Synthetic Material Abdominal Swabs Reduce Activation of Platelets and Leukocytes Compared to Cotton Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Abdominal Swab Materials
2.2. Preparation of Sample Swabs for in Vitro Tests
2.3. Blood Sampling for in Vitro Tests
2.4. Monocyte Activation Test (MAT)
2.5. Patients for the Clinical Study
2.6. Plasma and Blood Sampling from Patients
2.7. Soluble Activation Markers
2.8. Cytokine and Chemokine Assessment
2.9. Statistics
3. Results
3.1. Preliminary In Vitro Test Shows Marked Differences between Materials
3.2. Leukocyte Counts Increased at the End of HLM Only in the Cotton Group
3.3. Hemocompatibility Parameters Differed for Leukocyte and Thrombocyte Activation
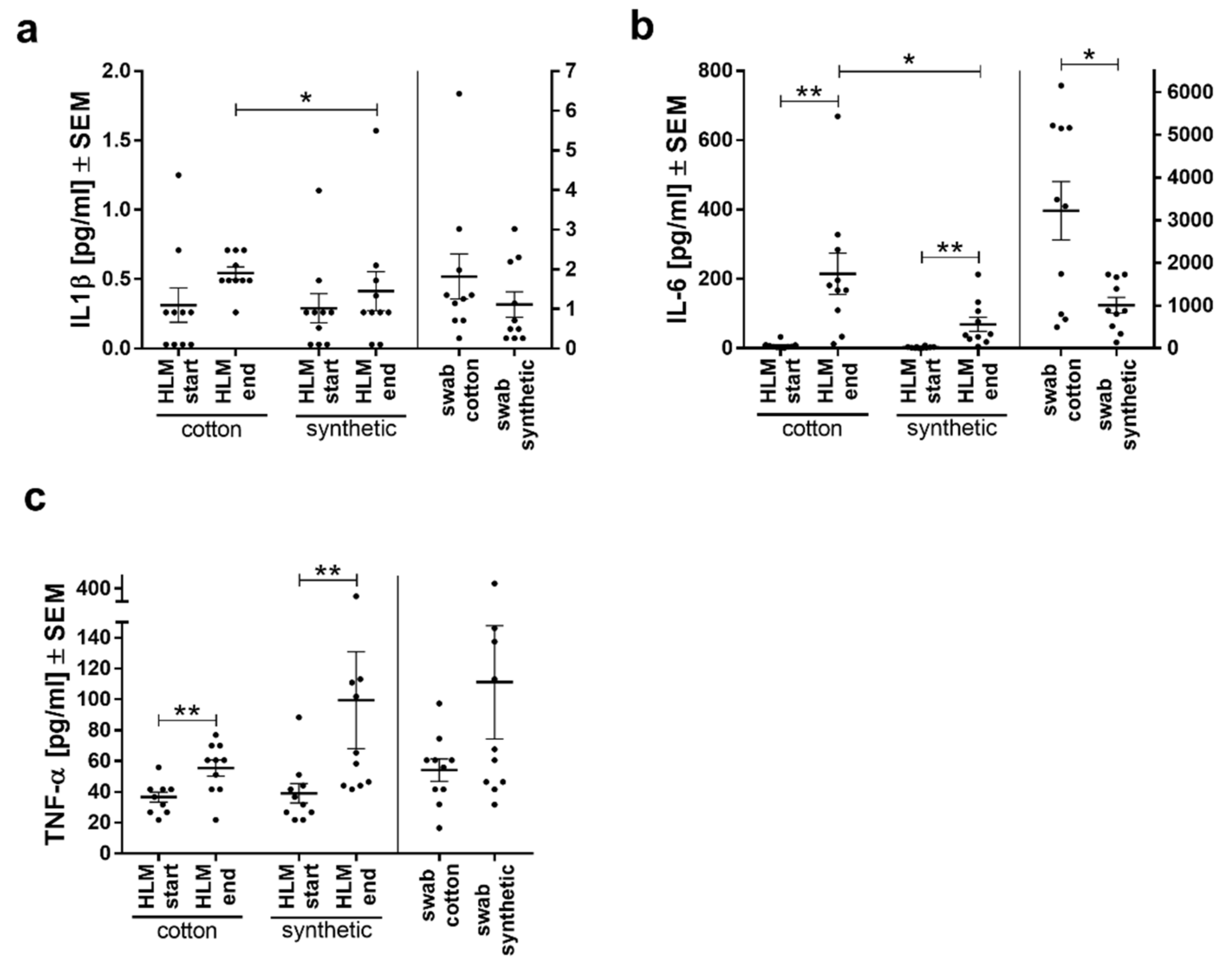
3.4. IL6 and IL1β Secretion was Lower with Synthetic Swabs
3.5. Levels of Chemokines MCP-1 and SDF1-α Reflect Inflammatory Processes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Criscitelli, T. Fast Facts for the Operating Room Nurse, Second Edition: An Orientation and Care Guide in a Nutshell; Springer Publishing Company: New York, NY, USA, 2018; p. 81. [Google Scholar]
- Reddy, K.R.; Davidonis, G.H.; Johnson, A.S.; Vinyard, B.T. Temperature regime and carbon dioxide enrichment alter cotton boll development and fiber properties. Agron. J. 1999, 91, 851–858. [Google Scholar] [CrossRef]
- Krajewski, S.; Hierlemann, T.; Neumann, B.; Nathan, T.; Abel, M.; Koggel, A.; Schlensak, C.; Wendel, H.P. Hypercoagulant abdominal swabs in cardiac surgery: Potential problems and background. Thorac Cardiovasc Surg 2016, 64, 589–595. [Google Scholar]
- Zhao, H.; Ma, H.; Meng, L.; Zhao, Z.; Quan, X.; Cheng, Z. Application of autologous blood cell salvage in off-pump coronary artery bypass graft operation. Heart Surg. Forum 2017, 20, E107–E110. [Google Scholar] [CrossRef]
- Tu, L.N.; Hsieh, L.; Kajimoto, M.; Charette, K.; Kibiryeva, N.; Forero, A.; Hampson, S.; Marshall, J.A.; O’brien, J.; Scatena, M. Shear stress associated with cardiopulmonary bypass induces expression of inflammatory cytokines and necroptosis in monocytes. JCI Insight 2021, 6, e141341. [Google Scholar] [CrossRef]
