Immobilized Soybean Peroxidase Hybrid Biocatalysts for Efficient Degradation of Various Emerging Pollutants
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Solvents, and Enzyme
2.2. Immobilization of SBP onto Photocatalytic Supports
2.3. Characterization of Photocatalytic Supports and Immobilized Enzyme
2.4. Effect of pH and Temperature on the Stability of Free and Immobilized SBP
2.5. Degradation Potential of Emerging Pollutants by Free and Immobilized Enzymes
2.6. Degradation Analysis by LC-MSMS Method
2.7. Recycling Ability of the Immobilized Biocatalyst
2.8. Statistical Analysis
3. Results and Discussion
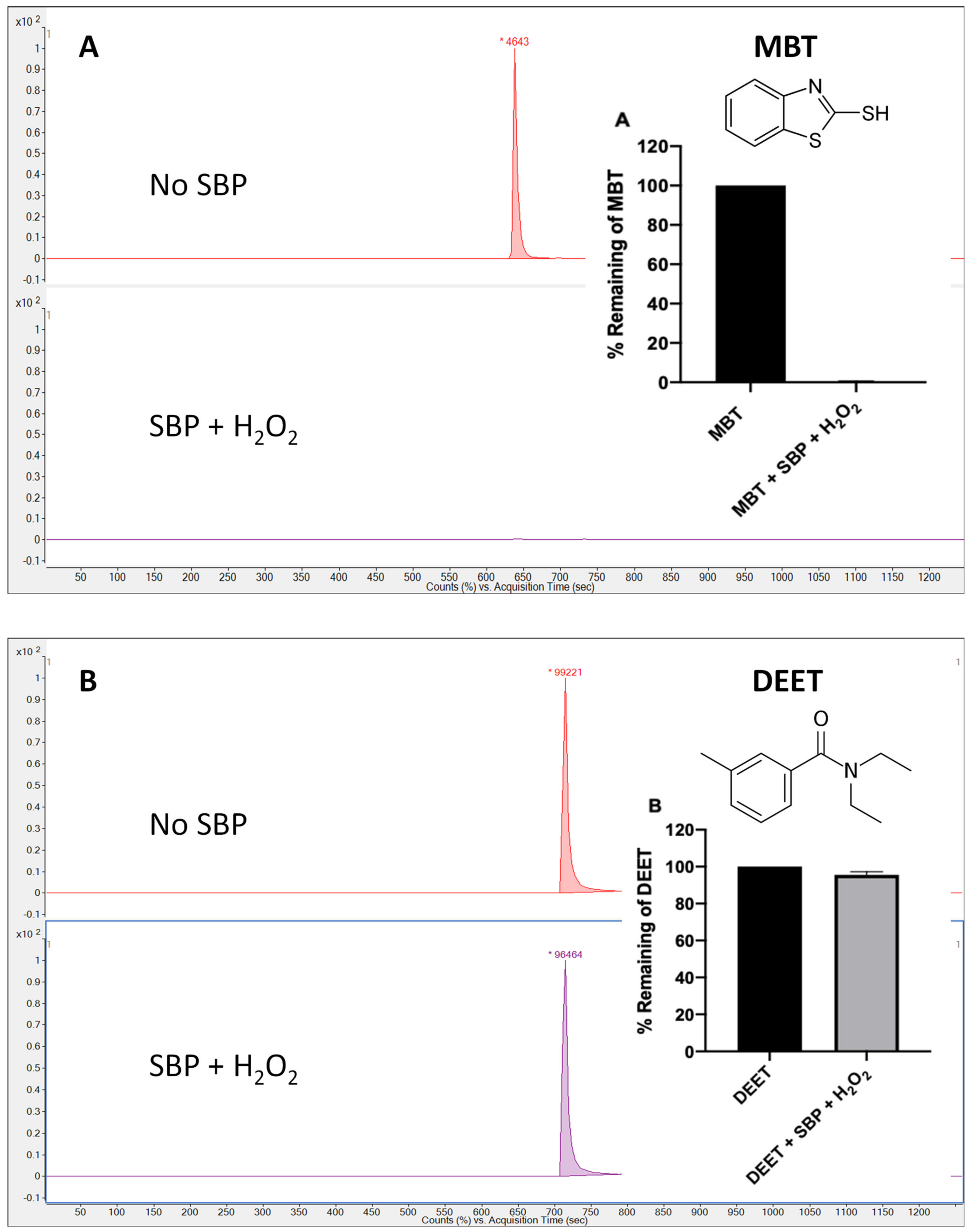
3.1. Soybean Peroxidase-Catalyzed Degradation of 21 Eps
3.2. Requirement of a Redox Mediator
3.3. Characterization of Immobilized Hybrid Biocatalysts
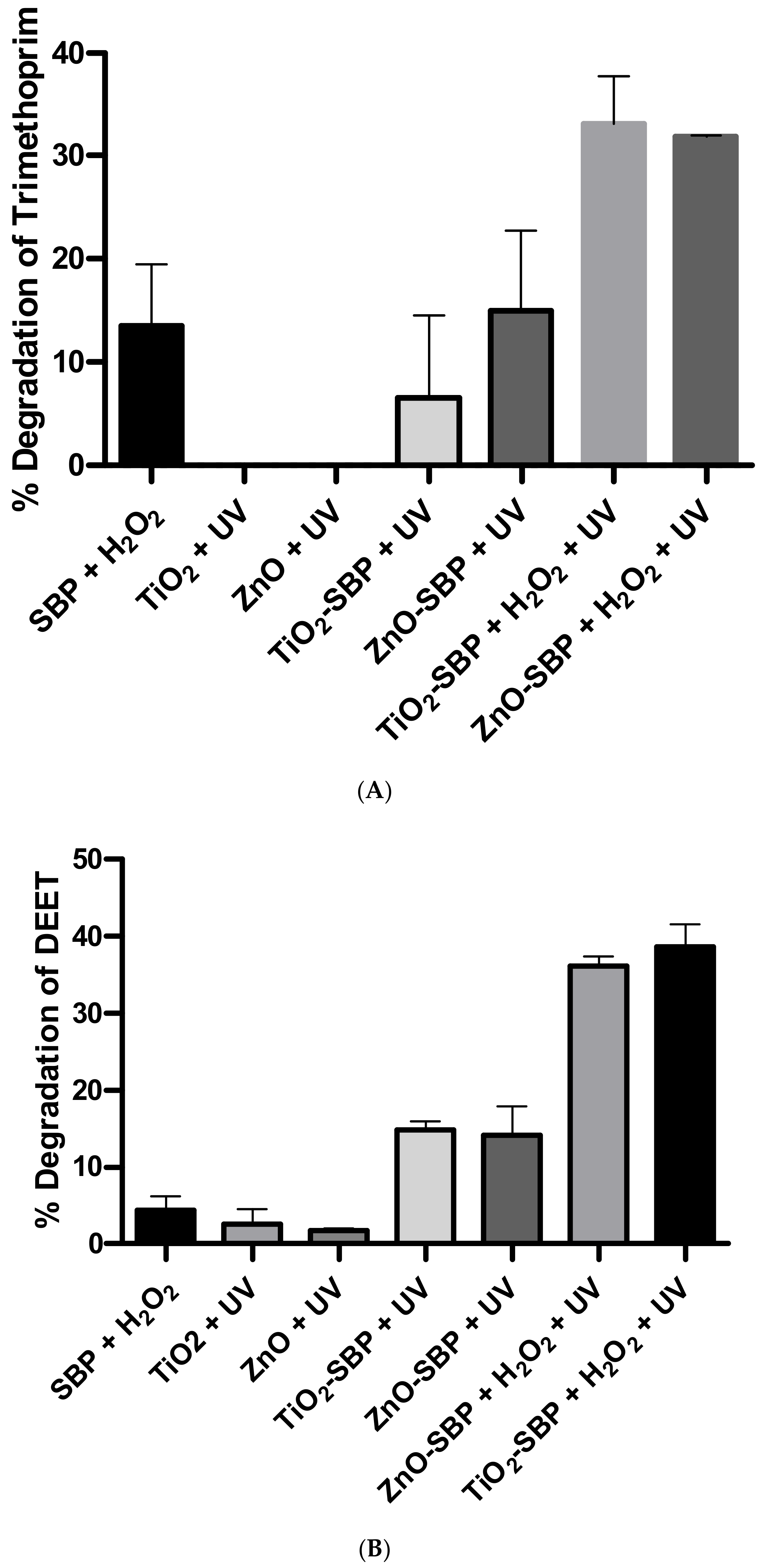
3.4. Degradation of 21 Eps by the Immobilized Hybrid Biocatalysts
3.5. Comparison between Free vs. Immobilized Enzyme
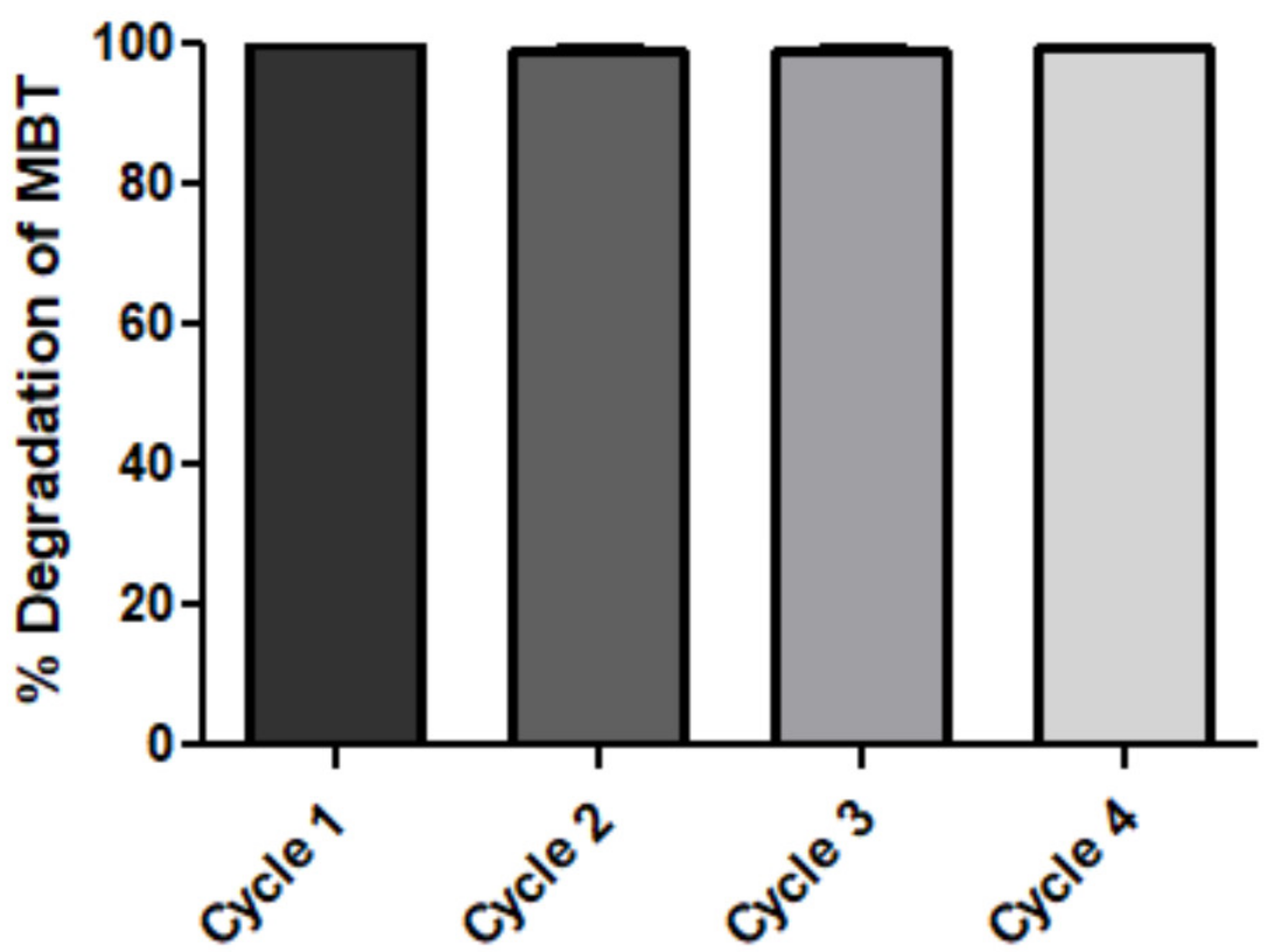
3.6. Reusability of Immobilized Enzyme
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sauvé, S.; Desrosiers, M. A Review of What Is an Emerging Contaminant. Chem. Cent. J. 2014, 8, 15. [Google Scholar] [CrossRef]
- Teodosiu, C.; Gilca, A.-F.; Barjoveanu, G.; Fiore, S. Emerging Pollutants Removal through Advanced Drinking Water Treatment: A Review on Processes and Environmental Performances Assessment. J. Clean. Prod. 2018, 197, 1210–1221. [Google Scholar] [CrossRef]
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging Organic Contaminants in Groundwater: A Review of Sources, Fate and Occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the Biological and Chemical Treatment Technologies for Emerging Contaminant Removal from Wastewater: A Critical Review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef]
- Tran, N.H.; Urase, T.; Ngo, H.H.; Hu, J.; Ong, S.L. Insight into Metabolic and Cometabolic Activities of Autotrophic and Heterotrophic Microorganisms in the Biodegradation of Emerging Trace Organic Contaminants. Bioresour. Technol. 2013, 146, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, D.; Baccar, R.; Jaabiri, I.; Bouzid, J.; Kallel, M.; Ayadi, H.; Zhou, J.L. Fate of Selected Estrogenic Hormones in an Urban Sewage Treatment Plant in Tunisia (North Africa). Sci. Total Environ. 2015, 505, 154–160. [Google Scholar] [CrossRef]
