Integrated Proteomic and Metabolomic Analysis of the Testes Characterizes BDE-47-Induced Reproductive Toxicity in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
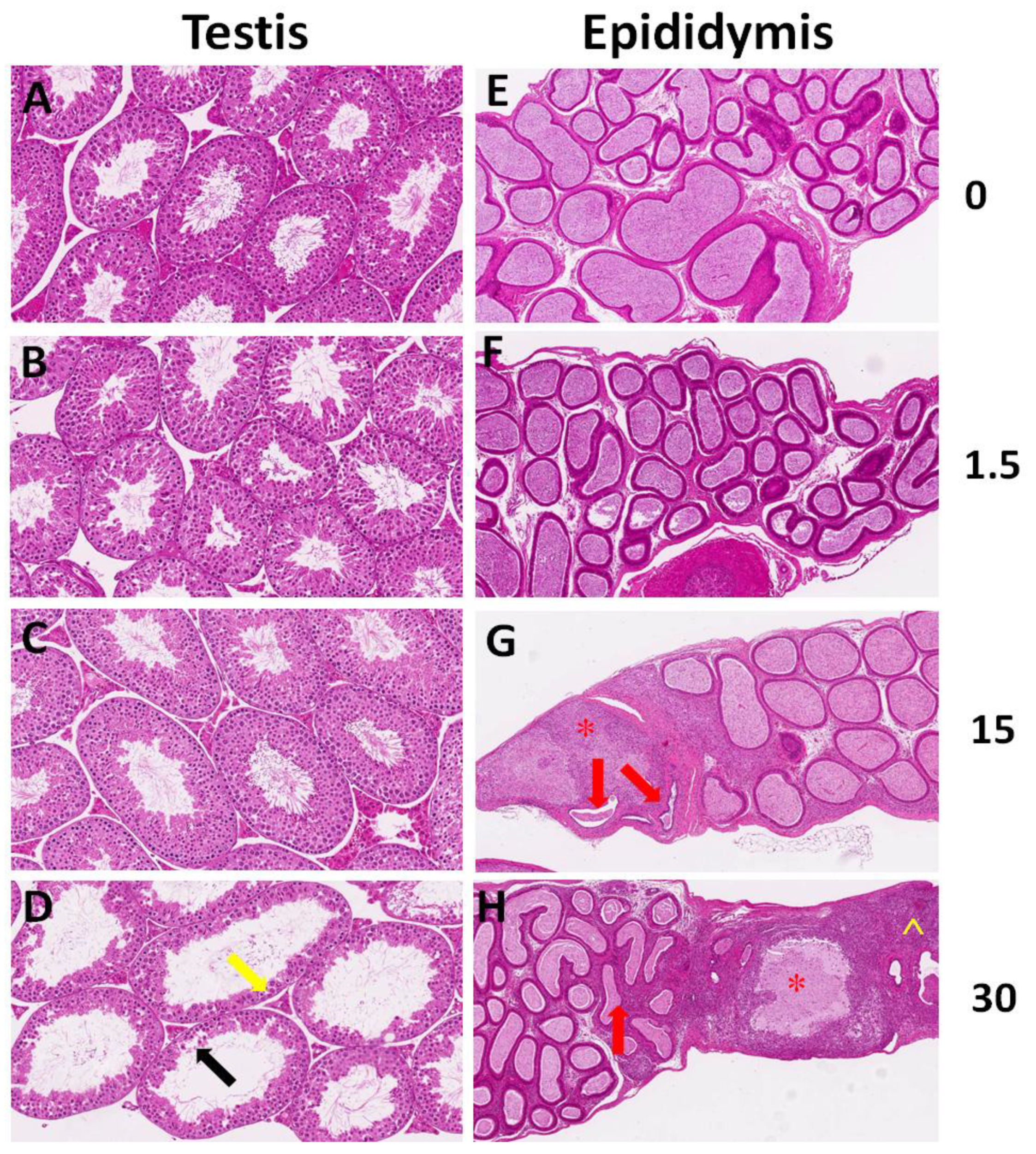
2.2. Histological Analysis
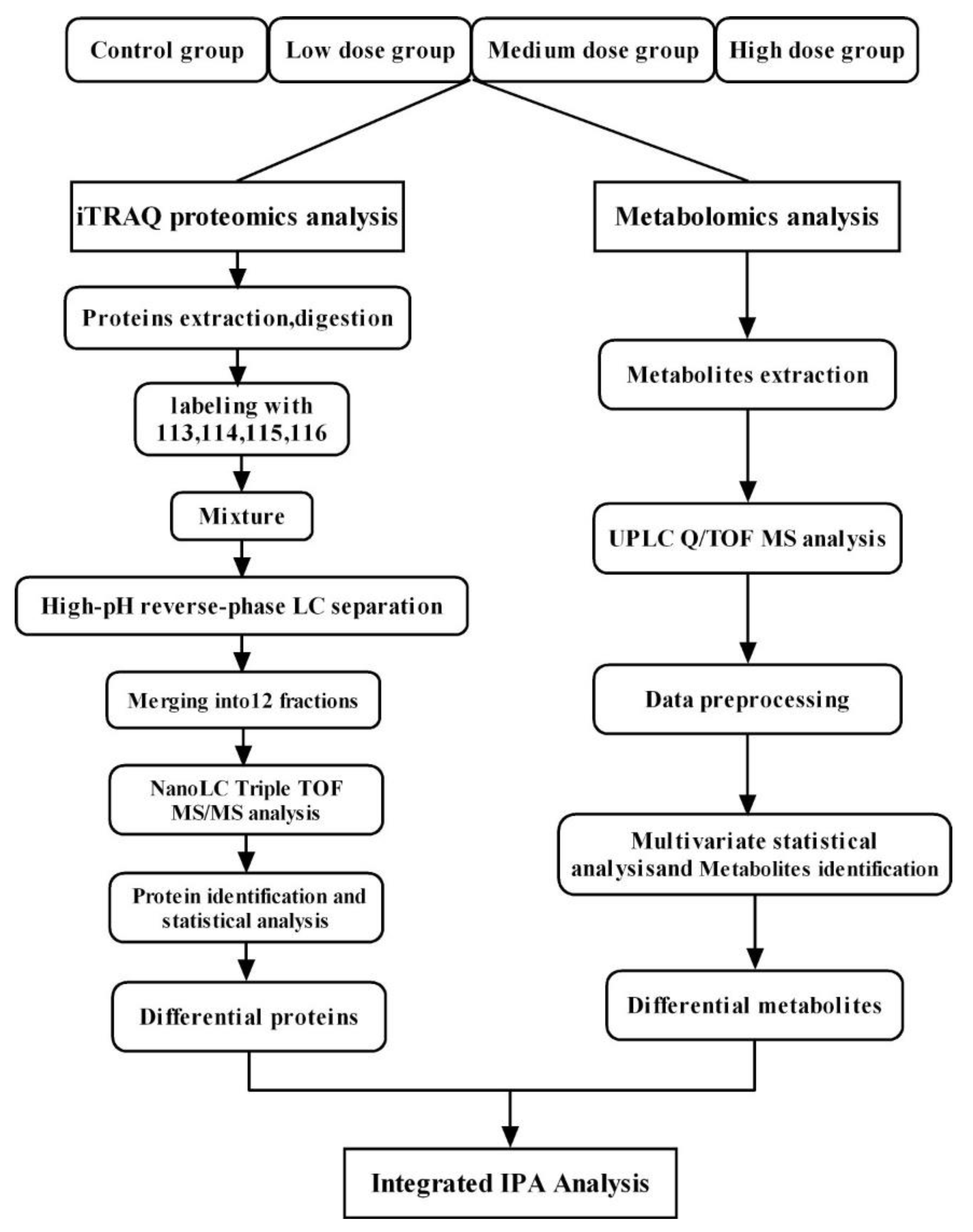
2.3. iTRAQ-Based Quantitative Proteomic Analysis
2.4. Metabolomic Analysis
2.5. Bioinformatics Analysis
2.6. Real-Time PCR
2.7. Statistical Analysis
3. Results
3.1. Histological Analysis
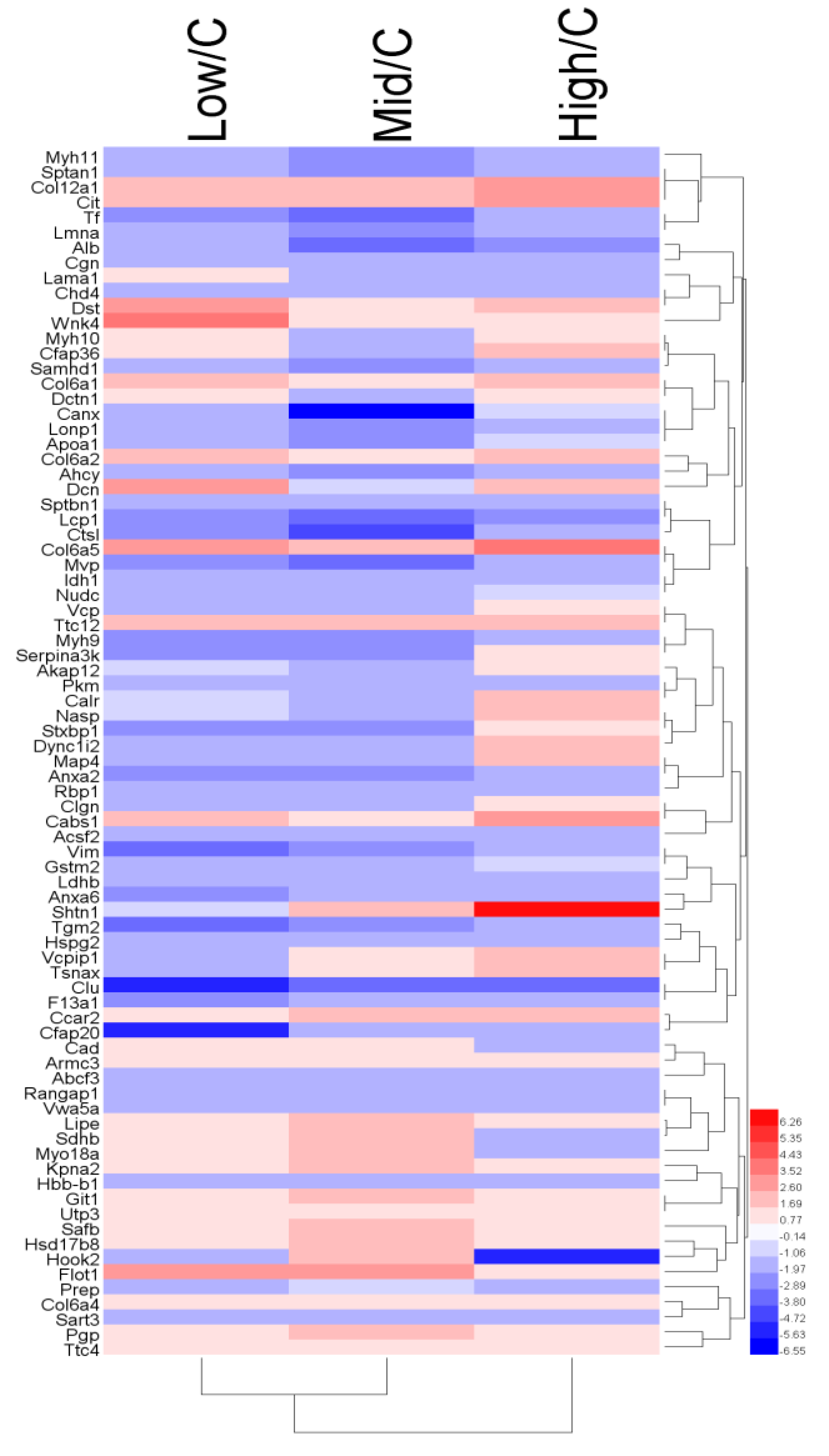
3.2. Proteome Analysis
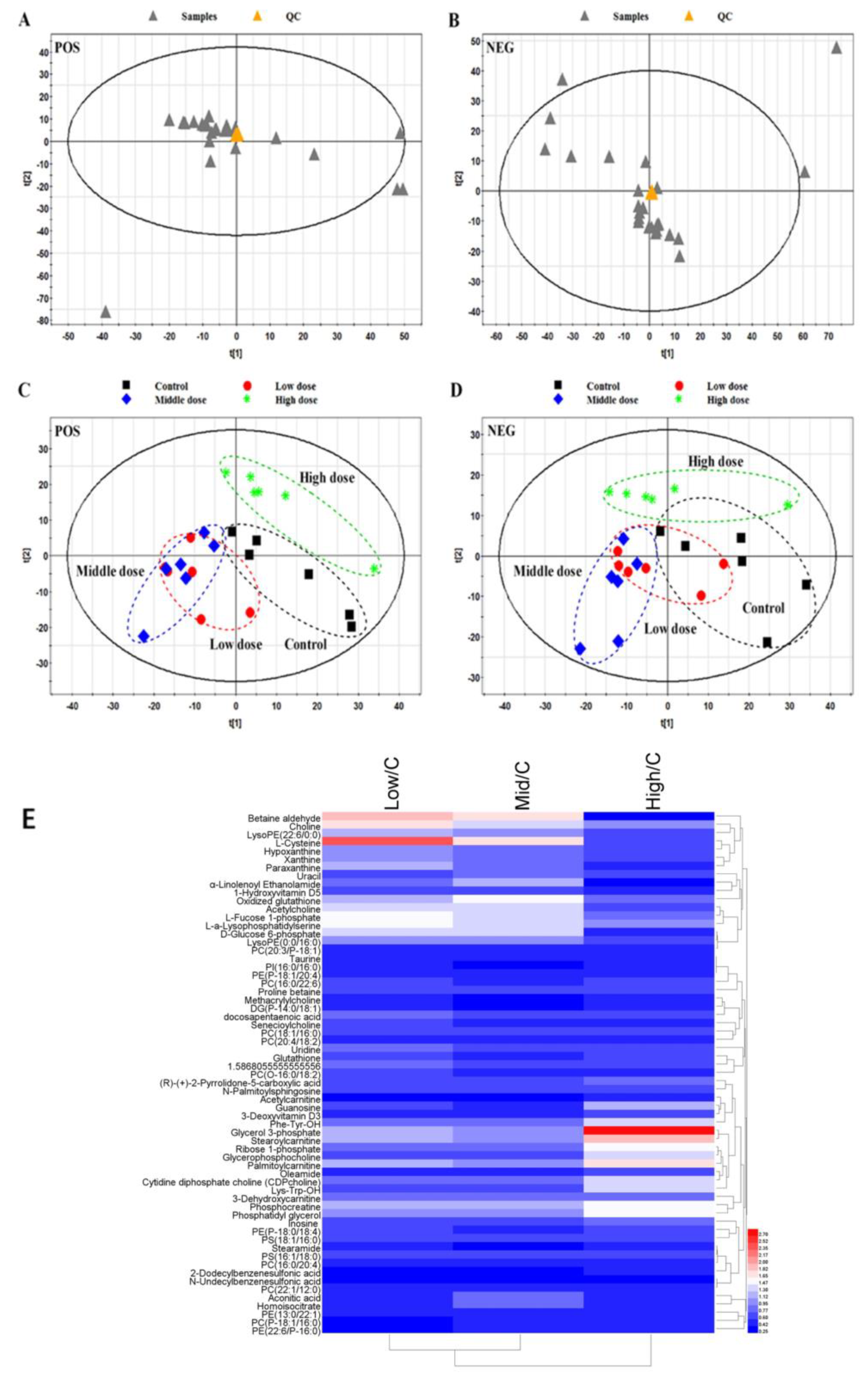
3.3. Metabolome Analysis
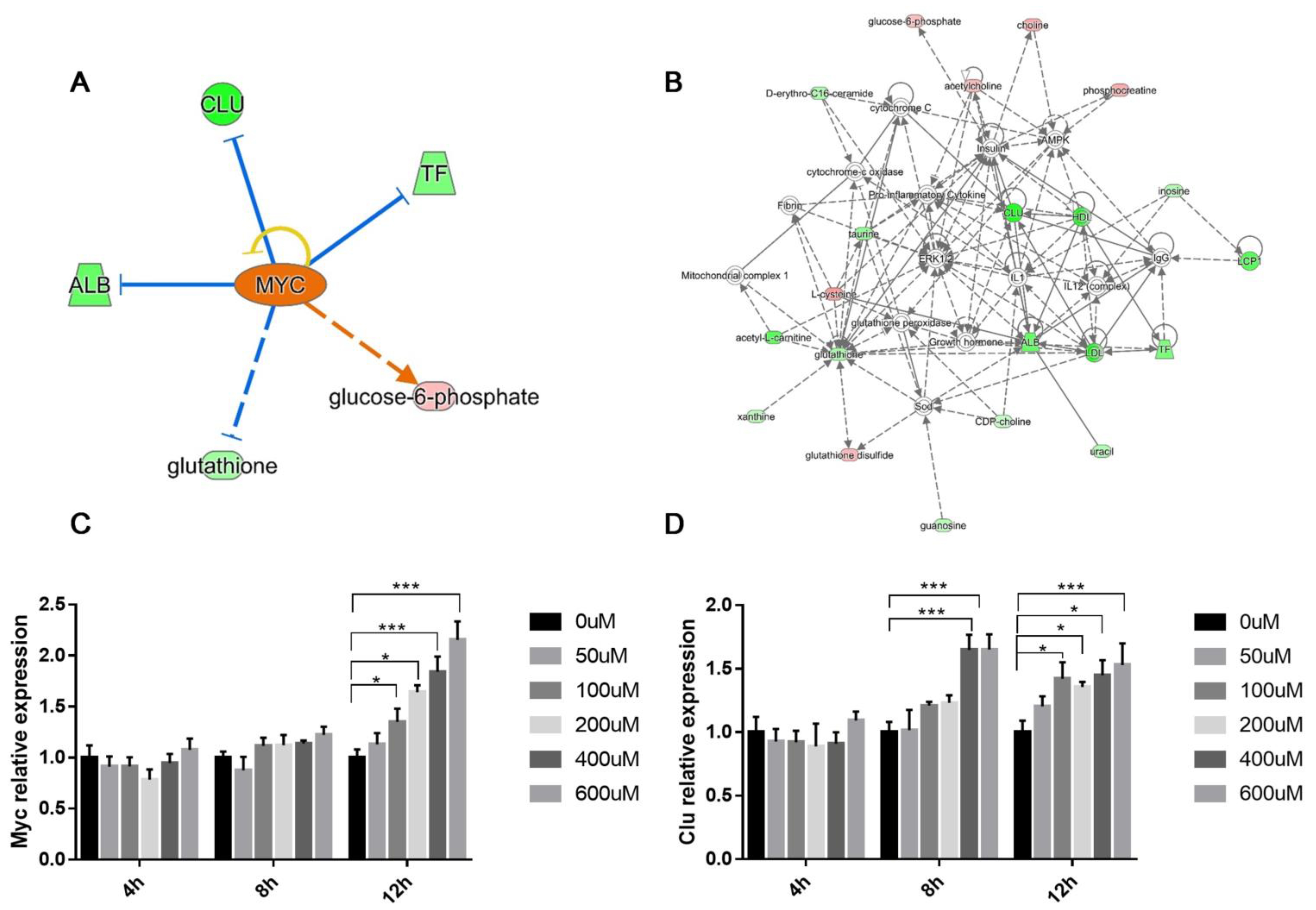
3.4. Integrated IPA Analysis and MYC-Activation in BDE-47-Induced Male Reproductive Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hites, R.A. Polybrominated Diphenyl Ethers in the Environment and in People: A Meta-Analysis of Concentrations. Environ. Sci. Technol. 2004, 38, 945–956. [Google Scholar] [CrossRef]
- Möller, A.; Xie, Z.; Sturm, R.; Ebinghaus, R. Polybrominated diphenyl ethers (PBDEs) and alternative brominated flame retardants in air and seawater of the European Arctic. Environ. Pollut. 2011, 159, 1577–1583. [Google Scholar] [CrossRef]
- McGrath, T.; Ball, A.S.; Clarke, B.O. Critical review of soil contamination by polybrominated diphenyl ethers (PBDEs) and novel brominated flame retardants (NBFRs); concentrations, sources and congener profiles. Environ. Pollut. 2017, 230, 741–757. [Google Scholar] [CrossRef]
