Ultrasonic Synthesis and Biomedical Application of Mn0.5Zn0.5ErxYxFe2−2xO4 Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Nanoparticles
2.2. Anti-Colon Cancer Activity
2.2.1. In Vitro Cytotoxicity
2.2.2. DAPI Staining
2.3. Antifungal Activity
2.3.1. Minimal Fungicidal Concentration (MFC)
2.3.2. Effect of Ultrasonicated NPs on Germ Tube Formation
3. Results and Discussion
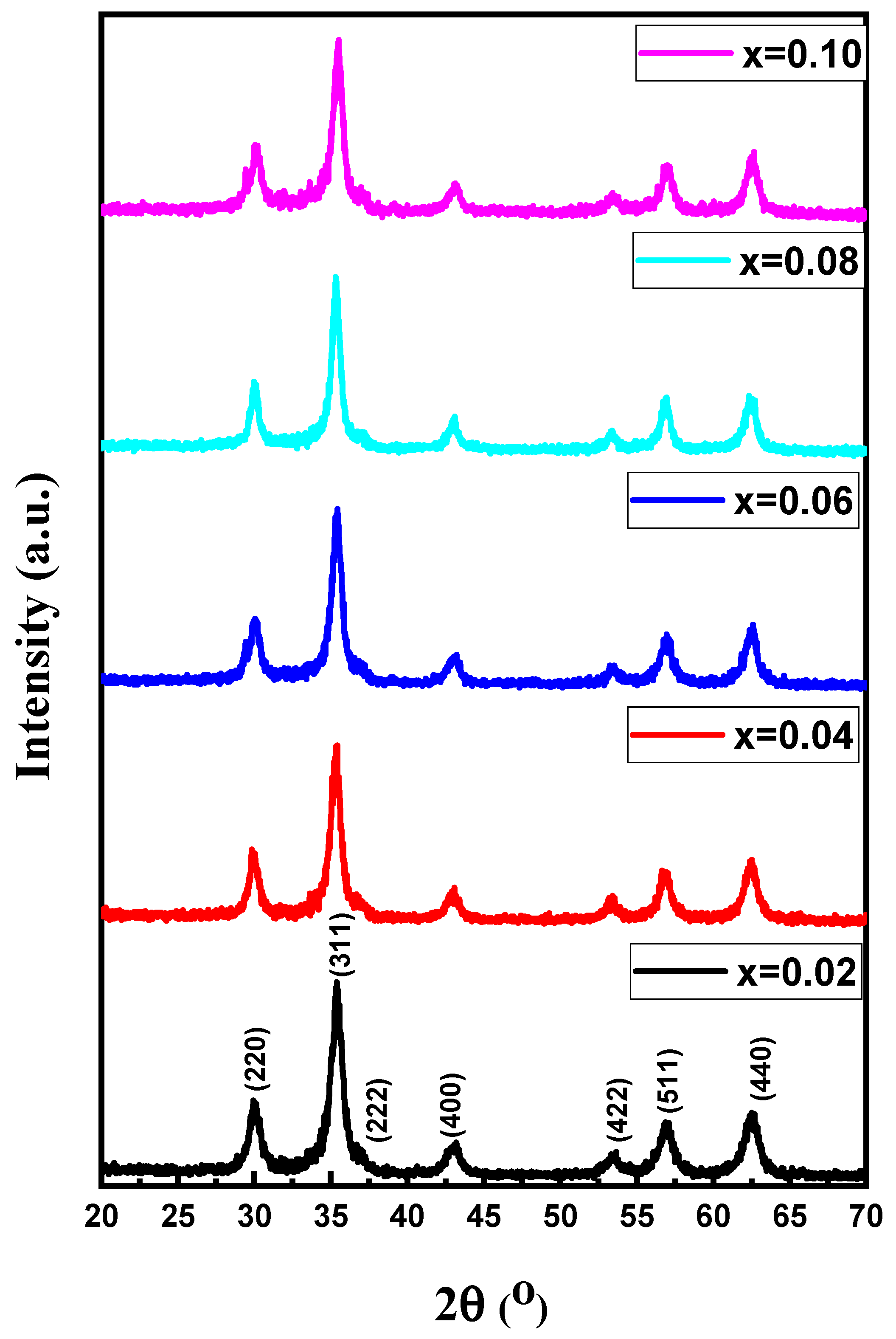
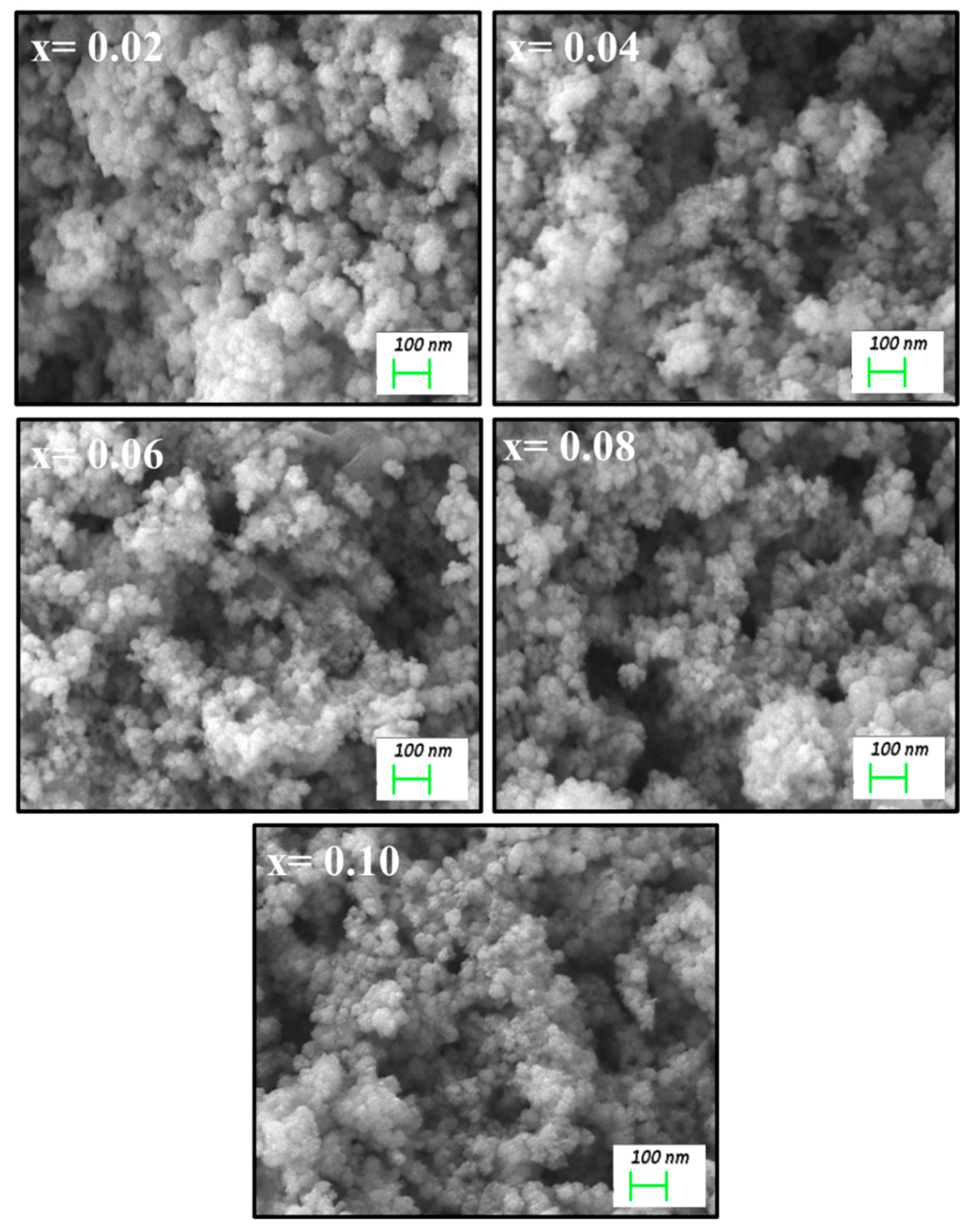
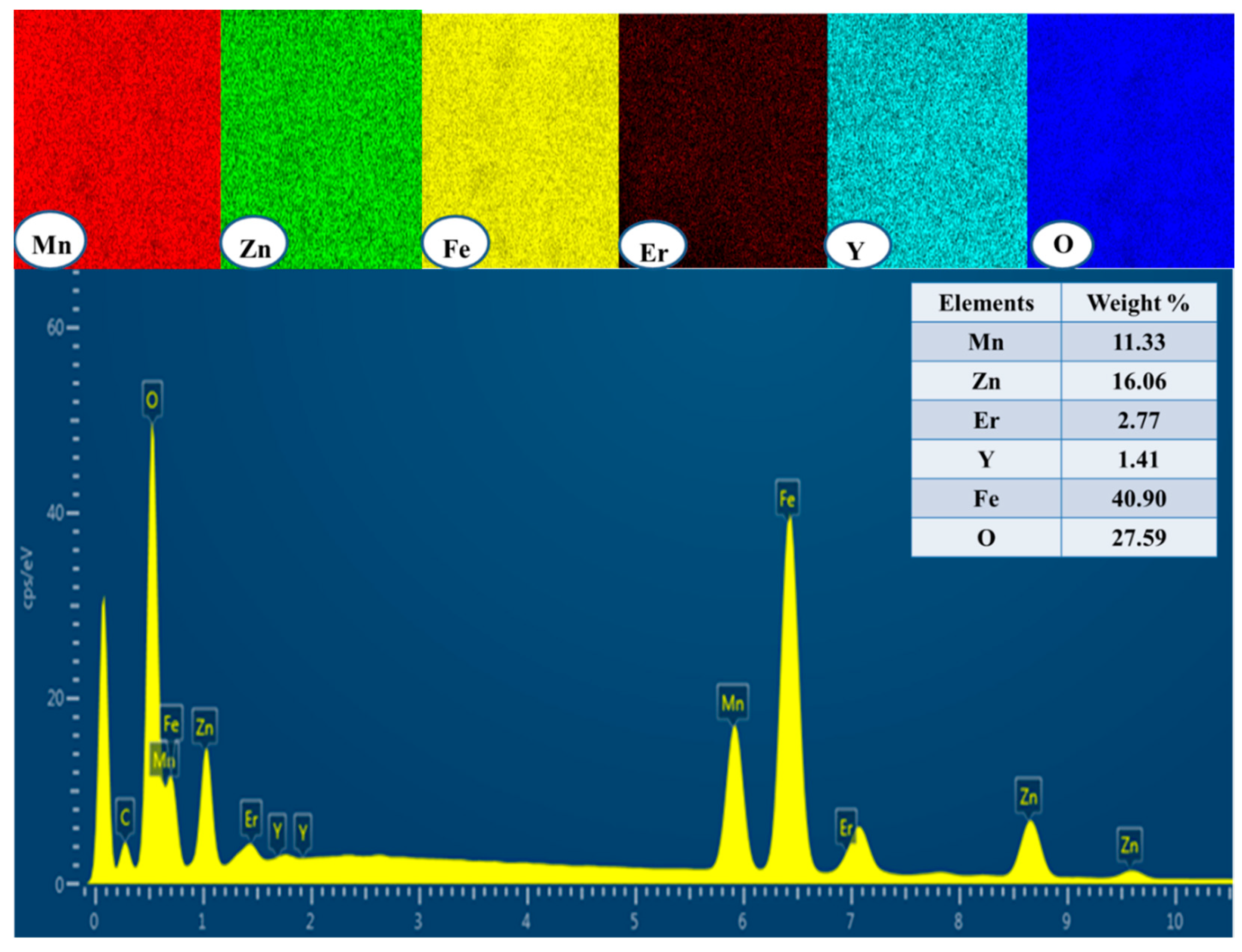
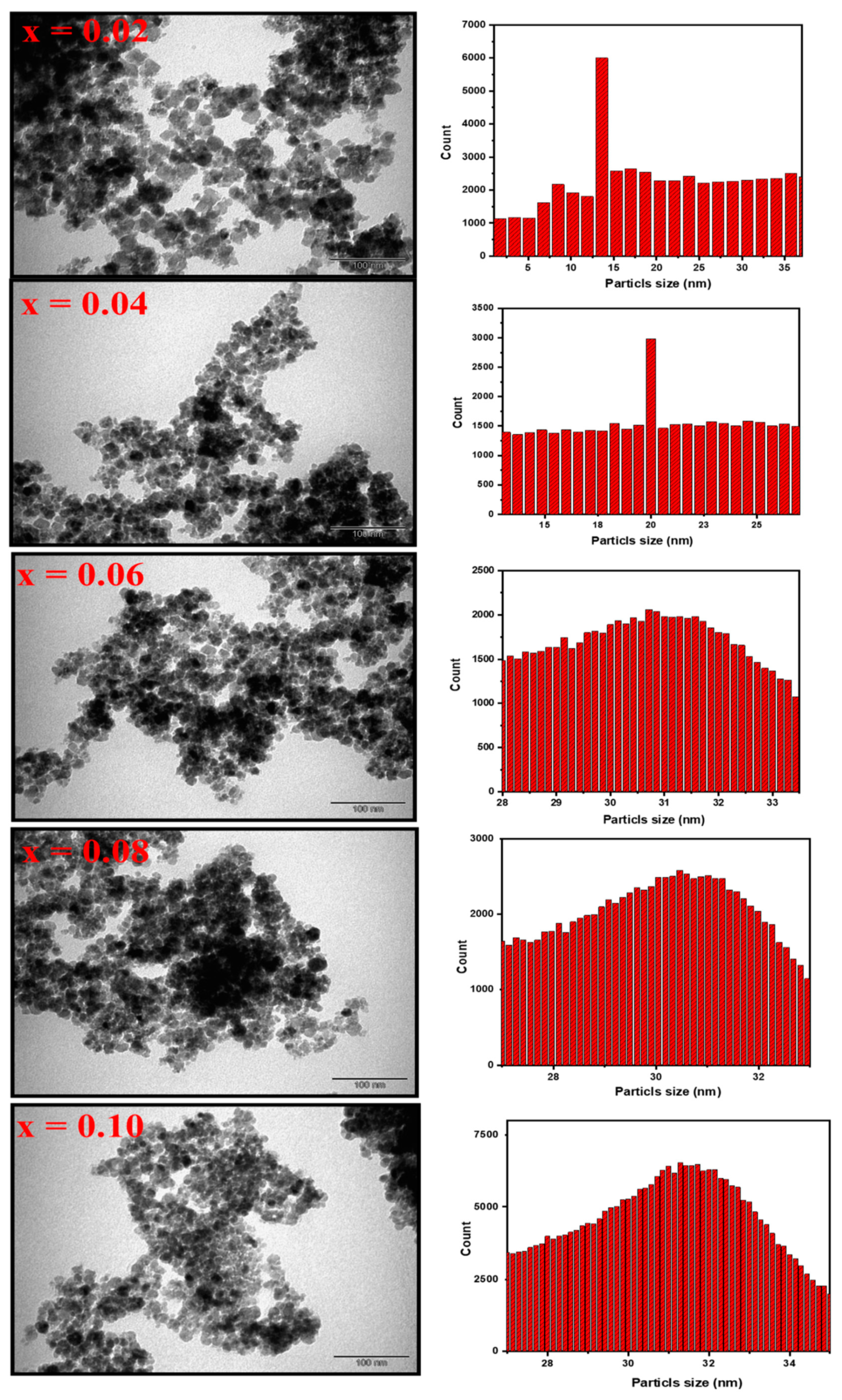
3.1. Morphological Analyses
3.2. Anticancer Activity
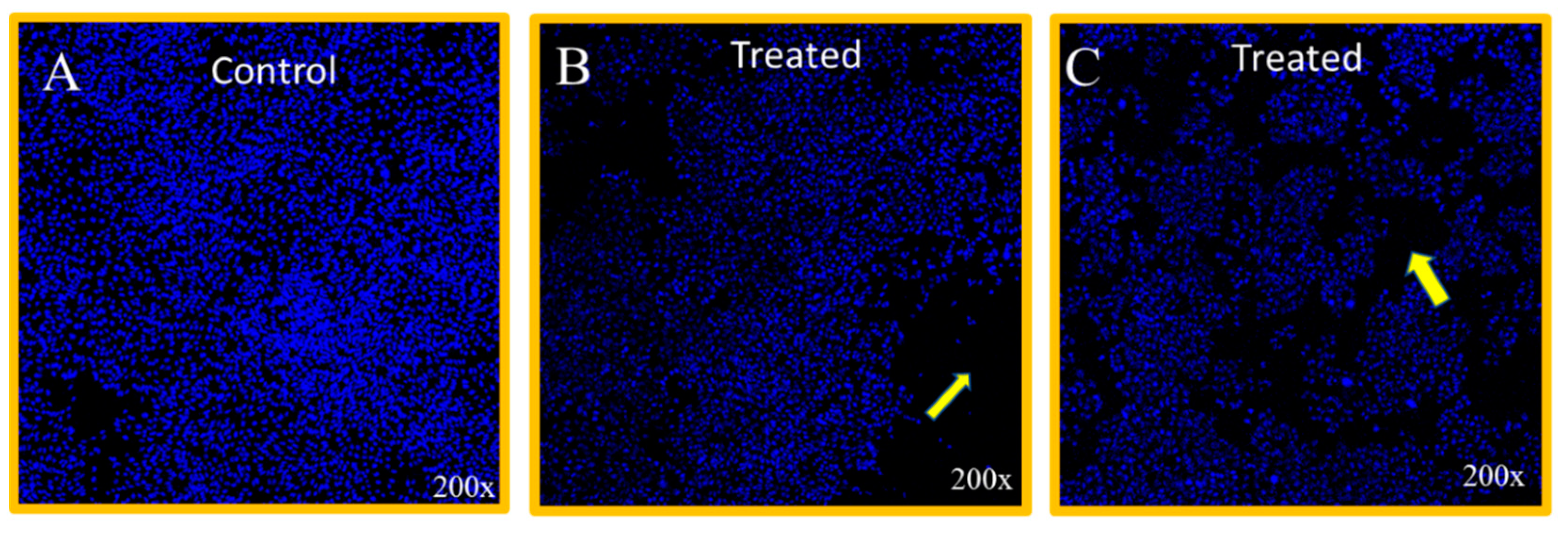
3.3. Cancer Cell Nuclear Disintegration
3.4. Antifungal Activities
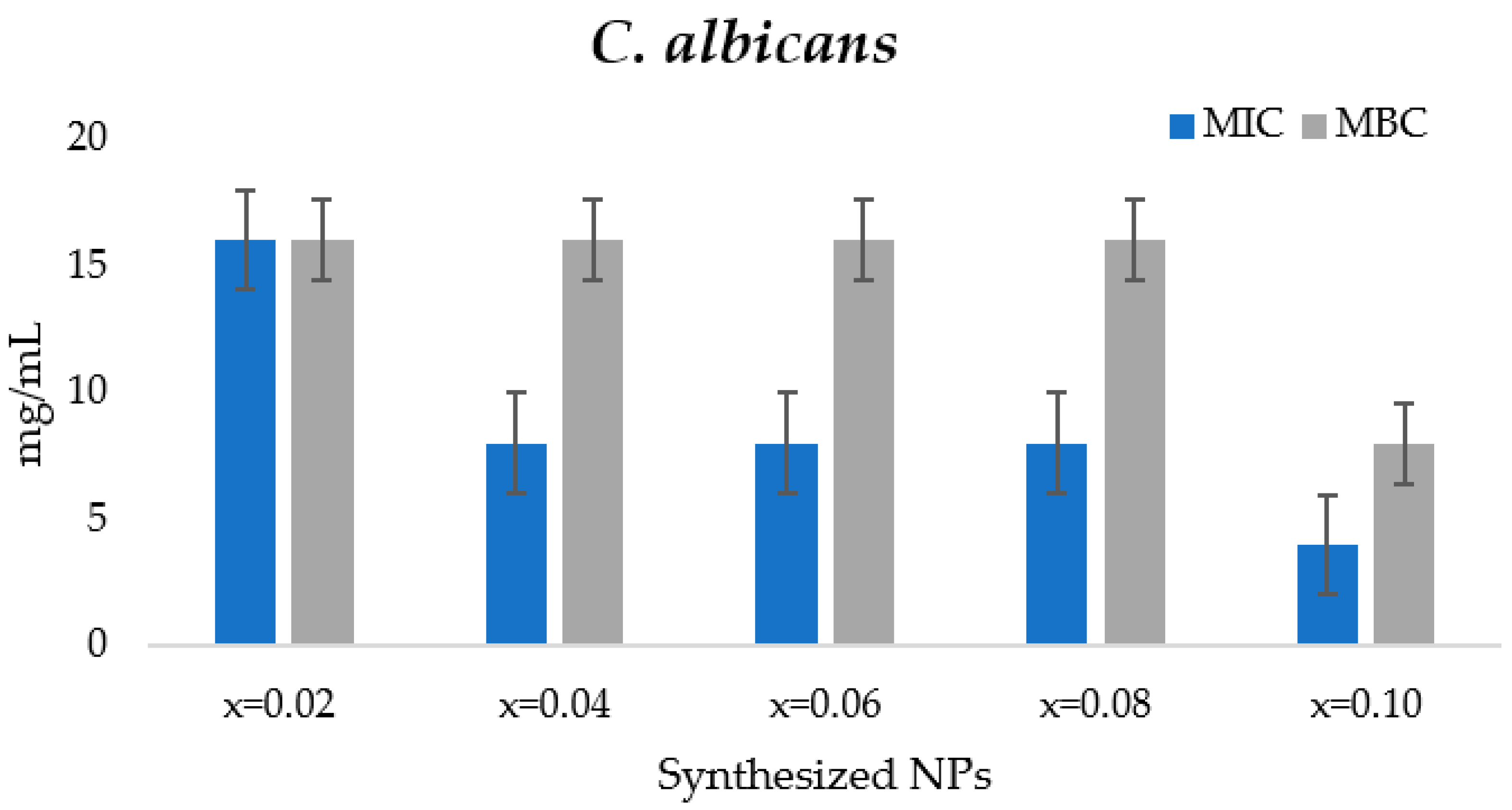
3.4.1. MIC and MFC Determination
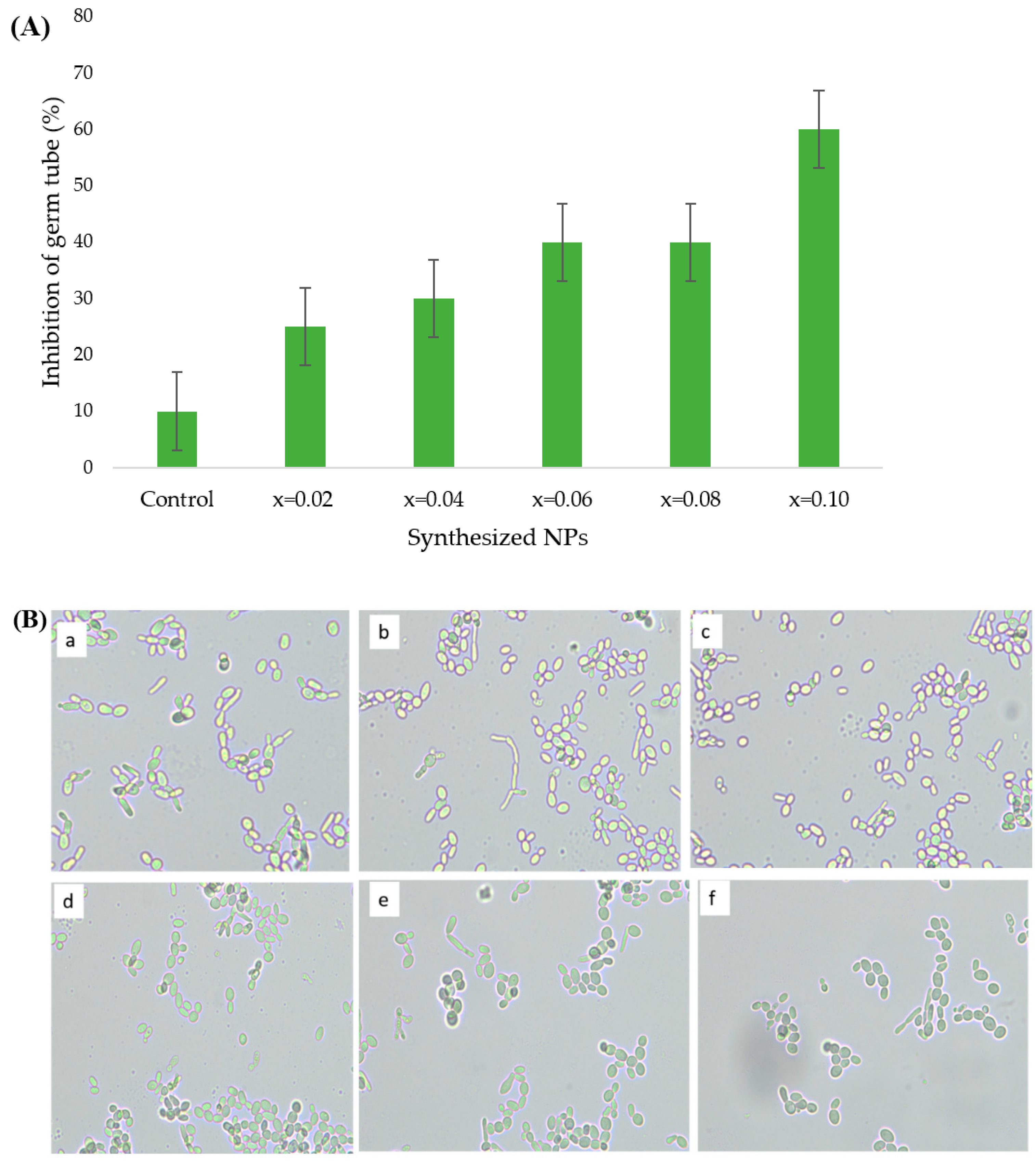
3.4.2. Effect of Ultrasonicated Mn0.5Zn0.5ErxYxFe2−2xO4 NPs on Germ Tube Formation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Aygar, G.; Kaya, M.; Özkan, N.; Kocabiyik, S.; Volkan, M. Preparation of silica coated cobalt ferrite magnetic nanoparticles for the purification of histidine-tagged proteins. J. Phys. Chem. Solids 2015, 87, 64–71. [Google Scholar] [CrossRef]
- Baharara, J.; Ramezani, T.; Divsalar, A.; Mousavi, M.; Seyedarabi, A. Induction of Apoptosis by Green Synthesized Gold Nanoparticles Through Activation of Caspase-3 and 9 in Human Cervical Cancer Cells. Avicenna J. Med. Biotechnol. 2016, 8, 75–83. [Google Scholar]