- Cinperi, N.C.; Ozturkb, E.; Yigit, N.O.; Kitis, M. Treatment of Woolen Textile Wastewater Using Membrane Bioreactor, Nanofiltration and Reverse Osmosis for Reuse in Production Processes. J. Clean. Prod. 2019, 233, 837–848. [Google Scholar] [CrossRef]
- Dias, N.C.; Bassin, J.P.; Geraldol, S.-A., Jr.; Dezotti, M. Ozonation of the Dye Reactive Red 239 and Biodegradation of Ozonation Products in a Moving-Bed Biofilm Reactor: Revealing Reaction Products and Degradation Pathways. Int. Biodeterior. Biodegrad. 2019, 144, 104742. [Google Scholar] [CrossRef]
- Gui, L.; Peng, J.; Li, P.; Penga, R.; Yua, P.; Luo, Y. Electrochemical Degradation of Dye on TiO2nanotube Array Constructed Anode. Chemosphere 2019, 235, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Gao, B.; Tian, X.; Yue, Q.; Zhang, P.; Shen, X.; Xu, X. Synthesis of Polyaluminium Chloride/Papermaking Sludge-Based Organic Polymer Composites for Removal of Disperse Yellow and Reactive Blue by Flocculation. Chemosphere 2019, 231, 337–348. [Google Scholar] [CrossRef]
- Wang, W.; Lua, T.; Chen, Y.; Tiana, G.; Sharma, V.K.; Zhu, Y.; Zonga, L.; Wang, A. Mesoporous silicate/carbon composites derived from dye-loaded palygorskite clay waste for efficient removal of organic contaminants. Sci. Total Environ. 2019, 696, 133955. [Google Scholar] [CrossRef]
- Al-Maqdi, K.A.; Hisaindee, S.M.; Rauf, M.A.; Ashraf, S.S. Comparative Degradation of a Thiazole Pollutant by an Advanced Oxidation Process and an Enzymatic Approach. Biomolecules 2017, 7, 64. [Google Scholar] [CrossRef]
- Gupta, V.K. Suhas Application of Low-Cost Adsorbents for Dye Removal—A Review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Mahamuni, N.N.; Adewuyi, Y.G. Advanced Oxidation Processes (AOPs) Involving Ultrasound for Waste Water Treatment: A Review with Emphasis on Cost Estimation. Ultrason. Sonochem. 2010, 17, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.A.; Salman Ashraf, S. Survey of Recent Trends in Biochemically Assisted Degradation of Dyes. Chem. Eng. J. 2012, 209, 520–530. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of Dyes in Textile Effluent: A Critical Review on Current Treatment Technologies with a Proposed Alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Okoh, A.I.; Nwodo, U.U. Aptitude of Oxidative Enzymes for Treatment of Wastewater Pollutants: A Laccase Perspective. Molecules 2019, 24, 2064. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Emerging Contaminants of High Concern and Their Enzyme-Assisted Biodegradation—A Review. Environ. Int. 2019, 124, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Almaqdi, K.A.; Morsi, R.; Alhayuti, B.; Alharthi, F.; Ashraf, S.S. LC-MSMS Based Screening of Emerging Pollutant Degradation by Different Peroxidases. BMC Biotechnol. 2019, 19, 83. [Google Scholar] [CrossRef]
- Alneyadi, A.H.; Rauf, M.A.; Ashraf, S.S. Oxidoreductases for the Remediation of Organic Pollutants in Water—A Critical Review. Crit. Rev. Biotechnol. 2018, 38, 971–988. [Google Scholar] [CrossRef]
- Morsi, R.; Bilal, M.; Iqbal, H.M.N.; Ashraf, S.S. Laccases and Peroxidases: The Smart, Greener and Futuristic Biocatalytic Tools to Mitigate Recalcitrant Emerging Pollutants. Sci. Total Environ. 2020, 714, 136572. [Google Scholar] [CrossRef]
- Mukherjee, D.; Bhattacharya, S.; Taylor, K.E.; Biswas, N. Enzymatic Treatment for Removal of Hazardous Aqueous Arylamines, 4,4′-Methylenedianiline and 4,4′-Thiodianiline. Chemosphere 2019, 235, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Rathner, R.; Petz, S.; Tasnádi, G.; Koller, M.; Ribitsch, V. Monitoring the Kinetics of Biocatalytic Removal of the Endocrine Disrupting Compound 17α-Ethinylestradiol from Differently Polluted Wastewater Bodies. J. Environ. Chem. Eng. 2017, 5, 1920–1926. [Google Scholar] [CrossRef]
- Pandey, K.; Singh, B.; Pandey, A.; Badruddin, I.; Pandey, S.; Mishra, V.; Jain, P. Application of Microbial Enzymes in Industrial Waste Water Treatment. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1243–1254. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Developments in Support Materials for Immobilization of Oxidoreductases: A Comprehensive Review. Adv. Colloid Interface Sci. 2018, 258, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ambatkar, M.; Usha, M. Enzymatic Treatment of Wastewater Containing Dyestuffs Using Different Delivery Systems. Sci. Rev. Chem. Commun. 2012, 2, 31–40. [Google Scholar]