- Edmunds, L.H.; Colman, R.; Niewiarowski, S. Blood-Surface Interactions during Cardiopulmonary Bypass. In Blood Use in Cardiac Surgery; Springer: Heidelberg, Germany, 1991; pp. 27–36. [Google Scholar]
- Gregoretti, S. Suction-induced hemolysis at various vacuum pressures: Implications for intraoperative blood salvage. Transfusion 1996, 36, 57–60. [Google Scholar] [CrossRef]
- Konig, G.; Waters, J.H. Washing and filtering of cell-salvaged blood–does it make autotransfusion safer? Transfus. Altern. Transfus. Med. 2012, 12, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, S.; Robertson, C.; Budak, A.B.; Gourlay, T. Comparative evaluation of blood salvage techniques in patients undergoing cardiac surgery with cardiopulmonary bypass. Perfusion 2018, 33, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, S.; Nielsen, C.H.; Andersen, L.W.; Bendtzen, K.; Tvede, M.; Steinbrüchel, D.A. Cell saver for on-pump coronary operations reduces systemic inflammatory markers: A randomized trial. Ann. Thorac. Surg. 2010, 89, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Taggart, D. Core Concepts in Cardiac Surgery; OUP OxfordL: Oxford, UK, 2018; p. 40. [Google Scholar]
- Hakim, R.M. Complement activation by biomaterials. Cardiovasc. Pathol. 1993, 2, 187–197. [Google Scholar] [CrossRef]
- Lander, H.; Zammert, M.; Fitzgerald, D. Anticoagulation management during cross-clamping and bypass. Best Pr. Res. Clin. Anaesthesiol 2016, 30, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, S.; Nathan, T.; Neumann, B.; Hoffmann, S.; Abel, M.; Koggel, A.; Schlensak, C.; Wendel, H.P. Simple clotting test to detect procoagulant abdominal swabs. J. Mater. Sci Mater. Med. 2015, 26, 106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trunk, S.; Mullerbader, P.; Hennig, U.; Abel, M.; Koggel, A.; Stang, K.; Altreuter, Y.; Steger, V.; Schlensak, C.; Wendel, H.P.; et al. Inflammatory potential of cotton-based surgically invasive devices: Implications for cardiac surgery. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, T. Polyurethane sheet: A potential substitute of surgical cotton gauze. J. Cardiothorac Surg 2011, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- European Council. European-Pharmacopoeia. Monocyte Activation Test. Edition 9.2; 2017; Chapter 2.6.30.