- Klinčić, D.; Dvoršćak, M.; Jagić, K.; Mendaš, G.; Romanić, S.H. Levels and distribution of polybrominated diphenyl ethers in humans and environmental compartments: A comprehensive review of the last five years of research. Environ. Sci. Pollut. Res. 2020, 27, 5744–5758. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, C.; Han, W.; Song, J.; Li, H.; Zhang, Y.; Jing, X.; Wu, W. Exposure pathways, levels and toxicity of polybromin-ated diphenyl ethers in humans: A review. Environ. Res. 2020, 187, 109531. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Parker, M.; Brown, S.E.; Cevik, S.E.; Guo, L.W.; Jensen, J.; Olmsted, A.; Portman, D.; Wu, H.; Suvorov, A. Peri-natal exposure to 2,2′,4′,4′-Tetrabromodiphenyl ether induces testicular toxicity in adult rats. Toxicology 2017, 389, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.V.; Evans, R.M.; Faust, M.; Kortenkamp, A. A Human Mixture Risk Assessment for Neurodevelopmental Toxicity Associated with Polybrominated Diphenyl Ethers Used as Flame Retardants. Environ. Health Perspect. 2017, 125, 087016. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, S.; Wan, Y.; Chang, H.; Zhang, X.; Hecker, M.; Jones, P.D.; Giesy, J.P. Polybrominated diphenyl ethers and their hydroxylated/methoxylated analogs: Environmental sources, metabolic relationships, and relative toxicities. Mar. Pollut. Bull. 2011, 63, 179–188. [Google Scholar] [CrossRef]