- Bilal, M.; Jing, Z.; Zhao, Y.; Iqbal, H.M.N. Immobilization of Fungal Laccase on Glutaraldehyde Cross-Linked Chitosan Beads and Its Bio-Catalytic Potential to Degrade Bisphenol A. Biocatal. Agric. Biotechnol. 2019, 19, 101174. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N.; Hu, H.; Wang, W.; Zhang, X. Enhanced Bio-Catalytic Performance and Dye Degradation Potential of Chitosan-Encapsulated Horseradish Peroxidase in a Packed Bed Reactor System. Sci. Total Environ. 2017, 575, 1352–1360. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M. Dye Decolorization and Detoxification Potential of Ca-Alginate Beads Immobilized Manganese Peroxidase. BMC Biotechnol. 2015, 15, 111. [Google Scholar] [CrossRef]
- Donadelli, J.A.; García Einschlag, F.S.; Laurenti, E.; Magnacca, G.; Carlos, L. Soybean Peroxidase Immobilized onto Silica-Coated Superparamagnetic Iron Oxide Nanoparticles: Effect of Silica Layer on the Enzymatic Activity. Colloids Surf. B Biointerfaces 2018, 161, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Tanaka, H.; Blas, B.L.; Noseñas, J.S.; Tokawa, T.; Ohsawa, S. Evaluation of ELISA with ABTS, 2-2′-Azino-Di-(3-Ethylbenzthiazoline Sulfonic Acid), as the Substrate of Peroxidase and Its Application to the Diagnosis of Schistosomiasis. Jpn. J. Exp. Med. 1984, 54, 131–138. [Google Scholar]
- Al-Maqdi, K.A.; Hisaindee, S.; Rauf, M.A.; Ashraf, S.S. Detoxification and Degradation of Sulfamethoxazole by Soybean Peroxidase and UV+ H2O2 Remediation Approaches. Chem. Eng. J. 2018, 352, 450–458. [Google Scholar] [CrossRef]
- Alneyadi, A.H.; Ashraf, S.S. Differential Enzymatic Degradation of Thiazole Pollutants by Two Different Peroxidases—A Comparative Study. Chem. Eng. J. 2016, 303, 529–538. [Google Scholar] [CrossRef]
- Gros, M.; Petrović, M.; Barceló, D. Development of a Multi-Residue Analytical Methodology Based on Liquid Chromatography–Tandem Mass Spectrometry (LC–MS/MS) for Screening and Trace Level Determination of Pharmaceuticals in Surface and Wastewaters. Talanta 2006, 70, 678–690. [Google Scholar] [CrossRef]
- Adelaja, O.; Keshavarz, T.; Kyazze, G. The Effect of Salinity, Redox Mediators and Temperature on Anaerobic Biodegradation of Petroleum Hydrocarbons in Microbial Fuel Cells. J. Hazard. Mater. 2015, 283, 211–217. [Google Scholar] [CrossRef]
- Pillard, D.A.; Cornell, J.S.; Dufresne, D.L.; Hernandez, M.T. Toxicity of Benzotriazole and Benzotriazole Derivatives to Three Aquatic Species. Water Res. 2001, 35, 557–560. [Google Scholar] [CrossRef]
- Hanif, M.; Lee, I.; Akter, J.; Islam, A.; Zahid, M.; Sapkota, K.; Hahn, J. Enhanced Photocatalytic and Antibacterial Performance of ZnO Nanoparticles Prepared by an Efficient Thermolysis Method. Catalysts 2019, 9, 608. [Google Scholar] [CrossRef]