- Ravinayagam, V.; Rehman, S. Zeolitic imidazolate framework-8 (ZIF-8) doped TiZSM-5 and Mesoporous carbon for antibacterial characterization. Saudi J. Biol. Sci. 2020, 27, 1726–1736. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, D.; Verduin, J.J.; Shah, M.; Sharma, S.B.; Poinern, G.E.J. A review of current research into the biogenic synthesis of metal and metal oxide nanoparticles via marine algae and seagrasses. J. Nanosci. 2017, 2017, 15. [Google Scholar] [CrossRef]
- Schöbel, J.; Burgard, M.; Hils, C.; Dersch, R.; Dulle, M.; Volk, K.; Karg, M.; Greiner, A.; Schmalz, H. Bottom-up meets top-down: Patchy hybrid nonwovens as an efficient catalysis platform. Angew. Chem. Int. Ed. 2017, 56, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, S. A combined self-assembly and calcination method for preparation of nanoparticles-assembled cobalt oxide nanosheets using graphene oxide as template and their application for non-enzymatic glucose biosensing. J. Colloid Interface Sci. 2017, 485, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Harzali, H.; Marzouki, A.; Saida, F.; Megriche, A.; Mgaidi, A. Structural magnetic and optical properties of nanosized Ni0.4Cu0.2Zn0.4R0.05Fe1.95O4 (R = Eu3+, Sm3+, Gd3+ and Pr3+) ferrites synthesized by co-precipitation method with ultrasound irradiation. J. Magn. Magn. Mater. 2018, 460, 89–94. [Google Scholar] [CrossRef]
- Harzali, F.; Saida, A.; Marzouki, A.; Megriche, F.B.; Espitalier, F. Structural and magnetic properties of nano-sized NiCuZn ferrites synthesized by co-precipitation method with ultrasound irradiation. J. Magn. Magn. Mater. 2016, 419, 50–56. [Google Scholar] [CrossRef]
