Current Understanding of the Structure and Function of Pentapeptide Repeat Proteins
Abstract
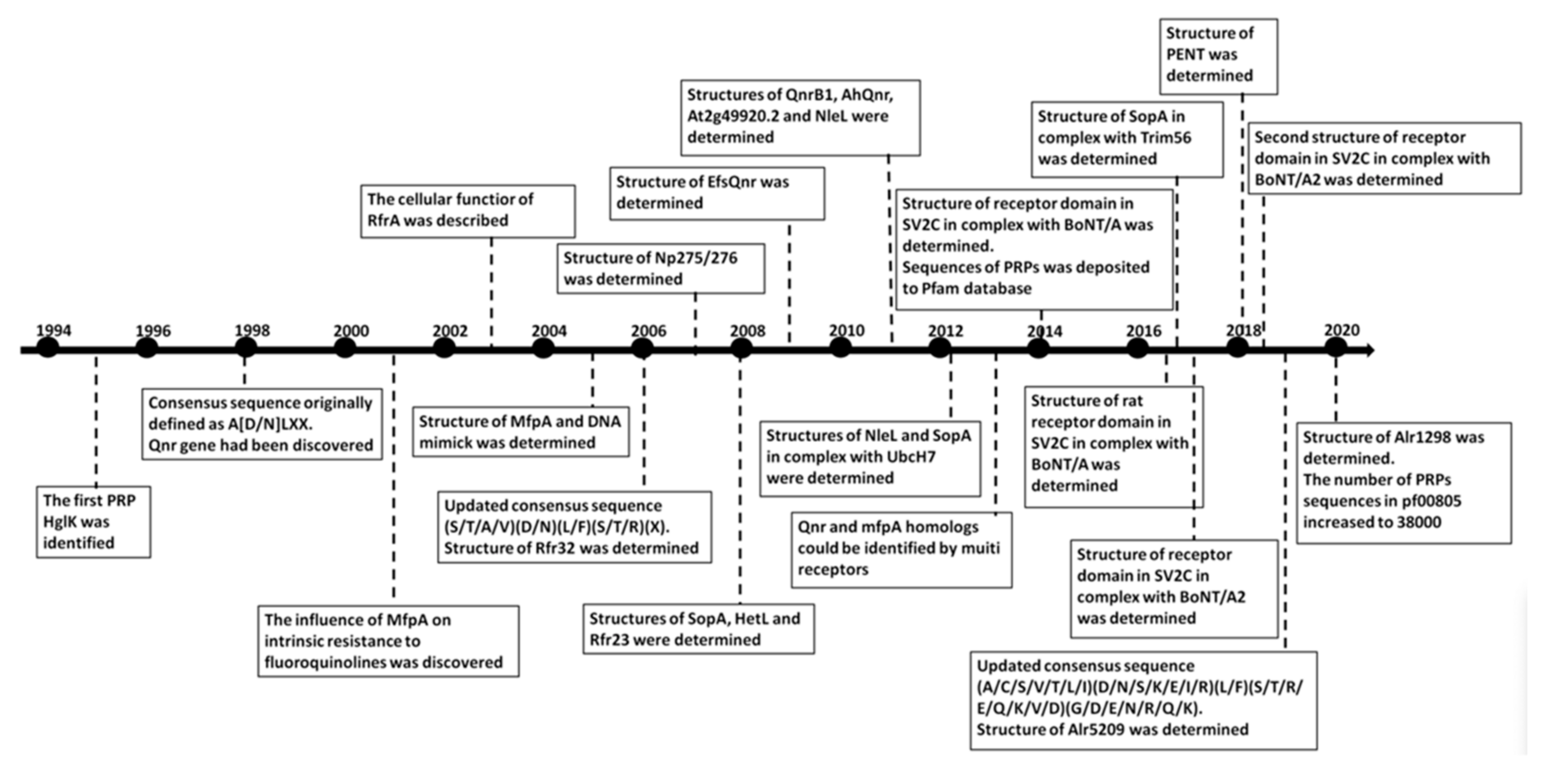
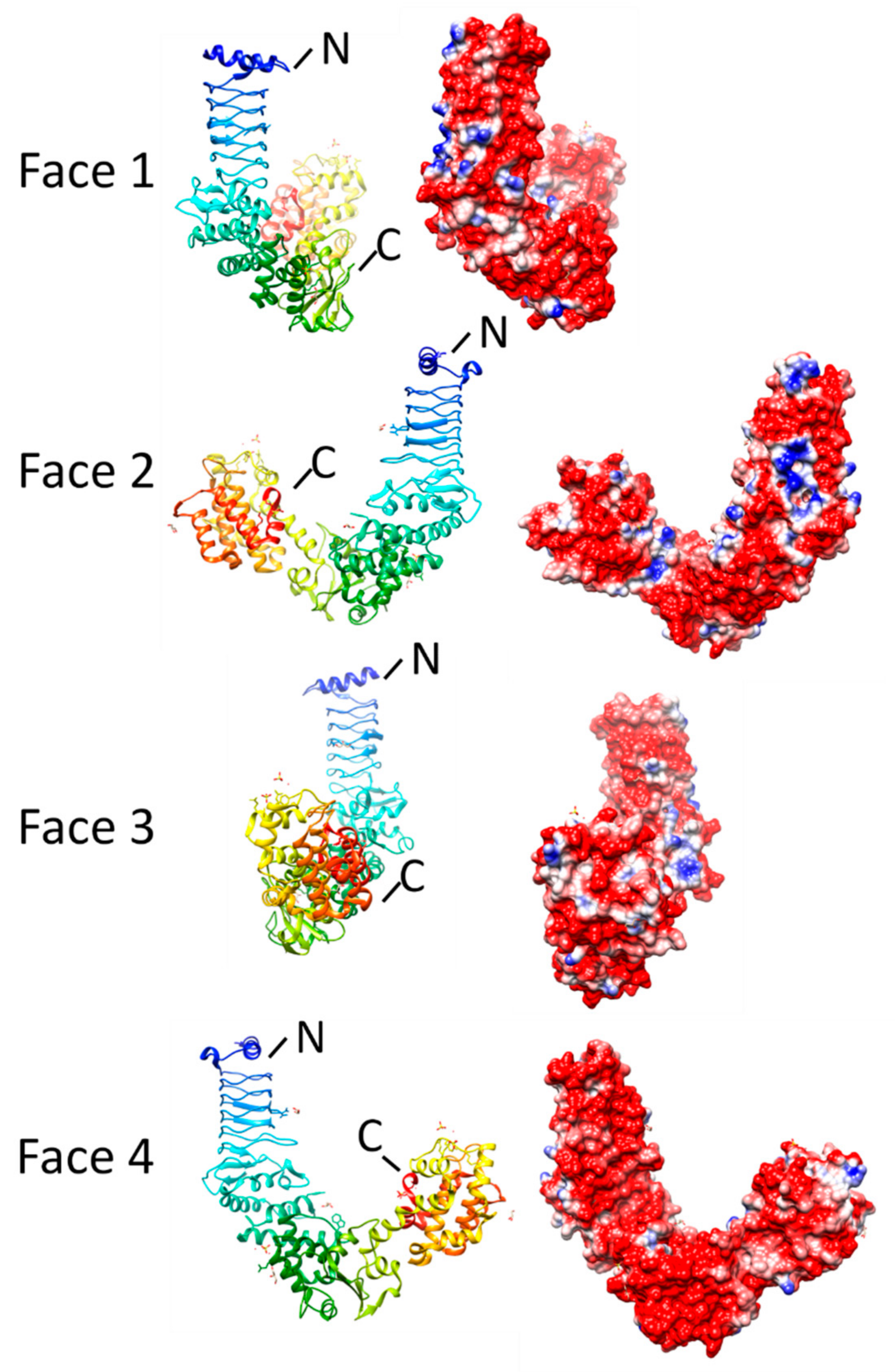
1. Introduction
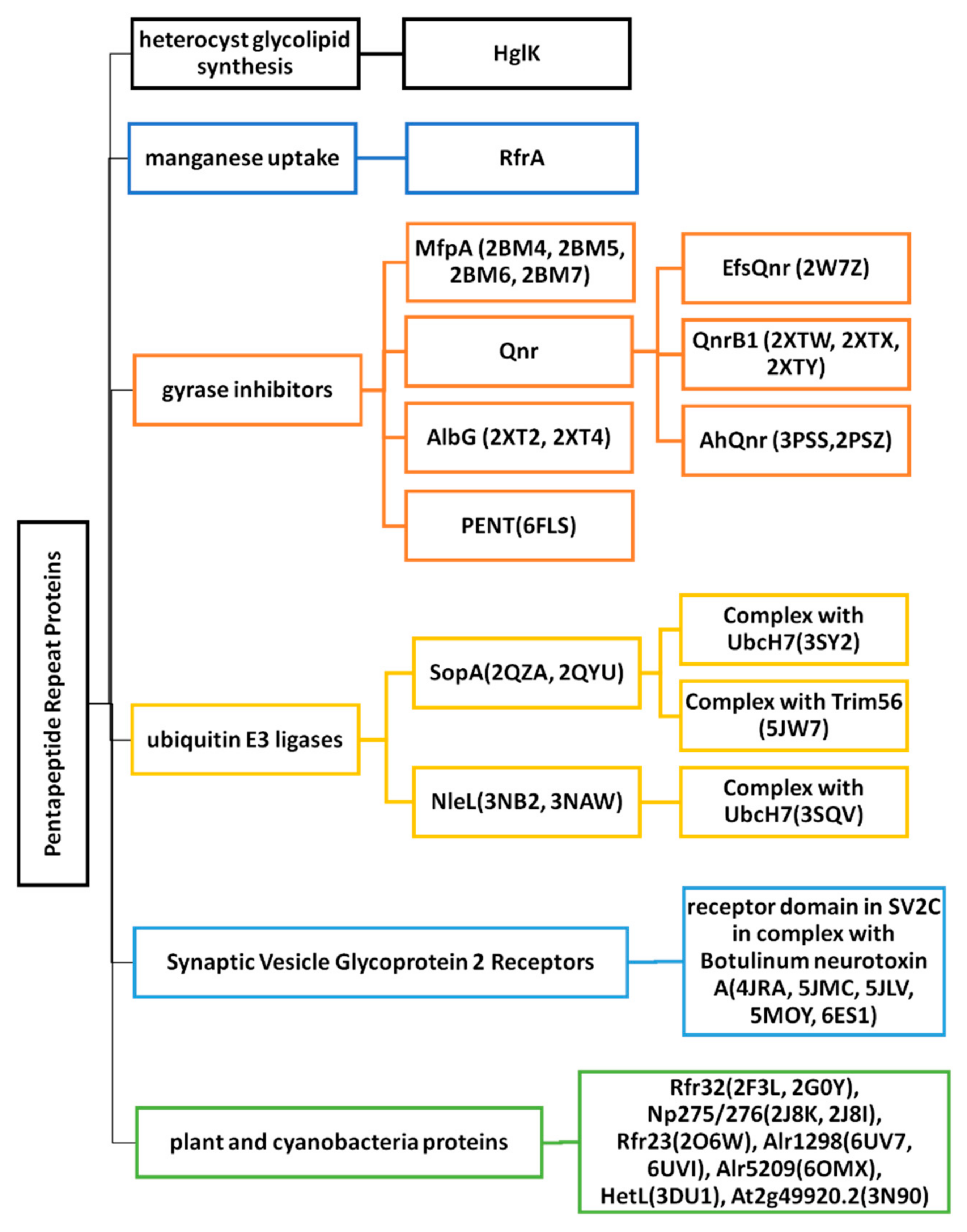
2. Cyanobacterial and Eubacterial PRPs with an Associated Biochemical or Cellular Function
2.1. Heterocyst Glycolipid Biosynthesis-HglK
2.2. Regulator of Manganese Uptake-RfrA
2.3. Gyrase Inhibitors
2.3.1. MfpA (2BM4, 2BM5, 2BM6 and 2BM7)