- Stoppelkamp, S.; Veseli, K.; Stang, K.; Schlensak, C.; Wendel, H.P.; Walker, T. Identification of predictive early biomarkers for sterile-sirs after cardiovascular surgery. PLoS ONE 2015, 10, e0135527. [Google Scholar] [CrossRef] [PubMed]
- Robert-Koch-Institut. Epidemiologisches Bulletin. Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2017/Ausgaben/37_17.pdf?__blob=publicationFile (accessed on 30 June 2021).
- Brauer, S.D.; Applegate Ii, R.L.; Jameson, J.J.; Hay, K.L.; Lauer, R.E.; Herrmann, P.C.; Bull, B.S. Association of plasma dilution with cardiopulmonary bypass-associated bleeding and morbidity. J. Cardiothorac. Vasc. Anesth. 2013, 27, 845–852. [Google Scholar] [CrossRef]
- Sniecinski, R.M.; Chandler, W.L. Activation of the hemostatic system during cardiopulmonary bypass. Anesth. Analg. 2011, 113, 1319–1333. [Google Scholar] [CrossRef]
- Wehlin, L.; Vedin, J.; Vaage, J.; Lundahl, J. Activation of complement and leukocyte receptors during on-and off pump coronary artery bypass surgery. Eur. J. Cardio-Thorac. Surg. 2004, 25, 35–42. [Google Scholar] [CrossRef]
- Plötz, F.B.; Van Oeveren, W.; Bartlett, R.H.; Wildevuur, C.R. Blood activation during neonatal extracorporeal life support. J. Thorac. Cardiovasc. Surg. 1993, 105, 823–832. [Google Scholar] [CrossRef]
- Leverett, L.; Hellums, J.; Alfrey, C.; Lynch, E. Red blood cell damage by shear stress. Biophys. J. 1972, 12, 257–273. [Google Scholar] [CrossRef]
- Watts, T.; Barigou, M.; Nash, G.B. Comparative rheology of the adhesion of platelets and leukocytes from flowing blood: Why are platelets so small? Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1483–H1494. [Google Scholar] [CrossRef]
- Sutton, S.; Patel, A.; Chase, V.; Schmidt, L.; Hunley, E.; Yancey, L.; Hebeler, R.; Cheung, E.; Henry Iii, A.; Meyers, T. Clinical benefits of continuous leukocyte filtration during cardiopulmonary bypass in patients undergoing valvular repair or replacement. Perfusion 2005, 20, 21–29. [Google Scholar] [CrossRef]
- Akramienė, D.; Kondrotas, A.; Didžiapetrienė, J.; Kėvelaitis, E. Effects of ß-glucans on the immune system. Medicina 2007, 43, 597. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Hong, J.T.; Kim, Y.; Han, S.-B. Stimulatory effect of β-glucans on immune cells. Immune Netw. 2011, 11, 191–195. [Google Scholar] [CrossRef]
- Vogt, L.M.; Boekschoten, M.V.; De Groot, P.J.; Faas, M.M.; De Vos, P. Cellulose alters the expression of nuclear factor kappa b-related genes and toll-like receptor-related genes in human peripheral blood mononuclear cells. J. Funct. Foods 2015, 18, 520–531. [Google Scholar] [CrossRef]
