4,6′-Anhydrooxysporidinone from Fusarium lateritium SSF2 Induces Autophagic and Apoptosis Cell Death in MCF-7 Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Isolation
2.2. Cell Culture
2.3. Assessment of Cell Viability
2.4. Immunocytochemistry
2.5. Western Blot Analysis
2.6. Quantitative Analysis for Apoptotic Cells
2.7. Cell Staining with Hoechst 33342
2.8. Statistical Analyses
3. Results
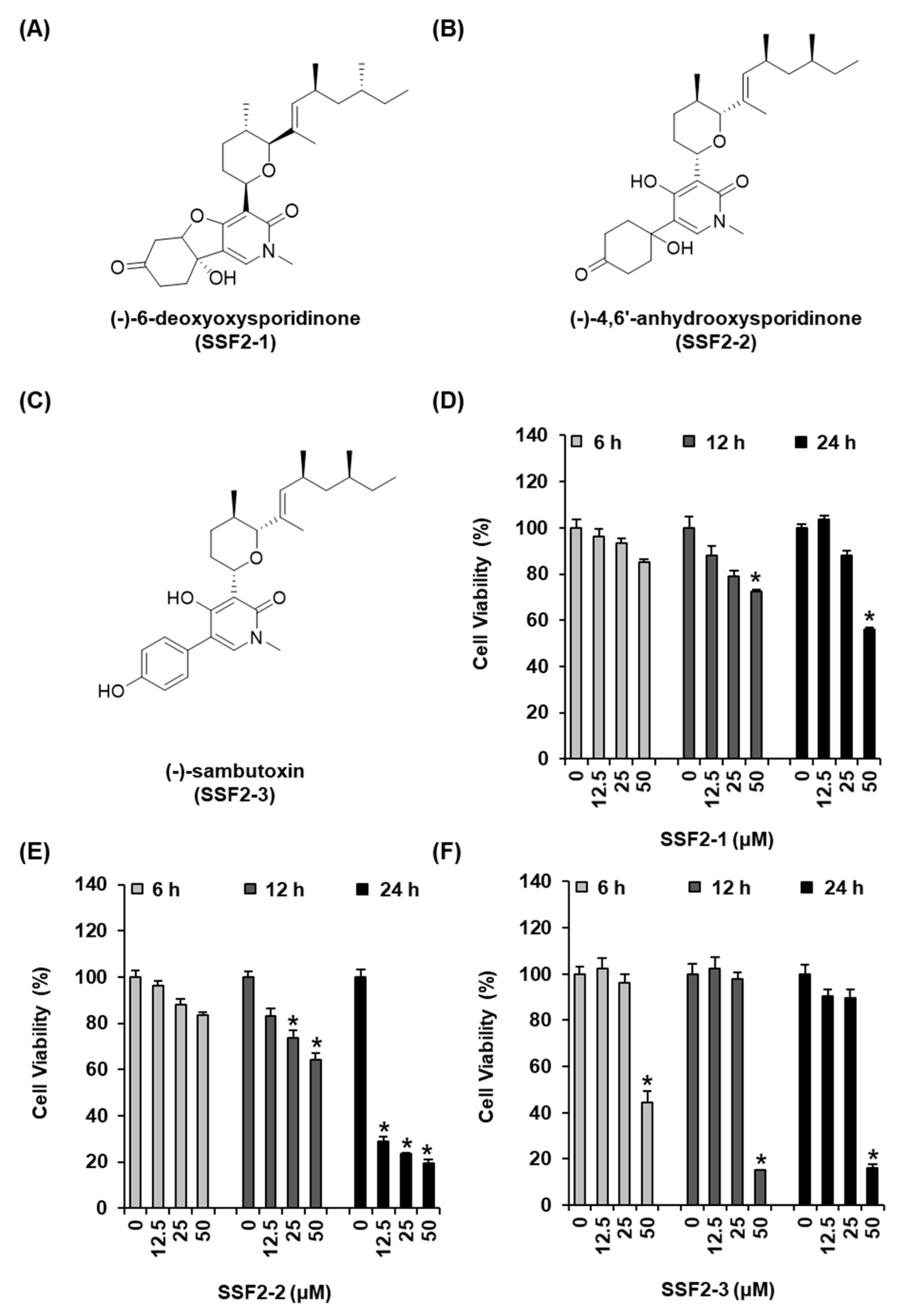
3.1. SSF2-2 Inhibits the Viability of MCF-7 Cells
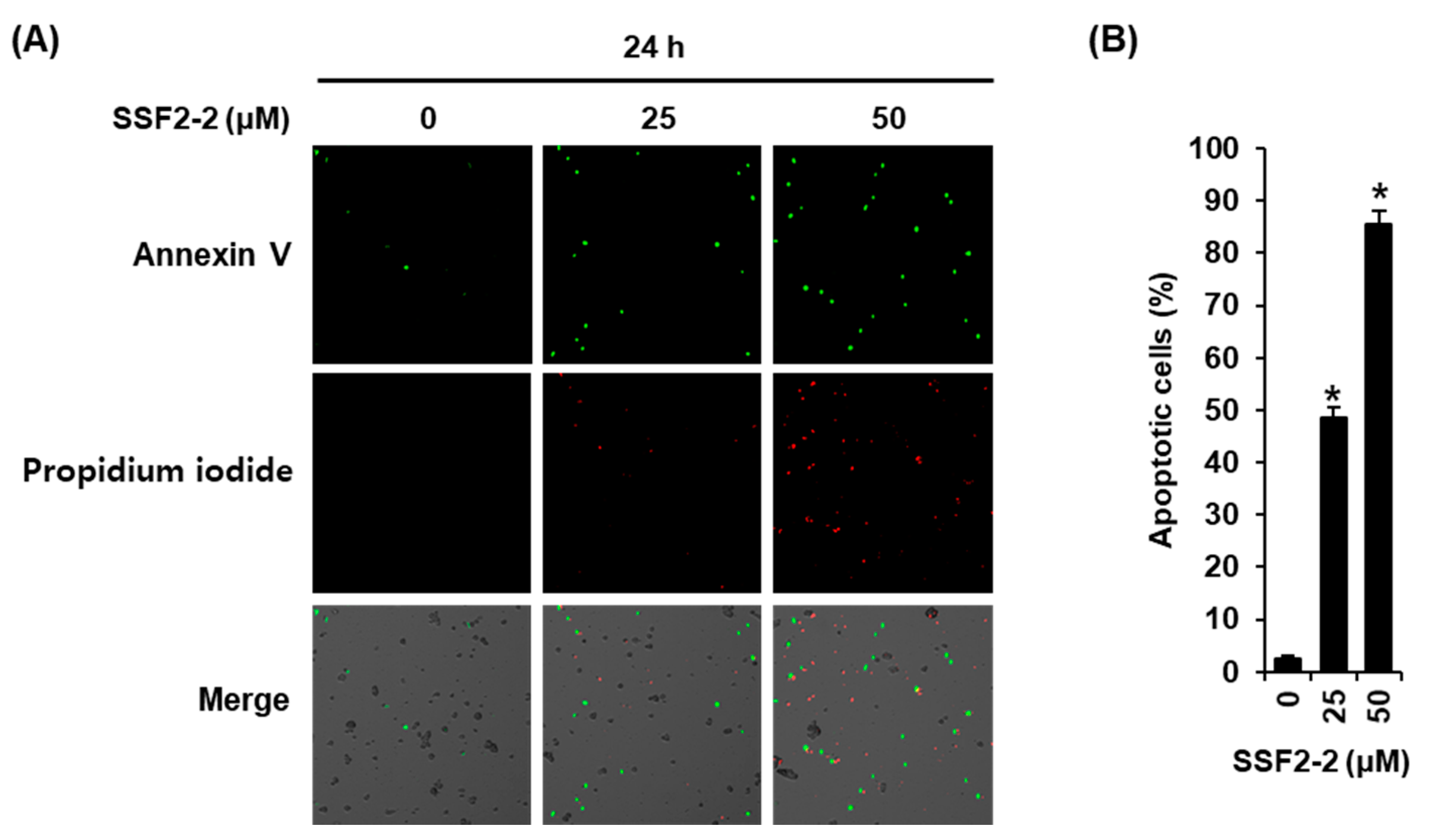
3.2. SSF2-2 Induces Apoptosis in MCF-7 Cells
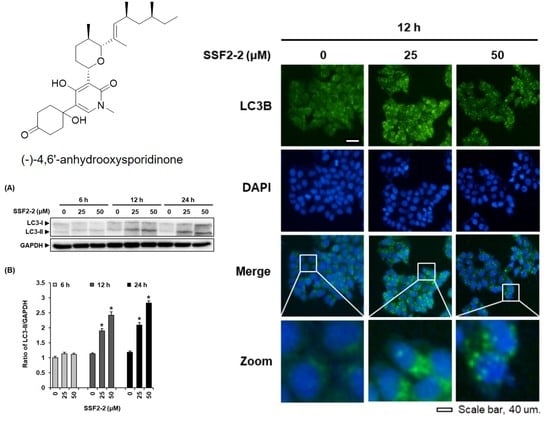
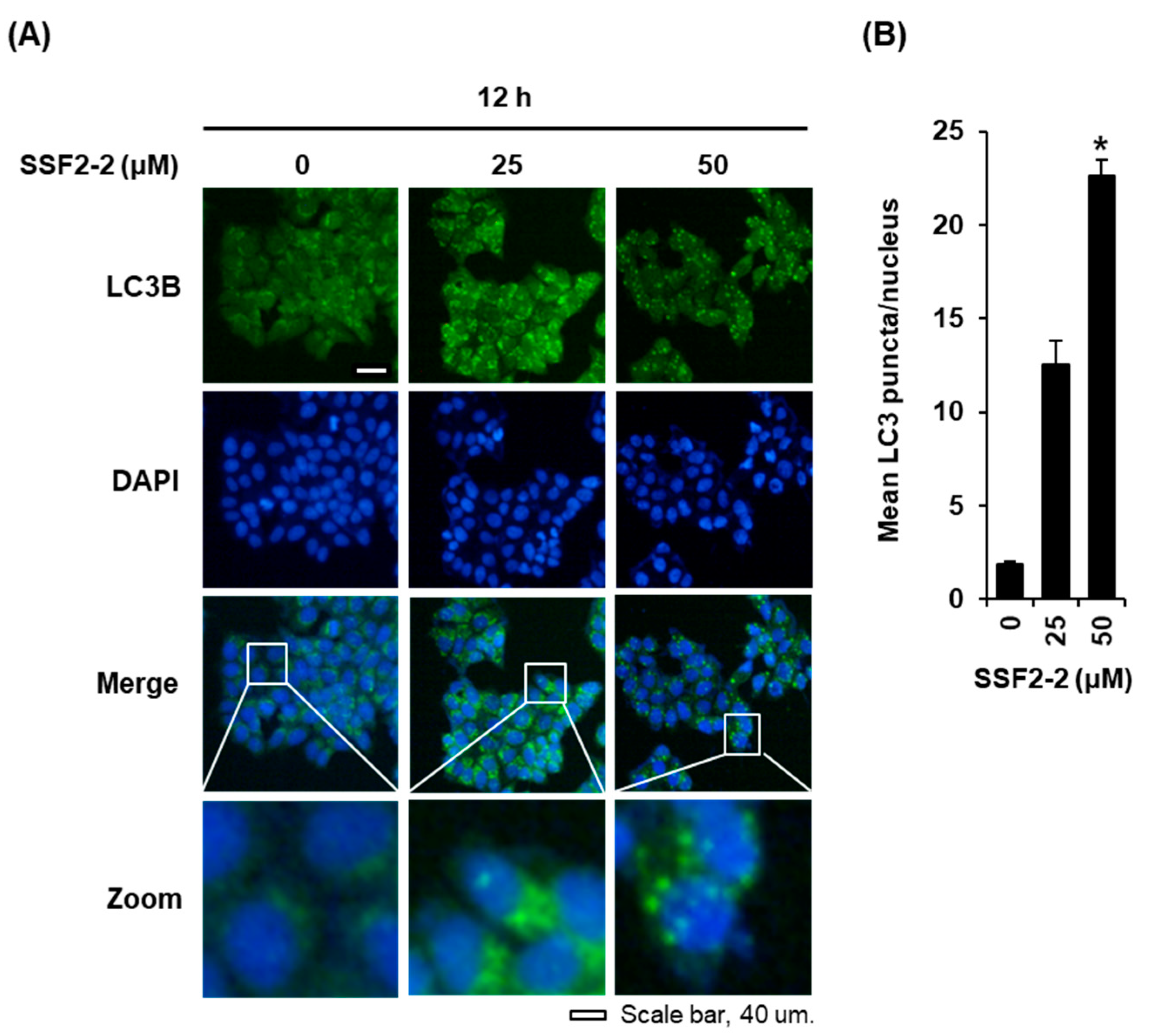
3.3. SSF2-2 Induces Autophgay in MCF-7 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global Trend of Breast Cancer Mortality Rate: A 25-Year Study. Asian Pac. J. Cancer Prev. 2019, 20, 2015. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef]
- Lee, D.; Lee, W.-Y.; Jung, K.; Kwon, Y.S.; Kim, D.; Hwang, G.S.; Kim, C.-E.; Lee, S.; Kang, K.S. The inhibitory effect of cordycepin on the proliferation of MCF-7 breast cancer cells, and its mechanism: An investigation using network pharmacology-based analysis. Biomolecules 2019, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.-Y.; Jo, M.S.; Lee, D.; Baek, S.-E.; Baek, J.; Yu, J.S.; Jo, J.; Yun, H.; Kang, K.S.; Yoo, J.-E.; et al. Dual effects of isoflavonoids from Pueraria lobata roots on estrogenic activity and anti-proliferation of MCF-7 human breast carcinoma cells. Bioorgan. Chem. 2019, 83, 135–144. [Google Scholar] [CrossRef]
