From the Matrix to the Nucleus and Back: Mechanobiology in the Light of Health, Pathologies, and Regeneration of Oral Periodontal Tissues
Abstract
1. Introduction
2. The ECM, Focal Adhesions and Adherens Junctions in Periodontal Health and Disease
3. Mechanotransduction to the Core: YAP/TAZ in the Periodontium
4. The Gist of the Matter: Nuclear Mechanotransduction
5. Porphyromonas gingivalis-Derived Proteases: A “Heavy Load” for the Periodontium
6. May the Force Be with You: MT and Its Implications for Periodontal Regeneration
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vogel, V. Unraveling the mechanobiology of extracellular matrix. Annu. Rev. Physiol. 2018, 80, 353–387. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Hillege, M.M.; Wüst, R.C.; Wu, G.; Jaspers, R.T. Synergistic short-term and long-term effects of TGF-β1 and 3 on collagen production in differentiating myoblasts. Biochem. Biophys. Res. Commun. 2021, 547, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, N.B.; Jurj, A.; Sorițău, O.; Lucaciu, O.P.; Dirzu, N.; Raduly, L.; Berindan-Neagoe, I.; Cenariu, M.; Boșca, B.A.; Campian, R.S. Cannabidiol and Vitamin D3 Impact on Osteogenic Differentiation of Human Dental Mesenchymal Stem Cells. Medicina 2020, 56, 607. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Debbi, L.; Zohar, B.; Samuel, R.; Arzi, R.S.; Fried, A.I.; Carmon, T.; Shevach, D.; Redenski, I.; Schlachet, I. Stimulating Extracellular Vesicles Production from Engineered Tissues by Mechanical Forces. Nano Lett. 2021, 21, 2497–2504. [Google Scholar] [CrossRef]
- Cicciù, M.; Fiorillo, L.; Cervino, G.; Habal, M.B. BMP Application as Grafting Materials for Bone Regeneration in the Craniofacial Surgery: Current Application and Future Directions by an RCT Analysis. J. Craniofac. Surg. 2020, 32, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-T.; Heuer, R.A.; Oleksijew, A.M.; Coots, K.S.; Roque, C.B.; Nella, K.T.; McGuire, T.L.; Matsuoka, A.J. An engineered three-dimensional stem cell niche in the inner ear by applying a nanofibrillar cellulose hydrogel with a sustained-release neurotrophic factor delivery system. Acta Biomater. 2020, 108, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Danish, A.; Gedschold, R.; Hinz, S.; Schiedel, A.C.; Thimm, D.; Bedner, P.; Steinhäuser, C.; Müller, C.E. A Cellular Assay for the Identification and Characterization of Connexin Gap Junction Modulators. Int. J. Mol. Sci. 2021, 22, 1417. [Google Scholar] [CrossRef]
- Imafuku, K.; Kamaguchi, M.; Natsuga, K.; Nakamura, H.; Shimizu, H.; Iwata, H. Zonula occludens-1 demonstrates a unique appearance in buccal mucosa over several layers. Cell Tissue Res. 2021, 1–12. [Google Scholar] [CrossRef]
- Patil, R.; Kale, A.D.; Mane, D.R.; Patil, D. Isolation, culture and characterization of primary cell lines of human buccal mucosal fibroblasts: A combination of explant enzamytic technique. J. Oral Maxillofac. Pathol. JOMFP 2020, 24, 68. [Google Scholar] [CrossRef]
- Fagalde, P.; Reininger, D. Oral tissues regeneration using intraoral mesenchymal stem cells. J. Clin. Exp. Dent. 2021, 13, e268. [Google Scholar] [CrossRef]
- Chukkapalli, S.S.; Lele, T.P. Periodontal cell mechanotransduction. Open Biol. 2018, 8, 180053. [Google Scholar] [CrossRef]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef]
- Zuidema, A.; Wang, W.; Sonnenberg, A. Crosstalk between Cell Adhesion Complexes in Regulation of Mechanotransduction. BioEssays 2020, 42, 2000119. [Google Scholar] [CrossRef]
- Naqvi, S.M.; McNamara, L.M. Stem Cell Mechanobiology and the Role of Biomaterials in Governing Mechanotransduction and Matrix Production for Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 1375. [Google Scholar] [CrossRef]
- Case, L.B.; Waterman, C.M. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat. Cell Biol. 2015, 17, 955–963. [Google Scholar] [CrossRef]
- Hatte, G.; Prigent, C.; Tassan, J.-P. Adherens junctions are involved in polarized contractile ring formation in dividing epithelial cells of Xenopus laevis embryos. Exp. Cell Res. 2021, 402, 112525. [Google Scholar] [CrossRef]
- Jasuja, H.; Kar, S.; Katti, D.R.; Katti, K. Perfusion bioreactor enabled fluid-derived shear stress conditions for novel bone metastatic prostate cancer testbed. Biofabrication 2021, 13, 035004. [Google Scholar] [CrossRef]
- Jaumouillé, V.; Cartagena-Rivera, A.X.; Waterman, C.M. Coupling of β 2 integrins to actin by a mechanosensitive molecular clutch drives complement receptor-mediated phagocytosis. Nat. Cell Biol. 2019, 21, 1357–1369. [Google Scholar] [CrossRef]
- Yu, D.; Wang, J.; Qian, K.-j.; Yu, J.; Zhu, H.-y. Effects of nanofibers on mesenchymal stem cells: Environmental factors affecting cell adhesion and osteogenic differentiation and their mechanisms. J. Zhejiang Univ. Sci. B 2020, 21, 871–884. [Google Scholar] [CrossRef]
- Liu, M.; Banerjee, R.; Rossa Jr, C.; D’Silva, N. RAP1-RAC1 signaling has an important role in adhesion and migration in HNSCC. J. Dent. Res. 2020, 99, 959–968. [Google Scholar] [CrossRef]
- Jacob, A.E.; Amack, J.D.; Turner, C.E. Paxillin genes and actomyosin contractility regulate myotome morphogenesis in zebrafish. Dev. Biol. 2017, 425, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Pence, L.J.; Kourtidis, A.; Feathers, R.W.; Haddad, M.T.; Sotiriou, S.; Decker, P.A.; Nassar, A.; Ocal, I.T.; Shah, S.S.; Anastasiadis, P.Z. PLEKHA7, an Apical Adherens Junction Protein, Suppresses Inflammatory Breast Cancer in the Context of High E-Cadherin and p120-Catenin Expression. Int. J. Mol. Sci. 2021, 22, 1275. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.E.; Sotomayor, M. Crystal structure of the nonclassical cadherin-17 N-terminus and implications for its adhesive binding mechanism. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2021, 77, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Ilina, O.; Gritsenko, P.G.; Syga, S.; Lippoldt, J.; La Porta, C.A.; Chepizhko, O.; Grosser, S.; Vullings, M.; Bakker, G.-J.; Starruß, J. Cell–cell adhesion and 3D matrix confinement determine jamming transitions in breast cancer invasion. Nat. Cell Biol. 2020, 22, 1103–1115. [Google Scholar] [CrossRef]
- Dahl, K.N.; Kahn, S.M.; Wilson, K.L.; Discher, D.E. The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J. Cell Sci. 2004, 117, 4779–4786. [Google Scholar] [CrossRef]
- Maurer, M.; Lammerding, J. The driving force: Nuclear mechanotransduction in cellular function, fate, and disease. Annu. Rev. Biomed. Eng. 2019, 21, 443–468. [Google Scholar] [CrossRef]
- Reynolds, N.; McEvoy, E.; Ghosh, S.; Pérez, J.A.P.; Neu, C.P.; McGarry, P. Image-derived modeling of nucleus strain amplification associated with chromatin heterogeneity. Biophys. J. 2021, 120, 1323–1332. [Google Scholar] [CrossRef]
- Owens, D.J.; Messéant, J.; Moog, S.; Viggars, M.; Ferry, A.; Mamchaoui, K.; Lacène, E.; Roméro, N.; Brull, A.; Bonne, G. Lamin-related congenital muscular dystrophy alters mechanical signaling and skeletal muscle growth. Int. J. Mol. Sci. 2021, 22, 306. [Google Scholar]
- Lin, J.D.; Ryder, M.; Kang, M.; Ho, S.P. Biomechanical pathways of dentoalveolar fibrous joints in health and disease. Periodontology 2000 2020, 82, 238–256. [Google Scholar] [CrossRef]
- Connizzo, B.; Sun, L.; Lacin, N.; Gendelman, A.; Solomonov, I.; Sagi, I.; Grodzinsky, A.; Naveh, G. Nonuniformity in Periodontal Ligament: Mechanics and Matrix Composition. J. Dent. Res. 2020, 100, 179–186. [Google Scholar] [CrossRef]
- Li, Z.; Yu, M.; Jin, S.; Wang, Y.; Luo, R.; Huo, B.; Liu, D.; He, D.; Zhou, Y.; Liu, Y. Stress distribution and collagen remodeling of periodontal ligament during orthodontic tooth movement. Front. Pharmacol. 2019, 10, 1263. [Google Scholar] [CrossRef]
- Takewaki, M.; Kajiya, M.; Takeda, K.; Sasaki, S.; Motoike, S.; Komatsu, N.; Matsuda, S.; Ouhara, K.; Mizuno, N.; Fujita, T. MSC/ECM cellular complexes induce periodontal tissue regeneration. J. Dent. Res. 2017, 96, 984–991. [Google Scholar] [CrossRef]
- Lundmark, A.; Johannsen, G.; Eriksson, K.; Kats, A.; Jansson, L.; Tervahartiala, T.; Rathnayake, N.; Åkerman, S.; Klinge, B.; Sorsa, T. Mucin 4 and matrix metalloproteinase 7 as novel salivary biomarkers for periodontitis. J. Clin. Periodontol. 2017, 44, 247–254. [Google Scholar] [CrossRef]
- Nakagawa, M.; Shirasugi, M.; Yamamoto, T.; Nakaya, T.; Kanamura, N. Long-term exposure to butyric acid induces excessive production of matrix metalloproteases in human gingival fibroblasts. Arch. Oral Biol. 2021, 123, 105035. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, Y.; Kim, M.-K.; Park, H.-R.; Kim, H.-J.; Bae, S.-K.; Bae, M.-K. Infection of Porphyromonas gingivalis Increases Phosphate-Induced Calcification of Vascular Smooth Muscle Cells. Cells 2020, 9, 2694. [Google Scholar] [CrossRef]
- Behm, C.; Nemec, M.; Blufstein, A.; Schubert, M.; Rausch-Fan, X.; Andrukhov, O.; Jonke, E. Interleukin-1β Induced Matrix Metalloproteinase Expression in Human Periodontal Ligament-Derived Mesenchymal Stromal Cells under In Vitro Simulated Static Orthodontic Forces. Int. J. Mol. Sci. 2021, 22, 1027. [Google Scholar] [CrossRef]
- Kamarajan, P.; Ateia, I.; Shin, J.M.; Fenno, J.C.; Le, C.; Zhan, L.; Chang, A.; Darveau, R.; Kapila, Y.L. Periodontal pathogens promote cancer aggressivity via TLR/MyD88 triggered activation of Integrin/FAK signaling that is therapeutically reversible by a probiotic bacteriocin. PLoS Pathog. 2020, 16, e1008881. [Google Scholar] [CrossRef]
- Li, Y.; Tan, X.; Zhao, X.; Xu, Z.; Dai, W.; Duan, W.; Huang, S.; Zhang, E.; Liu, J.; Zhang, S. Composition and function of oral microbiota between gingival squamous cell carcinoma and periodontitis. Oral Oncol. 2020, 107, 104710. [Google Scholar] [CrossRef]
- Batool, H.; Nadeem, A.; Kashif, M.; Shahzad, F.; Tahir, R.; Afzal, N. Salivary levels of IL-6 and IL-17 could be an indicator of disease severity in patients with calculus associated chronic periodontitis. BioMed Res. Int. 2018, 2018, 8531961. [Google Scholar] [CrossRef]
- Arroyo, R.; López, S.; Romo, E.; Montoya, G.; Hoz, L.; Pedraza, C.; Garfias, Y.; Arzate, H. Carboxy-Terminal Cementum Protein 1-Derived Peptide 4 (cemp1-p4) Promotes Mineralization through wnt/β-catenin Signaling in Human Oral Mucosa Stem Cells. Int. J. Mol. Sci. 2020, 21, 1307. [Google Scholar] [CrossRef]
- Martins, L.; Amorim, B.R.; Salmon, C.R.; Leme, A.F.P.; Kantovitz, K.R.; Nociti Jr, F.H. Novel LRAP-binding partner revealing the plasminogen activation system as a regulator of cementoblast differentiation and mineral nodule formation in vitro. J. Cell. Physiol. 2020, 235, 4545–4558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y.; Xu, M.; Zheng, J.; Xu, Y.; Chen, G.; Guo, Q.; Tian, W.; Guo, W. The Dual Effects of Reactive Oxygen Species on the Mandibular Alveolar Bone Formation in SOD1 Knockout Mice: Promotion or Inhibition. Oxidative Med. Cell. Longev. 2021, 2021, 8847140. [Google Scholar] [CrossRef] [PubMed]
- Denes, B.J.; Ait-Lounis, A.; Wehrle-Haller, B.; Kiliaridis, S. Core matrisome protein signature during periodontal ligament maturation from pre-occlusal eruption to occlusal function. Front. Physiol. 2020, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Zvackova, I.; Matalova, E.; Lesot, H. Regulators of collagen fibrillogenesis during molar development in the mouse. Front. Physiol. 2017, 8, 554. [Google Scholar] [CrossRef]
- Kurylo, M.P.; Grandfield, K.; Marshall, G.W.; Altoe, V.; Aloni, S.; Ho, S.P. Effect of proteoglycans at interfaces as related to location, architecture, and mechanical cues. Arch. Oral Biol. 2016, 63, 82–92. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Q.; Zheng, Y.; Nan, L.; Liao, N.; Mo, S. Potential Role of Integrin α5β1/Focal Adhesion Kinase (FAK) and Actin Cytoskeleton in the Mechanotransduction and Response of Human Gingival Fibroblasts Cultured on a 3-Dimension Lactide-Co-Glycolide (3D PLGA) Scaffold. Med Sci. Monit. Int. Med J. Exp. Clin. Res. 2020, 26, e921621–e921626. [Google Scholar]
- Hetmanski, J.H.; Jones, M.C.; Chunara, F.; Schwartz, J.-M.; Caswell, P.T. Combinatorial mathematical modelling approaches to interrogate rear retraction dynamics in 3D cell migration. PLoS Comput. Biol. 2021, 17, e1008213. [Google Scholar] [CrossRef]
- Kao, T.-W.; Chiou, A.; Lin, K.-H.; Liu, Y.-S.; Lee, O.K.-S. Alteration of 3D Matrix Stiffness Regulates Viscoelasticity of Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 2441. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef]
- Hegedűs, O.; Juriga, D.; Sipos, E.; Voniatis, C.; Juhász, Á.; Idrissi, A.; Zrínyi, M.; Varga, G.; Jedlovszky-Hajdú, A.; Nagy, K.S. Free thiol groups on poly (aspartamide) based hydrogels facilitate tooth-derived progenitor cell proliferation and differentiation. PLoS ONE 2019, 14, e0226363. [Google Scholar] [CrossRef]