2.3.2. EfsQnr (2W7Z)
2.3.3. QnrB1 (2XTW, 2XTX, and 2XTY)
2.3.4. AhQnr (3PSS and 3PSZ)
2.3.5. AlbG (2XT2 and 2XT4)
2.3.6. PENT (6FLS)
2.4. Ubiquitin E3 Ligases
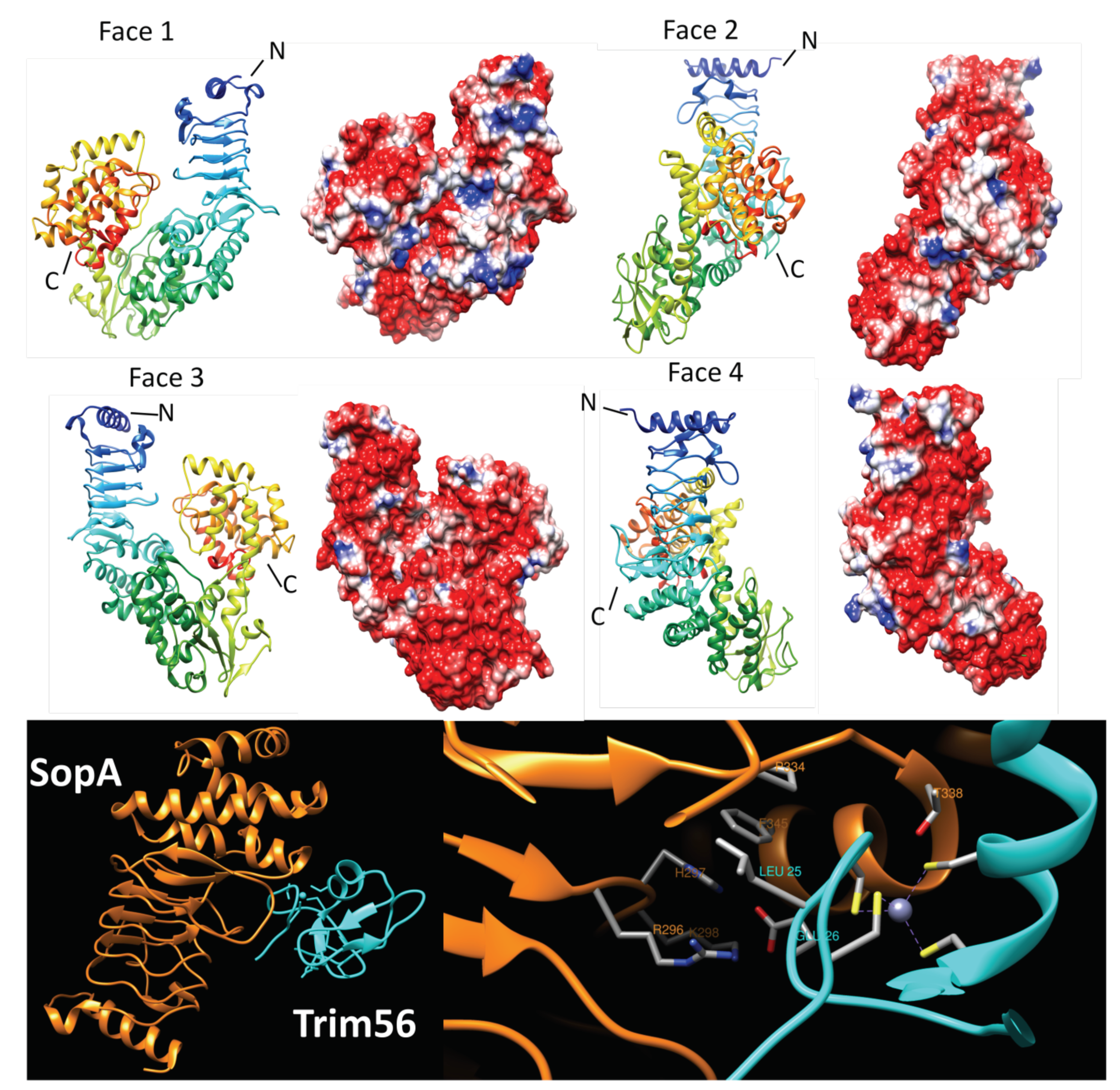
2.4.1. SopA (2QYU, 2QZA, 3SY2 and 5JW7)
2.4.2. NleL (3NB2, 3NAW, and 3SQV)
2.5. Synaptic Vesicle Glycoprotein 2 Receptors
SV2C-LD (4JRA, 5JMC, 5JLV, 5MOY, and 6ES1)
3. Cyanobacterial and Plant PRPs with Three-Dimensional Structures but Unknown Function
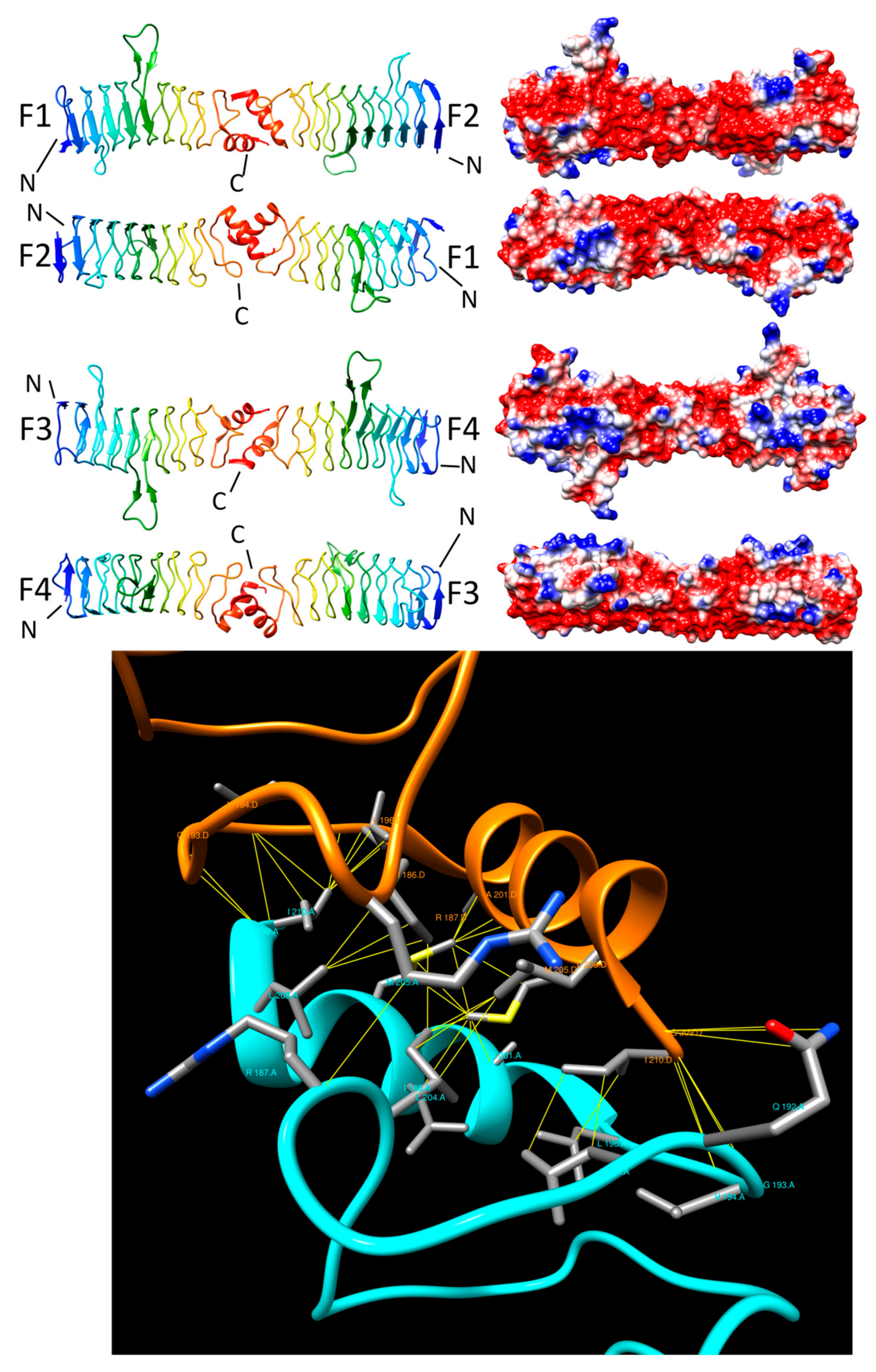