- Köffel, R.; Meshcheryakova, A.; Warszawska, J.; Hennig, A.; Wagner, K.; Jörgl, A.; Gubi, D.; Moser, D.; Hladik, A.; Hoffmann, U. Monocytic cell differentiation from band-stage neutrophils under inflammatory conditions via mkk6 activation. Blood 2014, 124, 2713–2724. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Infection, fever, and exogenous and endogenous pyrogens: Some concepts have changed. J. Endotoxin Res. 2004, 10, 201–222. [Google Scholar]
- Fennrich, S.; Hennig, U.; Toliashvili, L.; Schlensak, C.; Wendel, H.P.; Stoppelkamp, S. More than 70 years of pyrogen detection: Current state and future perspectives. Altern. Lab. Anim. 2016, 44, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Sahni, A.; Sahni, S.K.; Francis, C.W. Endothelial cell activation by il-1β in the presence of fibrinogen requires αvβ3. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2222–2227. [Google Scholar] [CrossRef] [PubMed]
- Gross, S.S.; Jaffe, E.A.; Levi, R.; Kilbourn, R.G. Cytokine-activated endothelial cells express an isotype of nitric oxide synthase which is tetrahydrobiopterin-dependent, calmodulin-independent and inhibited by arginine analogs with a rank-order of potency characteristic of activated macrophages. Biochem. Biophys. Res. Commun. 1991, 178, 823–829. [Google Scholar] [CrossRef]
- Hottz, E.D.; Monteiro, A.P.T.; Bozza, F.A.; Bozza, P.T. Inflammasome in platelets: Allying coagulation and inflammation in infectious and sterile diseases? Mediat. Inflamm. 2015. [Google Scholar] [CrossRef]
- Mansour, A.; Roussel, M.; Gaussem, P.; Nédelec-Gac, F.; Pontis, A.; Flécher, E.; Bachelot-Loza, C.; Gouin-Thibault, I. Platelet functions during extracorporeal membrane oxygenation. Platelet–leukocyte aggregates analyzed by flow cytometry as a promising tool to monitor platelet activation. J. Clin. Med. 2020, 9, 2361. [Google Scholar] [CrossRef] [PubMed]
- Brugger, W.; Mocklin, W.; Heimfeld, S.; Berenson, R.J.; Mertelsmann, R.; Kanz, L. Ex vivo expansion of enriched peripheral blood cd34+ progenitor cells by stem cell factor, interleukin-1 beta (il-1 beta), il-6, il-3, interferon-gamma, and erythropoietin. Blood 1993, 81, 579–584. [Google Scholar] [CrossRef]
- Wachtfogel, Y.T.; Kucich, U.; James, H.L.; Scott, C.F.; Schapira, M.; Zimmerman, M.; Cohen, A.B.; Colman, R. Human plasma kallikrein releases neutrophil elastase during blood coagulation. J. Clin. Investig. 1983, 72, 1672–1677. [Google Scholar] [CrossRef]
- Lacy, P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin. Immunol. 2006, 2, 98. [Google Scholar] [CrossRef]
- Taub, D.D.; Anver, M.; Oppenheim, J.J.; Longo, D.L.; Murphy, W.J. T lymphocyte recruitment by interleukin-8 (il-8). Il-8-induced degranulation of neutrophils releases potent chemoattractants for human t lymphocytes both in vitro and in vivo. J. Clin. Investig. 1996, 97, 1931–1941. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of il-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Flaumenhaft, R. Platelet α-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-N.; Bergeron, A.L.; Yu, Q.; Sun, C.; Mcintire, L.V.; López, J.A.; Dong, J.-F. Platelet aggregation and activation under complex patterns of shear stress. Thromb. Haemost. 2002, 88, 817–821. [Google Scholar] [CrossRef]