- Feld, L.; Kellerman, L.; Mukherjee, A.; Livne, A.; Bouchbinder, E.; Wolfenson, H. Cellular contractile forces are nonmechanosensitive. Sci. Adv. 2020, 6, eaaz6997. [Google Scholar] [CrossRef]
- Reyes-Ramos, A.M.; Álvarez-García, Y.R.; Solodin, N.; Almodovar, J.; Alarid, E.T.; Torres-Garcia, W.; Domenech, M. Collagen I Fibrous Substrates Modulate the Proliferation and Secretome of Estrogen Receptor-Positive Breast Tumor Cells in a Hormone-Restricted Microenvironment. ACS Biomater. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Wei, D.; Li, C.; Ye, J.; Xiang, F.; Liu, J. Extracellular Collagen Mediates Osteosarcoma Progression Through an Integrin α2β1/JAK/STAT3 Signaling Pathway. Cancer Manag. Res. 2020, 12, 12067. [Google Scholar] [CrossRef]
- Xing, Q.; Parvizi, M.; Higuita, M.L.; Griffiths, L.G. Basement membrane proteins modulate cell migration on bovine pericardium extracellular matrix scaffold. Sci. Rep. 2021, 11, 4607. [Google Scholar] [CrossRef]
- Al-Yafeai, Z.; Orr, A.W. Quantification of integrin activation and ligation in adherent cells. In The Integrin Interactome; Springer: New York, NY, USA, 2021; pp. 17–25. [Google Scholar]
- Ye, Y.; Zhang, R.; Feng, H. Fibronectin promotes tumor cells growth and drugs resistance through a CDC42-YAP-dependent signaling pathway in colorectal cancer. Cell Biol. Int. 2020, 44, 1840–1849. [Google Scholar] [CrossRef]
- Roy, S.; Spinali, K.; Schmuck, E.G.; Kink, J.A.; Hematti, P.; Raval, A.N. Cardiac fibroblast derived matrix-educated macrophages express VEGF and IL-6, and recruit mesenchymal stromal cells. J. Immunol. Regen. Med. 2020, 10, 100033. [Google Scholar]
- Sugahara, M.; Nakaoki, Y.; Yamaguchi, A.; Hashimoto, K.; Miyamoto, Y. Vitronectin is involved in the morphological transition of neurites in retinoic acid-induced neurogenesis of neuroblastoma cell line neuro2a. Neurochem. Res. 2019, 44, 1621–1635. [Google Scholar] [CrossRef]
- Jakhu, H.; Gill, G.; Singh, A. Role of integrins in wound repair and its periodontal implications. J. Oral Biol. Craniofac. Res. 2018, 8, 122–125. [Google Scholar] [CrossRef]
- Jang, A.; Wang, B.; Ustriyana, P.; Gansky, S.A.; Maslenikov, I.; Useinov, A.; Prevost, R.; Ho, S.P. Functional adaptation of interradicular alveolar bone to reduced chewing loads on dentoalveolar joints in rats. Dent. Mater. 2021, 37, 486–495. [Google Scholar] [CrossRef]
- Husari, A.; Steinberg, T.; Dieterle, M.P.; Prucker, O.; Rühe, J.; Jung, B.; Tomakidi, P. On the relationship of YAP and FAK in hMSCs and osteosarcoma cells: Discrimination of FAK modulation by nuclear YAP depletion or YAP silencing. Cell. Signal. 2019, 63, 109382. [Google Scholar] [CrossRef]
- Belgardt, E.; Steinberg, T.; Husari, A.; Dieterle, M.P.; Hülter-Hassler, D.; Jung, B.; Tomakidi, P. Force-responsive Zyxin modulation in periodontal ligament cells is regulated by YAP rather than TAZ. Cell. Signal. 2020, 72, 109662. [Google Scholar] [CrossRef]
- Gao, W.-J.; Liu, J.-X.; Xie, Y.; Luo, P.; Liu, Z.-Q.; Liu, L.; Zhou, H. Suppression of macrophage migration by down-regulating Src/FAK/P130Cas activation contributed to the anti-inflammatory activity of sinomenine. Pharmacol. Res. 2021, 167, 105513. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, H.; Tai, Y.; Xue, Y.; Wei, Y.; Wang, K.; Zhao, Q.; Wang, S.; Kong, D.; Midgley, A.C. Design and Evaluation of a Polypeptide that Mimics the Integrin Binding Site for EDA Fibronectin to Block Profibrotic Cell Activity. Int. J. Mol. Sci. 2021, 22, 1575. [Google Scholar] [CrossRef]
- Boujemaa-Paterski, R.; Martins, B.; Eibauer, M.; Beales, C.T.; Geiger, B.; Medalia, O. Talin-activated vinculin interacts with branched actin networks to initiate bundles. eLife 2020, 9, e53990. [Google Scholar] [CrossRef]
- Damiano-Guercio, J.; Kurzawa, L.; Mueller, J.; Dimchev, G.; Schaks, M.; Nemethova, M.; Pokrant, T.; Brühmann, S.; Linkner, J.; Blanchoin, L. Loss of Ena/VASP interferes with lamellipodium architecture, motility and integrin-dependent adhesion. eLife 2020, 9, e55351. [Google Scholar] [CrossRef]
- Hsiao, B.-Y.; Chen, C.-H.; Chi, H.-Y.; Yen, P.-R.; Yu, Y.-Z.; Lin, C.-H.; Pang, T.-L.; Lin, W.-C.; Li, M.-L.; Yeh, Y.-C. Human Costars Family Protein ABRACL Modulates Actin Dynamics and Cell Migration and Associates with Tumorigenic Growth. Int. J. Mol. Sci. 2021, 22, 2037. [Google Scholar] [CrossRef]
- Roopnarine, O.; Thomas, D.D. Mechanistic analysis of actin-binding compounds that affect the kinetics of cardiac myosin-actin interaction. J. Biol. Chem. 2021, 196, 100471. [Google Scholar] [CrossRef]
- Mani, S.; Katkar, H.H.; Voth, G.A. Compressive and Tensile Deformations Alter ATP Hydrolysis and Phosphate Release Rates in Actin Filaments. J. Chem. Theory Comput. 2021, 17, 1900–1913. [Google Scholar] [CrossRef]
- Zeng, Y.; Cao, Y.; Liu, L.; Zhao, J.; Zhang, T.; Xiao, L.; Jia, M.; Tian, Q.; Yu, H.; Chen, S. SEPT9_i1 regulates human breast cancer cell motility through cytoskeletal and RhoA/FAK signaling pathway regulation. Cell Death Dis. 2019, 10, 720. [Google Scholar] [CrossRef]
- Kurotsu, S.; Sadahiro, T.; Fujita, R.; Tani, H.; Yamakawa, H.; Tamura, F.; Isomi, M.; Kojima, H.; Yamada, Y.; Abe, Y. Soft Matrix Promotes Cardiac Reprogramming via Inhibition of YAP/TAZ and Suppression of Fibroblast Signatures. Stem Cell Rep. 2020, 15, 612–628. [Google Scholar] [CrossRef]
- Nikoloudaki, G.; Snider, P.; Simmons, O.; Conway, S.J.; Hamilton, D.W. Periostin and matrix stiffness combine to regulate myofibroblast differentiation and fibronectin synthesis during palatal healing. Matrix Biol. 2020, 94, 31–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rens, E.G.; Edelstein-Keshet, L. Spots, stripes, and spiral waves in models for static and motile cells. J. Math. Biol. 2021, 82, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Ugawa, Y.; Yamamoto, T.; Kawamura, M.; Yamashiro, K.; Shimoe, M.; Tomikawa, K.; Hongo, S.; Maeda, H.; Takashiba, S. Rho-kinase regulates extracellular matrix-mediated osteogenic differentiation of periodontal ligament cells. Cell Biol. Int. 2017, 41, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Ugawa, Y.; Yamashiro, K.; Shimoe, M.; Tomikawa, K.; Hongo, S.; Kochi, S.; Ideguchi, H.; Maeda, H.; Takashiba, S. Osteogenic differentiation regulated by Rho-kinase in periodontal ligament cells. Differentiation 2014, 88, 33–41. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Ren, H. Profilin promotes formin-mediated actin filament assembly and vesicle transport during polarity formation in pollen. Plant Cell 2021. [Google Scholar] [CrossRef]
- Matarrese, P.; Vona, R.; Ascione, B.; Paggi, M.G.; Mileo, A.M. Physical Interaction between HPV16E7 and the Actin-Binding Protein Gelsolin Regulates Epithelial-Mesenchymal Transition via HIPPO-YAP Axis. Cancers 2021, 13, 353. [Google Scholar] [CrossRef]
- Choi, C.K.; Vicente-Manzanares, M.; Zareno, J.; Whitmore, L.A.; Mogilner, A.; Horwitz, A.R. Actin and α-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol. 2008, 10, 1039–1050. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ugawa, Y.; Kawamura, M.; Yamashiro, K.; Kochi, S.; Ideguchi, H.; Takashiba, S. Modulation of microenvironment for controlling the fate of periodontal ligament cells: The role of Rho/ROCK signaling and cytoskeletal dynamics. J. Cell Commun. Signal. 2018, 12, 369–378. [Google Scholar] [CrossRef]
- Romero, S.; Le Clainche, C.; Gautreau, A.M. Actin polymerization downstream of integrins: Signaling pathways and mechanotransduction. Biochem. J. 2020, 477, 1–21. [Google Scholar] [CrossRef]
- Ramirez, I.; Gholkar, A.A.; Velasquez, E.F.; Guo, X.; Tofig, B.; Damoiseaux, R.; Torres, J.Z. The myosin regulatory light chain Myl5 localizes to mitotic spindle poles and is required for proper cell division. Cytoskeleton 2021, 78, 23–35. [Google Scholar] [CrossRef]
- Jarvis, K.J.; Bell, K.M.; Loya, A.K.; Swank, D.M.; Walcott, S. Force-velocity and tension transient measurements from Drosophila jump muscle reveal the necessity of both weakly-bound cross-bridges and series elasticity in models of muscle contraction. Arch. Biochem. Biophys. 2021, 701, 108809. [Google Scholar] [CrossRef]
- Salomon, J.; Gaston, C.; Magescas, J.; Duvauchelle, B.; Canioni, D.; Sengmanivong, L.; Mayeux, A.; Michaux, G.; Campeotto, F.; Lemale, J. Contractile forces at tricellular contacts modulate epithelial organization and monolayer integrity. Nat. Commun. 2017, 8, 13998. [Google Scholar] [CrossRef]
- Schreiber, C.; Amiri, B.; Heyn, J.C.; Rädler, J.O.; Falcke, M. On the adhesion–velocity relation and length adaptation of motile cells on stepped fibronectin lanes. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Santa-Cruz Mateos, C.; Valencia-Expósito, A.; Palacios, I.M.; Martín-Bermudo, M.D. Integrins regulate epithelial cell shape by controlling the architecture and mechanical properties of basal actomyosin networks. PLoS Genet. 2020, 16, e1008717. [Google Scholar] [CrossRef]
- Uçar, M.C.; Lipowsky, R. Collective force generation by molecular motors is determined by strain-induced unbinding. Nano Lett. 2019, 20, 669–676. [Google Scholar] [CrossRef]
- Cong, J.; Fang, B.; Wang, Q.; Su, Y.; Gu, T.; Luo, T. The mechanobiology of actin cytoskeletal proteins during cell–cell fusion. J. R. Soc. Interface 2019, 16, 20190022. [Google Scholar] [CrossRef]
- Van Helvert, S.; Friedl, P. Strain stiffening of fibrillar collagen during individual and collective cell migration identified by AFM nanoindentation. ACS Appl. Mater. Interfaces 2016, 8, 21946–21955. [Google Scholar] [CrossRef]
- Wu, C.; Bauer, J.; Juliano, R.; McDonald, J. The alpha 5 beta 1 integrin fibronectin receptor, but not the alpha 5 cytoplasmic domain, functions in an early and essential step in fibronectin matrix assembly. J. Biol. Chem. 1993, 268, 21883–21888. [Google Scholar] [CrossRef]
- Attia, M.S.; Alblowi, J.A. Effect of Subantimicrobial Dose Doxycycline Treatment on Gingival Crevicular Fluid Levels of MMP-9 and MMP-13 in Periodontitis Stage 2, Grade B in Subjects with Type 2 Diabetes Mellitus. J. Immunol. Res. 2020, 2020, 2807259. [Google Scholar] [CrossRef]
- Sato, T.; Verma, S.; Andrade, C.D.C.; Omeara, M.; Campbell, N.; Wang, J.S.; Cetinbas, M.; Lang, A.; Ausk, B.J.; Brooks, D.J. A FAK/HDAC5 signaling axis controls osteocyte mechanotransduction. Nat. Commun. 2020, 11, 3282. [Google Scholar] [CrossRef]
- Li, X.; Ominsky, M.S.; Niu, Q.T.; Sun, N.; Daugherty, B.; D’Agostin, D.; Kurahara, C.; Gao, Y.; Cao, J.; Gong, J. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J. Bone Miner. Res. 2008, 23, 860–869. [Google Scholar] [CrossRef]
- Muhamed, I.; Wu, J.; Sehgal, P.; Kong, X.; Tajik, A.; Wang, N.; Leckband, D.E. E-cadherin-mediated force transduction signals regulate global cell mechanics. J. Cell Sci. 2016, 129, 1843–1854. [Google Scholar] [CrossRef]
- Krischak, A.; Kowaliuk, J.; Sarsarshahi, S.; Dörr, W.; Kleiter, M. Effect of irradiation on the expression of E-cadherin and β-catenin in early and late radiation sequelae of the urinary bladder and its modulation by NF-κB inhibitor thalidomide. Strahlenther. Onkol. 2021, 197, 537–546. [Google Scholar] [CrossRef]
- Kluger, C.; Braun, L.; Sedlak, S.M.; Pippig, D.A.; Bauer, M.S.; Miller, K.; Milles, L.F.; Gaub, H.E.; Vogel, V. Different vinculin binding sites use the same mechanism to regulate directional force transduction. Biophys. J. 2020, 118, 1344–1356. [Google Scholar] [CrossRef]
- Chandran, R.; Kale, G.; Philippe, J.-M.; Lecuit, T.; Mayor, S. Distinct actin-dependent nanoscale assemblies underlie the dynamic and hierarchical organization of E-cadherin. Curr. Biol. 2021, 31, 1726–1736.e4. [Google Scholar] [CrossRef]
- Huang, S.-C.; Liang, J.Y.; Vu, L.V.; Faye, H.Y.; Ou, A.C.; Ou, J.P.; Zhang, H.S.; Burnett, K.M.; Benz, E.J., Jr. Epithelial-specific isoforms of protein 4.1 R promote adherens junction assembly in maturing epithelia. J. Biol. Chem. 2020, 295, 191–211. [Google Scholar] [CrossRef]
- Ishiyama, N.; Sarpal, R.; Wood, M.N.; Barrick, S.K.; Nishikawa, T.; Hayashi, H.; Kobb, A.B.; Flozak, A.S.; Yemelyanov, A.; Fernandez-Gonzalez, R. Force-dependent allostery of the α-catenin actin-binding domain controls adherens junction dynamics and functions. Nat. Commun. 2018, 9, 5121. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Chen, C.-C.; Hsueh, Y.-J.; Hung, L.-M.; Ma, D.H.-K.; Chen, H.-C.; Len, W.-B.; Meir, Y.-J.J. Extraneous E-Cadherin Engages the Deterministic Process of Somatic Reprogramming through Modulating STAT3 and Erk1/2 Activity. Cells 2021, 10, 284. [Google Scholar] [CrossRef]
- Ma, Y.-C.; Yang, Z.-S.; Ma, L.-Q.; Shu, R.; Zou, C.-G.; Zhang, K.-Q. YAP in epithelium senses gut barrier loss to deploy defenses against pathogens. PLoS Pathog. 2020, 16, e1008766. [Google Scholar] [CrossRef]
