Troponin T Mutation as a Cause of Left Ventricular Systolic Dysfunction in a Young Patient with Previous Surgical Correction of Aortic Coarctation
Abstract
1. Introduction
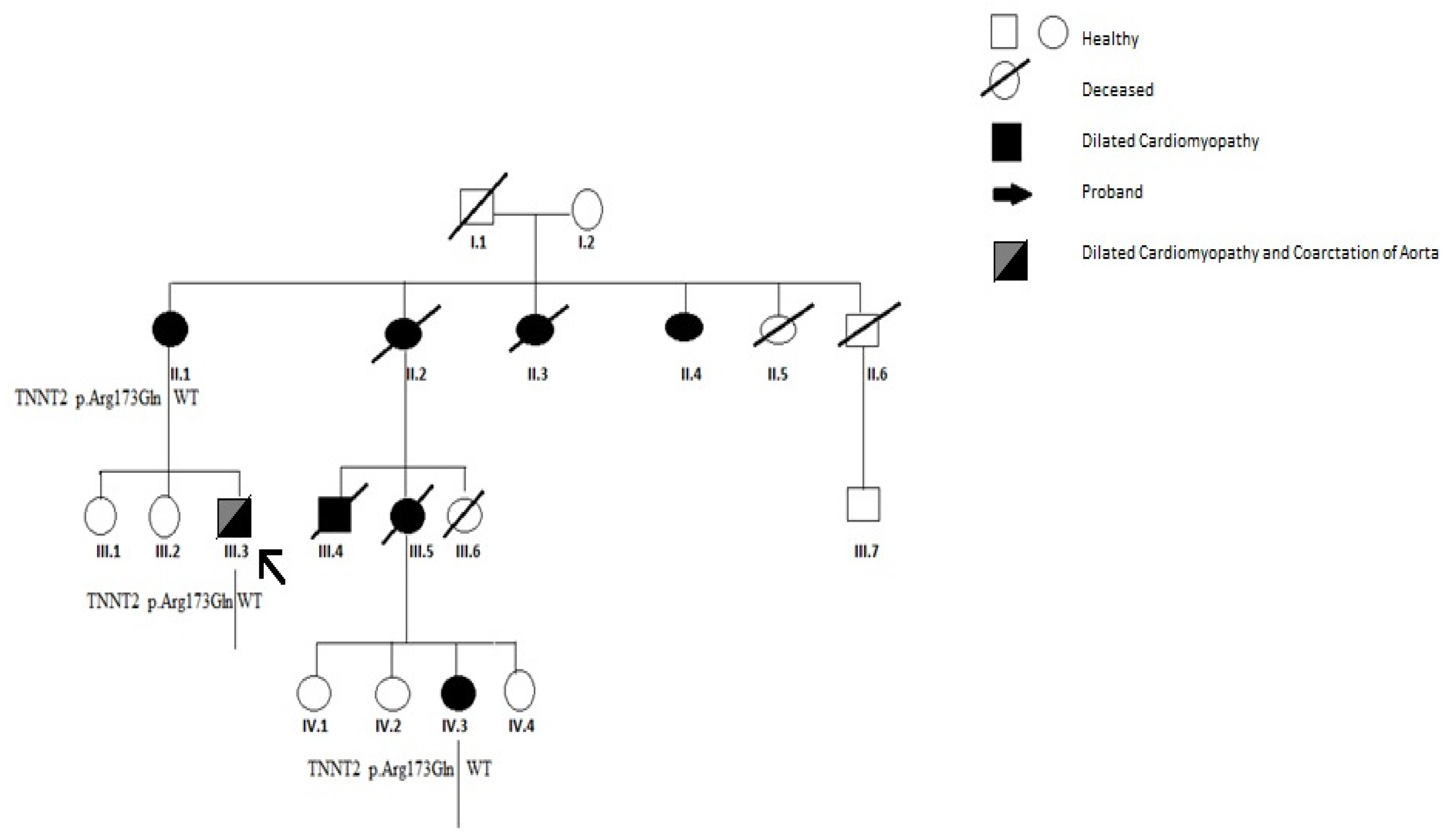
2. Case Report
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, M.L.; Burkhart, H.M.; Connolly, H.M.; Dearani, J.A.; Cetta, F.; Li, Z.; Oliver, W.C.; Warnes, C.A.; Schaff, H.V. Coarctation of the aorta: Lifelong surveillance is mandatory following surgical repair. J. Am. Coll. Cardiol. 2013, 62, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.Y.; Babu-Narayan, S.V.; Kempny, A.; Uebing, A.; Montanaro, C.; Shore, D.F.; D’Udekem, Y.; Gatzoulis, M.A. Long-term mortality and cardiovascular burden for adult survivors of coarctation of the aorta. Heart 2019, 105, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.E.; Awad, M.; Hassan, W.; Al Kadhi, Y.; Shoukri, M.; Fadley, F. Long-term outcome (up to 15 years) of balloon angioplasty of discrete native coarctation of the aorta in adolescents and adults. J. Am. Coll. Cardiol. 2004, 43, 1062–1067. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moonsamy, P.; Bhatt, A.B.; Mohan, N.; Masaki, F.; Serguei, M.; David, A.; D’Alessandro, G.T.; Thoralf, M.S.; Duke, E.C.; Arminder, J. Long term follow-up of patients with coarctation of the aorta. J. Am. Coll. Cardiol. 2020, 75, 609. [Google Scholar] [CrossRef]
- Hwang, M.S.; Chu, J.J.; Chang, Y.S.; Su, W.J. Dilated cardiomyopathy: An unusual presentation of aortic coarctation in an infant. Cardiology 2006, 106, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Héctor, B.; John, G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Veli-Pekka, H.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- ClinVar Database (ClinVar-NCBI-NIH). Available online: https://www.ncbi.nlm.nih.gov/clinvar/variation/43649/ (accessed on 16 April 2021).