- Østerud, B.; Bjørklid, E. Sources of tissue factor. Semin. Thromb. Hemost. 2006, 32, 11–23. [Google Scholar] [CrossRef]
- Kambas, K.; Mitroulis, I.; Apostolidou, E.; Girod, A.; Chrysanthopoulou, A.; Pneumatikos, I.; Skendros, P.; Kourtzelis, I.; Koffa, M.; Kotsianidis, I. Autophagy mediates the delivery of thrombogenic tissue factor to neutrophil extracellular traps in human sepsis. PLoS ONE 2012. [Google Scholar] [CrossRef]
- Zelaya, H.; Rothmeier, A.; Ruf, W. Tissue factor at the crossroad of coagulation and cell signaling. J. Thromb. Haemost. 2018, 16, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, R.; Siedlecki, C.A.; Vogler, E.A. Autoactivation of blood factor xii at hydrophilic and hydrophobic surfaces. Biomaterials 2006, 27, 4325–4332. [Google Scholar] [CrossRef]
- Green, D. Coagulation cascade. Hemodial. Int. 2006, 10, S2–S4. [Google Scholar] [CrossRef] [PubMed]
- Mandle, R.; Kaplan, A. Hageman factor substrates. Human plasma prekallikrein: Mechanism of activation by hageman factor and participation in hageman factor-dependent fibrinolysis. J. Biol. Chem. 1977, 252, 6097–6104. [Google Scholar] [CrossRef]
- Wiggins, R.C.; Bouma, B.N.; Cochrane, C.G.; Griffin, J.H. Role of high-molecular-weight kininogen in surface-binding and activation of coagulation factor xi and prekallikrein. Proc. Natl. Acad. Sci. USA 1977, 74, 4636–4640. [Google Scholar] [CrossRef]
- Dray, A.; Perkins, M. Bradykinin and inflammatory pain. Trends Neurosci. 1993, 16, 99–104. [Google Scholar] [CrossRef]
- Gouault-Heilmann, M.; Huet, Y.; Contant, G.; Payen, D.; Bloch, G.; Rapin, M. Cardiopulmonary bypass with a low-molecular-weight heparin fraction. Lancet 1983, 322, 1374. [Google Scholar] [CrossRef]
- Massonnet-Castel, S.; Pelissier, E.; Dreyfus, G.; Deloche, A.; Abry, B.; Guibourt, P.; Terrier, E.; Passelecq, J.; Jaulmes, B.; Carpentier, A. Low-molecular-weight heparin in extracorporeal circulation. Lancet 1984, 323, 1182–1183. [Google Scholar] [CrossRef]
- Dinarello, C.A. Blocking il-1 in systemic inflammation. J. Exp. Med. 2005, 201, 1355–1359. [Google Scholar] [CrossRef]
- Norris, C.A.; He, M.; Kang, L.-I.; Ding, M.Q.; Radder, J.E.; Haynes, M.M.; Yang, Y.; Paranjpe, S.; Bowen, W.C.; Orr, A. Synthesis of il-6 by hepatocytes is a normal response to common hepatic stimuli. PLoS ONE 2014, 9, e96053. [Google Scholar] [CrossRef] [PubMed]