- Monster, J.L.; Donker, L.; Vliem, M.J.; Win, Z.; Matthews, H.K.; Cheah, J.S.; Yamada, S.; de Rooij, J.; Baum, B.; Gloerich, M. An asymmetric junctional mechanoresponse coordinates mitotic rounding with epithelial integrity. J. Cell Biol. 2021, 220. [Google Scholar] [CrossRef]
- Lim, J.C.; Bae, S.H.; Lee, G.; Ryu, C.J.; Jang, Y.J. Activation of β-catenin by TGF-β1 promotes ligament-fibroblastic differentiation and inhibits cementoblastic differentiation of human periodontal ligament cells. STEM CELLS 2020, 38, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- Arun, R.; Hemalatha, R.; Arun, K.; Kumar, T. E-cadherin and CD1a expression in gingival epithelium in periodontal health, disease and post-treatment. Indian J. Dent. Res. 2010, 21, 396. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sharma, S.; Batra, M.; Abidullah, M.; Bhuvinder, S.; Katragadda, P. Role of E-cadherin in Progression of Oral Squamous Cell Carcinoma: A Retrospective Immunohistochemical Study. J. Contemp. Dent. Pract. 2018, 19, 1105–1110. [Google Scholar] [PubMed]
- Abe-Yutori, M.; Chikazawa, T.; Shibasaki, K.; Murakami, S. Decreased expression of E-cadherin by Porphyromonas gingivalis-lipopolysaccharide attenuates epithelial barrier function. J. Periodontal Res. 2017, 52, 42–50. [Google Scholar] [CrossRef]
- Sowmya, S.; Rao, R.S.; Prasad, K. Development of clinico-histopathological predictive model for the assessment of metastatic risk of oral squamous cell carcinoma. J. Carcinog. 2020, 19, 2. [Google Scholar] [CrossRef]
- Johnson, C.L.; Merryman, W.D. Side-specific valvular endothelial-interstitial cell mechano-communication via cadherin-11. J. Biomech. 2021, 119, 110253. [Google Scholar] [CrossRef]
- Piao, S.; Inglehart, R.C.; Scanlon, C.S.; Russo, N.; Banerjee, R.; D’Silva, N.J. CDH 11 inhibits proliferation and invasion in head and neck cancer. J. Oral Pathol. Med. 2017, 46, 89–97. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Kou, X.; Yang, R.; Liu, D.; Wang, X.; Song, Y.; Cao, H.; He, D.; Gan, Y. Cadherin-11 modulates cell morphology and collagen synthesis in periodontal ligament cells under mechanical stress. Angle Orthod. 2017, 87, 193–199. [Google Scholar] [CrossRef]
- Row, S.; Liu, Y.; Alimperti, S.; Agarwal, S.K.; Andreadis, S.T. Cadherin-11 is a novel regulator of extracellular matrix synthesis and tissue mechanics. J. Cell Sci. 2016, 129, 2950–2961. [Google Scholar] [CrossRef]
- Alimperti, S.; You, H.; George, T.; Agarwal, S.K.; Andreadis, S.T. Cadherin-11 regulates both mesenchymal stem cell differentiation into smooth muscle cells and the development of contractile function in vivo. J. Cell Sci. 2014, 127, 2627–2638. [Google Scholar]
- Steinmetz, E.L.; Dewald, D.N.; Walldorf, U. Drosophila Homeodomain-Interacting Protein Kinase (Hipk) Phosphorylates the Hippo/Warts Signalling Effector Yorkie. Int. J. Mol. Sci. 2021, 22, 1862. [Google Scholar] [CrossRef]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef]
- Dobrokhotov, O.; Samsonov, M.; Sokabe, M.; Hirata, H. Mechanoregulation and pathology of YAP/TAZ via Hippo and non-Hippo mechanisms. Clin. Transl. Med. 2018, 7, 23. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Dong, J.; Pan, D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 2003, 114, 445–456. [Google Scholar] [CrossRef]
- Sharma, J.; Antenos, M.; Madan, P. A Comparative Analysis of Hippo Signaling Pathway Components during Murine and Bovine Early Mammalian Embryogenesis. Genes 2021, 12, 281. [Google Scholar] [CrossRef]
- Oh, J.-E.; Kim, H.; Kang, H.K.; Chung, C.-P.; Park, W.H.; Min, B.-M. α3β1 integrin promotes cell survival via multiple interactions between 14-3-3 isoforms and proapoptotic proteins. Exp. Cell Res. 2009, 315, 3187–3200. [Google Scholar] [CrossRef]
- Elbediwy, A.; Vincent-Mistiaen, Z.I.; Spencer-Dene, B.; Stone, R.K.; Boeing, S.; Wculek, S.K.; Cordero, J.; Tan, E.H.; Ridgway, R.; Brunton, V.G. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development 2016, 143, 1674–1687. [Google Scholar]
- Park, J.; Kim, J.S.; Nahm, J.H.; Kim, S.-K.; Lee, D.-H.; Lim, D.-S. WWC1 and NF2 Prevent the Development of Intrahepatic Cholangiocarcinoma by Regulating YAP/TAZ Activity through LATS in Mice. Mol. Cells 2020, 43, 491. [Google Scholar]
- Li, T.; Guo, T.; Liu, H.; Jiang, H.; Wang, Y. Platelet-derived growth factor-BB mediates pancreatic cancer malignancy via regulation of the Hippo/Yes-associated protein signaling pathway. Oncol. Rep. 2021, 45, 83–94. [Google Scholar] [CrossRef]
- Maziarz, M.; Federico, A.; Zhao, J.; Dujmusic, L.; Zhao, Z.; Monti, S.; Varelas, X.; Garcia-Marcos, M. Naturally occurring hotspot cancer mutations in Gα13 promote oncogenic signaling. J. Biol. Chem. 2020, 295, 16897–16904. [Google Scholar] [CrossRef]
- Strippoli, R.; Sandoval, P.; Moreno-Vicente, R.; Rossi, L.; Battistelli, C.; Terri, M.; Pascual-Antón, L.; Loureiro, M.; Matteini, F.; Calvo, E. Caveolin1 and YAP drive mechanically induced mesothelial to mesenchymal transition and fibrosis. Cell Death Dis. 2020, 11, 647. [Google Scholar] [CrossRef]
- Kang, P.H.; Schaffer, D.V.; Kumar, S. Angiomotin links ROCK and YAP signaling in mechanosensitive differentiation of neural stem cells. Mol. Biol. Cell 2020, 31, 386–396. [Google Scholar] [CrossRef]
- Pagliari, S.; Vinarsky, V.; Martino, F.; Perestrelo, A.R.; De La Cruz, J.O.; Caluori, G.; Vrbsky, J.; Mozetic, P.; Pompeiano, A.; Zancla, A. YAP–TEAD1 control of cytoskeleton dynamics and intracellular tension guides human pluripotent stem cell mesoderm specification. Cell Death Differ. 2021, 28, 1193–1207. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Xie, Z.; Chen, L.; Sinkemani, A.; Yu, H.; Wu, X. AMOT 130 linking F-actin to YAP is involved in intervertebral disc degeneration. Cell Prolif. 2018, 51, e12492. [Google Scholar] [CrossRef]
- Low, B.C.; Pan, C.Q.; Shivashankar, G.; Bershadsky, A.; Sudol, M.; Sheetz, M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014, 588, 2663–2670. [Google Scholar] [CrossRef]
- Hu, J.K.-H.; Du, W.; Shelton, S.J.; Oldham, M.C.; DiPersio, C.M.; Klein, O.D. An FAK-YAP-mTOR signaling axis regulates stem cell-based tissue renewal in mice. Cell Stem Cell 2017, 21, 91–106.e106. [Google Scholar] [CrossRef]
- Marikawa, Y.; Alarcon, V.B. RHOA activity in expanding blastocysts is essential to regulate HIPPO-YAP signaling and to maintain the trophectoderm-specific gene expression program in a ROCK/actin filament-independent manner. MHR Basic Sci. Reprod. Med. 2019, 25, 43–60. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, Q.; Wang, M.; Irani, S.; Li, X.; Zhang, Q.; Meng, F.; Liu, S.; Zhang, F.; Wu, L. The protein phosphatase PPM1A dephosphorylates and activates YAP to govern mammalian intestinal and liver regeneration. PLoS Biol. 2021, 19, e3001122. [Google Scholar] [CrossRef]
- Samarakoon, R.; Chitnis, S.S.; Higgins, S.P.; Higgins, C.E.; Krepinsky, J.C.; Higgins, P.J. Redox-induced Src kinase and caveolin-1 signaling in TGF-β1-initiated SMAD2/3 activation and PAI-1 expression. PLoS ONE 2011, 6, e22896. [Google Scholar] [CrossRef]
- Li, C.-Y.; Hu, J.; Lu, H.; Lan, J.; Du, W.; Galicia, N.; Klein, O.D. αE-catenin inhibits YAP/TAZ activity to regulate signalling centre formation during tooth development. Nat. Commun. 2016, 7, 12133. [Google Scholar] [CrossRef]
- Alisafaei, F.; Jokhun, D.S.; Shivashankar, G.; Shenoy, V.B. Regulation of nuclear architecture, mechanics, and nucleocytoplasmic shuttling of epigenetic factors by cell geometric constraints. Proc. Natl. Acad. Sci. USA 2019, 116, 13200–13209. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, M.; Lin, J.; Hu, C. Hippo/TEAD4 signaling pathway as a potential target for the treatment of breast cancer. Oncol. Lett. 2021, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gu, W.; Wang, Q.; Fu, X.; Wang, Y.; Xu, X.; Wen, Y. C-MYC and BCL-2 mediate YAP-regulated tumorigenesis in OSCC. Oncotarget 2018, 9, 668. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Remue, E.; Meerschaert, K.; Vanloo, B.; Boucherie, C.; Gfeller, D.; Bader, G.D.; Sidhu, S.S.; Vandekerckhove, J.; Gettemans, J. Functional complexes between YAP2 and ZO-2 are PDZ domain-dependent, and regulate YAP2 nuclear localization and signalling. Biochem. J. 2010, 432, 461–478. [Google Scholar] [CrossRef]
- Oka, T.; Schmitt, A.; Sudol, M. Opposing roles of angiomotin-like-1 and zona occludens-2 on pro-apoptotic function of YAP. Oncogene 2012, 31, 128–134. [Google Scholar] [CrossRef]
- Domínguez-Calderón, A.; Ávila-Flores, A.; Ponce, A.; López-Bayghen, E.; Calderón-Salinas, J.-V.; Luis Reyes, J.; Chávez-Munguía, B.; Segovia, J.; Angulo, C.; Ramírez, L. ZO-2 silencing induces renal hypertrophy through a cell cycle mechanism and the activation of YAP and the mTOR pathway. Mol. Biol. Cell 2016, 27, 1581–1595. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, S.; Lin, Q.; Wang, X.-P. YAP regulates the expression of Hoxa1 and Hoxc13 in mouse and human oral and skin epithelial tissues. Mol. Cell. Biol. 2015, 35, 1449–1461. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, Y.; Hsu, C.-W.; Martinez-Traverso, I.M.; Zhang, M.; Bai, Y.; Ishii, M.; Maxson, R.E.; Olson, E.N.; Dickinson, M.E. Yap and Taz play a crucial role in neural crest-derived craniofacial development. Development 2016, 143, 504–515. [Google Scholar] [CrossRef]
- Kaku, M.; Komatsu, Y.; Mochida, Y.; Yamauchi, M.; Mishina, Y.; Ko, C.-C. Identification and characterization of neural crest-derived cells in adult periodontal ligament of mice. Arch. Oral Biol. 2012, 57, 1668–1675. [Google Scholar] [CrossRef]
- Dong, T.; Sun, X.; Jin, H. Role of YAP1 gene in proliferation, osteogenic differentiation, and apoptosis of human periodontal ligament stem cells induced by TNF-α. J. Periodontol. 2020. [Google Scholar] [CrossRef]
- Komatsu, N.; Kajiya, M.; Motoike, S.; Takewaki, M.; Horikoshi, S.; Iwata, T.; Ouhara, K.; Takeda, K.; Matsuda, S.; Fujita, T. Type I collagen deposition via osteoinduction ameliorates YAP/TAZ activity in 3D floating culture clumps of mesenchymal stem cell/extracellular matrix complexes. Stem Cell Res. Ther. 2018, 9, 342. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.-K.; Chang, M.-L.; Wan, Z.-Q.; Han, G.-L. Cyclic stretch enhances osteogenic differentiation of human periodontal ligament cells via YAP activation. BioMed Res. Int. 2018, 2018, 2174824. [Google Scholar] [CrossRef]
- Wang, C.; Gu, W.; Sun, B.; Zhang, Y.; Ji, Y.; Xu, X.; Wen, Y. CTHRC1 promotes osteogenic differentiation of periodontal ligament stem cells by regulating TAZ. J. Mol. Histol. 2017, 48, 311–319. [Google Scholar] [CrossRef]
- Sun, B.; Wen, Y.; Wu, X.; Zhang, Y.; Qiao, X.; Xu, X. Expression pattern of YAP and TAZ during orthodontic tooth movement in rats. J. Mol. Histol. 2018, 49, 123–131. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, B.; Hu, R.; Tong, X.; Zhang, M.; Xu, C.; He, Z.; Zhao, Y.; Deng, H. TAZ contributes to osteogenic differentiation of periodontal ligament cells under tensile stress. J. Periodontal Res. 2020, 55, 152–160. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, X.N.; Lu, Y.; Wu, P.; Zhao, H.G.; Li, Q.L.; Xu, Y.H. miR-140 inhibits osteogenic differentiation of human periodontal ligament fibroblasts through ras homolog gene family, member A-transcriptional co-activator with PDZ-binding motif pathway. Kaohsiung J. Med Sci. 2021, 37, 38–46. [Google Scholar] [CrossRef]
- Hu, P.; Gao, Q.; Zheng, H.; Tian, Y.; Zheng, G.; Yao, X.; Zhang, J.; Wu, X.; Sui, L. The Role and Activation Mechanism of TAZ in Hierarchical Microgroove/Nanopore Topography-Mediated Regulation of Stem Cell Differentiation. Int. J. Nanomed. 2021, 16, 1021. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, L.; Chen, L.; Jiang, J.; Zhou, X.; Wang, M.; Fan, Y. Static magnetic field regulates proliferation, migration, differentiation, and YAP/TAZ activation of human dental pulp stem cells. J. Tissue Eng. Regen. Med. 2018, 12, 2029–2040. [Google Scholar] [CrossRef]
- Kim, S.C.; Im, W.; Shim, J.Y.; Kim, S.-K.; Kim, B.J. Static magnetic field controls cell cycle in cultured human glioblastoma cells. Cytotechnology 2016, 68, 2745–2751. [Google Scholar] [CrossRef]
- Jouni, F.J.; Abdolmaleki, P.; Behmanesh, M.; Movahedin, M. An in vitro study of the impact of 4mT static magnetic field to modify the differentiation rate of rat bone marrow stem cells into primordial germ cells. Differentiation 2014, 87, 230–237. [Google Scholar] [CrossRef]
- Izzo, L.; Tunesi, M.; Boeri, L.; Laganà, M.; Giordano, C.; Raimondi, M.T. Influence of the static magnetic field on cell response in a miniaturized optically accessible bioreactor for 3D cell culture. Biomed. Microdevices 2019, 21, 531. [Google Scholar] [CrossRef]