- Slimani, Y.; Almessiere, M.A.; Korkmaz, A.D.; Guner, S.; Güngüneş, H.; Sertkol, M.; Manikandan, A.; Yildiz, A.; Akhtar, S.; Shirsath, S.E.; et al. Ni0.4Cu0.2Zn0.4TbxFe2−xO4 nanospinel ferrites: Ultrasonic synthesis and physical properties. Ultrason. Sonochem. 2019, 59, 104757. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuřitka, I.; Vilcakova, J.; Havlica, J.; Kalina, L.; Urbánek, P.; Machovsky, M.; Skoda, D.; Masař, M.; Holek, M. Sonochemical synthesis of Gd3+ doped CoFe2O4 spinel ferrite nanoparticles and its physical properties. Ultrason. Sonochem. 2018, 40, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Mahulkar, A.V.; Riedel, C.; Gogate, P.R.; Neis, U.; Pandit, A.B. Effect of dissolved gas on efficacy of sonochemical reactors for microbial cell disruption: Experimental and numerical analysis. Ultrason. Sonochem. 2009, 16, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Sonia, M.M.L.; Anand, S.; Vinosel, V.M.; Janifer, M.A.; Pauline, S.; Manikandan, A. Effect of lattice strain on structure, morphology and magneto-dielectric properties of spinel NiGdxFe2−xO4 ferrite nano-crystallites synthesized by sol-gel route. J. Magn. Magn. Mater. 2018, 466, 238–251. [Google Scholar] [CrossRef]
- Hedayatnasab, Z.; Abnisa, F.; Daud, W.M. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater. Des. 2017, 123, 174–196. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Guner, S.; Sertkol, M.; Korkmaz, A.D.; Shirsath, S.E.; Baykal, A. Sonochemical synthesis and physical properties of Co0.3Ni0.5Mn0.2EuxFe2−xO4 nano-spinel ferrites. Ultrason. Sonochem. 2019, 58, 104–654. [Google Scholar]