- Janowski, M. Functional diversity of sdf-1 splicing variants. Cell Adhes. Migr. 2009, 3, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Mccandless, E.E.; Budde, M.; Lees, J.R.; Dorsey, D.; Lyng, E.; Klein, R.S. Il-1r signaling within the central nervous system regulates cxcl12 expression at the blood-brain barrier and disease severity during experimental autoimmune encephalomyelitis. J. Immunol. 2009, 183, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Seizer, P.; Borst, O.; Schönberger, T.; Mack, A.; Geisler, T.; Langer, H.F.; May, A.E.; Vogel, S.; Lang, F. Sdf-1α induces differential trafficking of cxcr4-cxcr7 involving cyclophilin a, cxcr7 ubiquitination and promotes platelet survival. FASEB J. 2014, 28, 2864–2878. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cheng, G.; Hao, M.; Zheng, J.; Zhou, X.; Zhang, J.; Taichman, R.S.; Pienta, K.J.; Wang, J. Cxcl12/cxcr4/cxcr7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010, 29, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Massberg, S.; Konrad, I.; SchüRzinger, K.; Lorenz, M.; Schneider, S.; Zohlnhoefer, D.; Hoppe, K.; Schiemann, M.; Kennerknecht, E.; Sauer, S. Platelets secrete stromal cell–derived factor 1α and recruit bone marrow–derived progenitor cells to arterial thrombi in vivo. J. Exp. Med. 2006, 203, 1221–1233. [Google Scholar] [CrossRef]
- Chatterjee, M.; Von Ungern-Sternberg, S.N.; Seizer, P.; Schlegel, F.; Büttcher, M.; Sindhu, N.; Müller, S.; Mack, A.; Gawaz, M. Platelet-derived cxcl12 regulates monocyte function, survival, differentiation into macrophages and foam cells through differential involvement of cxcr4–cxcr7. Cell Death Dis. 2015, 6, e1989. [Google Scholar] [CrossRef]
- Clancy, D.M.; Sullivan, G.P.; Moran, H.B.; Henry, C.M.; Reeves, E.P.; Mcelvaney, N.G.; Lavelle, E.C.; Martin, S.J. Extracellular neutrophil proteases are efficient regulators of il-1, il-33, and il-36 cytokine activity but poor effectors of microbial killing. Cell Rep. 2018, 22, 2937–2950. [Google Scholar] [CrossRef]
- Hernández, M.R.; Galán, A.M.; Cases, A.; Lopez-Pedret, J.; Pereira, A.; Tonda, R.; Bozzo, J.; Escolar, G.; Ordinas, A. Biocompatibility of cellulosic and synthetic membranes assessed by leukocyte activation. Am. J. Nephrol. 2004, 24, 235–241. [Google Scholar] [CrossRef]
- Castelli, W.A.; Nasjleti, C.F.; Diaz-Perez, R.; Caffesse, R.G. Cheek mucosa response to silk, cotton, and nylon suture materials. Oral Surg. Oral Med. Oral Pathol. 1978, 45, 186–189. [Google Scholar] [CrossRef]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Jang, J.; Go, W.-S. Continuous photografting of hema onto polypropylene fabrics with benzophenone photoinitiator. Fibers Polym. 2008, 9, 375–379. [Google Scholar] [CrossRef]




| Abdominal Swabs | Material | Lot | Company | Size |
|---|---|---|---|---|
| Telasorb white | cotton | 299900005 | Paul Hartmann AG | 20 × 30 cm |
| BARRIER special non-woven abdominal swabs white | synthetic | 17395462 | Mölnlycke Health Care GmbH | 40 × 40 cm |
| Patients | Cotton | Synthetic | p Values |
|---|---|---|---|
| Number | 10 | 10 (1 death day 5) | |