- Kwak, J.H.; Park, J.Y.; Lee, D.; Kwak, J.Y.; Park, E.H.; Kim, K.H.; Park, H.-J.; Kim, H.Y.; Jang, H.J.; Ham, J.; et al. Inhibitory effects of ginseng sapogenins on the proliferation of triple negative breast cancer MDA-MB-231 cells. Bioorgan. Med. Chem. Lett. 2014, 24, 5409–5412. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, Y.H.; Lee, K.H.; Lee, B.S.; Alishir, A.; Ko, Y.-J.; Kang, K.S.; Kim, K.H. Aviculin Isolated from Lespedeza cuneata Induce Apoptosis in Breast Cancer Cells through Mitochondria-Mediated Caspase Activation Pathway. Molecules 2020, 25, 1708. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Lee, D.; Baek, S.-E.; Kim, K.H.; Kang, K.S.; Jang, T.S.; Lee, H.L.; Song, J.H.; Yoo, J.-E. Cytotoxic effect of sanguiin H-6 on MCF-7 and MDA-MB-231 human breast carcinoma cells. Bioorgan. Med. Chem. Lett. 2017, 27, 4389–4392. [Google Scholar] [CrossRef]
- Lee, D.; Park, S.; Choi, S.; Kim, S.H.; Kang, K.S. In Vitro Estrogenic and Breast Cancer Inhibitory Activities of Chemical Constituents Isolated from Rheum undulatum L. Molecules 2018, 23, 1215. [Google Scholar] [CrossRef]
- Knaapen, M.W.; Davies, M.J.; De Bie, M.; Haven, A.J.; Martinet, W.; Kockx, M.M. Apoptotic versus autophagic cell death in heart failure. Cardiovasc. Res. 2001, 51, 304–312. [Google Scholar] [CrossRef]
- Giridharan, P.; Verekar, S.A.; Khanna, A.; Mishra, P.D.; Deshmukh, S.K. Anticancer activity of sclerotiorin, isolated from an endophytic fungus Cephalotheca faveolata Yaguchi, Nishim. & Udagawa. Indian J. Exp. Biol. 2012, 50, 464–468. [Google Scholar]