- Khan, F.A.; Akhtar, S.; Almofty, S.A.; Almohazey, D.; Alomari, M. FMSP-Nanoparticles Induced Cell Death on Human Breast Adenocarcinoma Cell Line (MCF-7 Cells): Morphometric Analysis. Biomolecules 2018, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Akhtar, S.; Almohazey, D.; Alomari, M.; Almofty, S.A.; Badr, I.; Elaissari, A. Targeted delivery of poly (methyl methacrylate) particles in colon cancer cells selectively attenuates cancer cell proliferation. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Ansari, M.A.; Alzohairy MAAlomary, M.N.; Jermy, B.R.; Shahzad, R.; Tashkandi, N.; Alsalem, Z.H. Antibacterial and Antifungal Activity of Novel Synthesized Neodymium-Substituted Cobalt Ferrite Nanoparticles for Biomedical Application. Processes 2019, 7, 714. [Google Scholar] [CrossRef]
- Jalal, M.; Ansari, M.A.; Ali, S.G.; Khan, H.M.; Rehman, S. Anticandidal activity of bioinspired ZnO NPs: Effect on growth, cell morphology and key virulence attributes of Candida species. Artif. Cells Nanomed. Biotechnol. 2018, 46, 912–925. [Google Scholar] [CrossRef]
- Al-Zharani, M.; Qurtam, A.A.; Daoush, W.M.; Eisa, M.H.; Aljarba, N.H.; Alkahtani, S.; Nasr, F.A. Antitumor effect of copper nanoparticles on human breast and colon malignancies. Environ. Sci. Pollut. Res. 2021, 28, 1587–1595. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Ichwan, S.J.; Al-Zikri, P.N.H.; Suriyah, W.H.; Soundharrajan, I.; Govindan, N.; Maniam, G.P.; Yusoff, M.M. In vitro anticancer activity of Au, Ag nanoparticles synthesized using Commelina nudiflora L. aqueous extract against HCT-116 colon cancer cells. Biol. Trace Elem. Res. 2016, 173, 297–305. [Google Scholar] [CrossRef]