- Hu, F.; Gong, Y.; Bian, Z.; Zhang, X.; Xu, B.; Zhang, J.; Shi, X.; Yu, Y.; Song, L. Comparison of three different types of two-implant-supported magnetic attachments on the stress distribution in edentulous mandible. Comput. Math. Methods Med. 2019, 2019, 6839517. [Google Scholar] [CrossRef]
- Alfarsi, M.A.; Shaik, S. Oral rehabilitation of a cleft palate patient with tooth-supported, telescopic magnetic overdenture. BMJ Case Rep. CP 2020, 13, e233777. [Google Scholar] [CrossRef]
- He, Y.; Xu, H.; Xiang, Z.; Yu, H.; Xu, L.; Guo, Y.; Tian, Y.; Shu, R.; Yang, X.; Xue, C. YAP regulates periodontal ligament cell differentiation into myofibroblast interacted with RhoA/ROCK pathway. J. Cell. Physiol. 2019, 234, 5086–5096. [Google Scholar] [CrossRef]
- Jia, L.; Gu, W.; Zhang, Y.; Jiang, B.; Qiao, X.; Wen, Y. Activated Yes-associated protein accelerates cell cycle, inhibits apoptosis, and delays senescence in human periodontal ligament stem cells. Int. J. Med Sci. 2018, 15, 1241. [Google Scholar] [CrossRef]
- Liu-Chittenden, Y.; Huang, B.; Shim, J.S.; Chen, Q.; Lee, S.-J.; Anders, R.A.; Liu, J.O.; Pan, D. Genetic and pharmacological disruption of the TEAD–YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012, 26, 1300–1305. [Google Scholar] [CrossRef]
- Morice, S.; Mullard, M.; Brion, R.; Dupuy, M.; Renault, S.; Tesfaye, R.; Royer, B.-L.; Ory, B.; Redini, F.; Verrecchia, F. The YAP/TEAD Axis as a New Therapeutic Target in Osteosarcoma: Effect of Verteporfin and CA3 on Primary Tumor Growth. Cancers 2020, 12, 3847. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Gu, K.; Li, A.; Fu, X.; Wang, Y.; Gu, W.; Wen, Y. Effect of YAP on an immortalized periodontal ligament stem cell line. Stem Cells Int. 2019, 2019, 6804036. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.Y.; Tang, J.; Casey, P.J.; Wang, M. Evaluating the Epithelial-Mesenchymal Program in Human Breast Epithelial Cells Cultured in Soft Agar Using a Novel Macromolecule Extraction Protocol. Cancers 2021, 13, 807. [Google Scholar] [CrossRef]
- Omori, H.; Nishio, M.; Masuda, M.; Miyachi, Y.; Ueda, F.; Nakano, T.; Sato, K.; Mimori, K.; Taguchi, K.; Hikasa, H. YAP1 is a potent driver of the onset and progression of oral squamous cell carcinoma. Sci. Adv. 2020, 6, eaay3324. [Google Scholar] [CrossRef]
- Shen, Y.; Pan, Y.; Guo, S.; Sun, L.; Zhang, C.; Wang, L. The roles of mechanosensitive ion channels and associated downstream MAPK signaling pathways in PDLC mechanotransduction. Mol. Med. Rep. 2020, 21, 2113–2122. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Estevez, M.; Gadalla, K.K.; Liñan-Barba, N.; Cobb, S.; Dev, K.K.; Sheridan, G.K. Inhibition of Piezo1 attenuates demyelination in the central nervous system. Glia 2020, 68, 356–375. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Fujii, S.; Matsumoto, S.; Tajiri, Y.; Kikuchi, A.; Kiyoshima, T. YAP signaling induces PIEZO1 to promote oral squamous cell carcinoma cell proliferation. J. Pathol. 2021, 253, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, X.; Jiang, J.; Xiao, B. Structural Designs and Mechanogating Mechanisms of the Mechanosensitive Piezo Channels. Trends Biochem. Sci. 2021, 46, 472–488. [Google Scholar] [CrossRef]
- Totaro, A.; Zhuang, Q.; Panciera, T.; Battilana, G.; Azzolin, L.; Brumana, G.; Gandin, A.; Brusatin, G.; Cordenonsi, M.; Piccolo, S. Cell phenotypic plasticity requires autophagic flux driven by YAP/TAZ mechanotransduction. Proc. Natl. Acad. Sci. USA 2019, 116, 17848–17857. [Google Scholar] [CrossRef]
- Pavel, M.; Renna, M.; Park, S.J.; Menzies, F.M.; Ricketts, T.; Füllgrabe, J.; Ashkenazi, A.; Frake, R.A.; Lombarte, A.C.; Bento, C.F. Contact inhibition controls cell survival and proliferation via YAP/TAZ-autophagy axis. Nat. Commun. 2018, 9, 2961. [Google Scholar] [CrossRef]
- Zainab, H.; Ameena Sultana, S. Stromal desmoplasia as a possible prognostic indicator in different grades of oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. JOMFP 2019, 23, 338. [Google Scholar]
- Matte, B.F.; Kumar, A.; Placone, J.K.; Zanella, V.G.; Martins, M.D.; Engler, A.J.; Lamers, M.L. Matrix stiffness mechanically conditions EMT and migratory behavior of oral squamous cell carcinoma. J. Cell Sci. 2019, 132, jcs224360. [Google Scholar] [CrossRef]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordóñez-Morán, P.; Clevers, H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef]
- Dou, C.; Liu, Z.; Tu, K.; Zhang, H.; Chen, C.; Yaqoob, U.; Wang, Y.; Wen, J.; Van Deursen, J.; Sicard, D. P300 acetyltransferase mediates stiffness-induced activation of hepatic stellate cells into tumor-promoting myofibroblasts. Gastroenterology 2018, 154, 2209–2221.e2214. [Google Scholar] [CrossRef]
- Nilsson, M.B.; Sun, H.; Robichaux, J.; Pfeifer, M.; McDermott, U.; Travers, J.; Diao, L.; Xi, Y.; Tong, P.; Shen, L. A YAP/FOXM1 axis mediates EMT-associated EGFR inhibitor resistance and increased expression of spindle assembly checkpoint components. Sci. Transl. Med. 2020, 12, eaaz4589. [Google Scholar] [CrossRef]
- Wei, W.; Xue, L.; Tan, L.; Liu, J.; Yang, Q.; Wang, J.; Yan, B.; Cai, Q.; Yang, L.; Yue, Y. Inhibition of yes-associated protein dephosphorylation prevents aggravated periodontitis with occlusal trauma. J. Periodontol. 2020. [Google Scholar] [CrossRef]
- Pan, W.; Yang, L.; Li, J.; Xue, L.; Wei, W.; Ding, H.; Deng, S.; Tian, Y.; Yue, Y.; Wang, M. Traumatic occlusion aggravates bone loss during periodontitis and activates Hippo-YAP pathway. J. Clin. Periodontol. 2019, 46, 438–447. [Google Scholar] [CrossRef]
- Dupont, S. Regulation of YAP/TAZ activity by mechanical cues: An experimental overview. Hippo Pathw. 2019, 183–202. [Google Scholar] [CrossRef]
- Bautista, M.; Fernandez, A.; Pinaud, F. A Micropatterning Strategy to Study Nuclear Mechanotransduction in Cells. Micromachines 2019, 10, 810. [Google Scholar] [CrossRef]
- Stephens, A.D.; Liu, P.Z.; Banigan, E.J.; Almassalha, L.M.; Backman, V.; Adam, S.A.; Goldman, R.D.; Marko, J.F. Chromatin histone modifications and rigidity affect nuclear morphology independent of lamins. Mol. Biol. Cell 2018, 29, 220–233. [Google Scholar] [CrossRef]
- Morel, V.; Lepicard, S.; Rey, A.N.; Parmentier, M.-L.; Schaeffer, L. Drosophila Nesprin-1 controls glutamate receptor density at neuromuscular junctions. Cell. Mol. Life Sci. 2014, 71, 3363–3379. [Google Scholar] [CrossRef]
- Kracklauer, M.P.; Banks, S.M.; Xie, X.; Wu, Y.; Fischer, J.A. Drosophila klaroid encodes a SUN domain protein required for Klarsicht localization to the nuclear envelope and nuclear migration in the eye. Fly 2007, 1, 75–85. [Google Scholar] [CrossRef]
- Ulianov, S.V.; Doronin, S.A.; Khrameeva, E.E.; Kos, P.I.; Luzhin, A.V.; Starikov, S.S.; Galitsyna, A.A.; Nenasheva, V.V.; Ilyin, A.A.; Flyamer, I.M. Nuclear lamina integrity is required for proper spatial organization of chromatin in Drosophila. Nat. Commun. 2019, 10, 1176. [Google Scholar] [CrossRef]
- Jahed, Z.; Hao, H.; Thakkar, V.; Vu, U.T.; Valdez, V.A.; Rathish, A.; Tolentino, C.; Kim, S.C.; Fadavi, D.; Starr, D.A. Role of KASH domain lengths in the regulation of LINC complexes. Mol. Biol. Cell 2019, 30, 2076–2086. [Google Scholar] [CrossRef]
- Gurusaran, M.; Davies, O.R. A molecular mechanism for LINC complex branching by structurally diverse SUN-KASH 6: 6 assemblies. eLife 2021, 10, e60175. [Google Scholar] [CrossRef] [PubMed]
- Alena, S.K.; Eva, B.; Aleš, K.; Emilie, L. Spatiotemporal Mislocalization of Nuclear Membrane-Associated Proteins in γ-Irradiation-Induced Senescent Cells. Cells 2020, 9, 999. [Google Scholar]
- Ketema, M.; Kreft, M.; Secades, P.; Janssen, H.; Sonnenberg, A. A Highlights from MBoC Selection: Nesprin-3 connects plectin and vimentin to the nuclear envelope of Sertoli cells but is not required for Sertoli cell function in spermatogenesis. Mol. Biol. Cell 2013, 24, 2454. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.C.; Quintremil, S.; Yi, J.; Vallee, R.B. Nesprin-2 recruitment of BicD2 to the nuclear envelope controls dynein/kinesin-mediated neuronal migration in vivo. Curr. Biol. 2020, 30, 3116–3129.e3114. [Google Scholar] [CrossRef]
- Holt, I.; Fuller, H.R.; Sewry, C.A.; Shirran, S.L.; Zhang, Q.; Shanahan, C.M.; Morris, G.E. Nesprin-1-alpha2 associates with kinesin at myotube outer nuclear membranes, but is restricted to neuromuscular junction nuclei in adult muscle. Sci. Rep. 2019, 9, 14202. [Google Scholar] [CrossRef]
- Porter, L.; Minaisah, R.-M.; Ahmed, S.; Ali, S.; Norton, R.; Zhang, Q.; Ferraro, E.; Molenaar, C.; Holt, M.; Cox, S. SUN1/2 are essential for RhoA/ROCK-regulated actomyosin activity in isolated vascular smooth muscle cells. Cells 2020, 9, 132. [Google Scholar] [CrossRef]
- Matsumoto, A.; Sakamoto, C.; Matsumori, H.; Katahira, J.; Yasuda, Y.; Yoshidome, K.; Tsujimoto, M.; Goldberg, I.G.; Matsuura, N.; Nakao, M. Loss of the integral nuclear envelope protein SUN1 induces alteration of nucleoli. Nucleus 2016, 7, 68–83. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, J.; Jeong, S.; Kang, S.-m.; Park, B.-J.; Ha, N.-C. Beta-strand-mediated dimeric formation of the Ig-like domains of human lamin A/C and B1. Biochem. Biophys. Res. Commun. 2021, 550, 191–196. [Google Scholar] [CrossRef]
- Parry, D.A.; Martin, C.-A.; Greene, P.; Marsh, J.A.; Blyth, M.; Cox, H.; Donnelly, D.; Greenhalgh, L.; Greville-Heygate, S.; Harrison, V. Heterozygous lamin B1 and lamin B2 variants cause primary microcephaly and define a novel laminopathy. Genet. Med. 2021, 23, 408–414. [Google Scholar] [CrossRef]
- Xie, W.; Chojnowski, A.; Boudier, T.; Lim, J.S.; Ahmed, S.; Ser, Z.; Stewart, C.; Burke, B. A-type lamins form distinct filamentous networks with differential nuclear pore complex associations. Curr. Biol. 2016, 26, 2651–2658. [Google Scholar] [CrossRef]
- Li, H.-P.; Liu, J.-T.; Chen, Y.-X.; Wang, W.-B.; Han, Y.; Yao, Q.-P.; Qi, Y.-X. Suppressed nuclear envelope proteins activate autophagy of vascular smooth muscle cells during cyclic stretch application. Biochim. Biophys. Acta BBA Mol. Cell Res. 2021, 1868, 118855. [Google Scholar] [CrossRef]
- Shimi, T.; Kittisopikul, M.; Tran, J.; Goldman, A.E.; Adam, S.A.; Zheng, Y.; Jaqaman, K.; Goldman, R.D. Structural organization of nuclear lamins A, C, B1, and B2 revealed by superresolution microscopy. Mol. Biol. Cell 2015, 26, 4075–4086. [Google Scholar] [CrossRef]
- Oldenburg, A.; Briand, N.; Sørensen, A.L.; Cahyani, I.; Shah, A.; Moskaug, J.Ø.; Collas, P. A lipodystrophy-causing lamin A mutant alters conformation and epigenetic regulation of the anti-adipogenic MIR335 locus. J. Cell Biol. 2017, 216, 2731–2743. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.E.M.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.-L.; et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017, 171, 1397–1410. [Google Scholar] [CrossRef]
- Serebryannyy, L.A.; Cruz, C.M.; De Lanerolle, P. A role for nuclear actin in HDAC 1 and 2 regulation. Sci. Rep. 2016, 6, 28460. [Google Scholar] [CrossRef]
- Philimonenko, V.V.; Zhao, J.; Iben, S.; Dingová, H.; Kyselá, K.; Kahle, M.; Zentgraf, H.; Hofmann, W.A.; de Lanerolle, P.; Hozák, P. Nuclear actin and myosin I are required for RNA polymerase I transcription. Nat. Cell Biol. 2004, 6, 1165–1172. [Google Scholar] [CrossRef]
- Hu, P.; Wu, S.; Hernandez, N. A role for β-actin in RNA polymerase III transcription. Genes Dev. 2004, 18, 3010–3015. [Google Scholar] [CrossRef]
- Sokolova, M.; Moore, H.M.; Prajapati, B.; Dopie, J.; Meriläinen, L.; Honkanen, M.; Matos, R.C.; Poukkula, M.; Hietakangas, V.; Vartiainen, M.K. Nuclear actin is required for transcription during Drosophila oogenesis. iScience 2018, 9, 63–70. [Google Scholar] [CrossRef]
- Wei, M.; Fan, X.; Ding, M.; Li, R.; Shao, S.; Hou, Y.; Meng, S.; Tang, F.; Li, C.; Sun, Y. Nuclear actin regulates inducible transcription by enhancing RNA polymerase II clustering. Sci. Adv. 2020, 6, eaay6515. [Google Scholar] [CrossRef]
- Plessner, M.; Melak, M.; Chinchilla, P.; Baarlink, C.; Grosse, R. Nuclear F-actin formation and reorganization upon cell spreading. J. Biol. Chem. 2015, 290, 11209–11216. [Google Scholar] [CrossRef]
- Lattanzi, G.; Cenni, V.; Marmiroli, S.; Capanni, C.; Mattioli, E.; Merlini, L.; Squarzoni, S.; Maraldi, N.M. Association of emerin with nuclear and cytoplasmic actin is regulated in differentiating myoblasts. Biochem. Biophys. Res. Commun. 2003, 303, 764–770. [Google Scholar] [CrossRef]
- Lombardi, M.L.; Jaalouk, D.E.; Shanahan, C.M.; Burke, B.; Roux, K.J.; Lammerding, J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J. Biol. Chem. 2011, 286, 26743–26753. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Louhghalam, A.; Lee, G.; Schafer, B.W.; Wirtz, D.; Kim, D.-H. Nuclear lamin A/C harnesses the perinuclear apical actin cables to protect nuclear morphology. Nat. Commun. 2017, 8, 2123. [Google Scholar] [CrossRef] [PubMed]