- Koul, M.; Meena, S.; Kumar, A.; Sharma, P.R.; Singamaneni, V.; Riyaz-Ul-Hassan, S.; Hamid, A.; Chaubey, A.; Prabhakar, A.; Gupta, P.; et al. Secondary Metabolites from Endophytic Fungus Penicillium pinophilum Induce ROS-Mediated Apoptosis through Mitochondrial Pathway in Pancreatic Cancer Cells. Planta Med. 2016, 82, 344–355. [Google Scholar] [CrossRef]
- Arora, D.; Sharma, N.; Singamaneni, V.; Sharma, V.; Kushwaha, M.; Abrol, V.; Guru, S.; Sharma, S.; Gupta, A.P.; Bhushan, S.; et al. Isolation and characterization of bioactive metabolites from Xylaria psidii, an endophytic fungus of the medicinal plant Aegle marmelos and their role in mitochondrial dependent apoptosis against pancreatic cancer cells. Phytomedicine 2016, 23, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wu, X.; Xiu, Z.; Liu, X.; Huang, B.; Hu, L.; Liu, J.; Zhou, Z.; Tang, X. Cytochalasin H isolated from mangrove-derived endophytic fungus induces apoptosis and inhibits migration in lung cancer cells. Oncol. Rep. 2018, 39, 2899–2905. [Google Scholar] [CrossRef]
- Wang, F.-Q.; Tong, Q.-Y.; Ma, H.-R.; Xu, H.-F.; Hu, S.; Ma, W.; Xue, Y.; Liu, J.-J.; Wang, J.-P.; Song, H.-P.; et al. Indole diketopiperazines from endophytic Chaetomium sp 88194 induce breast cancer cell apoptotic death. Sci. Rep. 2015, 5, 1–9, srep09294. [Google Scholar] [CrossRef] [PubMed]
- Bashyal, B.P.; Wijeratne, E.M.K.; Faeth, S.H.; Gunatilaka, A.A.L. Globosumones A−C, Cytotoxic Orsellinic Acid Esters from the Sonoran Desert Endophytic FungusChaetomium globosum1. J. Nat. Prod. 2005, 68, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Choi, H.G.; Hwang, J.H.; Shim, S.H.; Kang, K.S. Neuroprotective