M.T.; Vo, T.A.T.; Hoang, T.X.; Cho, S. Graphene integrated hydrogels based biomaterials in photothermal biomedicine. Nanomaterials 2021, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Bellet, P.; Gasparotto, M.; Pressi, S.; Fortunato, A.; Scapin, G.; Mba, M.; Menna, E.; Filippini, F. Graphene-based scaffolds for regenerative medicine. Nanomaterials 2021, 11, 404. [Google Scholar] [CrossRef]
- Liu, Y.; Lyu, Y.; Hu, Y.; An, J.; Chen, R.; Chen, M.; Du, J.; Hou, C. Novel Graphene Oxide Nanohybrid Doped Methacrylic Acid Hydrogels for Enhanced Swelling Capability and Cationic Adsorbability. Polymers 2021, 13, 1112. [Google Scholar] [CrossRef]
- Zhao, X.; Zou, X.; Ye, L. Controlled pH- and glucose-responsive drug release behavior of cationic chitosan based nano-composite hydrogels by using graphene oxide as drug nanocarrier. J. Ind. Eng. Chem. 2017, 49, 36–45. [Google Scholar] [CrossRef]
- More, M.P.; Chitalkar, R.V.; Bhadane, M.S.; Dhole, S.D.; Patil, A.G.; Patil, P.O.; Deshmukh, P.K. Development of graphene-drug nanoparticle based supramolecular self assembled pH sensitive hydrogel as potential carrier for targeting MDR tuberculosis. Mater. Technol. 2019, 34, 324–335. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Owonubi, S.J.; Jayaramudu, J.; Sadiku, E.R.; Ray, S.S. Targeted drug delivery potential of hydrogel biocomposites containing partially and thermally reduced graphene oxide and natural polymers prepared via green process. Colloid Polym. Sci. 2014, 293, 409–420. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Li, L.; Yu, F.; Li, J.; Liu, L.; Fang, B.; Xia, L. Photothermally triggered biomimetic drug delivery of Teriparatide via reduced graphene oxide loaded chitosan hydrogel for osteoporotic bone regeneration. Chem. Eng. J. 2020, 413. [Google Scholar] [CrossRef]
- Lu, Y.J.; Lan, Y.H.; Chuang, C.C.; Lu, W.T.; Chan, L.Y.; Hsu, P.W.; Chen, J.P. Injectable thermo-sensitive chitosan hydrogel containing CPT-11-loaded EGFR-targeted graphene oxide and SLP2 shRNA for localized drug/gene delivery in glioblastoma therapy. Int. J. Mol. Sci. 2020, 21, 7111. [Google Scholar] [CrossRef]
- Ali, L.; Tanzil, S.; Rehman, U.; Khan, M. Synthesis of graphene oxide doped poly(2-acrylamido-2-methyl propane sulfonic acid) [GO@p(AMPS)] composite hydrogel with pseudo-plastic thixotropic behavior. Polym. Bull. 2019. [Google Scholar] [CrossRef]
- Patarroyo, J.L.; Florez-Rojas, J.S.; Pradilla, D.; Valderrama-Rincón, J.D.; Cruz, J.C.; Reyes, L.H. Formulation and characterization of gelatin-based hydrogels for the encapsulation of kluyveromyces lactis-Applications in packed-bed reactors and probiotics delivery in humans. Polymers 2020, 12, 1287. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, M.; Ren, J.; Wang, J.; Fan, L.; Xu, Q. Preparation and swelling properties of graphene oxide/poly(acrylic acid-co-acrylamide) super-absorbent hydrogel nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2012, 401, 97–106. [Google Scholar] [CrossRef]
- Yan, X.; Yang, J.; Chen, F.; Zhu, L.; Tang, Z.; Qin, G.; Chen, Q.; Chen, G. Mechanical properties of gelatin/polyacrylamide/graphene oxide nanocomposite double-network hydrogels. Compos. Sci. Technol. 2018, 163, 81–88. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, J.S.; Bennett, M.D.; Rayburn, A.L.; Galbraith, D.W.; Price, H.J. Reference standards for determination of DNA content of plant nuclei. Am. J. Bot. 1999, 86, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Reyes Ortega, F.; Rodríguez, G.; Rosa Aguilar, M.; García-Sanmartín, J.; Martínez, A.; San Román, J. Comportamiento reológico de geles biodegradables para aplicaciones en medicina regenerativa. Soc. Ibér. Biomec. Biomater. 2012, 20, 7–19. [Google Scholar]
- Piao, Y.; Chen, B. Synthesis and mechanical properties of double cross-linked gelatin-graphene oxide hydrogels. Int. J. Biol. Macromol. 2017, 101, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon N. Y. 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Sahraei, R.; Ghaemy, M. Synthesis of modified gum tragacanth/graphene oxide composite hydrogel for heavy metal ions removal and preparation of silver nanocomposite for antibacterial activity. Carbohydr. Polym. 2017, 157, 823–833. [Google Scholar] [CrossRef]