- Mohajershojaei, K.; Mahmoodi, N.M.; Khosravi, A. Immobilization of Laccase Enzyme onto Titania Nanoparticle and Decolorization of Dyes from Single and Binary Systems. Biotechnol. Bioprocess Eng. 2015, 20, 109–116. [Google Scholar] [CrossRef]
- Tummino, M.L.; Tolardo, V.; Malandrino, M.; Sadraei, R.; Magnacca, G.; Laurenti, E. A Way to Close the Loop: Physicochemical and Adsorbing Properties of Soybean Hulls Recovered After Soybean Peroxidase Extraction. Front. Chem. 2020, 8. [Google Scholar] [CrossRef]
- Singh, B.R. Basic Aspects of the Technique and Applications of Infrared Spectroscopy of Peptides and Proteins. In Infrared Analysis of Peptides and Proteins; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1999; Volume 750, pp. 2–37. ISBN 978-0-8412-3636-3. [Google Scholar]
- Calza, P.; Zacchigna, D.; Laurenti, E. Degradation of Orange Dyes and Carbamazepine by Soybean Peroxidase Immobilized on Silica Monoliths and Titanium Dioxide. Environ. Sci. Pollut. Res. 2016, 23, 23742–23749. [Google Scholar] [CrossRef] [PubMed]
- Calza, P.; Avetta, P.; Rubulotta, G.; Sangermano, M.; Laurenti, E. TiO2-Soybean Peroxidase Composite Materials as a New Photocatalytic System. Chem. Eng. J. 2014, 239, 87–92. [Google Scholar] [CrossRef]
- Sarro, M.; Gule, N.P.; Laurenti, E.; Gamberini, R.; Paganini, M.C.; Mallon, P.E.; Calza, P. ZnO-Based Materials and Enzymes Hybrid Systems as Highly Efficient Catalysts for Recalcitrant Pollutants Abatement. Chem. Eng. J. 2018, 334, 2530–2538. [Google Scholar] [CrossRef]
- Wang, M.; Shi, H.; Wu, D.; Han, H.; Zhang, J.; Xing, Z.; Wang, S.; Li, Q. Glutaraldehyde Cross-Linking of Immobilized Thermophilic Esterase on Hydrophobic Macroporous Resin for Application in Poly(ε-Caprolactone) Synthesis. Molecules 2014, 19, 9838–9849. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, S.; Neppolian, B.; Shankar, M.V.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar Photocatalytic Degradation of Azo Dye: Comparison of Photocatalytic Efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Asgher, M.; Noreen, S.; Bilal, M. Enhancement of catalytic, reusability, and long-term stability features of Trametes versicolor IBL-04 laccase immobilized on different polymers. Int. J. Biol. Macromol. 2017, 95, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yu, S.; Li, J.; Mo, T.; Li, P. Enhancement of the Catalytic Activity and Stability of Immobilized Aminoacylase Using Modified Magnetic Fe3O4 Nanoparticles. Chem. Eng. J. 2016, 286, 216–222. [Google Scholar] [CrossRef]