- Gnanavel, V.; Palanichamy, V.; Roopan, S.M. Biosynthesis and characterization of copper oxide nanoparticles and its anticancer activity on human colon cancer cell lines (HCT-116). J. Photochem. Photobiol. B Biol. 2017, 171, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Malaikolundhan, H.; Mookkan, G.; Krishnamoorthi, G.; Matheswaran, N.; Alsawalha, M.; Veeraraghavan, V.P.; Krishna Mohan, S.; Di, A. Anticarcinogenic effect of gold nanoparticles synthesized from Albizia lebbeck on HCT-116 colon cancer cell lines. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Shagholani, H.; Ghoreishi, S.M. Improvement of interaction between PVA and chitosan via magnetite nanoparticles for drug delivery application. Int. J. Biol. Macromol. 2015, 78, 130–136. [Google Scholar] [CrossRef]
- Leng, J.; Li, J.; Ren, J.; Deng, L.; Lin, C. Star–block copolymer micellar nanocomposites with Mn, Zn-doped nano-ferrite as superparamagnetic MRI contrast agent for tumor imaging. Mater. Lett. 2015, 152, 185–188. [Google Scholar] [CrossRef]
- Shiva, I.; Zhohreh, S.; Seyed Mohammad, A.; Shahriar, M. Induction of growth arrest in colorectal cancer cells by cold plasma and gold nanoparticles. Arch. Med. Sci. 2015, 11, 1286–1295. [Google Scholar]