| Age (years) | 66.7 ± 7.0 | 68.9 ± 7.5 | 0.885 |
| Gender (male/female) | 7/3 | 6/4 | 0.639 |
| Height (cm) | 170.2 ± 10.1 | 169.3 ± 8.7 | 0.544 |
| Weight (kg) | 82.1 ±14.2 | 75.5 ± 20.1 | 0.244 |
| BMI (kg/m²) | 28.7 ± 4.7 | 25.9 ± 5.1 | 0.761 |
| Body surface area (m2) | 1.9 ± 0.2 | 1.9 0.3 | 0.272 |
| Hospitalization (days) | 13.4 ± 5.7 | 11.0 ± 3.5 | 0.118 |
| Duration of surgery (min) | 245.7 ± 47.0 | 229.0 ± 50.5 | 0.675 |
| Duration of bypass (min) | 133.7 ±45.7 | 103.6 ± 37.7 | 0.357 |
| Duration of aortic clamping (min) | 101.4 ± 36.1 | 76.3 ± 31.9 | 0.704 |
| Intensive postoperative treatment (days) | 2.0 ± 2.1 | 1.4 ± 1.2 | 0.305 |
| Comorbidities | |||
| BMI (kg/m²) <25 | 20% | 30% | |
| BMI (kg/m²) ≥ 25-<30 | 50% | 50% | |
| BMI (kg/m²) ≥30-<35 | 20% | 20% | |
| BMI (kg/m²) ≥35-<40 | 10% | ||
| Diabetes mellitus | 20% | 50% | |
| (Arterial) hypertension | 60% | 70% | |
| Inflammatory markers (post-surgery) | |||
| Maximal CRP (mg/dl) | 14.57 ± 5.88 | 12.16 ± 3.32 | 0.275 |
| CRP at discharge (mg/dl) | 4.26 ± 2.23 | 4.49 ± 2.67 | 0.855 |
| Patients with PCT >2 (ng/mL) | 2 | 1 | |
| SIRS symptoms (1 day post-surgery) | 5/10 | 2/10 | |
| SOFA score (1 day post-surgery) | 5.6 ± 3.5 | 4.2 ± 2.3 | 0.302 |
| Cotton | Synthetic | |
|---|---|---|
| +0.1 EU/mL liquid LPS | 42.3% | 21.8% |
| +0.1 EU/mL impregnated LPS | 0% | 2.2% |
| +1.0 EU/mL impregnated LPS | 0% | 17.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerling, K.; Herrmann, L.M.; Salewski, C.; Wolf, M.; Müllerbader, P.; Siegel-Axel, D.; Wendel, H.P.; Schlensak, C.; Avci-Adali, M.; Stoppelkamp, S. Synthetic Material Abdominal Swabs Reduce Activation of Platelets and Leukocytes Compared to Cotton Materials. Biomolecules 2021, 11, 1023. https://doi.org/10.3390/biom11071023
Gerling K, Herrmann LM, Salewski C, Wolf M, Müllerbader P, Siegel-Axel D, Wendel HP, Schlensak C, Avci-Adali M, Stoppelkamp S. Synthetic Material Abdominal Swabs Reduce Activation of Platelets and Leukocytes Compared to Cotton Materials. Biomolecules. 2021; 11(7):1023. https://doi.org/10.3390/biom11071023
Chicago/Turabian StyleGerling, Katharina, Lisa Maria Herrmann, Christoph Salewski, Melanie Wolf, Pia Müllerbader, Dorothea Siegel-Axel, Hans Peter Wendel, Christian Schlensak, Meltem Avci-Adali, and Sandra Stoppelkamp. 2021. "Synthetic Material Abdominal Swabs Reduce Activation of Platelets and Leukocytes Compared to Cotton Materials" Biomolecules 11, no. 7: 1023. https://doi.org/10.3390/biom11071023
APA StyleGerling, K., Herrmann, L. M., Salewski, C., Wolf, M., Müllerbader, P., Siegel-Axel, D., Wendel, H. P., Schlensak, C., Avci-Adali, M., & Stoppelkamp, S. (2021). Synthetic Material Abdominal Swabs Reduce Activation of Platelets and Leukocytes Compared to Cotton Materials. Biomolecules, 11(7), 1023. https://doi.org/10.3390/biom11071023