- Lamm, N.; Read, M.N.; Nobis, M.; Van Ly, D.; Page, S.G.; Masamsetti, V.P.; Timpson, P.; Biro, M.; Cesare, A.J. Nuclear F-actin counteracts nuclear deformation and promotes fork repair during replication stress. Nat. Cell Biol. 2020, 22, 1460–1470. [Google Scholar] [CrossRef]
- Cao, X.; Lin, Y.; Driscoll, T.P.; Franco-Barraza, J.; Cukierman, E.; Mauck, R.L.; Shenoy, V.B. A chemomechanical model of matrix and nuclear rigidity regulation of focal adhesion size. Biophys. J. 2015, 109, 1807–1817. [Google Scholar] [CrossRef]
- Chancellor, T.; Lee, J.; Thodeti, C.K.; Lele, T. Actomyosin tension exerted on the nucleus through nesprin-1 connections influences endothelial cell adhesion, migration, and cyclic strain-induced reorientation. Biophys. J. 2010, 99, 115–123. [Google Scholar] [CrossRef]
- Hoffman, L.M.; Smith, M.A.; Jensen, C.C.; Yoshigi, M.; Blankman, E.; Ullman, K.S.; Beckerle, M.C. Mechanical stress triggers nuclear remodeling and the formation of transmembrane actin nuclear lines with associated nuclear pore complexes. Mol. Biol. Cell 2020, 31, 1774–1787. [Google Scholar] [CrossRef]
- Ren, J.; Li, Y.; Hu, S.; Liu, Y.; Tsao, S.W.; Lau, D.; Luo, G.; Tsang, C.M.; Lam, R.H. Nondestructive quantification of single-cell nuclear and cytoplasmic mechanical properties based on large whole-cell deformation. Lab Chip 2020, 20, 4175–4185. [Google Scholar] [CrossRef]
- Guilluy, C.; Osborne, L.D.; Van Landeghem, L.; Sharek, L.; Superfine, R.; Garcia-Mata, R.; Burridge, K. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol. 2014, 16, 376–381. [Google Scholar] [CrossRef]
- Buxboim, A.; Swift, J.; Irianto, J.; Spinler, K.R.; Dingal, P.D.P.; Athirasala, A.; Kao, Y.-R.C.; Cho, S.; Harada, T.; Shin, J.-W. Matrix elasticity regulates lamin-A, C phosphorylation and turnover with feedback to actomyosin. Curr. Biol. 2014, 24, 1909–1917. [Google Scholar] [CrossRef]
- Tifft, K.E.; Bradbury, K.A.; Wilson, K.L. Tyrosine phosphorylation of nuclear-membrane protein emerin by Src, Abl and other kinases. J. Cell Sci. 2009, 122, 3780–3790. [Google Scholar] [CrossRef]
- Bera, M.; Kotamarthi, H.C.; Dutta, S.; Ray, A.; Ghosh, S.; Bhattacharyya, D.; Ainavarapu, S.R.K.; Sengupta, K. Characterization of Unfolding Mechanism of Human Lamin a Ig Fold by Single-Molecule Force Spectroscopy Implications in EDMD. Biochemistry 2014, 53, 7247–7258. [Google Scholar] [CrossRef]
- Yao, M.; Qiu, W.; Liu, R.; Efremov, A.; Cong, P.; Seddiki, R.; Payre, M.; Lim, C.; Ladoux, B.; Mege, R.-M.; et al. Force-dependent conformational switch of α-catenin controls vinculin binding. Nat. Commun. 2014, 5, 4525. [Google Scholar] [CrossRef]
- Owens, D.J.; Fischer, M.; Jabre, S.; Moog, S.; Mamchaoui, K.; Butler-Browne, G.; Coirault, C. Lamin mutations cause increased YAP nuclear entry in muscle stem cells. Cells 2020, 9, 816. [Google Scholar] [CrossRef]
- Stephens, A.D.; Banigan, E.J.; Adam, S.A.; Goldman, R.D.; Marko, J.F. Chromatin and lamin A determine two different mechanical response regimes of the cell nucleus. Mol. Biol. Cell 2017, 28, 1984–1996. [Google Scholar] [CrossRef]
- Nava, M.M.; Miroshnikova, Y.A.; Biggs, L.C.; Whitefield, D.B.; Metge, F.; Boucas, J.; Vihinen, H.; Jokitalo, E.; Li, X.; Arcos, J.M.G. Heterochromatin-driven nuclear softening protects the genome against mechanical stress-induced damage. Cell 2020, 181, 800–817.e822. [Google Scholar] [CrossRef]
- Quang Le, H.; Ghatak, S.; Chloé Yeung, C.-Y.; Tellkamp, F.; Günschmann, C.; Dieterich, C.; Yeroslaviz, A.; Habermann, B.; Pombo, A.; Niessen, C.M.; et al. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol. 2016, 18, 864–875. [Google Scholar]
- Tajik, A.; Zhang, Y.; Wei, F.; Sun, J.; Jia, Q.; Zhou, W.; Singh, R.; Khanna, N.; Belmont, A.S.; Wang, N. Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater. 2016, 15, 1287–1296. [Google Scholar] [CrossRef]
- Gadban, N.; Weinberg, E.; Zoabi, A.; Ashkenazi, M.; Yaffe, A.; Binderman, I. Strain reduction of human gingival fibroblasts induces the ATP pathway. J. Interdiscipl. Med. Dent. Sci. 2015, 3. [Google Scholar] [CrossRef]
- Denes, B.J.; Bolton, C.; Illsley, C.; Kok, W.; Walker, J.; Poetsch, A.; Tredwin, C.; Kiliaridis, S.; Hu, B. Notch coordinates periodontal ligament maturation through regulating Lamin, A. J. Dent. Res. 2019, 98, 1357–1366. [Google Scholar] [CrossRef]
- Shah, P.P.; Lv, W.; Rhoades, J.H.; Poleshko, A.; Abbey, D.; Caporizzo, M.A.; Linares-Saldana, R.; Heffler, J.G.; Sayed, N.; Thomas, D. Pathogenic LMNA variants disrupt cardiac lamina-chromatin interactions and de-repress alternative fate genes. Cell Stem Cell 2021, 28, 938–954.e9. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Mishima, H.; Barc, J.; Takahashi, M.P.; Hirono, K.; Terada, S.; Kowase, S.; Sato, T.; Mukai, Y.; Yui, Y. Cardiac Emerinopathy: A Nonsyndromic Nuclear Envelopathy With Increased Risk of Thromboembolic Stroke Due to Progressive Atrial Standstill and Left Ventricular Noncompaction. Circ. Arrhythmia Electrophysiol. 2020, 13, e008712. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.J.; Goodsell, K.; Grogan, M.; Ackerman, M.J. LMNA-mediated arrhythmogenic right ventricular cardiomyopathy and charcot-marie-tooth type 2B1: A patient-discovered unifying diagnosis. J. Cardiovasc. Electrophysiol. 2016, 27, 868–871. [Google Scholar] [CrossRef]
- Huang, J.; Wan, Q.; Zou, Y.; Wang, L.; Pan, Y. Familial dilated cardiomyopathy caused by a novel variant in the Lamin A/C gene: A case report. BMC Cardiovasc. Disord. 2020, 20, 423. [Google Scholar] [CrossRef] [PubMed]
- Erdos, M.R.; Cabral, W.A.; Tavarez, U.L.; Cao, K.; Gvozdenovic-Jeremic, J.; Narisu, N.; Zerfas, P.M.; Crumley, S.; Boku, Y.; Hanson, G. A targeted antisense therapeutic approach for Hutchinson–Gilford progeria syndrome. Nat. Med. 2021, 27, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Peric-Hupkes, D.; Meuleman, W.; Pagie, L.; Bruggeman, S.W.; Solovei, I.; Brugman, W.; Gräf, S.; Flicek, P.; Kerkhoven, R.M.; van Lohuizen, M. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol. Cell 2010, 38, 603–613. [Google Scholar] [CrossRef]
- Killaars, A.R.; Walker, C.J.; Anseth, K.S. Nuclear mechanosensing controls MSC osteogenic potential through HDAC epigenetic remodeling. Proc. Natl. Acad. Sci. USA 2020, 117, 21258–21266. [Google Scholar] [CrossRef] [PubMed]
- Mozzetta, C.; Tedesco, F.S. Challenging the “chromatin hypothesis” of cardiac laminopathies with LMNA mutant iPS cells. J. Cell Biol. 2019, 218, 2826–2828. [Google Scholar] [CrossRef]
- Swift, J.; Ivanovska, I.; Buxboim, A.; Harada, T.; Dingal, P.; Pinter, J.; Pajerowski, J.; Spinler, K.; Shin, J.; Manorama, T.; et al. Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-Directed Differentiation. Science 2013, 341, 1240104. [Google Scholar] [CrossRef]
- Torvaldson, E.; Kochin, V.; Eriksson, J.E. Phosphorylation of lamins determine their structural properties and signaling functions. Nucleus 2015, 6, 166–171. [Google Scholar] [CrossRef]
- Cho, S.; Vashisth, M.; Abbas, A.; Majkut, S.; Vogel, K.; Xia, Y.; Ivanovska, I.L.; Irianto, J.; Tewari, M.; Zhu, K. Mechanosensing by the lamina protects against nuclear rupture, DNA damage, and cell-cycle arrest. Dev. Cell 2019, 49, 920–935.e925. [Google Scholar] [CrossRef]
- VanGompel, M.J.; Nguyen, K.C.; Hall, D.H.; Dauer, W.T.; Rose, L.S. A novel function for the Caenorhabditis elegans torsin OOC-5 in nucleoporin localization and nuclear import. Mol. Biol. Cell 2015, 26, 1752–1763. [Google Scholar] [CrossRef]
- Li, J.; Levin, D.S.; Kim, A.J.; Pappas, S.S.; Dauer, W.T. TorsinA restoration in a mouse model identifies a critical therapeutic window for DYT1 dystonia. J. Clin. Investig. 2021. [Google Scholar] [CrossRef] [PubMed]
- Gill, N.K.; Ly, C.; Kim, P.H.; Saunders, C.A.; Fong, L.G.; Young, S.G.; Luxton, G.; Rowat, A.C. DYT1 dystonia patient-derived fibroblasts have increased deformability and susceptibility to damage by mechanical forces. Front. Cell Dev. Biol. 2019, 7, 103. [Google Scholar] [CrossRef]
- Huelgas-Morales, G.; Sanders, M.; Mekonnen, G.; Tsukamoto, T.; Greenstein, D. Decreased mechanotransduction prevents nuclear collapse in a Caenorhabditis elegans laminopathy. Proc. Natl. Acad. Sci. USA 2020, 117, 31301–31308. [Google Scholar] [CrossRef]
- Maloney, W. The integral role of the dentist in treating individuals with Hutchinson-Gilford progeria syndrome. DENTISTRY 2010, 1, WMC00446. [Google Scholar]
- Reichert, C.; Gölz, L.; Götz, W.; Wolf, M.; Deschner, J.; Jäger, A. Dental and craniofacial characteristics in a patient with Hutchinson–Gilford progeria syndrome. J. Orofac. Orthop. Fortschr. Kieferorthopädie 2014, 75, 251–263. [Google Scholar] [CrossRef]
- Bengtsson, V.W.; Persson, G.R.; Berglund, J.S.; Renvert, S. Periodontitis related to cardiovascular events and mortality: A long-time longitudinal study. Clin. Oral Investig. 2021, 25, 4085–4095. [Google Scholar] [CrossRef]
- Almeida-Santos, A.; Martins-Mendes, D.; Gayà-Vidal, M.; Pérez-Pardal, L.; Beja-Pereira, A. Characterization of the oral microbiome of medicated type-2 diabetes patients. Front. Microbiol. 2021, 12, 56. [Google Scholar] [CrossRef]
- Feng, Y.-K.; Wu, Q.-L.; Peng, Y.-W.; Liang, F.-Y.; You, H.-J.; Feng, Y.-W.; Li, G.; Li, X.-J.; Liu, S.-H.; Li, Y.-C.; et al. gingivalis impairs gut permeability and mediates immune responses associated with neurodegeneration in LRRK2 R1441G mice. J. Neuroinflammation 2020, 17, 347. [Google Scholar] [CrossRef]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontology 2000 2020, 83, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Groeger, S.E.; Meyle, J. Epithelial barrier and oral bacterial infection. Periodontology 2000 2015, 69, 46–67. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.M.; Ling, M.R.; Insall, R.; Kalna, G.; Spengler, J.; Grant, M.M.; Chapple, I.L. Impaired neutrophil directional chemotactic accuracy in chronic periodontitis patients. J. Clin. Periodontol. 2015, 42, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mortensen, M.S.; Schjørring, S.; Trivedi, U.; Vestergaard, G.; Stokholm, J.; Bisgaard, H.; Krogfelt, K.A.; Sørensen, S.J. Amplicon sequencing provides more accurate microbiome information in healthy children compared to culturing. Commun. Biol. 2019, 2, 291. [Google Scholar] [CrossRef]
- Arweiler, N.B.; Auschill, T.M.; Heumann, C.; Hellwig, E.; Al-Ahmad, A. Influence of Probiotics on the Salivary Microflora Oral Streptococci and Their Integration into Oral Biofilm. Antibiotics 2020, 9, 803. [Google Scholar] [CrossRef]
- Moore, W.; Moore, L.V. The bacteria of periodontal diseases. Periodontology 2000 1994, 5, 66–77. [Google Scholar] [CrossRef]
- Diaz, P.I.; Chalmers, N.I.; Rickard, A.H.; Kong, C.; Milburn, C.L.; Palmer, R.J.; Kolenbrander, P.E. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl. Environ. Microbiol. 2006, 72, 2837–2848. [Google Scholar] [CrossRef]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef]
- Hong, B.-Y.; Araujo, M.V.F.; Strausbaugh, L.D.; Terzi, E.; Ioannidou, E.; Diaz, P.I. Microbiome profiles in periodontitis in relation to host and disease characteristics. PLoS ONE 2015, 10, e0127077. [Google Scholar] [CrossRef]
- Souto, G.R.; Queiroz-Junior, C.M.; de Abreu, M.H.N.G.; Costa, F.O.; Mesquita, R.A. Pro-inflammatory, Th1, Th2, Th17 cytokines and dendritic cells: A cross-sectional study in chronic periodontitis. PLoS ONE 2014, 9, e91636. [Google Scholar] [CrossRef]
- Özkavaf, A.; Aras, H.; Huri, C.B.; Mottaghian-Dini, F.; Tözüm, T.F.; Etikan, I.; Yamalik, N.; Çaglayan, F. Relationship between the quantity of gingival crevicular fluid and clinical periodontal status. J. Oral Sci. 2000, 42, 231–238. [Google Scholar] [CrossRef]
- Sandholm, L. Proteases and their inhibitors in chronic inflammatory periodontal disease. J. Clin. Periodontol. 1986, 13, 19–26. [Google Scholar] [CrossRef]
- Laugisch, O.; Schacht, M.; Guentsch, A.; Kantyka, T.; Sroka, A.; Stennicke, H.; Pfister, W.; Sculean, A.; Potempa, J.; Eick, S. Periodontal pathogens affect the level of protease inhibitors in gingival crevicular fluid. Mol. Oral Microbiol. 2012, 27, 45–56. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Laino, L.; D’Amico, C.; Mauceri, R.; Tozum, T.F.; Gaeta, M.; Cicciù, M. Porphyromonas gingivalis, periodontal and systemic implications: A systematic review. Dent. J. 2019, 7, 114. [Google Scholar] [CrossRef]
- Nunes, J.M.; Fillis, T.; Page, M.J.; Venter, C.; Lancry, O.; Kell, D.B.; Windberger, U.; Pretorius, E. Gingipain R1 and lipopolysaccharide from Porphyromonas gingivalis have major effects on blood clot morphology and mechanics. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Katz, J.; Yang, Q.-B.; Zhang, P.; Potempa, J.; Travis, J.; Michalek, S.M.; Balkovetz, D.F. Hydrolysis of epithelial junctional proteins by Porphyromonas gingivalis gingipains. Infect. Immun. 2002, 70, 2512–2518. [Google Scholar] [CrossRef]