- Mytych, J.; Lewinska, A.; Zebrowski, J.; Wnuk, M. Gold nanoparticles promote oxidant-mediated activation of NF-κB and 53BP1 recruitment-based adaptive response in human astrocytes. Biomed. Res. Int. 2015, 304575. [Google Scholar] [CrossRef]
- Lin-Wei, W.; Ai-Ping, Q.; Wen-Lou, L.; Jia-Mei, C.; Jing-Ping, Y.; Han, W.; Yan, L.; Juan, L. Quantum dots-based double imaging combined with organic dye imaging to establish an automatic computerized method for cancer Ki67 measurement. Sci. Rep. 2016, 6, 20564. [Google Scholar]
- Abbasi, A.Z.; Gordijo, C.R.; Amini, M.A.; Maeda, A.; Rauth, A.M.; DaCosta, R.S.; Wu, X.Y. Hybrid manganese dioxide nanoparticles potentiate radiation therapy by modulating tumor hypoxia. Cancer Res. 2016, 76, 6643–6656. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Q.; Liang, C.; Yang, Z.; Zhang, L.; Yi, X.; Dong, Z.; Chao, Y.; Chen, Y.; Liu, Z. Albumin-templated biomineralizing growth of composite nanoparticles as smart nano-theranostics for enhanced radiotherapy of tumors. Nanoscale 2017, 9, 14826–14835. [Google Scholar] [CrossRef]
- Abdel-Ghany, S.; Raslan, S.; Tombuloglu, H.; Shamseddin, A.; Cevik, E.; Said, O.A.; Madyan, E.F.; Senel, M.; Bozkurt, A.; Rehman, S.; et al. Vorinostat-loaded titanium oxide nanoparticles (anatase) induce G2/M cell cycle arrest in breast cancer cells via PALB2 upregulation. 3 Biotech. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Akhtar, S.; Rehman, S.; Asiri, S.M.; Khan, F.A.; Baig, U.; Hakeem, A.S.; Gondal, M.A. Evaluation of bioactivities of zinc oxide, cadmium sulfide and cadmium sulfide loaded zinc oxide nanostructured materials prepared by nanosecond pulsed laser. Mater. Sci. Eng. C 2020, 116, 111–156. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Asiri SMKhan, F.A.; Jermy, B.R.; Khan, H.; Akhtar, S.; Jindan, R.A.; Khan, K.M.; Qurashi, A. Biocompatible Tin Oxide Nanoparticles: Synthesis, Antibacterial, Anticandidal and Cytotoxic Activities. ChemistrySelect 2019, 4, 4013–4017. [Google Scholar] [CrossRef]
- Park, S.; Cho, B.B.; Anusha, J.R.; Jung, S.; Justin Raj, C.; Kim, B.C.; Yu, K.H. Synthesis of 64Cu-radiolabeled folate-conjugated iron oxide nanoparticles for cancer diagnosis. J. Nanosci. 2020, 20, 2040–2044. [Google Scholar] [CrossRef] [PubMed]
- Dedkova, K.; Kuznikova, L.; Pavelek, L.; Matejova, K.; Kupkova, J.; Cech Barabaszova, K.; Vana, R.; Burda, J.; Vlcek, J.; Cvejn, D.; et al. Daylight induced antibacterial activity of gadolinium oxide, samarium oxide and erbium oxide nanoparticles and their aquatic toxicity. Mater. Chem. Phys. 2017, 197, 226–235. [Google Scholar] [CrossRef]
- Ficociello, G.; De Caris, M.G.; Trillò, G.; Cavallini, D.; Sarto, M.S.; Uccelletti, D.; Mancini, P. Anti-Candidal Activity and In Vitro Cytotoxicity Assessment of Graphene Nanoplatelets Decorated with Zinc Oxide Nanorods. Nanomaterials 2018, 8, 752. [Google Scholar] [CrossRef] [PubMed]
- Tronchin, G.; Bouchara, J.P.; Robert, R.; Senet, J.M. Adherence of Candida albicans germ tubes to plastic: Ultrastructural and molecular studies of fibrillar adhesins. Infect. Immun. 1988, 56, 1987–1993. [Google Scholar] [CrossRef] [PubMed]

| x | IC50 (HCT-116 | IC50 (HEK-293) |