- Allah, H.; Mohamed, S.T.; Sakhawy, E.; Kamel, S. Carboxymethyl Cellulose—Grafted Graphene Oxide/Polyethylene Glycol for Efficient Ni (II) Adsorption. J. Polym. Environ. 2021, 29, 859–870. [Google Scholar]
- Das, L.; Das, P.; Bhowal, A.; Bhattachariee, C. Synthesis of hybrid hydrogel nano-polymer composite using Graphene oxide, Chitosan and PVA and its application in waste water treatment. Environ. Technol. Innov. 2020, 18. [Google Scholar] [CrossRef]
- Sarkar, N.; Sahoo, G.; Swain, S.K. Nanoclay sandwiched reduced graphene oxide filled macroporous polyacrylamide-agar hybrid hydrogel as an adsorbent for dye decontamination. Nano-Struct. Nano-Objects 2020, 23. [Google Scholar] [CrossRef]
- Zhang, X.J.; Cai, W.B.; Hao, L.Y.; Hu, X.H.; Wei, X.J.; Wang, X.Y.; Lin, Q. Preparation of thermo/pH-sensitive reduced graphene oxide interpenetrating hydrogel nanocomposites for co-delivery of paclitaxel and epirubicin. Mater. Technol. 2018, 33, 245–252. [Google Scholar] [CrossRef]
- Kumar, A.; Zo, S.M.; Kim, J.H.; Kim, S.C.; Han, S.S. Enhanced physical, mechanical, and cytocompatibility behavior of polyelectrolyte complex hydrogels by reinforcing halloysite nanotubes and graphene oxide. Compos. Sci. Technol. 2019, 175, 35–45. [Google Scholar] [CrossRef]
- Jafarigol, E.; Salehi, M.B.; Mortaheb, H.R. Preparation and assessment of electro-conductive poly(acrylamide-co-acrylic acid) carboxymethyl cellulose/reduced graphene oxide hydrogel with high viscoelasticity. Chem. Eng. Res. Des. 2020, 162, 74–84. [Google Scholar] [CrossRef]
- Chang, Z.; Chen, Y.; Tang, S.; Yang, J.; Chen, Y.; Chen, S.; Li, P.; Yang, Z. Construction of chitosan/polyacrylate/graphene oxide composite physical hydrogel by semi-dissolution/acidification/sol-gel transition method and its simultaneous cationic and anionic dye adsorption properties. Carbohydr. Polym. 2020, 229, 1–10. [Google Scholar] [CrossRef]
- Sarkar, N.; Sahoo, G.; Swain, S.K. Graphene quantum dot decorated magnetic graphene oxide filled polyvinyl alcohol hybrid hydrogel for removal of dye pollutants. J. Mol. Liq. 2020, 302. [Google Scholar] [CrossRef]
- Youssef, A.M.; Hasanin, M.S.; El-Aziz, M.E.A.; Turky, G.M. Conducting chitosan/hydroxylethyl cellulose/polyaniline bionanocomposites hydrogel based on graphene oxide doped with Ag-NPs. Int. J. Biol. Macromol. 2021, 167, 1435–1444. [Google Scholar] [CrossRef]
- Jafarigol, E.; Afshar Ghotli, R.; Hajipour, A.; Pahlevani, H.; Baghban Salehi, M. Tough dual-network GAMAAX hydrogel for the efficient removal of cadmium and nickle ions in wastewater treatment applications. J. Ind. Eng. Chem. 2021, 94, 352–360. [Google Scholar] [CrossRef]
- Tarashi, S.; Nazockdast, H.; Sodeifian, G. Reinforcing effect of graphene oxide on mechanical properties, self-healing performance and recoverability of double network hydrogel based on κ-carrageenan and polyacrylamide. Polymer 2019, 183. [Google Scholar] [CrossRef]
- Shah, S.A.; Kulhanek, D.; Sun, W.; Zhao, X.; Yu, S.; Parviz, D.; Lutkenhaus, J.L.; Green, M.J. Aramid nanofiber-reinforced three-dimensional graphene hydrogels for supercapacitor electrodes. J. Colloid Interface Sci. 2020, 560, 581–588. [Google Scholar] [CrossRef]
- Sharma, B. Viscoelastic investigation of graphene oxide grafted PVA biohybrid using ostwald modeling for packaging applications. Polym. Test. 2020, 91. [Google Scholar] [CrossRef]
- Hasda, A.M.; Vuppaladadium, S.S.R.; Qureshi, D.; Prasad, G.; Mohanty, B.; Banerjee, I.; Shaikh, H.; Anis, A.; Sarkar, P.; Pal, K. Graphene oxide reinforced nanocomposite oleogels improves corneal permeation of drugs. J. Drug Deliv. Sci. Technol. 2020, 60. [Google Scholar] [CrossRef]