- Feghali, K.; Grenier, D. Priming effect of fibronectin fragments on the macrophage inflammatory response: Potential contribution to periodontitis. Inflammation 2012, 35, 1696–1705. [Google Scholar] [CrossRef]
- Lindemann, W.R.; Mijalis, A.J.; Alonso, J.L.; Borbat, P.P.; Freed, J.H.; Arnaout, M.A.; Pentelute, B.L.; Ortony, J.H. Conformational dynamics in extended RGD-containing peptides. Biomacromolecules 2020, 21, 2786–2794. [Google Scholar] [CrossRef]
- Sela, M.N.; Babitski, E.; Steinberg, D.; Kohavi, D.; Rosen, G. Degradation of collagen-guided tissue regeneration membranes by proteolytic enzymes of Porphyromonas gingivalis and its inhibition by antibacterial agents. Clin. Oral Implant. Res. 2009, 20, 496–502. [Google Scholar] [CrossRef]
- Mo, W.; Luo, H.; Wu, J.; Xu, N.; Zhang, F.; Qiu, Q.; Zhu, W.; Liang, M. Gingipains promote RANKL-induced osteoclastogenesis through the enhancement of integrin β3 in RAW264. 7 cells. J. Mol. Histol. 2020, 51, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Hu, C.-C.; Wu, Y.-Y.; Ueng, S.W.; Chang, C.-H.; Chen, M.-F. Ibudilast Mitigates Delayed Bone Healing Caused by Lipopolysaccharide by Altering Osteoblast and Osteoclast Activity. Int. J. Mol. Sci. 2021, 22, 1169. [Google Scholar] [CrossRef] [PubMed]
- Okahashi, N.; Inaba, H.; Nakagawa, I.; Yamamura, T.; Kuboniwa, M.; Nakayama, K.; Hamada, S.; Amano, A. Porphyromonas gingivalis induces receptor activator of NF-κB ligand expression in osteoblasts through the activator protein 1 pathway. Infect. Immun. 2004, 72, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Miyamoto, Y.; Yoshimura, K.; Yamada, A.; Takami, M.; Suzawa, T.; Hoshino, M.; Imamura, T.; Akiyama, C.; Yasuhara, R. Porphyromonas gingivalis-derived lysine gingipain enhances osteoclast differentiation induced by tumor necrosis factor-α and interleukin-1β but suppresses that by interleukin-17A: Importance of proteolytic degradation of osteoprotegerin by lysine gingipain. J. Biol. Chem. 2014, 289, 15621–15630. [Google Scholar] [CrossRef] [PubMed]
- Tamashunas, A.C.; Katiyar, A.; Zhang, Q.; Purkayastha, P.; Singh, P.K.; Chukkapalli, S.S.; Lele, T.P. Osteoprotegerin is sensitive to actomyosin tension in human periodontal ligament fibroblasts. J. Cell. Physiol. 2021, 236, 5715–5724. [Google Scholar] [CrossRef]
- Bergsma, A.; Ganguly, S.S.; Wiegand, M.E.; Dick, D.; Williams, B.O.; Miranti, C.K. Regulation of cytoskeleton and adhesion signaling in osteoclasts by tetraspanin CD82. Bone Rep. 2019, 10, 100196. [Google Scholar] [CrossRef]
- Hočevar, K.; Vizovišek, M.; Wong, A.; Kozieł, J.; Fonović, M.; Potempa, B.; Lamont, R.J.; Potempa, J.; Turk, B. Proteolysis of Gingival Keratinocyte Cell Surface Proteins by Gingipains Secreted from Porphyromonas gingivalis–Proteomic Insights Into Mechanisms Behind Tissue Damage in the Diseased Gingiva. Front. Microbiol. 2020, 11, 722. [Google Scholar] [CrossRef]
- Ruggiero, S.; Cosgarea, R.; Potempa, J.; Potempa, B.; Eick, S.; Chiquet, M. Cleavage of extracellular matrix in periodontitis: Gingipains differentially affect cell adhesion activities of fibronectin and tenascin-C. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2013, 1832, 517–526. [Google Scholar] [CrossRef]
- Qiu, Q.; Zhang, F.; Wu, J.; Xu, N.; Liang, M. Gingipains disrupt F-actin and cause osteoblast apoptosis via integrin β1. J. Periodontal Res. 2018, 53, 762–776. [Google Scholar] [CrossRef]
- Aliko, A.; Kamińska, M.; Bergum, B.; Gawron, K.; Benedyk, M.; Lamont, R.J.; Malicki, S.; Delaleu, N.; Potempa, J.; Mydel, P. Impact of Porphyromonas gingivalis peptidylarginine deiminase on bacterial biofilm formation, epithelial cell invasion, and epithelial cell transcriptional landscape. Sci. Rep. 2018, 8, 14144. [Google Scholar] [CrossRef]
- Kinane, J.A.; Benakanakere, M.R.; Zhao, J.; Hosur, K.B.; Kinane, D.F. Porphyromonas gingivalis influences actin degradation within epithelial cells during invasion and apoptosis. Cell. Microbiol. 2012, 14, 1085–1096. [Google Scholar] [CrossRef]
- Sheets, S.M.; Potempa, J.; Travis, J.; Fletcher, H.M.; Casiano, C.A. Gingipains from Porphyromonas gingivalis W83 synergistically disrupt endothelial cell adhesion and can induce caspase-independent apoptosis. Infect. Immun. 2006, 74, 5667–5678. [Google Scholar] [CrossRef]
- Bugueno, I.M.; Batool, F.; Keller, L.; Kuchler-Bopp, S.; Benkirane-Jessel, N.; Huck, O. Porphyromonas gingivalis bypasses epithelial barrier and modulates fibroblastic inflammatory response in an in vitro 3D spheroid model. Sci. Rep. 2018, 8, 14914. [Google Scholar] [CrossRef]
- Takeuchi, H.; Sasaki, N.; Yamaga, S.; Kuboniwa, M.; Matsusaki, M.; Amano, A. Porphyromonas gingivalis induces penetration of lipopolysaccharide and peptidoglycan through the gingival epithelium via degradation of junctional adhesion molecule 1. PLoS Pathog. 2019, 15, e1008124. [Google Scholar] [CrossRef]
- Eick, S.; Gadzo, N.; Tacchi, M.; Sculean, A.; Potempa, J.; Stavropoulos, A. Gingipains impair attachment of epithelial cell to dental titanium abutment surfaces. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2019, 107, 2549–2556. [Google Scholar] [CrossRef]
- Verrelli, D.I.; Albijanic, B. A comparison of methods for measuring the induction time for bubble–particle attachment. Miner. Eng. 2015, 80, 8–13. [Google Scholar] [CrossRef]
- Inaba, H.; Sugita, H.; Kuboniwa, M.; Iwai, S.; Hamada, M.; Noda, T.; Morisaki, I.; Lamont, R.J.; Amano, A. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of pro MMP 9 and its activation. Cell. Microbiol. 2014, 16, 131–145. [Google Scholar] [CrossRef]
- Abdulkareem, A.; Shelton, R.; Landini, G.; Cooper, P.; Milward, M. Potential role of periodontal pathogens in compromising epithelial barrier function by inducing epithelial-mesenchymal transition. J. Periodontal Res. 2018, 53, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Huck, O.; Mulhall, H.; Rubin, G.; Kizelnik, Z.; Iyer, R.; Perpich, J.D.; Haque, N.; Cani, P.D.; de Vos, W.M.; Amar, S. Akkermansia muciniphila reduces Porphyromonas gingivalis-induced inflammation and periodontal bone destruction. J. Clin. Periodontol. 2020, 47, 202–212. [Google Scholar] [CrossRef]
- Bhalla, U.S.; Iyengar, R. Emergent properties of networks of biological signaling pathways. Science 1999, 283, 381–387. [Google Scholar] [CrossRef]
- Antmen, E.; Demirci, U.; Hasirci, V. Micropatterned Surfaces Expose the Coupling between Actin Cytoskeleton-Lamin/Nesprin and Nuclear Deformability of Breast Cancer Cells with Different Malignancies. Adv. Biol. 2021, 5, 2000048. [Google Scholar] [CrossRef]
- Jung, Y.; Lee, S.-H.; Kim, S.-H.; Lim, J.C.; Kim, S.H. Synthesis and characterization of the biodegradable and elastic terpolymer poly (glycolide-co-L-lactide-co-ϵ-caprolactone) for mechano-active tissue engineering. J. Biomater. Sci. Polym. Ed. 2013, 24, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Hörner, M.; Raute, K.; Hummel, B.; Madl, J.; Creusen, G.; Thomas, O.S.; Christen, E.H.; Hotz, N.; Gübeli, R.J.; Engesser, R. Phytochrome-based extracellular matrix with reversibly tunable mechanical properties. Adv. Mater. 2019, 31, 1806727. [Google Scholar] [CrossRef] [PubMed]
- Kortsmit, J.; Davies, N.H.; Miller, R.; Macadangdang, J.R.; Zilla, P.; Franz, T. The effect of hydrogel injection on cardiac function and myocardial mechanics in a computational post-infarction model. Comput. Methods Biomech. Biomed. Eng. 2013, 16, 1185–1195. [Google Scholar] [CrossRef]
- Wu, B.; Fu, Y.; Shi, H.; Yan, B.; Lu, R.; Ma, S.; Markert, B. Tensile testing of the mechanical behavior of the human periodontal ligament. Biomed. Eng. Online 2018, 17, 172. [Google Scholar] [CrossRef]
- Uhlir, R.; Mayo, V.; Lin, P.H.; Chen, S.; Lee, Y.-T.; Hershey, G.; Lin, F.-C.; Ko, C.-C. Biomechanical characterization of the periodontal ligament: Orthodontic tooth movement. Angle Orthod. 2017, 87, 183–192. [Google Scholar] [CrossRef]
- Pfisterer, K.; Lumicisi, B.; Parsons, M. Imaging of Human Cancer Cells in 3D Collagen Matrices. Bio Protoc. 2021, 11, e3889. [Google Scholar] [CrossRef]
- Maeda, E.; Tsutsumi, T.; Kitamura, N.; Kurokawa, T.; Gong, J.P.; Yasuda, K.; Ohashi, T. Significant increase in Young’s modulus of ATDC5 cells during chondrogenic differentiation induced by PAMPS/PDMAAm double-network gel: Comparison with induction by insulin. J. Biomech. 2014, 47, 3408–3414. [Google Scholar] [CrossRef]
- Ettelt, V.; Belitsky, A.; Lehnert, M.; Loidl-Stahlhofen, A.; Epple, M.; Veith, M. Enhanced selective cellular proliferation by multi-biofunctionalization of medical implant surfaces with heterodimeric BMP-2/6, fibronectin, and FGF-2. J. Biomed. Mater. Res. Part. A 2018, 106, 2910–2922. [Google Scholar] [CrossRef]
- Doyle, A.D.; Carvajal, N.; Jin, A.; Matsumoto, K.; Yamada, K.M. Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat. Commun. 2015, 6, 8720. [Google Scholar] [CrossRef]
- Qi, L.; Shi, C.; Li, J.; Xu, S.; Han, Y.; Li, J.; Zhang, L. Yes-associated protein promotes cell migration via activating Wiskott-Aldrich syndrome protein family member 1 in oral squamous cell carcinoma. J. Oral Pathol. Med. 2019, 48, 290–298. [Google Scholar] [CrossRef]
- Wolf, K.; Te Lindert, M.; Krause, M.; Alexander, S.; Te Riet, J.; Willis, A.L.; Hoffman, R.M.; Figdor, C.G.; Weiss, S.J.; Friedl, P. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 2013, 201, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Babensee, J.E. Controlled Delivery of Immunomodulators from a Biomaterial Scaffold Niche to Induce a Tolerogenic Phenotype in Human Dendritic Cells. ACS Biomater. Sci. Eng. 2020, 6, 4062–4076. [Google Scholar] [CrossRef] [PubMed]
- Kara, A.; Koçtürk, S.; Bilici, G.; Havitcioglu, H. Development of biological meniscus scaffold: Decellularization method and recellularization with meniscal cell population derived from mesenchymal stem cells. J. Biomater. Appl. 2021, 0885328220981189. [Google Scholar] [CrossRef]
- Amler, A.-K.; Thomas, A.; Tüzüner, S.; Lam, T.; Geiger, M.-A.; Kreuder, A.-E.; Palmer, C.; Nahles, S.; Lauster, R.; Kloke, L. 3D bioprinting of tissue-specific osteoblasts and endothelial cells to model the human jawbone. Sci. Rep. 2021, 11, 4876. [Google Scholar] [CrossRef]
- Zeng, W.-Y.; Ning, Y.; Huang, X. Advanced technologies in periodontal tissue regeneration based on stem cells: Current status and future perspectives. J. Dent. Sci. 2020, 16, 501–507. [Google Scholar] [CrossRef]
- Lepsky, V.R.; Natan, S.; Tchaicheeyan, O.; Kolel, A.; Zussman, M.; Zilberman, M.; Lesman, A. FITC-Dextran Release from Cell-Embedded Fibrin Hydrogels. Biomolecules 2021, 11, 337. [Google Scholar] [CrossRef]
- Selig, M.; Lauer, J.C.; Hart, M.L.; Rolauffs, B. Mechanotransduction and Stiffness-Sensing: Mechanisms and Opportunities to Control Multiple Molecular Aspects of Cell Phenotype as a Design Cornerstone of Cell-Instructive Biomaterials for Articular Cartilage Repair. Int. J. Mol. Sci. 2020, 21, 5399. [Google Scholar] [CrossRef]
- Ayuningtyas, F.D.; Kim, M.-H.; Kino-Oka, M. Muscle lineage switching by migratory behaviour-driven epigenetic modifications of human mesenchymal stem cells on a dendrimer-immobilized surface. Acta Biomater. 2020, 106, 170–180. [Google Scholar] [CrossRef]
- Merrett, K.; Wan, F.; Lee, C.-J.; Harden, J.L. Enhanced Collagen-like Protein for Facile Biomaterial Fabrication. ACS Biomater. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Guo, J.; Kim, Y.; Xie, V.; Smith, B.; Watson, E.; Lam, J.; Pearce, H.; Engel, P.; Mikos, A. Modular, tissue-specific, and biodegradable hydrogel cross-linkers for tissue engineering. Sci. Adv. 2019, 5, eaaw7396. [Google Scholar] [CrossRef]
- Guo, J.L.; Li, A.; Kim, Y.S.; Xie, V.Y.; Smith, B.T.; Watson, E.; Bao, G.; Mikos, A.G. Click functionalized, tissue-specific hydrogels for osteochondral tissue engineering. J. Biomed. Mater. Res. Part. A 2020, 108, 684–693. [Google Scholar] [CrossRef]
- Yuan, Y.; Loh, Y.-h.E.; Han, X.; Feng, J.; Ho, T.-V.; He, J.; Jing, J.; Groff, K.; Wu, A.; Chai, Y. Spatiotemporal cellular movement and fate decisions during first pharyngeal arch morphogenesis. Sci. Adv. 2020, 6, eabb0119. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable polymeric drug delivery devices: Classification, manufacture, materials, and clinical applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef]
- Hernández Martinez, V.M.; Garcia Benavides, L.; Totsuka Sutto, S.E.; Cardona Muñoz, E.G.; Campos Bayardo, T.I.; Pascoe Gonzalez, S. Effectiveness of degradable and non-degradable implants to close large septal perforations in an experimental model. J. Plast. Surg. Hand Surg. 2016, 50, 222–226. [Google Scholar] [CrossRef]