- Watanabe, I.; Sakai, S.I. Environmental release and behavior of brominated flame retardants. Environ. Int. 2004, 29, 665–682. [Google Scholar] [CrossRef]
- Ji, K.; Choi, K.; Giesy, J.P.; Musarrat, J.; Takeda, S. Genotoxicity of Several Polybrominated Diphenyl Ethers (PBDEs) and Hydroxylated PBDEs, and Their Mechanisms of Toxicity. Environ. Sci. Technol. 2011, 45, 5003–5008. [Google Scholar] [CrossRef]
- Huang, S.C.; Giordano, G.; Costa, L.G. Comparative cytotoxicity and intracellular accumulation of five polybro-minated diphenyl ether congeners in mouse cerebellar granule neurons. Toxicol. Sci. 2010, 114, 124–132. [Google Scholar] [CrossRef]
- Abdelouahab, N.; AinMelk, Y.; Takser, L. Polybrominated diphenyl ethers and sperm quality. Reprod. Toxicol. 2011, 31, 546–550. [Google Scholar] [CrossRef]
- Harley, K.G.; Marks, A.R.; Chevrier, J.; Bradman, A.; Sjödin, A.; Eskenazi, B. PBDE concentrations in women’s serum and fecundability. Environ. Health Perspect. 2010, 118, 699–704. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Li, L.; Liu, D.; Li, L.; Tang, C.; Li, J. Adverse effects of 2, 2′, 4, 4′-tetrabromodiphenyl ether on se-men quality and spermatogenesis in male mice. Bull. Environ. Contam. Toxicol. 2013, 90, 51–54. [Google Scholar] [CrossRef]