Effect of Tricyclic Pyridine Alkaloids from Fusarium lateritium SSF2, against Glutamate-Induced Oxidative Stress and Apoptosis in the HT22 Hippocampal Neuronal Cell Line. Antioxidants 2020, 9, 1115. [Google Scholar] [CrossRef]
- Kim, H.; Choi, P.; Kim, T.; Kim, Y.; Song, B.G.; Park, Y.-T.; Choi, S.-J.; Yoon, C.H.; Lim, W.-C.; Ko, H.; et al. Ginsenosides Rk1 and Rg5 inhibit transforming growth factor-β1-induced epithelial-mesenchymal transition and suppress migration, invasion, anoikis resistance, and development of stem-like features in lung cancer. J. Ginseng Res. 2021, 45, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Hwang, H.; Nah, S.-Y.; Rhim, H. Gintonin stimulates autophagic flux in primary cortical astrocytes. J. Ginseng Res. 2020, 44, 67–78. [Google Scholar] [CrossRef]
- Ryu, Y.-S.; Hyun, J.-W.; Chung, H.-S. Fucoidan induces apoptosis in A2058 cells through ROS-exposed activation of MAPKs signaling pathway. Nat. Prod. Sci. 2020, 26, 191–199. [Google Scholar]
- Sun, W.-J.; Zhu, H.-T.; Zhang, T.-Y.; Zhang, M.-Y.; Wang, D.; Yang, C.-R.; Zhang, Y.-X.; Zhang, Y.-J. Two New Alkaloids from Fusarium tricinctum SYPF 7082, an Endophyte from the Root of Panax notoginseng. Nat. Prod. Bioprospecting 2018, 8, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-X.; Li, S.-F.; Zhao, F.; Dai, H.-Q.; Bao, L.; Ding, R.; Gao, H.; Zhang, L.-X.; Wen, H.-A.; Liu, H.