- Colaris, M.J.; de Boer, M.; van der Hulst, R.R.; Tervaert, J.W.C. Two hundreds cases of ASIA syndrome following silicone implants: A comparative study of 30 years and a review of current literature. Immunol. Res. 2017, 65, 120–128. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.Y.; Jeon, W.-J.; Lee, J.; Ha, I.-H. Evaluation of the effects of differences in silicone hardness on rat model of lumbar spinal stenosis. PLoS ONE 2021, 16, e0251464. [Google Scholar] [CrossRef]
- Sharma, S.; Mandhani, A.; Bose, S.; Basu, B. Dynamically crosslinked polydimethylsiloxane-based polyurethanes with contact-killing antimicrobial properties as implantable alloplasts for urological reconstruction. Acta Biomater. 2021. [Google Scholar] [CrossRef]
- Valipour, F.; Valipour, F.; Rahbarghazi, R.; Navali, A.M.; Rashidi, M.R.; Davaran, S. Novel hybrid polyester-polyacrylate hydrogels enriched with platelet-derived growth factor for chondrogenic differentiation of adipose-derived mesenchymal stem cells in vitro. J. Biol. Eng. 2021, 15, 6. [Google Scholar] [CrossRef]
- Khandaker, M.; Kotturi, H.; Progri, H.; Tummala, S.; Nikfarjam, S.; Rao, P.; Hosna, A.U.; Arasu, D.T.; Williams, W.; Haleem, A. In vitro and in vivo effect of polycaprolactone nanofiber coating on polyethylene glycol diacrylate scaffolds for intervertebral disc repair. Biomed. Mater. 2021. [Google Scholar] [CrossRef]
- Milojević, M.; Harih, G.; Vihar, B.; Vajda, J.; Gradišnik, L.; Zidarič, T.; Stana Kleinschek, K.; Maver, U.; Maver, T. Hybrid 3D Printing of Advanced Hydrogel-Based Wound Dressings with Tailorable Properties. Pharmaceutics 2021, 13, 564. [Google Scholar] [CrossRef]
- Ferrández-Montero, A.; Eguiluz, A.; Vazquez, E.; Guerrero, J.D.; Gonzalez, Z.; Sanchez-Herencia, A.J.; Ferrari, B. Controlled SrR Delivery by the Incorporation of Mg Particles on Biodegradable PLA-Based Composites. Polymers 2021, 13, 1061. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Zhang, H.; Tang, Z.; Cui, X.; Li, X.; Liang, J.; Wang, Q.; Fan, Y.; Zhang, X. Solubilized Cartilage ECM Facilitates the Recruitment and Chondrogenesis of Endogenous BMSCs in Collagen Scaffolds for Enhancing Microfracture Treatment. ACS Appl. Mater. Interfaces 2021. [Google Scholar] [CrossRef]
- Rahoui, N.; Jiang, B.; Taloub, N.; Huang, Y.D. Spatio-temporal control strategy of drug delivery systems based nano structures. J. Control. Release 2017, 255, 176–201. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Sareethammanuwat, M.; Apinyauppatham, K.; Boonyuen, S. Characteristics and biologic effects of thermosensitive quercetin-chitosan/collagen hydrogel on human periodontal ligament stem cells. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2021. [Google Scholar] [CrossRef]
- Johnson, A.; Kong, F.; Miao, S.; Lin, H.-T.V.; Thomas, S.; Huang, Y.-C.; Kong, Z.-L. Therapeutic effects of antibiotics loaded cellulose nanofiber and κ-carrageenan oligosaccharide composite hydrogels for periodontitis treatment. Sci. Rep. 2020, 10, 18037. [Google Scholar]
- Asparuhova, M.B.; Stähli, A.; Guldener, K.; Sculean, A. A Novel Volume-Stable Collagen Matrix Induces Changes in the Behavior of Primary Human Oral Fibroblasts, Periodontal Ligament, and Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 4051. [Google Scholar] [CrossRef]
- Chen, H.; Dai, Y.; Cui, J.; Yin, X.; Feng, W. Carbon Monoxide Releasing Molecule-3 Enhances Osteogenic Differentiation of Human Periodontal Ligament Stem Cells by Carbon Monoxide Release. Drug Des. Dev. Ther. 2021, 15, 1691. [Google Scholar] [CrossRef]
- Peng, W.; Ren, S.; Zhang, Y.; Fan, R.; Zhou, Y.; Li, L.; Xu, X.; Xu, Y. MgO Nanoparticles-Incorporated PCL/Gelatin-Derived Coaxial Electrospinning Nanocellulose Membranes for Periodontal Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 216. [Google Scholar] [CrossRef]
- Synytsya, A.; Poučková, P.; Zadinová, M.; Troshchynska, Y.; Štětina, J.; Synytsya, A.; Saloň, I.; Král, V. Hydrogels based on low-methoxyl amidated citrus pectin and flaxseed gum formulated with tripeptide glycyl-l-histidyl-l-lysine improve the healing of experimental cutting wounds in rats. Int. J. Biol. Macromol. 2020, 165, 3156–3168. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, J.; Zhao, L.; Deng, J.; Li, Q. A simvastatin-releasing scaffold with periodontal ligament stem cell sheets for periodontal regeneration. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800019900094. [Google Scholar] [CrossRef]
- Panzeri, S.; Arosio, D.; Gazzola, S.; Belvisi, L.; Civera, M.; Potenza, D.; Vasile, F.; Kemker, I.; Ertl, T.; Sewald, N. Cyclic RGD and isoDGR Integrin Ligands Containing cis-2-amino-1-cyclopentanecarboxylic (cis-β-ACPC) Scaffolds. Molecules 2020, 25, 5966. [Google Scholar] [CrossRef] [PubMed]
- Almonte-Becerril, M.; Gimeno-LLuch, I.; Villarroya, O.; Benito-Jardón, M.; Kouri, J.B.; Costell, M. Genetic abrogation of the fibronectin-α5β1 integrin interaction in articular cartilage aggravates osteoarthritis in mice. PLoS ONE 2018, 13, e0198559. [Google Scholar] [CrossRef] [PubMed]
- Massam-Wu, T.; Chiu, M.; Choudhury, R.; Chaudhry, S.S.; Baldwin, A.K.; McGovern, A.; Baldock, C.; Shuttleworth, C.A.; Kielty, C.M. Assembly of fibrillin microfibrils governs extracellular deposition of latent TGFβ. J. Cell Sci. 2010, 123, 3006–3018. [Google Scholar] [CrossRef] [PubMed]
- Khorolsuren, Z.; Lang, O.; Pallinger, E.; Foldes, A.; Szabolcs, G.G.; Varga, G.; Mezo, G.; Vag, J.; Kohidai, L. Functional and cell surface characteristics of periodontal ligament cells (PDLCs) on RGD-synthetic polypeptide conjugate coatings. J. Periodontal Res. 2020, 55, 713–723. [Google Scholar] [CrossRef]
- Matsugami, D.; Murakami, T.; Yoshida, W.; Imamura, K.; Bizenjima, T.; Seshima, F.; Saito, A. Treatment with functionalized designer self-assembling peptide hydrogels promotes healing of experimental periodontal defects. J. Periodontal Res. 2021, 56, 162–172. [Google Scholar] [CrossRef]
- Etemadi Sh, M.; Hsieh, N.-C.; Movahed Mohammadi, S.S.; Momeni, S.; Razavi, S.M.; Alizargar, J. Histological and Radiological Evaluation of Low-Intensity Pulsed Ultrasound Versus Whole Body Vibration on Healing of Mandibular Bone Defects in Rats. Medicina 2020, 56, 457. [Google Scholar] [CrossRef]
- Li, H.; Deng, Y.; Tan, M.; Feng, G.; Kuang, Y.; Li, J.; Song, J. Low-intensity pulsed ultrasound upregulates osteogenesis under inflammatory conditions in periodontal ligament stem cells through unfolded protein response. Stem Cell Res. Ther. 2020, 11, 215. [Google Scholar]
- Shobara, K.; Ogawa, T.; Shibamoto, A.; Miyashita, M.; Ito, A.; Sitalaksmi, R.M. Osteogenic effect of low-intensity pulsed ultrasound and whole-body vibration on peri-implant bone. An experimental in vivo study. Clin. Oral Implant. Res. 2021, 32, 641–650. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Y.; Wu, L.; Li, S. Effects of collimated and focused low-intensity pulsed ultrasound stimulation on the mandible repair in rabbits. Ann. Transl. Med. 2020, 8, 98. [Google Scholar] [CrossRef]
- Shimizu, T.; Fujita, N.; Tsuji-Tamura, K.; Kitagawa, Y.; Fujisawa, T.; Tamura, M.; Sato, M. Osteocytes as main responders to low-intensity pulsed ultrasound treatment during fracture healing. Sci. Rep. 2021, 11, 10298. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, Y.; Li, J.; Zhao, C.; Song, J. Low-intensity pulsed ultrasound promotes alveolar bone regeneration in a periodontal injury model. Ultrasonics 2018, 90, 166–172. [Google Scholar] [CrossRef]
- Li, H.; Zhou, J.; Zhu, M.; Ying, S.; Li, L.; Chen, D.; Li, J.; Song, J. Low-intensity pulsed ultrasound promotes the formation of periodontal ligament stem cell sheets and ectopic periodontal tissue regeneration. J. Biomed. Mater. Res. Part. A 2021, 109, 1101–1112. [Google Scholar] [CrossRef]
- Li, Y.; Sun, C.; Feng, G.; He, Y.; Li, J.; Song, J. Low-intensity pulsed ultrasound activates autophagy in periodontal ligament cells in the presence or absence of lipopolysaccharide. Arch. Oral Biol. 2020, 117, 104769. [Google Scholar] [CrossRef]
- Ying, S.; Tan, M.; Feng, G.; Kuang, Y.; Chen, D.; Li, J.; Song, J. Low-intensity Pulsed Ultrasound regulates alveolar bone homeostasis in experimental Periodontitis by diminishing Oxidative Stress. Theranostics 2020, 10, 9789. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, M.; Li, J.; Hu, B.; Jiang, D.; Huang, H.; Song, J. LIPUS inhibited the expression of inflammatory factors and promoted the osteogenic differentiation capacity of hPDLCs by inhibiting the NF-κB signaling pathway. J. Periodontal Res. 2020, 55, 125–140. [Google Scholar] [CrossRef]
- Alshihah, N.; Alhadlaq, A.; El-Bialy, T.; Aldahmash, A.; Bello, I. The effect of low intensity pulsed ultrasound on dentoalveolar structures during orthodontic force application in diabetic ex-vivo model. Arch. Oral Biol. 2020, 119, 104883. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, Y.; Xiong, Y.; Wang, B.; Guo, Y.; Gong, P.; Zhang, L. Low-intensity pulsed ultrasound improves osseointegration of dental implant in mice by inducing local neuronal production of αCGRP. Arch. Oral Biol. 2020, 115, 104736. [Google Scholar] [CrossRef]
- Callhoff, J.; Dietrich, T.; Chubrieva, M.; Klotsche, J.; Zink, A. A patient-reported questionnaire developed in a German early arthritis cohort to assess periodontitis in patients with rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 197. [Google Scholar] [CrossRef]
- Strassburg, S.; Caduc, M.; Stark, G.B.; Jedrusik, N.; Tomakidi, P.; Steinberg, T.; Simunovic, F.; Finkenzeller, G. In vivo evaluation of an electrospun gelatin nonwoven mat for regeneration of epithelial tissues. J. Biomed. Mater. Res. Part A 2019, 107, 1605–1614. [Google Scholar] [CrossRef]
- Schulz, S.; Angarano, M.; Fabritius, M.; Mülhaupt, R.; Dard, M.; Obrecht, M.; Tomakidi, P.; Steinberg, T. Nonwoven-based gelatin/polycaprolactone membrane proves suitability in a preclinical assessment for treatment of soft tissue defects. Tissue Eng. Part. A 2014, 20, 1935–1947. [Google Scholar] [CrossRef]
- Jedrusik, N.; Steinberg, T.; Husari, A.; Volk, L.; Wang, X.; Finkenzeller, G.; Strassburg, S.; Tomakidi, P. Gelatin nonwovens-based epithelial morphogenesis involves a signaling axis comprising EGF-receptor, MAP kinases ERK 1/2, and β1 integrin. J. Biomed. Mater. Res. Part. A 2019, 107, 663–677. [Google Scholar] [CrossRef]
- Jedrusik, N.; Meyen, C.; Finkenzeller, G.; Stark, G.B.; Meskath, S.; Schulz, S.D.; Steinberg, T.; Eberwein, P.; Strassburg, S.; Tomakidi, P. Nanofibered gelatin-based nonwoven elasticity promotes epithelial Histogenesis. Adv. Healthc. Mater. 2018, 7, 1700895. [Google Scholar] [CrossRef]
- Dunnwald, M.; Tomanek-Chalkley, A.; Alexandrunas, D.; Fishbaugh, J.; Bickenbach, J. Isolating a pure population of epidermal stem cells for use in tissue engineering. Exp. Dermatol. 2001, 10, 45–54. [Google Scholar] [CrossRef]
- Franzè, E.; Monteleone, I.; Laudisi, F.; Rizzo, A.; Dinallo, V.; Di Fusco, D.; Colantoni, A.; Ortenzi, A.; Giuffrida, P.; Di Carlo, S. Cadherin-11 is a Regulator of intestinal fibrosis. J. Crohn’s Colitis 2020, 14, 406–417. [Google Scholar] [CrossRef]
- Niu, C.; Yang, P.; Yao, B. Engineering Protease-Resistant and Highly Active Phytases. In Inositol Phosphates; Springer: New York, NY, USA, 2020; pp. 155–162. [Google Scholar]
- Frank, C.F.; Hostetter, M.K. Cleavage of E-cadherin: A mechanism for disruption of the intestinal epithelial barrier by Candida albicans. Transl. Res. 2007, 149, 211–222. [Google Scholar] [CrossRef]
- Oh, C.; Kim, H.J.; Kim, H.-M. Vitamin D maintains E-cadherin intercellular junctions by downregulating MMP-9 production in human gingival keratinocytes treated by TNF-α. J. Periodontal Implant. Sci. 2019, 49, 270. [Google Scholar] [CrossRef]
- Jin, C.; Lee, G.; Oh, C.; Kim, H.J.; Kim, H.-M. Substrate roughness induces the development of defective E-cadherin junctions in human gingival keratinocytes. J. Periodontal Implant. Sci. 2017, 47, 116. [Google Scholar] [CrossRef]
- Bischoff, M.C.; Lieb, S.; Renkawitz-Pohl, R.; Bogdan, S. Filopodia-based contact stimulation of cell migration drives tissue morphogenesis. Nat. Commun. 2021, 12, 791. [Google Scholar] [CrossRef] [PubMed]
- Dyberg, C.; Fransson, S.; Andonova, T.; Sveinbjörnsson, B.; Lännerholm-Palm, J.; Olsen, T.K.; Forsberg, D.; Herlenius, E.; Martinsson, T.; Brodin, B. Rho-associated kinase is a therapeutic target in neuroblastoma. Proc. Natl. Acad. Sci. USA 2017, 114, E6603–E6612. [Google Scholar] [CrossRef]
- Macks, C.; Jeong, D.; Lee, J.S. Local delivery of RhoA siRNA by PgP nanocarrier reduces inflammatory response and improves neuronal cell survival in a rat TBI model. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102343. [Google Scholar] [CrossRef]