- Huang, S.; Cui, Y.; Guo, X.; Wang, L.; Li, S.; Lu, Y.; Bi, Y.; Huang, X.; Lin, M.; Xia, Y.; et al. 2,2′,4,4′-Tetrabromodiphenyl ether disrupts spermatogenesis, impairs mitochondrial function and induces apoptosis of early leptotene spermatocytes in rats. Reprod. Toxicol. 2015, 51, 114–124. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, X.; Xu, A.; Tian, Y.; Wang, Y.; Zhang, Y.; Gu, Q.; Wang, S.; Wang, Z. Toxicity of polybrominated diphenyl ethers (PBDEs) on rodent male reproductive system: A systematic review and meta-analysis of randomized control studies. Sci. Total Environ. 2020, 720, 137419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, X.; Sun, Z.; Dong, H.; Qiu, L.; Gu, J.; Zhou, J.; Wang, X.; Wang, S.L. Cytochrome P450 3A1 medi-ates 2, 2′, 4, 4′-tetrabromodiphenyl ether-induced reduction of spermatogenesis in adult rats. PLoS ONE 2013, 8, e66301. [Google Scholar] [CrossRef]
- Zota, A.R.; Park, J.-S.; Wang, Y.; Petreas, M.; Zoeller, R.T.; Woodruff, T. Polybrominated Diphenyl Ethers, Hydroxylated Polybrominated Diphenyl Ethers, and Measures of Thyroid Function in Second Trimester Pregnant Women in California. Environ. Sci. Technol. 2011, 45, 7896–7905. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liu, C.; Chen, Q.; Zhou, B. En-docrine disruption and reproduction impairment in zebrafish after long-term exposure to DE-71. Environ. Toxicol. Chem. 2014, 33, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Tavazoie, S.; Hughes, J.D.; Campbell, M.J.; Cho, R.J.; Church, G.M. Systematic determination of genetic network architecture. Nat. Genet. 1999, 22, 281–285. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living sys-tems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef]
- Alexovič, M.; Urban, P.L.; Tabani, H.; Sabo, J. Recent advances in robotic protein sample preparation for clinical analysis and other biomedical applications. Clin. Chim. Acta 2020, 507, 104–116. [Google Scholar] [CrossRef]
- Aslam, B.; Basit, M.; Nisar, M.A.; Khurshid, M.; Rasool, M.H. Proteomics: Technologies and Their Applications. J. Chromatogr. Sci. 2017, 55, 182–196. [Google Scholar] [CrossRef]
- Rauniyar, N.; Yates, J.R., III. Isobaric labeling-based relative quantification in shotgun proteomics. J. Proteome Res. 2014, 13, 5293–5309. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef]
- Zhao, H.; Du, H.; Liu, M.; Gao, S.; Li, N.; Chao, Y.; Li, R.; Chen, W.; Lou, Z.; Dong, X. Integrative Proteomics–Metabolomics Strategy for Pathological Mechanism of Vascular Depression Mouse Model. J. Proteome Res. 2017, 17, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Marín, Á.; Collado-Romero, M.; Ramirez-Boo, M.; Arce, C.; Garrido, J.J. Biological pathway analysis by ArrayUnlock and Ingenuity Pathway Analysis. BMC Proc. 2009, 3, S6. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Wang, Y.; Liu, Z.; Cheng, H.; Xue, Y. HemI: A Toolkit for Illustrating Heatmaps. PLoS ONE 2014, 9, e111988. [Google Scholar] [CrossRef]
- Smetanová, S.; Riedl, J.; Zitzkat, D.; Altenburger, R.; Busch, W. High-throughput concentration-response analysis for omics datasets. Environ. Toxicol. Chem. 2015, 34, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yan, M.; Ge, R.; Cheng, C.Y. Crosstalk between Sertoli and Germ Cells in Male Fertility. Trends Mol. Med. 2020, 26, 215–231. [Google Scholar] [CrossRef]
- Miner, J.H.; Li, C.; Mudd, J.L.; Go, G.; Sutherland, A.E. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development 2004, 131, 2247–2256. [Google Scholar] [CrossRef]
- Camargo, M.; Intasqui, P.; Bertolla, R.P. Understanding the seminal plasma proteome and its role in male fertility. Basic Clin. Androl. 2018, 28, 1–12. [Google Scholar] [CrossRef]
- Uetani, N.; Yamaura, K.; Sato, K. Expression in situ of c-myc mRNA and c-myc protein during spermatogenesis in the adult mouse. Cell Biol. Int. 1994, 18, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.S.; Vilagrasa, X. Biphasic c-Myc protein expression during gossypol-induced apoptosis in rat spermatocytes. Contraception 1998, 57, 117–123. [Google Scholar] [CrossRef]