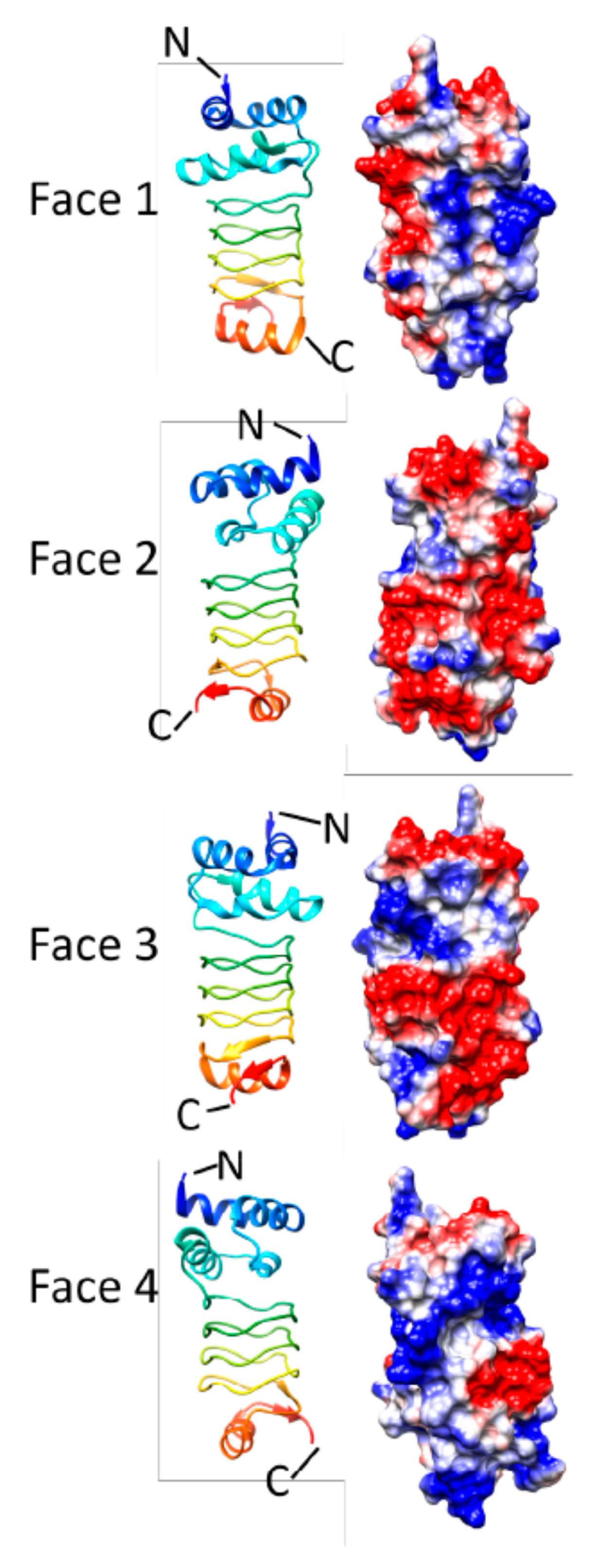
3.1. HetL (3DU1)
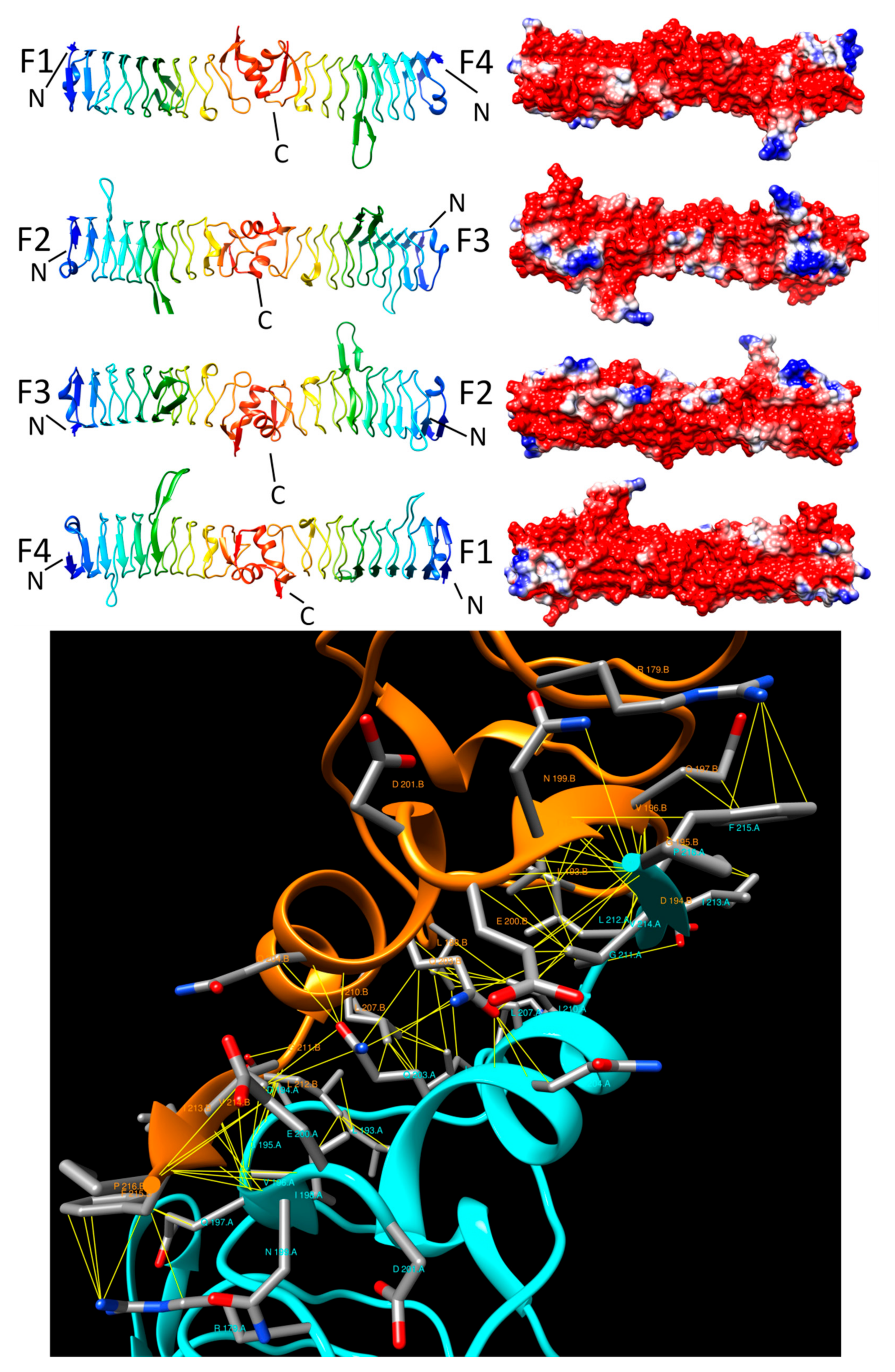
3.2. Alr1298 (6UV7 and 6UVI)
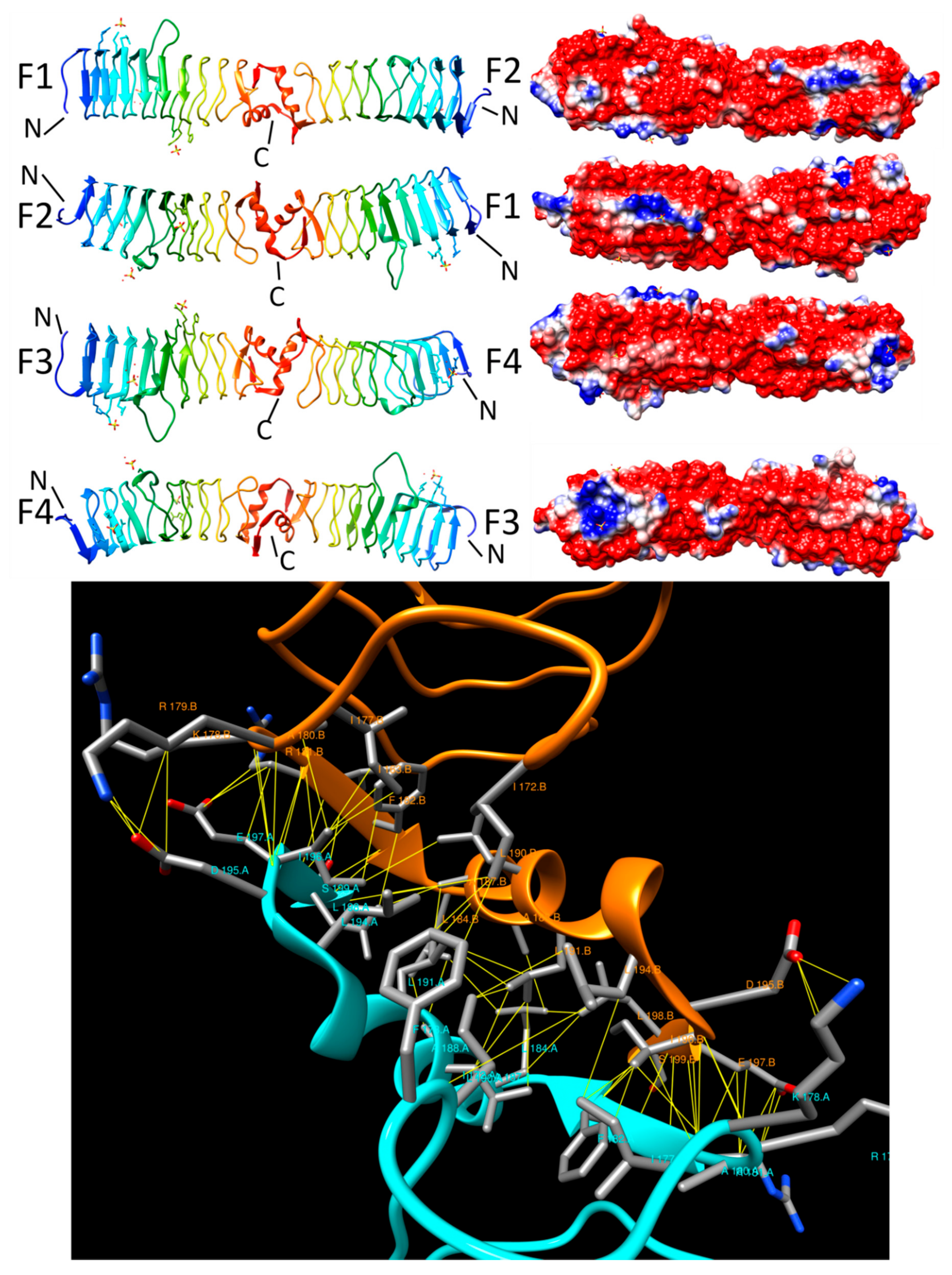
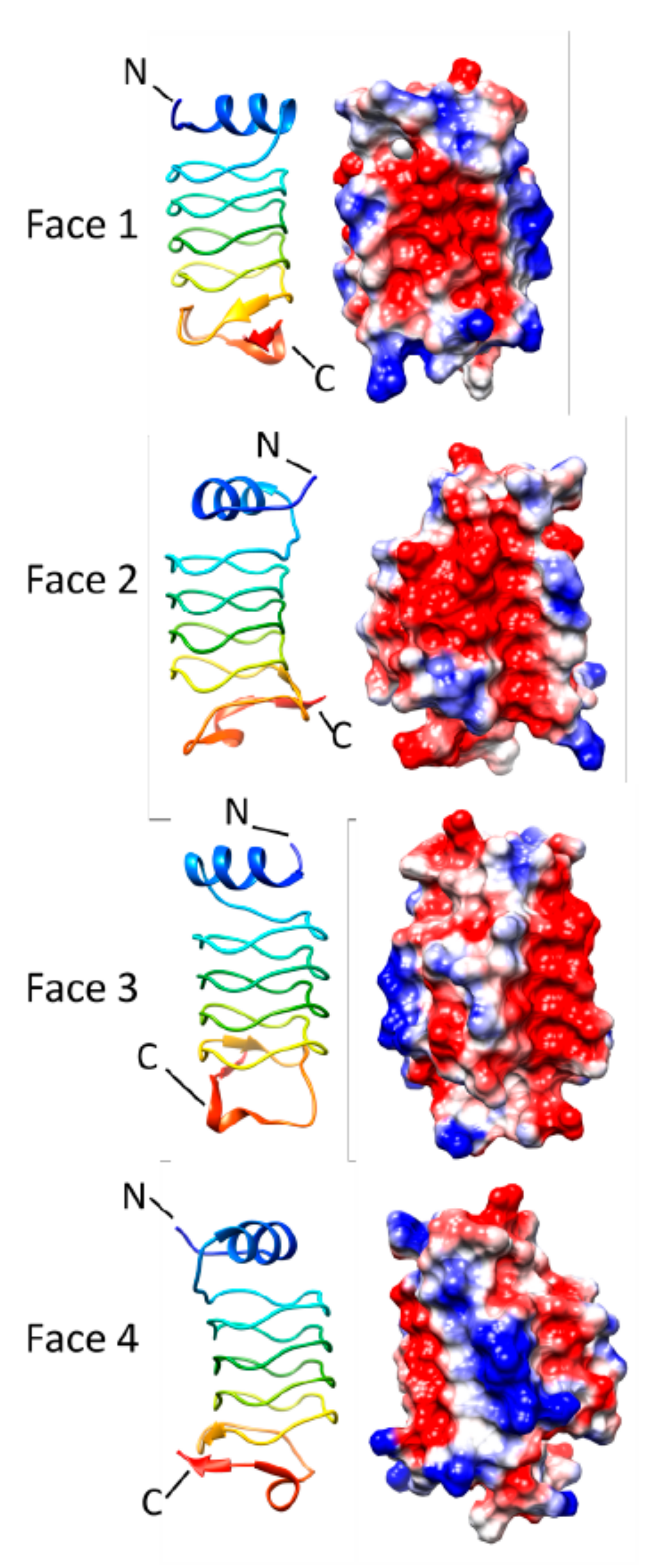
3.3. Alr5209 (6OMX)
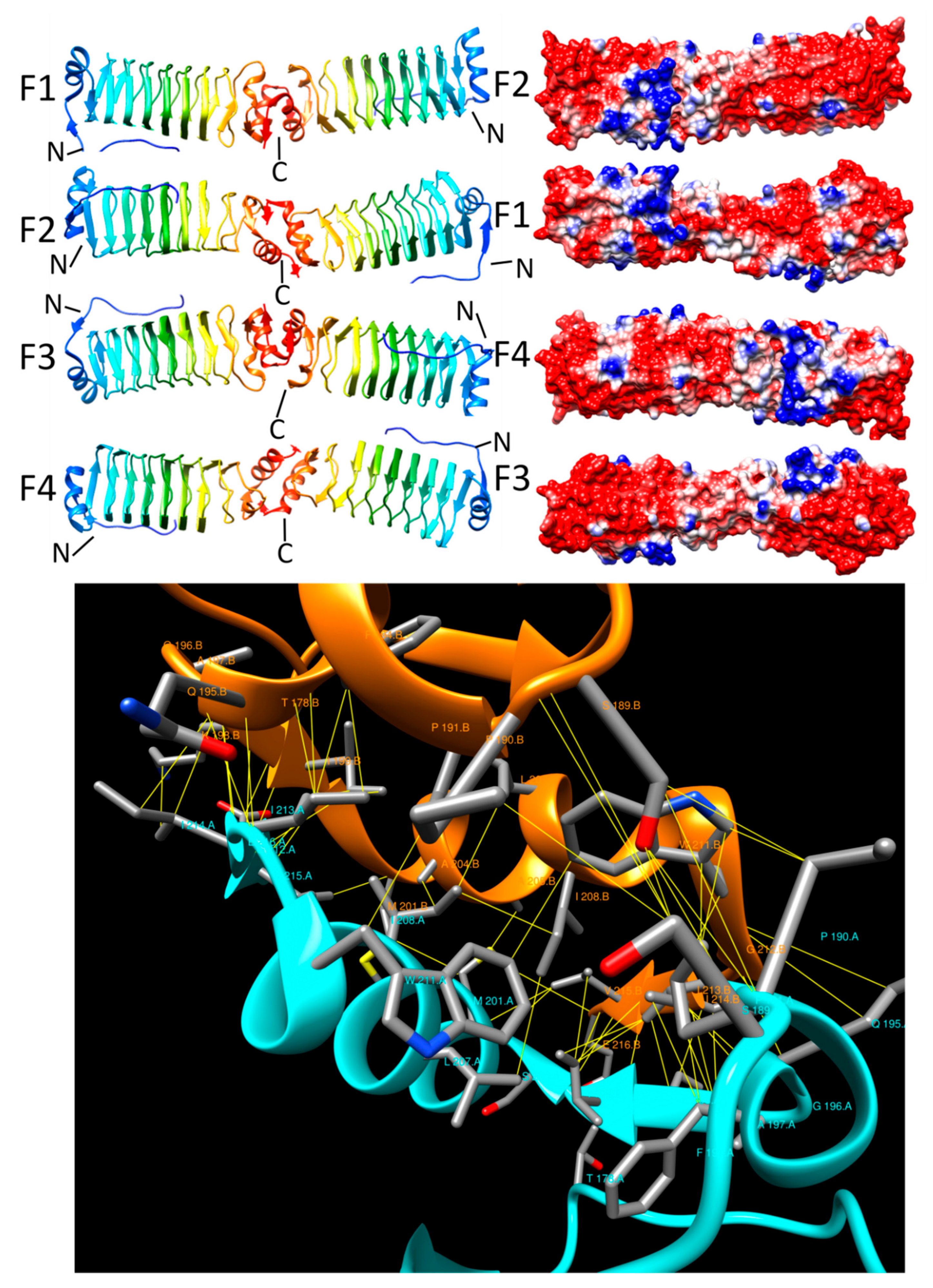
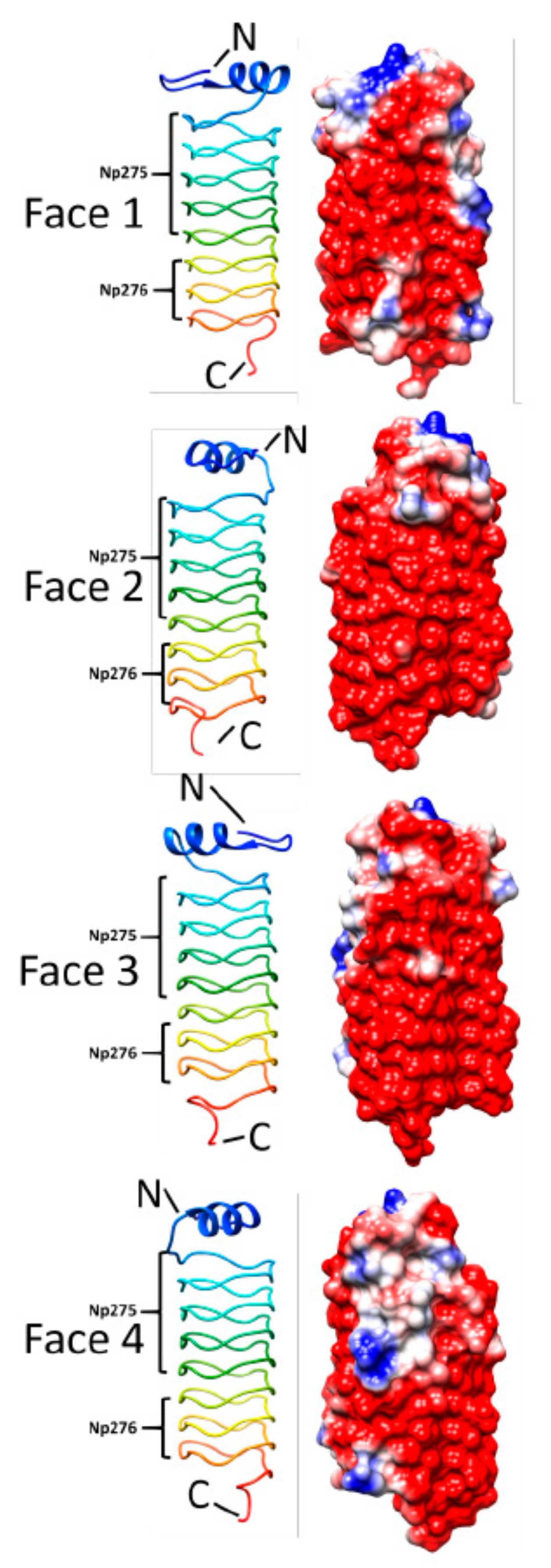
3.4. Np275/Np276 (2J8K and 2J8I)
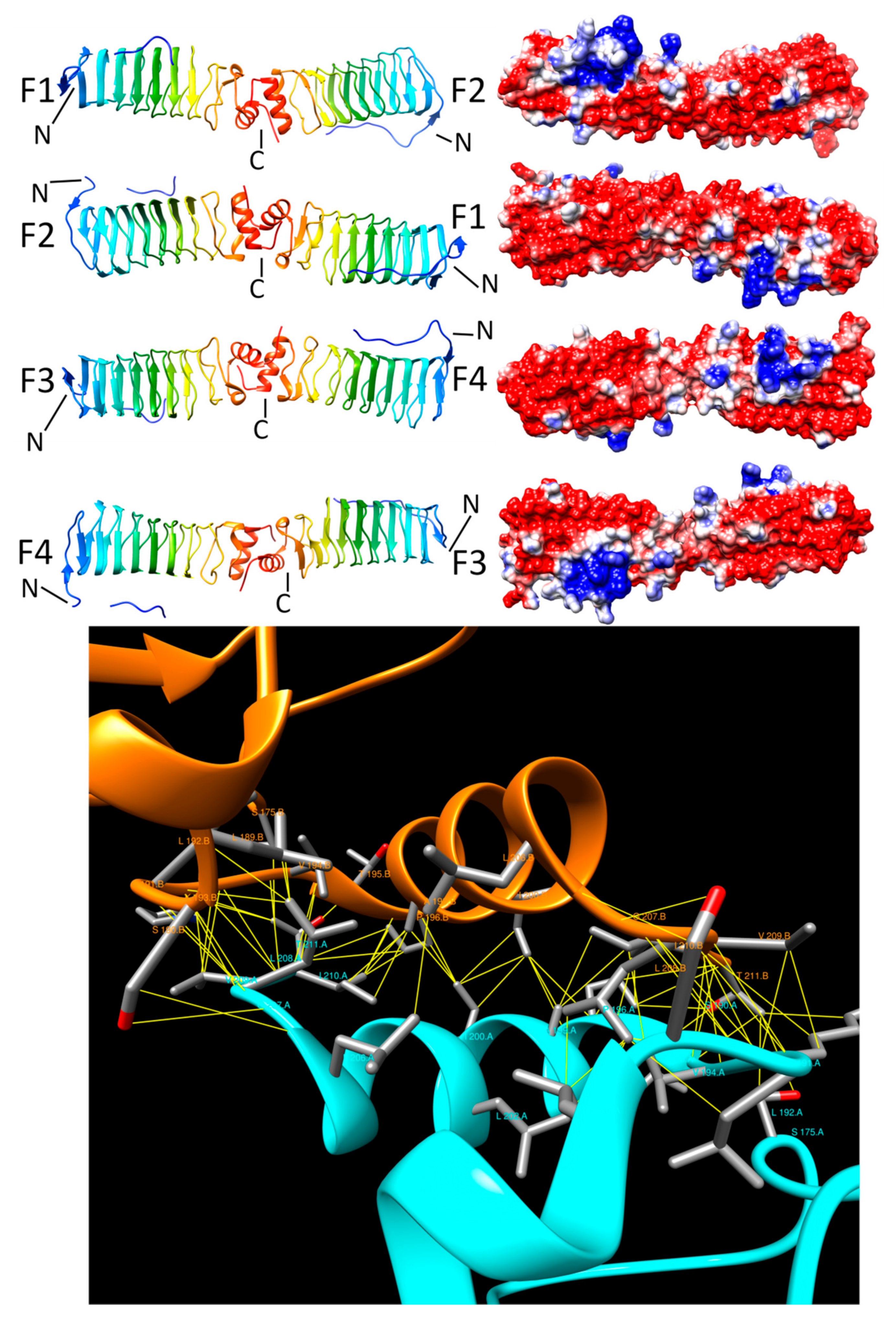
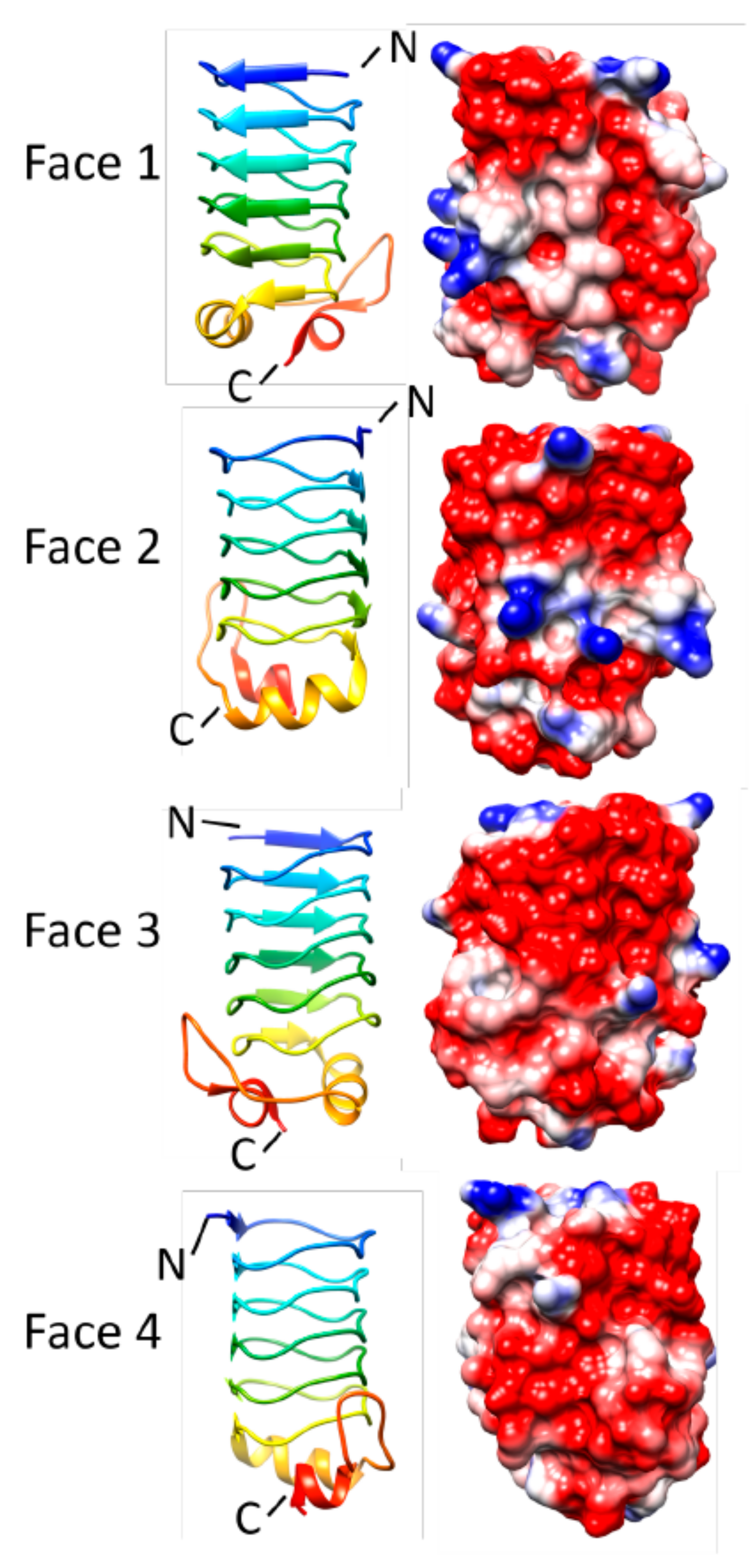
3.5. Rfr32 (2F3L and 2G0Y)