-W. Chemical constituents from endophytic fungus Fusarium oxysporum. Fitoterapia 2011, 82, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Burns, A.M.; Liu, M.X.; Faeth, S.H.; Gunatilaka, A.L. Search for cell motility and angiogenesis inhibitors with potential anticancer activity: Beauvericin and other constituents of two endophytic strains of Fusarium oxysporum. J. Nat. Prod. 2007, 70, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 1–9. [Google Scholar] [CrossRef]
- Jänicke, R.U. MCF-7 breast carcinoma cells do not express caspase-3. Breast Cancer Res. Treat. 2009, 117, 219–221. [Google Scholar] [CrossRef]
- Yang, X.H.; Sladek, T.L.; Liu, X.; Butler, B.R.; Froelich, C.J.; Thor, A.D. Reconstitution of caspase 3 sensitizes MCF-7 breast cancer cells to doxorubicin- and etoposide-induced apoptosis. Cancer Res. 2001, 61, 348–354. [Google Scholar]
- Mc Gee, M.M.; Hyland, E.; Campiani, G.; Ramunno, A.; Nacci, V.; Zisterer, D. Caspase-3 is not essential for DNA fragmentation in MCF-7 cells during apoptosis induced by the pyrrolo-1,5-benzoxazepine, PBOX-6. FEBS Lett. 2002, 515, 66–70. [Google Scholar] [CrossRef]
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.; Iyer, S.; Smulson, M. Role of poly (ADP-ribose) polymerase (PARP) cleavage in apoptosis: Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999, 274, 22932–22940. [Google Scholar] [CrossRef] [PubMed]
- Eisenberglerner, A.; Bialik, S.; Simon, H.-U.; Kimchi, A. Life and death partners: Apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009, 16, 966–975. [Google Scholar] [CrossRef]
- Shimizu, S. Autophagic Cell Death and Cancer Chemotherapeutics. In Innovative Medicine; Springer: Berlin/Heidelberg, Germany, 2015; pp. 219–226. [Google Scholar]