- Gestrelius, S.; Mattiasson, B.; Mosbach, K. On the Regulation of the Activity of Immobilized Enzymes. Microenvironmental Effects of Enzyme-Generated PH Changes. Eur. J. Biochem. 1973, 36, 89–96. [Google Scholar] [CrossRef] [PubMed]



| Emerging Pollutant | SBP | SBP + HOBT | ||
|---|---|---|---|---|
| % Degradation | % Degradation | |||
| Group A | 1 | MBT | 99.4 ± 0.1 | 98.7 ± 0.3 |
| 2 | Meloxicam | 98.8 ± 0.2 | 99.0 ± 0.3 | |
| 3 | Caffeic acid | 88.2 ± 2.4 | 100.0 ± 0.1 | |
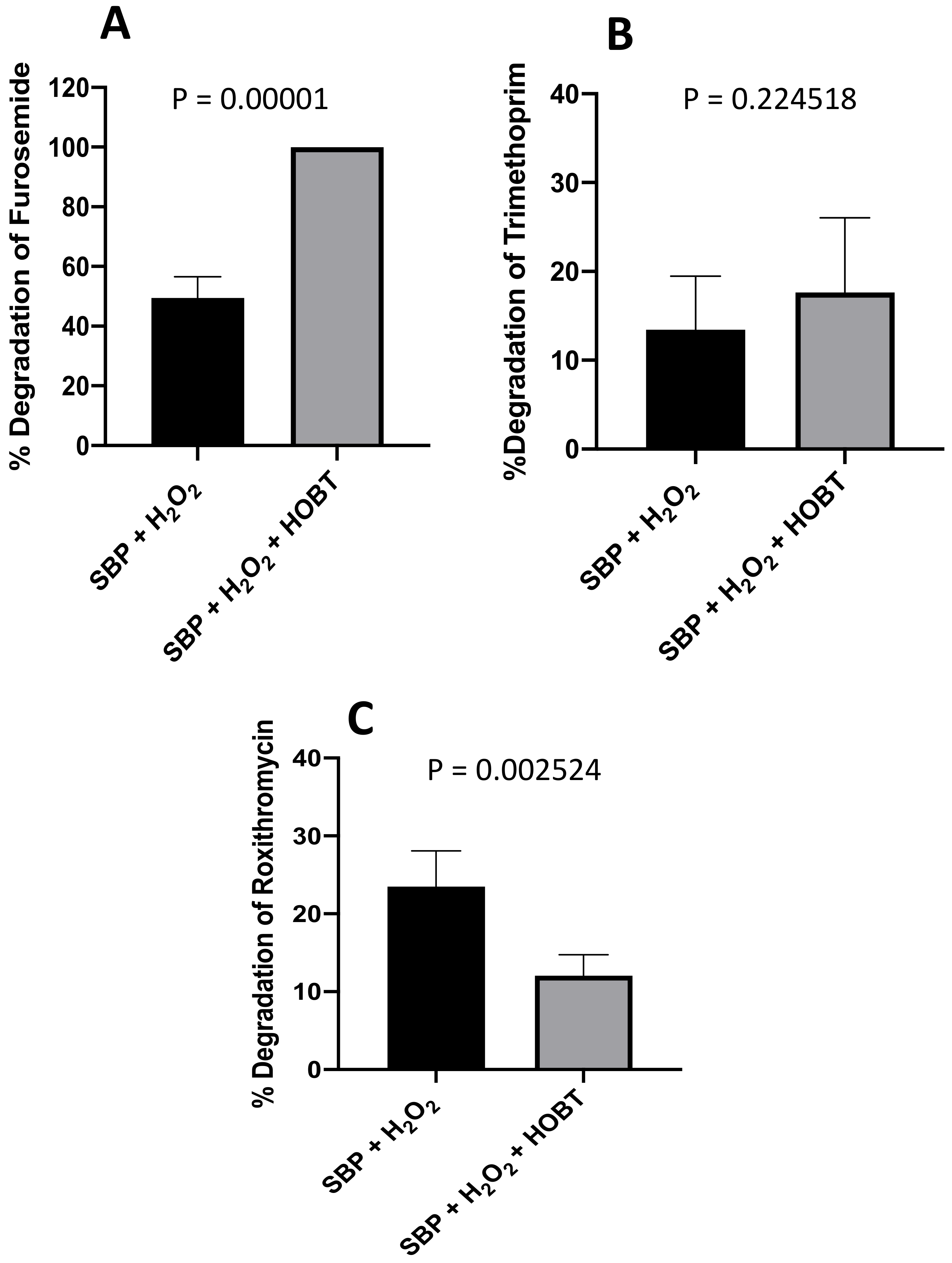
| Group B | 4 | Furosemide | 49.5 ± 7.0 | 100.0 ± 0.1 |
| 5 | SMX | 38.5 ± 6.9 | 99.3 ± 0.3 | |
| Group C | 6 | Roxithromycin | 23.5 ± 4.6 | 12.0 ± 2.7 |
| Group D | 7 | Caffeine | 16.7 ± 8.5 | 21.8 ± 1.3 |
| 8 | Ibuprofen | 16.6 ± 5.3 | 13.8 ± 8.9 | |
| 9 | Atenolol | 13.5 ± 5.8 | 6.9 ± 9.5 | |
| 10 | Trimethoprim | 13.4 ± 6.0 | 17.6 ± 8.4 | |
| Group E | 11 | Prometryn | 8.1 ± 7.3 | 4.3 ± 5.6 |
| 12 | Hydrochlorothiazide | 5.9 ± 3.4 | 5.5 ± 5.3 | |
| 13 | DEET | 4.4 ± 1.7 | 5.4 ± 1.4 | |
| 14 | Venlafaxine-HCl | 4.4 ± 3.1 | 3.8 ± 2.4 | |
| 15 | MCPA | 4.1 ± 6.4 | 6.1 ± 6.3 | |
| 16 | Phenytoin | 3.7 ± 6.7 | 0.0 ± 4.2 | |
| 17 | Lincomycin-HCl | 2.6 ± 7.9 | 5.8 ± 8.1 | |
| 18 | Norfloxacin | 2.5 ± 4.8 | 5.5 ± 3.6 | |
| 19 | Cimetidine | 2.1 ± 7.8 | 8.7 ± 7.7 | |
| 20 | Fluometuron | 1.8 ± 4.6 | 2.5 ± 4.5 | |
| 21 | Thiabendazole | 0.0 ± 0.1 | 4.3 ± 3.9 |
| Emerging Pollutant | TiO2-SBP | TiO2-SBP + HOBT | ||
|---|---|---|---|---|
| % Degradation | % Degradation | |||
| Group A | 1 | Caffeic acid | 100.0 ± 0.1 | 100.0 ± 0.1 |
| 2 | MBT | 100.0 ± 0.1 | 100.0 ± 0.1 | |
| 3 | Caffeine | 60.2 ± 4.1 | 57.2 ± 4.6 | |
| Group B | 4 | Meloxicam | 26.8 ± 4.4 | 99.8 ± 0.1 |
| 5 | Furosemide | 15.7 ± 5.5 | 100.0 ± 0.1 | |
| 6 | SMX | 9.7 ± 1.5 | 37.9 ± 3.2 | |
| 7 | DEET | 3.7 ± 2.8 | 17.6 ± 2.8 | |
| 8 | MCPA | 3.1 ± 3.8 | 11.5 ± 7.1 | |
| 9 | Lincomycin-HCl | 2.8 ± 1.4 | 15.1 ± 4.9 | |
| 10 | Hydrochlorothiazide | 0.0 ± 0.1 | 14.3 ± 7.5 | |
| Group C | 11 | Norfloxacin | 45.1 ± 1.3 | 0.0 ± 0.1 |
| Group D | 12 | Ibuprofen | 32.2 ± 8.0 | 39.5 ± 5.3 |
| 13 | Cimetidine | 24.4 ± 2.0 | 19.3 ± 2.5 | |
| 14 | Roxithromycin | 24.4 ± 7.3 | 17.7 ± 3.4 | |
| Group E | 15 | Prometryn | 9.4 ± 5 | 9.2 ± 5.4 |