- Meyer, N.; Penn, L.Z. Reflecting on 25 years with MYC. Nat. Rev. Cancer 2008, 8, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, T.; Tao, Q.; Tan, W.; Chen, H.; Liu, W. Bta-miR-34b inhibits proliferation and promotes apoptosis via the MEK/ERK pathway by targeting MAP2K1 in bovine primary Sertoli cells. J. Anim. Sci. 2020, 98, skaa313. [Google Scholar] [CrossRef] [PubMed]
- Ran, M.; Luo, H.; Gao, H.; Tang, X.; Chen, Y.; Zeng, X.; Chen, B. miR-362 knock-down promotes proliferation and inhibits apoptosis in porcine immature Sertoli cells by targeting the RMI1 gene. Reprod. Domest. Anim. 2020, 55, 547–558. [Google Scholar] [CrossRef]
- Meroni, S.B.; Galardo, M.N.; Rindone, G.; Gorga, A.; Riera, M.F.; Cigorraga, S.B. Molecular mechanisms and signaling pathways involved in sertoli cell proliferation. Front. Endocrinol. 2019, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Tanaka, T.; Ogonuki, N.; Ogura, A.; Morimoto, H.; Cheng, P.F. Myc/Mycn-mediated glycolysis enhances mouse spermatogonial stem cell self-renewal. Genes Dev. 2016, 30, 2637–2648. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, A.; Buzzard, J.W.; Reddy, H.; Kang, M.H.; Rhee, D.J.; Pattabiraman, P.P. Regulation and role of clusterin, a secretory chaperone protein, on intraocular pressure. Investig. Ophthalmol. Vis. Sci. 2020, 61, 3208. [Google Scholar]