- Salahuddin, N.; Wilson, A.D.; Rao, P.S. An unusual presentation of coarctation of the aorta in infancy: Role of balloon angioplasty in the critically ill infant. Am. Heart J. 1991, 122, 1772–1775. [Google Scholar] [CrossRef]
- Alehan, D.; Kafali, G.; Demircin, M. Middle aortic syndrome as a cause of dilated cardiomyopathy. Anadolu Kardiyol. Derg. 2004, 4, 178–180. [Google Scholar]
- Aǧaç, M.T.; Acar, Z.; Akdemir, R.; Korkmaz, L.; Kiris, A.; Akyuz, A.R.; Erkan, H. Dilated cardiomyopathy secondary to coarctation of the aorta was completely resolved after stent implantation. Cardiovasc. J. Afr. 2012, 23, 6. [Google Scholar] [CrossRef]
- Alcibar, J.; Peña, N.; Oñate, A.; Gochi, R.; Barrenechea, J.I. Stent implantation in an adult with coarctation of the aorta in the presence of advanced secondary heart failure. Texas Heart Inst. J. 1999, 26, 143–147. [Google Scholar]
- Pauly, D.F.; Morss, S.E.; Tanio, J.W.; Irani, K.; Cameron, D.E.; Schulman, S.P.; Hare, J.M. Reduced left ventricular dimension and normalized atrial natriuretic hormone level after repair of aortic coarctation in an adult. Clin. Cardiol. 1999, 22, 33–235. [Google Scholar] [CrossRef] [PubMed]
- Raffel, O.C.; Abraham, A.; Ruygrok, P.N.; Finucane, A.K.; McGeorge, A.D.; French, R.L. Cardiac transplantation and aortic coarctation repair in severe heart failure. Asian Cardiovasc. Thorac. Ann. 2006, 14, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Kenny, D.; Polson, J.; Martin, R.P.; Paton, J.F.R.; Wolf, A.R. Hypertension and coarctation of the aorta: An inevitable consequence of developmental pathophysiology. Hypertens Res. 2011, 34, 543–547. [Google Scholar] [CrossRef]
- Frey, N.; Olson, E.N. Cardiac Hypertrophy: The Good, the Bad, and the Ugly. Annu. Rev. Physiol. 2003, 65, 45–79. [Google Scholar] [CrossRef] [PubMed]
- De Acetis, M.; Notte, A.; Accornero, F.; Selvetella, G.; Brancaccio, M.; Vecchione, C.; Sbroggiò, M.; Collino, F.; Pacchioni, B.; Lanfranchi, G.; et al. Cardiac overexpression of melusin protects from dilated cardiomyopathy due to long-standing pressure overload. Circ. Res. 2005, 96, 1087–1094. [Google Scholar] [CrossRef]
- Brancaccio, M.; Fratta, L.; Notte, A.; Hirsch, E.; Poulet, R.; Guazzone, S.; De Acetis, M.; Vecchione, C.; Marino, G.; Altruda, F. Melusin a muscle-specific integrin β1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload. Nat. Med. 2003, 9, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2019, 139, E637–E697. [Google Scholar] [CrossRef]
- Jáchymová, M.; Muravská, A.; Paleček, T.; Kuchynka, P.; Řeháková, H.; Magage, S.; Král, A.; Zima, T.; Horký, K.; Linhart, A. Genetic variation screening of TNNT2 gene in a cohort of patients with hypertrophic and dilated cardiomyopathy. Physiol. Res. 2012, 61, 169–175. [Google Scholar] [CrossRef]
- Millat, G.; Bouvagnet, P.; Chevalier, P.; Sebbag, L.; Dulac, A.; Dauphin, C.; Jouk, P.S.; Delrue, M.A.; Thambo, J.B.; Le Metayer, P. Clinical and mutational spectrum in a cohort of 105 unrelated patients with dilated cardiomyopathy. Eur. J. Med. Genet. 2011, 54, e570–e575. [Google Scholar] [CrossRef]