|---|---|---|
| 0.02 | 0.88 µg/mL | No inhibition |
| 0.04 | 2.40 µg/mL | No inhibition |
| 0.06 | 0.85 µg/mL | No inhibition |
| 0.08 | 0.78 µg/mL | No inhibition |
| 0.10 | 0.45 µg/mL | No inhibition |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, S.; Almessiere, M.A.; A. Al-Suhaimi, E.; Hussain, M.; Yousuf Bari, M.; Mehmood Ali, S.; Al-Jameel, S.S.; Slimani, Y.; Khan, F.A.; Baykal, A. Ultrasonic Synthesis and Biomedical Application of Mn0.5Zn0.5ErxYxFe2−2xO4 Nanoparticles. Biomolecules 2021, 11, 703. https://doi.org/10.3390/biom11050703
Rehman S, Almessiere MA, A. Al-Suhaimi E, Hussain M, Yousuf Bari M, Mehmood Ali S, Al-Jameel SS, Slimani Y, Khan FA, Baykal A. Ultrasonic Synthesis and Biomedical Application of Mn0.5Zn0.5ErxYxFe2−2xO4 Nanoparticles. Biomolecules. 2021; 11(5):703. https://doi.org/10.3390/biom11050703
Chicago/Turabian StyleRehman, Suriya, Munirah A. Almessiere, Ebtesam A. Al-Suhaimi, Mehwish Hussain, Maha Yousuf Bari, Syed Mehmood Ali, Suhailah S. Al-Jameel, Yassine Slimani, Firdos Alam Khan, and Abdulhadi Baykal. 2021. "Ultrasonic Synthesis and Biomedical Application of Mn0.5Zn0.5ErxYxFe2−2xO4 Nanoparticles" Biomolecules 11, no. 5: 703. https://doi.org/10.3390/biom11050703
APA StyleRehman, S., Almessiere, M. A., A. Al-Suhaimi, E., Hussain, M., Yousuf Bari, M., Mehmood Ali, S., Al-Jameel, S. S., Slimani, Y., Khan, F. A., & Baykal, A. (2021). Ultrasonic Synthesis and Biomedical Application of Mn0.5Zn0.5ErxYxFe2−2xO4 Nanoparticles. Biomolecules, 11(5), 703. https://doi.org/10.3390/biom11050703