- Dere, E.; Spade, D.J.; Hall, S.J.; Altemus, A.; Smith, J.D.; Phillips, J.A.; Moffit, J.S.; Blanchard, K.T.; Boekelheide, K. Identification of sperm mRNA biomarkers associated with testis injury during preclinical testing of pharmaceutical compounds. Toxicol. Appl. Pharmacol. 2017, 320, 1–7. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Gao, S.; Zhao, H.; Wang, L.; Cao, Y.; Xi, J.; Zhang, X.; Dong, X.; Luan, Y. Integrated Proteomic and Metabolomic Analysis of the Testes Characterizes BDE-47-Induced Reproductive Toxicity in Mice. Biomolecules 2021, 11, 821. https://doi.org/10.3390/biom11060821
Xu L, Gao S, Zhao H, Wang L, Cao Y, Xi J, Zhang X, Dong X, Luan Y. Integrated Proteomic and Metabolomic Analysis of the Testes Characterizes BDE-47-Induced Reproductive Toxicity in Mice. Biomolecules. 2021; 11(6):821. https://doi.org/10.3390/biom11060821
Chicago/Turabian StyleXu, Liang, Songyan Gao, Hongxia Zhao, Liupeng Wang, Yiyi Cao, Jing Xi, Xinyu Zhang, Xin Dong, and Yang Luan. 2021. "Integrated Proteomic and Metabolomic Analysis of the Testes Characterizes BDE-47-Induced Reproductive Toxicity in Mice" Biomolecules 11, no. 6: 821. https://doi.org/10.3390/biom11060821
APA StyleXu, L., Gao, S., Zhao, H., Wang, L., Cao, Y., Xi, J., Zhang, X., Dong, X., & Luan, Y. (2021). Integrated Proteomic and Metabolomic Analysis of the Testes Characterizes BDE-47-Induced Reproductive Toxicity in Mice. Biomolecules, 11(6), 821. https://doi.org/10.3390/biom11060821