- Hershberger, R.E.; Pinto, J.R.; Parks, S.B.; Kushner, J.D.; Duanxiang, L.; Ludwigsen, S.; Cowan, J.; Morales, A.S.; Parvatiyar, M.; Potter, J.D. Clinical and functional Characterization of TNNT2 mutations identified in patients with dilated cardiomyopathy. Circ. Cardiovasc. Genet. 2009, 2, 306–313. [Google Scholar] [CrossRef]
- Kamisago, M.; Sharma, S.D.; DePalma, S.R.; Solomon, S.; Sharma, P.; McDonough, B.; Smoot, L.; Mullen, M.P.; Woolf, P.K.; Wigle, E.D.; et al. Mutations in Sarcomere Protein Genes as a Cause of Dilated Cardiomyopathy. N. Engl. J. Med. 2000, 343, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; Canedo, P.; Campelo, M.; Moura, B.; Leite, S.; Baixia, M.; Belo, A.; Rocha-Gonçalves, F.; Machado, J.C.; Silva-Cardoso, J. Genetic Variants Are Not Rare in ICD Candidates with Dilated Cardiomyopathy: Time for Next-Generation Sequencing? Cardiol. Res. Pract. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S. Molecular pathogenic mechanisms of cardiomyopathies caused by mutations in cardiac troponin T. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2007; Volume 592, pp. 227–239. [Google Scholar]
- Crispell, K.A.; Wray, A.; Ni, H.; Nauman, D.J.; Hershberger, R.E. Clinical profiles of four large pedigrees with familial dilated cardiomyopathy: Preliminary recommendations for clinical practice. J. Am. Coll. Cardiol. 1999, 34, 837–847. [Google Scholar] [CrossRef][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caiazza, M.; Lioncino, M.; Monda, E.; Di Fraia, F.; Verrillo, F.; Pacileo, R.; Amodio, F.; Rubino, M.; Cirillo, A.; Fusco, A.; et al. Troponin T Mutation as a Cause of Left Ventricular Systolic Dysfunction in a Young Patient with Previous Surgical Correction of Aortic Coarctation. Biomolecules 2021, 11, 696. https://doi.org/10.3390/biom11050696
Caiazza M, Lioncino M, Monda E, Di Fraia F, Verrillo F, Pacileo R, Amodio F, Rubino M, Cirillo A, Fusco A, et al. Troponin T Mutation as a Cause of Left Ventricular Systolic Dysfunction in a Young Patient with Previous Surgical Correction of Aortic Coarctation. Biomolecules. 2021; 11(5):696. https://doi.org/10.3390/biom11050696
Chicago/Turabian StyleCaiazza, Martina, Michele Lioncino, Emanuele Monda, Francesco Di Fraia, Federica Verrillo, Roberta Pacileo, Federica Amodio, Marta Rubino, Annapaola Cirillo, Adelaide Fusco, and et al. 2021. "Troponin T Mutation as a Cause of Left Ventricular Systolic Dysfunction in a Young Patient with Previous Surgical Correction of Aortic Coarctation" Biomolecules 11, no. 5: 696. https://doi.org/10.3390/biom11050696
APA StyleCaiazza, M., Lioncino, M., Monda, E., Di Fraia, F., Verrillo, F., Pacileo, R., Amodio, F., Rubino, M., Cirillo, A., Fusco, A., Romeo, E., Scatteia, A., Dellegrottaglie, S., Calabrò, P., Sarubbi, B., Baban, A., Frisso, G., Russo, M. G., & Limongelli, G. (2021). Troponin T Mutation as a Cause of Left Ventricular Systolic Dysfunction in a Young Patient with Previous Surgical Correction of Aortic Coarctation. Biomolecules, 11(5), 696. https://doi.org/10.3390/biom11050696