- Fogli, S.; Stefanelli, F.; Battolla, B.; Bianchi, F.; Breschi, M.C.; Mattii, L. Salbutamol inhibits RhoA activation in normal but not in desensitized bronchial smooth muscle cells. J. Pharm. Pharmacol. 2015, 67, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Sato-Ohira, S.; Tanihara, H.; Inoue, T. RhoA activation decreases phagocytosis of trabecular meshwork cells. Curr. Eye Res. 2020, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Goto, R.; Nishida, E.; Kobayashi, S.; Aino, M.; Ohno, T.; Iwamura, Y.; Kikuchi, T.; Hayashi, J.-i.; Yamamoto, G.; Asakura, M. Gelatin Methacryloyl–Riboflavin (GelMA–RF) Hydrogels for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 1635. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang, J.Y.; Ballance, W.C.; Sutton, B.P.; Shin, D.H.; Jang, K.H.; Shin, M.; Kong, H.; Kim, J.W. Fabrication of cell penetrating peptide-conjugated bacterial cellulose nanofibrils with remarkable skin adhesion and water retention performance. Int. J. Pharm. 2021, 600, 120476. [Google Scholar] [CrossRef] [PubMed]
- Tone, Y.; Mamchaoui, K.; Tsoumpra, M.K.; Hashimoto, Y.; Terada, R.; Maruyama, R.; Gait, M.J.; Arzumanov, A.A.; McClorey, G.; Imamura, M. Immortalized Canine Dystrophic Myoblast Cell Lines for Development of Peptide-Conjugated Splice-Switching Oligonucleotides. Nucleic Acid Ther. 2021, 31, 172–181. [Google Scholar] [CrossRef]
- Unkart, J.T.; Chen, S.L.; Wapnir, I.L.; González, J.E.; Harootunian, A.; Wallace, A.M. Intraoperative tumor detection using a ratiometric activatable fluorescent peptide: A first-in-human phase 1 study. Ann. Surg. Oncol. 2017, 24, 3167–3173. [Google Scholar] [CrossRef]
- Lulla, R.R.; Goldman, S.; Yamada, T.; Beattie, C.W.; Bressler, L.; Pacini, M.; Pollack, I.F.; Fisher, P.G.; Packer, R.J.; Dunkel, I.J. Phase I trial of p28 (NSC745104), a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in pediatric patients with recurrent or progressive central nervous system tumors: A Pediatric Brain Tumor Consortium Study. Neuro Oncol. 2016, 18, 1319–1325. [Google Scholar] [CrossRef]
- Zhang, G.; Dai, S.; Chen, Y.; Wang, H.; Chen, T.; Shu, Q.; Chen, S.; Shou, L.; Cai, X. Aqueous extract of Taxus chinensis var. mairei regulates the Hippo-YAP pathway and promotes apoptosis of non-small cell lung cancer via ATF3 in vivo and in vitro. Biomed. Pharmacother. 2021, 138, 111506. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Feng, X.; Tian, H.; Fu, X.; Gu, W.; Wen, Y. Metformin inhibits mTOR and c-Myc by decreasing YAP protein expression in OSCC cells. Oncol. Rep. 2021, 45, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.H.; Lai, T.Y.; Takahashi, K.; Wong, T.Y.; Chen, L.-J.; Ruamviboonsuk, P.; Tan, C.S.; Lee, W.K.; Cheung, C.M.G.; Ngah, N.F. Comparison of ranibizumab with or without Verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: The EVEREST II Randomized Clinical Trial. JAMA Ophthalmol. 2020, 138, 935–942. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, X.; Feng, W.; Yu, Y.; Jeong, K.; Guo, W.; Lu, Y.; Mills, G.B. Verteporfin inhibits YAP function through up-regulating 14-3-3σ sequestering YAP in the cytoplasm. Am. J. Cancer Res. 2016, 6, 27–37. [Google Scholar] [PubMed]
- Zhou, L.; Wang, Q.; Zhang, H.; Li, Y.; Xie, S.; Xu, M. YAP Inhibition by Nuciferine via AMPK-Mediated Downregulation of HMGCR Sensitizes Pancreatic Cancer Cells to Gemcitabine. Biomolecules 2019, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Cassino, T.R.; Drowley, L.; Okada, M.; Beckman, S.A.; Keller, B.; Tobita, K.; LeDuc, P.R.; Huard, J. Mechanical loading of stem cells for improvement of transplantation outcome in a model of acute myocardial infarction: The role of loading history. Tissue Eng. Part A 2012, 18, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Götschi, T.; Schulz, N.; Snedeker, J.G.; Hanimann, J.; Franchi, M.V.; Spörri, J. Three-Dimensional Mapping of Shear Wave Velocity in Human Tendon: A Proof of Concept Study. Sensors 2021, 21, 1655. [Google Scholar] [CrossRef]
- Wang, Z.; Weng, Y.; Ishihara, Y.; Odagaki, N.; Hlaing, E.E.H.; Izawa, T.; Okamura, H.; Kamioka, H. Loading history changes the morphology and compressive force-induced expression of receptor activator of nuclear factor kappa B ligand/osteoprotegerin in MLO-Y4 osteocytes. PeerJ 2020, 8, e10244. [Google Scholar] [CrossRef]
- Bidan, C.M.; Fratzl, M.; Coullomb, A.; Moreau, P.; Lombard, A.H.; Wang, I.; Balland, M.; Boudou, T.; Dempsey, N.M.; Devillers, T. Magneto-active substrates for local mechanical stimulation of living cells. Sci. Rep. 2018, 8, 1464. [Google Scholar] [CrossRef]
- Kojima, T.; Husari, A.; Dieterle, M.P.; Fontaine, S.; Prucker, O.; Tomakidi, P.; Rühe, J. PnBA/PDMAA-Based Iron-Loaded Micropillars Allow for Discrete Cell Adhesion and Analysis of Actuation-Related Molecular Responses. Adv. Mater. Interfaces 2020, 7, 1901806. [Google Scholar] [CrossRef]
- Chatelet, M.; Afota, F.; Savoldelli, C. Review of bone graft and implant survival rate: A comparison between autogenous bone block versus guided bone regeneration. J. Stomatol. Oral Maxillofac. Surg. 2021. [Google Scholar] [CrossRef]
- Yu, D.; Huang, C.; Jiang, C.; Zhu, H. Features of a simvastatin-loaded multi-layered co-electrospun barrier membrane for guided bone regeneration. Exp. Ther. Med. 2021, 22, 713. [Google Scholar] [CrossRef]
- Porrelli, D.; Mardirossian, M.; Musciacchio, L.; Pacor, M.; Berton, F.; Crosera, M.; Turco, G. Antibacterial Electrospun Polycaprolactone Membranes Coated with Polysaccharides and Silver Nanoparticles for Guided Bone and Tissue Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 17255–17267. [Google Scholar] [CrossRef]
- De Souza Balbinot, G.; da Cunha Bahlis, E.A.; Visioli, F.; Leitune, V.C.B.; Soares, R.M.D.; Collares, F.M. Polybutylene-adipate-terephthalate and niobium-containing bioactive glasses composites: Development of barrier membranes with adjusted properties for guided bone regeneration. Mater. Sci. Eng. C 2021, 125, 112115. [Google Scholar] [CrossRef]
- Küçüktürkmen, B.; Öz, U.C.; Toptaş, M.; Devrim, B.; Saka, O.M.; Bilgili, H.; Deveci, M.S.; Ünsal, E.; Bozkır, A. Development of Zoledronic Acid Containing Biomaterials for Enhanced Guided Bone Regeneration. J. Pharm. Sci. 2021. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; Manzano-Moreno, F.J.; Toledano, M.; Osorio, R.; Medina-Castillo, A.L.; Costela-Ruiz, V.J.; Ruiz, C. Doxycycline-doped membranes induced osteogenic gene expression on osteoblastic cells. J. Dent. 2021, 109, 103676. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; Manzano-Moreno, F.J.; Toledano, M.; Medina-Castillo, A.L.; Costela-Ruiz, V.J.; Ruiz, C.; Osorio, R. Doxycycline-Doped Polymeric Membranes Induced Growth, Differentiation and Expression of Antigenic Phenotype Markers of Osteoblasts. Polymers 2021, 13, 1063. [Google Scholar] [CrossRef]
- Naenni, N.; Stucki, L.; Huesler, J.; Schneider, D.; Hämmerle, C.H.; Jung, R.E.; Thoma, D.S. Implants sites with concomitant bone regeneration using a resorbable or non-resorbable membrane result in stable marginal bone levels and similar profilometric outcomes over 5 years. Clin. Oral Implant. Res. 2021. [Google Scholar] [CrossRef]
- Li, J.; Yan, J.-F.; Wan, Q.-Q.; Shen, M.-J.; Ma, Y.-X.; Gu, J.-T.; Gao, P.; Tang, X.-Y.; Yu, F.; Chen, J.-H. Matrix stiffening by self-mineralizable guided bone regeneration. Acta Biomater. 2021, 125, 112–125. [Google Scholar] [CrossRef]
- Barajaa, M.A.; Nair, L.S.; Laurencin, C.T. Bioinspired scaffold designs for regenerating musculoskeletal tissue interfaces. Regen. Eng. Transl. Med. 2020, 6, 451–483. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, Y.; Yao, B.; Li, Z.; Song, W.; Wang, Y.; Duan, X.; Yuan, X.; Fu, X. Biophysical and Biochemical Cues of Biomaterials Guide Mesenchymal Stem Cell Behaviors. Front. Cell Dev. Biol. 2021, 9, 397. [Google Scholar]
- De Medeiros, G.; Kromm, D.; Balazs, B.; Norlin, N.; Günther, S.; Izquierdo, E.; Ronchi, P.; Komoto, S.; Krzic, U.; Schwab, Y. Cell and tissue manipulation with ultrashort infrared laser pulses in light-sheet microscopy. Sci. Rep. 2020, 10, 1942. [Google Scholar]
- Ma, Y.; Lin, M.; Huang, G.; Li, Y.; Wang, S.; Bai, G.; Lu, T.J.; Xu, F. 3D spatiotemporal mechanical microenvironment: A hydrogel-based platform for guiding stem cell fate. Adv. Mater. 2018, 30, 1705911. [Google Scholar] [CrossRef]
- Richter, B.; Hahn, V.; Bertels, S.; Claus, T.K.; Wegener, M.; Delaittre, G.; Barner-Kowollik, C.; Bastmeyer, M. Guiding cell attachment in 3D microscaffolds selectively functionalized with two distinct adhesion proteins. Adv. Mater. 2017, 29, 1604342. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Liu, C. The horizon of materiobiology: A perspective on material-guided cell behaviors and tissue engineering. Chem. Rev. 2017, 117, 4376–4421. [Google Scholar] [CrossRef]
- Kang, E.-S.; Kim, D.-S.; Suhito, I.R.; Lee, W.; Song, I.; Kim, T.-H. Two-dimensional material-based bionano platforms to control mesenchymal stem cell differentiation. Biomater. Res. 2018, 22, 10. [Google Scholar] [CrossRef]
- Suhito, I.R.; Han, Y.; Kim, D.-S.; Son, H.; Kim, T.-H. Effects of two-dimensional materials on human mesenchymal stem cell behaviors. Biochem. Biophys. Res. Commun. 2017, 493, 578–584. [Google Scholar] [CrossRef]
- Song, J.; Michas, C.; Chen, C.S.; White, A.E.; Grinstaff, M.W. Controlled Cell Alignment Using Two-Photon Direct Laser Writing-Patterned Hydrogels in 2D and 3D. Macromol. Biosci. 2021, 21, 2100051. [Google Scholar] [CrossRef]
- Cheng, D.; Jayne, R.K.; Tamborini, A.; Eyckmans, J.; White, A.E.; Chen, C.S. Studies of 3D directed cell migration enabled by direct laser writing of curved wave topography. Biofabrication 2019, 11, 021001. [Google Scholar] [CrossRef]
- Dietrich, M.; Le Roy, H.; Brückner, D.B.; Engelke, H.; Zantl, R.; Rädler, J.O.; Broedersz, C.P. Guiding 3D cell migration in deformed synthetic hydrogel microstructures. Soft Matter 2018, 14, 2816–2826. [Google Scholar] [CrossRef]
- Odde, D.J.; Renn, M.J. Laser-guided direct writing of living cells. Biotechnol. Bioeng. 2000, 67, 312–318. [Google Scholar] [CrossRef]
- KehrIbar, D.; Özgen, M.; YolbaŞ, S.; Yildirim, A.; Önalan, E.; ÇİftÇİ, O.; Özercan, İ.; Koca, S. The inhibition of Src kinase suppresses the production of matrix metalloproteinases in from synovial fibroblasts and inhibits MAPK and STATs pathways. Turk. J. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Darwish, I.A.; Khalil, N.Y.; Alsaif, N.A.; Herqash, R.N.; Sayed, A.Y.; Abdel-Rahman, H.M. Charge-Transfer Complex of Linifanib with 2, 3-dichloro-3, 5-dicyano-1, 4-benzoquinone: Synthesis, Spectroscopic Characterization, Computational Molecular Modelling and Application in the Development of Novel 96-microwell Spectrophotometric Assay. Drug Des. Dev. Ther. 2021, 15, 1167. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol. Res. 2019, 144, 19–50. [Google Scholar] [CrossRef] [PubMed]
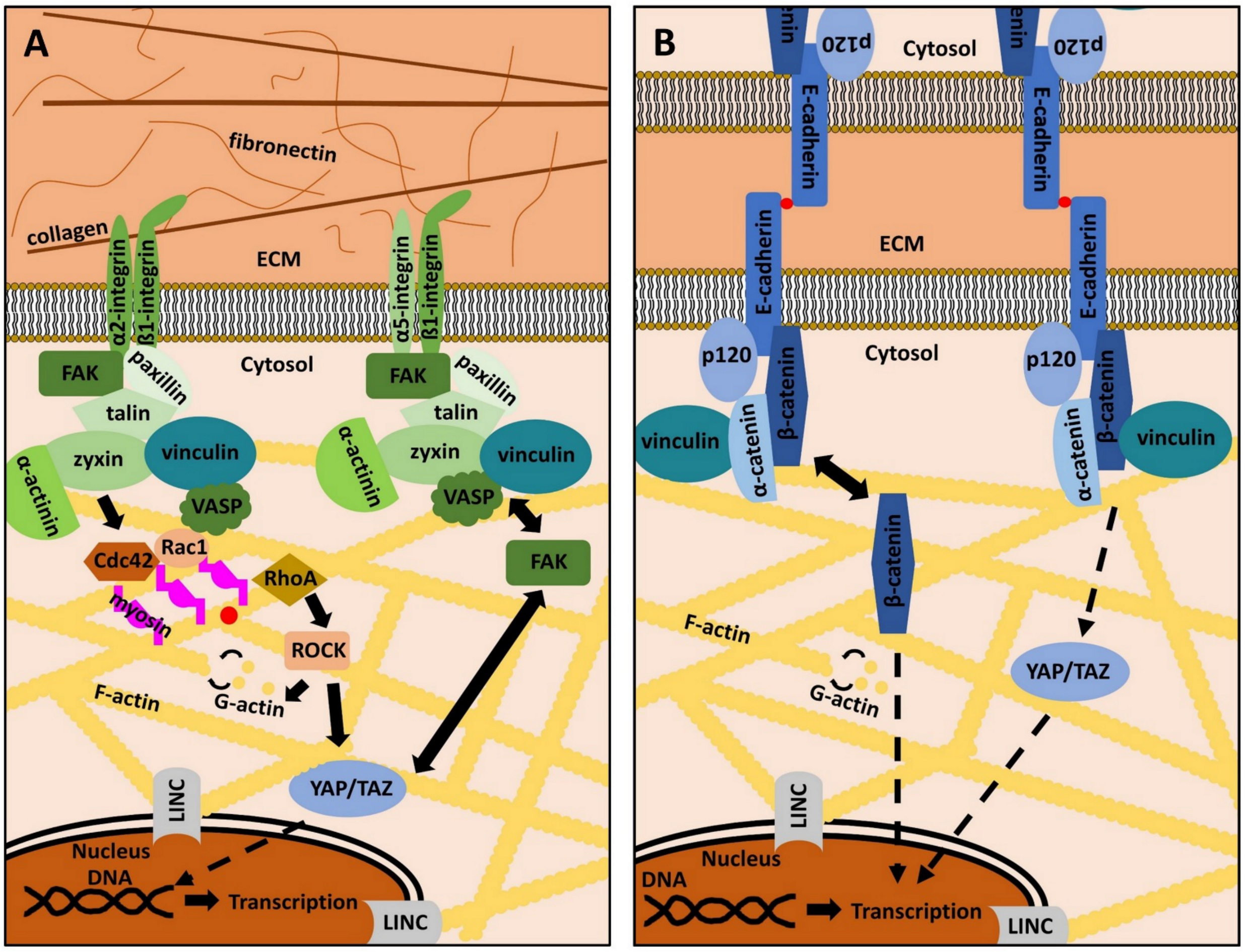
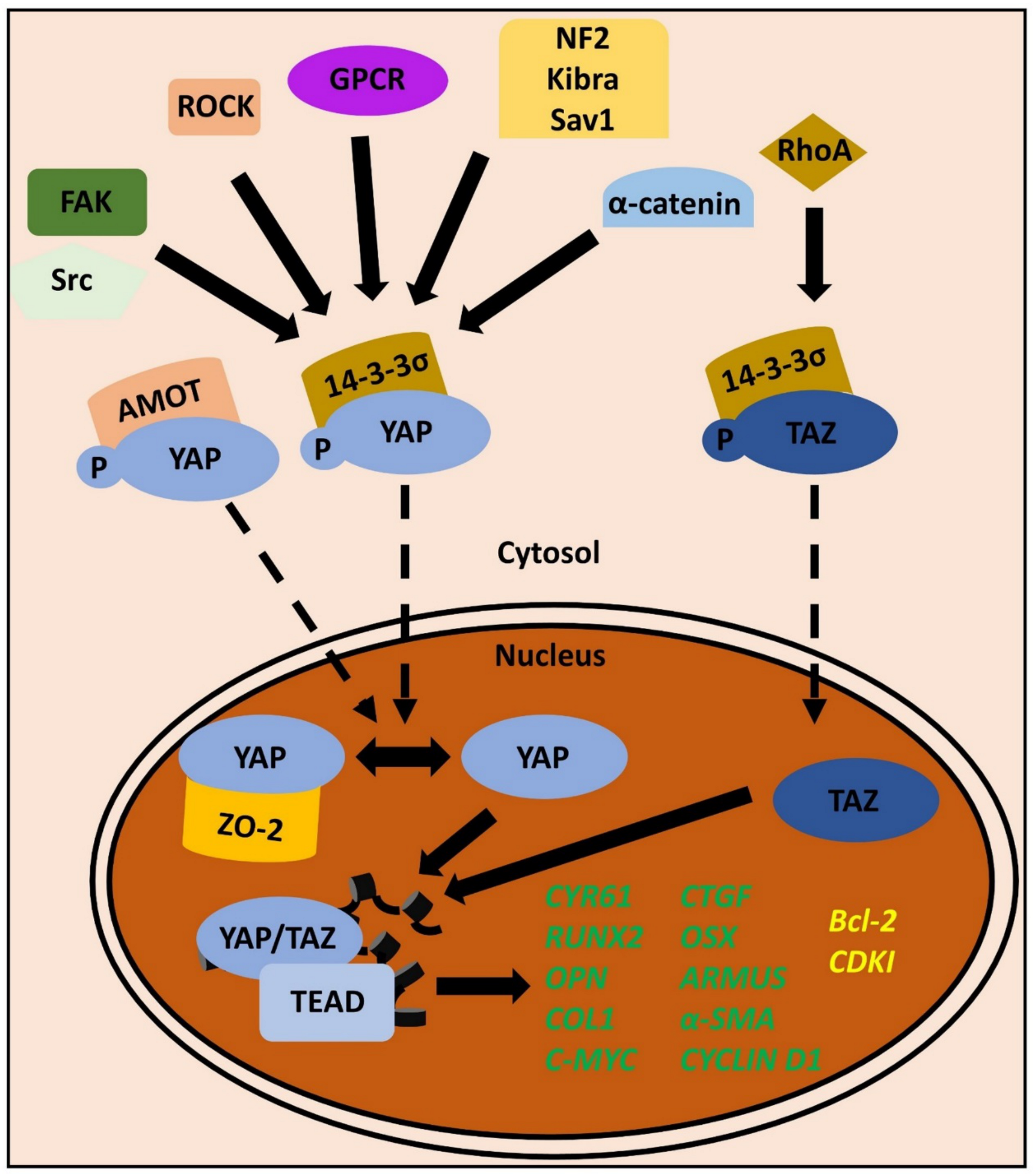
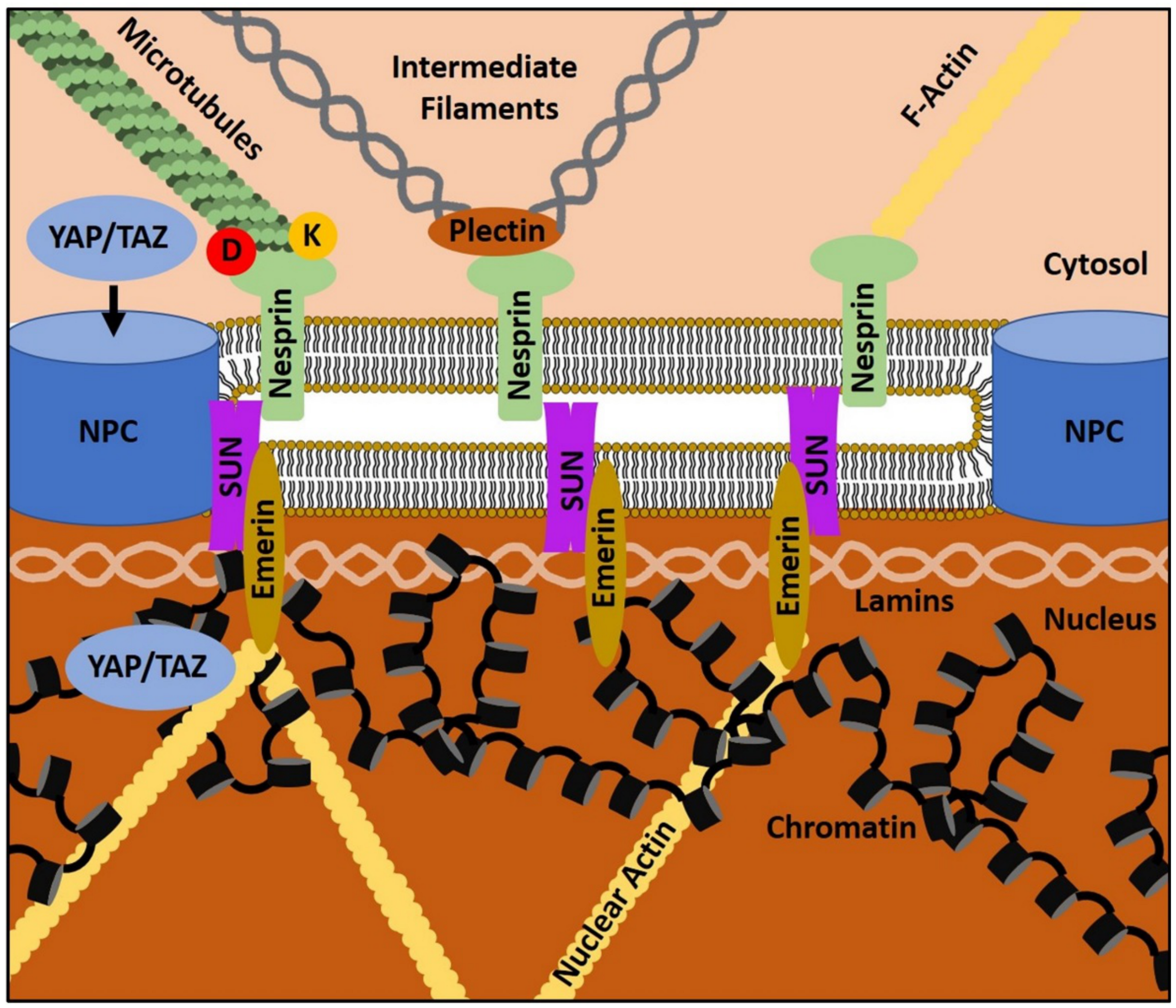
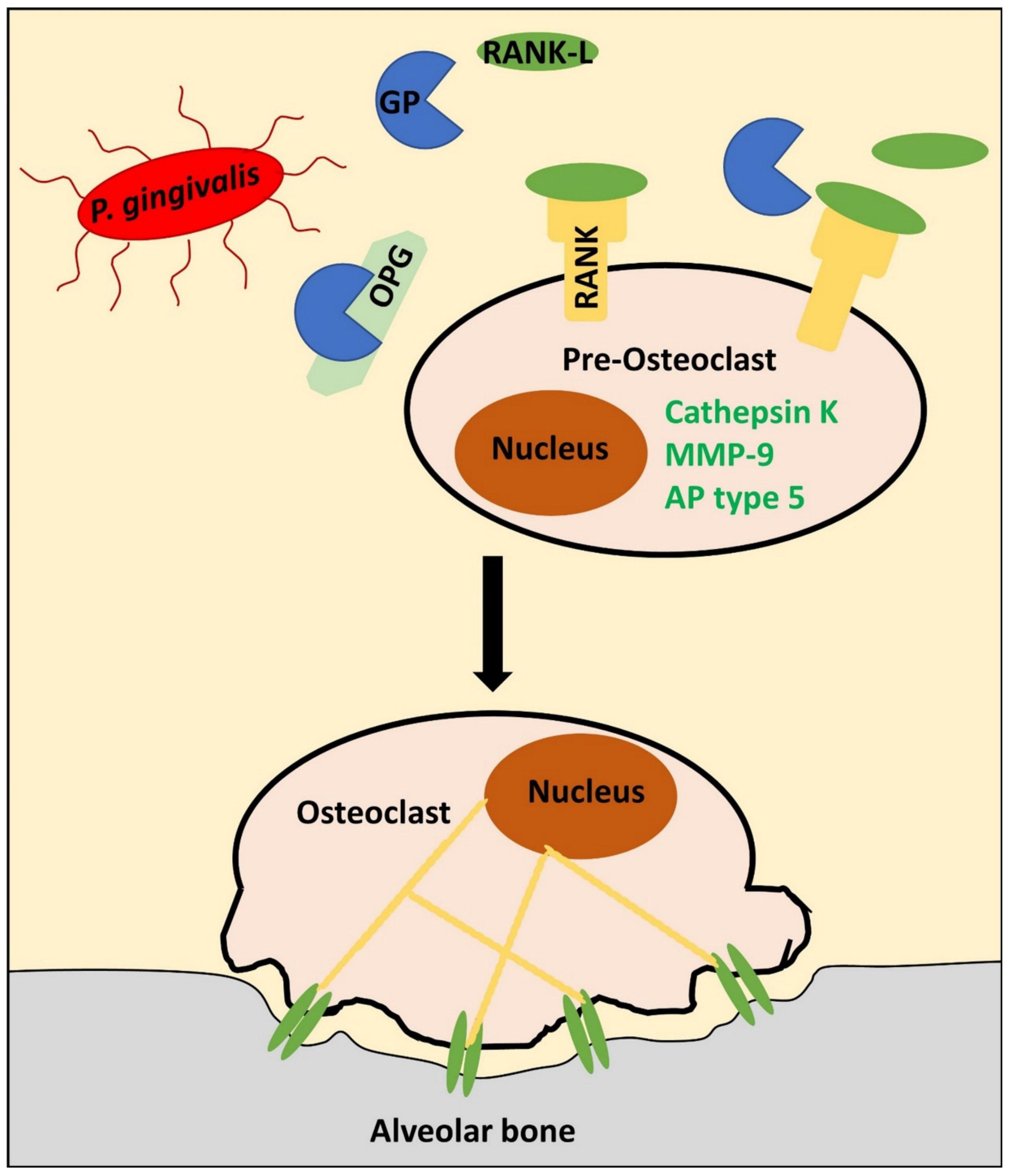
| Integrin Heterodimers | α1β1 | α2β1 | α3β1 | α5β1 | αVβ1 | αVβ3 | αVβ5 |
|---|---|---|---|---|---|---|---|
| Ligand(s) | Different collagens (e.g. type I) | Different collagens (e.g. type I) | laminins | fibronectin | fibronectin | fibronectin | vitronectin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieterle, M.P.; Husari, A.; Steinberg, T.; Wang, X.; Ramminger, I.; Tomakidi, P. From the Matrix to the Nucleus and Back: Mechanobiology in the Light of Health, Pathologies, and Regeneration of Oral Periodontal Tissues. Biomolecules 2021, 11, 824. https://doi.org/10.3390/biom11060824
Dieterle MP, Husari A, Steinberg T, Wang X, Ramminger I, Tomakidi P. From the Matrix to the Nucleus and Back: Mechanobiology in the Light of Health, Pathologies, and Regeneration of Oral Periodontal Tissues. Biomolecules. 2021; 11(6):824. https://doi.org/10.3390/biom11060824
Chicago/Turabian StyleDieterle, Martin Philipp, Ayman Husari, Thorsten Steinberg, Xiaoling Wang, Imke Ramminger, and Pascal Tomakidi. 2021. "From the Matrix to the Nucleus and Back: Mechanobiology in the Light of Health, Pathologies, and Regeneration of Oral Periodontal Tissues" Biomolecules 11, no. 6: 824. https://doi.org/10.3390/biom11060824
APA StyleDieterle, M. P., Husari, A., Steinberg, T., Wang, X., Ramminger, I., & Tomakidi, P. (2021). From the Matrix to the Nucleus and Back: Mechanobiology in the Light of Health, Pathologies, and Regeneration of Oral Periodontal Tissues. Biomolecules, 11(6), 824. https://doi.org/10.3390/biom11060824