- Kim, H.; Williams, D.; Qiu, Y.; Song, Z.; Yang, Z.; Kimler, V.; Goldberg, A.; Zhang, R.; Yang, Z.; Chen, X.; et al. Regulation of hepatic autophagy by stress-sensing transcription factor CREBH. FASEB J. 2019, 33, 7896–7914. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Kang, J.; Fu, C. The independence of and associations among apoptosis, autophagy, and necrosis. Signal Transduct. Target. Ther. 2018, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A. Apoptosis and autophagy: Regulatory connections between two supposedly different processes. Apoptosis 2008, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Shim, S.; Kang, K. 4,6′-Anhydrooxysporidinone from Fusarium lateritium SSF2 Induces Autophagic and Apoptosis Cell Death in MCF-7 Breast Cancer Cells. Biomolecules 2021, 11, 869. https://doi.org/10.3390/biom11060869
Lee D, Shim S, Kang K. 4,6′-Anhydrooxysporidinone from Fusarium lateritium SSF2 Induces Autophagic and Apoptosis Cell Death in MCF-7 Breast Cancer Cells. Biomolecules. 2021; 11(6):869. https://doi.org/10.3390/biom11060869
Chicago/Turabian StyleLee, Dahae, Sanghee Shim, and Kisung Kang. 2021. "4,6′-Anhydrooxysporidinone from Fusarium lateritium SSF2 Induces Autophagic and Apoptosis Cell Death in MCF-7 Breast Cancer Cells" Biomolecules 11, no. 6: 869. https://doi.org/10.3390/biom11060869
APA StyleLee, D., Shim, S., & Kang, K. (2021). 4,6′-Anhydrooxysporidinone from Fusarium lateritium SSF2 Induces Autophagic and Apoptosis Cell Death in MCF-7 Breast Cancer Cells. Biomolecules, 11(6), 869. https://doi.org/10.3390/biom11060869