- Luo, H.; Dong, J.; Yao, F.; Yang, Z.; Li, W.; Wang, J.; Xu, X.; Hu, J.; Wan, Y. Layer-by-Layer Assembled Bacterial Cellulose/Graphene Oxide Hydrogels with Extremely Enhanced Mechanical Properties. Nano-Micro Lett. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Nath, J.; Shekhar, S.; Dolui, S.K. Artificial Nacre-based Chitosan/Graphene Oxide-Mg Hydrogel with Significant Mechanical Strength and Shape Memory Effect. Polym. Sci. Ser. A 2021, 63, 123–132. [Google Scholar] [CrossRef]
- di Luca, M.; Vittorio, O.; Cirillo, G.; Curcio, M.; Czuban, M.; Voli, F.; Farfalla, A.; Hampel, S.; Nicoletta, F.P.; Iemma, F. Electro-responsive graphene oxide hydrogels for skin bandages: The outcome of gelatin and trypsin immobilization. Int. J. Pharm. 2018, 546, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xiao, L.; Zhou, J.; Li, X.; Liu, J.; Zeng, M. Mechanical enhancement of graphene oxide-filled chitosan-based composite hydrogels by multiple mechanisms. J. Mater. Sci. 2020, 55, 14690–14701. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, Y.; Teng, L.; Lu, B.; Ren, L.; Wang, Y. Antimicrobial colloidal hydrogels assembled by graphene oxide and thermo-sensitive nanogels for cell encapsulation. J. Colloid Interface Sci. 2018, 513, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, G.; Zhao, Z.; Sun, L.; Zou, M.; Ren, J.; Zhao, Y. Responsive graphene oxide hydrogel microcarriers for controllable cell capture and release. Sci. China Mater. 2018, 61, 1314–1324. [Google Scholar] [CrossRef] [Green Version]
- Breuer, L.; Raue, M.; Strobel, M.; Mang, T.; Schöning, M.J.; Thoelen, R.; Wagner, T. Hydrogels with incorporated graphene oxide as light-addressable actuator materials for cell culture environments in lab-on-chip systems. Phys. Status Solidi Appl. Mater. Sci. 2016, 213, 1520–1525. [Google Scholar] [CrossRef]
- Ligorio, C.; Zhou, M.; Wychowaniec, J.K.; Zhu, X.; Bartlam, C.; Miller, A.F.; Vijayaraghavan, A.; Hoyland, J.A.; Saiani, A. Graphene oxide containing self-assembling peptide hybrid hydrogels as a potential 3D injectable cell delivery platform for intervertebral disc repair applications. Acta Biomater. 2019, 92, 92–103. [Google Scholar] [CrossRef]
- Liu, P.; Shao, H.; Kong, Y.; Wang, D. Effect of graphene oxide exposure on intestinal Wnt signaling in nematode Caenorhabditis elegans. J. Environ. Sci. 2020, 88, 200–208. [Google Scholar] [CrossRef]



| 0.0 mg/mL GO | 0.1 mg/mL GO | 0.2 mg/mL GO | ||||
|---|---|---|---|---|---|---|
| Treatment | Mean | SD | Mean | SD | Mean | SD |
| None | 6.880 | 1.907 | 11.863 | 1.707 | 19.807 | 4.478 |
| 72 h bioreactor | 7.516 | 1.495 | 5.321 | 0.923 | 11.834 | 0.572 |
| Saliva | 10.913 | 0.902 | 6.444 | 2.486 | 6.014 | 2.524 |
| Stomach | 13.853 | 3.406 | 2.369 | 0.300 | 4.000 | 2.286 |
| Small intestine | 1.950 | 1.393 | 4.174 | 1.355 | 6.105 | 2.072 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patarroyo, J.L.; Fonseca, E.; Cifuentes, J.; Salcedo, F.; Cruz, J.C.; Reyes, L.H. Gelatin-Graphene Oxide Nanocomposite Hydrogels for Kluyveromyces lactis Encapsulation: Potential Applications in Probiotics and Bioreactor Packings. Biomolecules 2021, 11, 922. https://doi.org/10.3390/biom11070922
Patarroyo JL, Fonseca E, Cifuentes J, Salcedo F, Cruz JC, Reyes LH. Gelatin-Graphene Oxide Nanocomposite Hydrogels for Kluyveromyces lactis Encapsulation: Potential Applications in Probiotics and Bioreactor Packings. Biomolecules. 2021; 11(7):922. https://doi.org/10.3390/biom11070922
Chicago/Turabian StylePatarroyo, Jorge Luis, Eduardo Fonseca, Javier Cifuentes, Felipe Salcedo, Juan C. Cruz, and Luis H. Reyes. 2021. "Gelatin-Graphene Oxide Nanocomposite Hydrogels for Kluyveromyces lactis Encapsulation: Potential Applications in Probiotics and Bioreactor Packings" Biomolecules 11, no. 7: 922. https://doi.org/10.3390/biom11070922
APA StylePatarroyo, J. L., Fonseca, E., Cifuentes, J., Salcedo, F., Cruz, J. C., & Reyes, L. H. (2021). Gelatin-Graphene Oxide Nanocomposite Hydrogels for Kluyveromyces lactis Encapsulation: Potential Applications in Probiotics and Bioreactor Packings. Biomolecules, 11(7), 922. https://doi.org/10.3390/biom11070922