| 16 | Venlafaxine-HCl | 6.2 ± 2.5 | 6.5 ± 5.5 | |
| 17 | Fluometuron | 5.7 ± 8.9 | 3.0 ± 9.6 | |
| 18 | Trimethoprim | 3.9 ± 3.8 | 7.0 ± 3.5 | |
| 19 | Phenytoin | 3.2 ± 1.9 | 7.4 ± 2.5 | |
| 20 | Thiabendazole | 0.2 ± 2.8 | 4.5 ± 3.2 | |
| 21 | Atenolol | 0.0 ± 0.1 | 0.2 ± 4.2 |
| Emerging Pollutant | ZnO-SBP | ZnO-SBP + HOBT | ||
|---|---|---|---|---|
| % Degradation | % Degradation | |||
| Group A | 1 | MBT | 100.0 ± 0.1 | 100.0 ± 0.1 |
| Group B | 2 | Caffeic acid | 54.4 ± 3.8 | 100.0 ± 0.1 |
| 3 | Hydrochlorothiazide | 19.0 ± 1.5 | 25.8 ± 3.7 | |
| 4 | SMX | 12.5 ± 2.4 | 31.9 ± 4.1 | |
| 5 | Meloxicam | 11.4 ± 6.9 | 100.0 ± 0.1 | |
| 6 | Lincomycin-HCl | 5.6 ± 3.6 | 14.9 ± 3.5 | |
| 7 | Cimetidine | 4.7 ± 1.0 | 11.3 ± 4.4 | |
| 8 | DEET | 3.0 ± 5.5 | 14.4 ± 2.2 | |
| 9 | Furosemide | 2.6 ± 7.1 | 100.0 ± 0.1 | |
| Group C | 10 | MCPA | 18.7 ± 7.8 | 1.6 ± 9.8 |
| Group D | 11 | Roxithromycin | 48.6 ± 4.9 | 37.4 ± 8.6 |
| 12 | Trimethoprim | 13.8 ± 9.4 | 17.8 ± 1.2 | |
| 13 | Phenytoin | 13.4 ± 3.3 | 10.3 ± 2.8 | |
| 14 | Ibuprofen | 13.1 ± 6.4 | 22.2 ± 8.8 | |
| 15 | Venlafaxine-HCl | 7.3 ± 5.7 | 11.0 ± 4.5 | |
| Group E | 16 | Prometryn | 5.6 ± 1.9 | 4.0 + 3.9 |
| 17 | Norfloxacin | 5.5 ± 3.0 | 5.5 ± 1.7 | |
| 18 | Thiabendazole | 4.8 ± 1.1 | 6.5 ± 1.4 | |
| 19 | Fluometuron | 2.8 ± 6.8 | 2.2 ± 7.1 | |
| 20 | Caffeine | 0.2 ± 9.6 | 1.5 ± 7.4 | |
| 21 | Atenolol | 0.0 ± 0.1 | 9.5 ± 4 |
| Emerging Pollutant | SBP | SBP-TiO2 | SBP-ZnO | ||||
|---|---|---|---|---|---|---|---|
| No HOBT | With HOBT | No HOBT | With HOBT | No HOBT | With HOBT | ||
| 1 | MBT | 99.4 ± 0.1 | 98.7 ± 0.3 | 100.0 ± 0.1 | 100.0 ± 0.1 | 100.0 ± 0.1 | 100.0 ± 0.1 |
| 2 | Meloxicam | 98.8 ± 0.2 | 99.0 ± 0.3 | 26.8 ± 4.4 | 99.8 ± 0.1 | 11.4 ± 6.9 | 100.0 ± 0.1 |
| 3 | Caffeic acid | 88.2 ± 2.4 | 100.0 ± 0.1 | 100.0 ± 0.1 | 100.0 ± 0.1 | 54.4 ± 3.8 | 100.0 ± 0.1 |
| 4 | Furosemide | 49.5 ± 7.0 | 100.0 ± 0.1 | 15.7 ± 5.5 | 100.0 ± 0.1 | 2.6 ± 7.1 | 100.0 ± 1.0 |
| 5 | SMX | 38.5 ± 6.9 | 99.3 ± 0.3 | 9.7 ± 1.5 | 37.9 ± 3.2 | 12.5 ± 2.4 | 31.9 ± 4.1 |
| 6 | Roxithromycin | 23.5 ± 4.6 | 12.0 ± 2.7 | 24.4 ± 7.3 | 17.7 ± 3.4 | 48.6 ± 4.9 | 37.4 ± 8.6 |
| 7 | Caffeine | 16.7 ± 8.5 | 21.8 ± 1.3 | 60.2 ± 4.1 | 57.2 ± 4.6 | 0.2 ± 9.6 | 1.5 ± 7.4 |
| 8 | Ibuprofen | 16.6 ± 5.3 | 13.8 ± 8.9 | 32.2 ± 8.0 | 39.5 ± 5.3 | 13.1 ± 6.4 | 22.2 ± 8.8 |
| 9 | Atenolol | 13.5 ± 5.8 | 6.9 ± 9.5 | 0.0 ± 0.1 | 0.2 ± 4.2 | 0.0 ± 0.1 | 9.5 ± 4.0 |
| 10 | Trimethoprim | 13.4 ± 6.0 | 17.6 ± 8.4 | 3.9 ± 3.8 | 7.0 ± 3.5 | 13.8 ± 9.4 | 17.8 ± 1.2 |
| 11 | Prometryn | 8.1 ± 7.3 | 4.3 ± 5.6 | 9.4 ± 5.0 | 9.2 ± 5.4 | 5.6 ± 1.9 | 4.0 ± 3.9 |
| 12 | Hydrochlorothiazide | 5.9 ± 3.4 | 5.5 ± 5.3 | 0.0 ± 0.1 | 14.3 ± 7.5 | 19.0 ± 1.5 | 25.8 ± 3.7 |
| 13 | DEET | 4.4 ± 1.7 | 5.4 ± 1.4 | 3.7 ± 2.8 | 17.6 ± 2.8 | 3.0 ± 5.5 | 14.4 ± 2.2 |
| 14 | Venlafaxine-HCl | 4.4 ± 3.1 | 3.8 ± 2.4 | 6.2 ± 2.5 | 6.5 ± 5.5 | 7.3 ± 5.7 | 11.0 ± 4.5 |
| 15 | MCPA | 4.1 ± 6.4 | 6.1 ± 6.3 | 3.1 ± 3.8 | 11.5 ± 7.1 | 18.7 ± 7.8 | 1.6 ± 9.8 |
| 16 | Phenytoin | 3.7 ± 6.7 | 0.0 ± 4.2 | 3.2 ± 1.9 | 7.4 ± 2.5 | 13.4 ± 3.3 | 10.3 ± 2.8 |
| 17 | Lincomycin-HCl | 2.6 ± 7.9 | 5.8 ± 8.1 | 2.8 ± 1.4 | 15.1 ± 4.9 | 5.6 ± 3.6 | 14.9 ± 3.5 |
| 18 | Norfloxacin | 2.5 ± 4.8 | 5.5 ± 3.6 | 45.1 ± 1.3 | 0.0 ± 0.1 | 5.5 ± 3.0 | 5.5 ± 1.7 |
| 19 | Cimetidine | 2.1 ± 7.8 | 8.7 ± 7.7 | 24.4 ± 2.0 | 19.3 ± 2.5 | 4.7 ± 1.0 | 11.3 ± 4.4 |
| 20 | Fluometuron | 1.8 ± 4.6 | 2.5 ± 4.5 | 5.7 ± 8.9 | 3.0 ± 9.6 | 2.8 ± 6.8 | 2.2 ± 7.1 |
| 21 | Thiabendazole | 0.0 ± 0.1 | 4.3 ± 3.9 | 0.2 ± 2.8 | 4.5 ± 3.2 | 4.8 ± 1.1 | 6.5 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morsi, R.; Al-Maqdi, K.A.; Bilal, M.; Iqbal, H.M.N.; Khaleel, A.; Shah, I.; Ashraf, S.S. Immobilized Soybean Peroxidase Hybrid Biocatalysts for Efficient Degradation of Various Emerging Pollutants. Biomolecules 2021, 11, 904. https://doi.org/10.3390/biom11060904
Morsi R, Al-Maqdi KA, Bilal M, Iqbal HMN, Khaleel A, Shah I, Ashraf SS. Immobilized Soybean Peroxidase Hybrid Biocatalysts for Efficient Degradation of Various Emerging Pollutants. Biomolecules. 2021; 11(6):904. https://doi.org/10.3390/biom11060904
Chicago/Turabian StyleMorsi, Rana, Khadega A. Al-Maqdi, Muhammad Bilal, Hafiz M. N. Iqbal, Abbas Khaleel, Iltaf Shah, and Syed Salman Ashraf. 2021. "Immobilized Soybean Peroxidase Hybrid Biocatalysts for Efficient Degradation of Various Emerging Pollutants" Biomolecules 11, no. 6: 904. https://doi.org/10.3390/biom11060904
APA StyleMorsi, R., Al-Maqdi, K. A., Bilal, M., Iqbal, H. M. N., Khaleel, A., Shah, I., & Ashraf, S. S. (2021). Immobilized Soybean Peroxidase Hybrid Biocatalysts for Efficient Degradation of Various Emerging Pollutants. Biomolecules, 11(6), 904. https://doi.org/10.3390/biom11060904