3.6. Rfr23 (2O6W)
3.7. At2g49920.2 (3N90)
3.8. Changes in PRP Gene Expression Levels Nostoc sp. st. PCC 7120 in Response to Nitrogen Deprivation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Black, K.; Buikema, W.J.; Haselkorn, R. The hglK gene is required for localization of heterocyst-specific glycolipids in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 1995, 177, 6440–6448. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Murzin, A.G.; Teichmann, S.A. Structure and distribution of pentapeptide repeats in bacteria. Protein Sci. 1998, 7, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, L.; Pascual, A.; Jacoby, G.A. Quinolone resistance from a transferable plasmid. Lancet 1998, 351, 797–799. [Google Scholar] [CrossRef]
- Rozwandowicz, M.; Brouwer, M.S.M.; Fischer, J.; Wagenaar, J.A.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.J.; Hordijk, J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef] [PubMed]
- Montero, C.; Mateu, G.; Rodriguez, R.; Takiff, H. Intrinsic resistance of Mycobacterium smegmatis to fluoroquinolones may be influenced by new pentapeptide protein MfpA. Antimicrob. Agents Chemother. 2001, 45, 3387–3392. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.S.; Vetting, M.W.; Roderick, S.L.; Mitchenall, L.A.; Maxwell, A.M.; Takiff, H.E.; Blanchard, J.S. A fluoroquinolone resistance protein from Mycobacterium tuberculosis that mimics DNA. Abstr. Pap. Am. Chem. Soc. 2005, 230, U538–U539. [Google Scholar] [CrossRef]
- Jacoby, G.A.; Hooper, D.C. Phylogenetic analysis of chromosomally determined qnr and related proteins. Antimicrob. Agents Chemother. 2013, 57, 1930–1934. [Google Scholar] [CrossRef]
- Chandler, L.E.; Bartsevich, V.V.; Pakrasi, H.B. Regulation of Manganese Uptake in Synechocystis 6803 by RfrA, a Member of a Novel Family of Proteins Containing a Repeated Five-Residues Domain. Biochemistry 2003, 42, 5508–5514. [Google Scholar] [CrossRef]
- Vetting, M.W.; Hegde, S.S.; Fajardo, J.E.; Fiser, A.; Roderick, S.L.; Takiff, H.E.; Mitchenall, L.A.; Maxwell, A. Pentapeptide repeat proteins. Biochemistry 2006, 5, 1–10. [Google Scholar] [CrossRef]
- Buchko, G.W. Pentapeptide Repeat Proteins and Cyanobacteria. Handb. Cyanobacteria Biochem. Biotechnol. Appl. 2009, 233–257. [Google Scholar]
- Shah, S.; Heddle, J.G. Squaring up to DNA: Pentapeptide repeat proteins and DNA mimicry. Appl. Microbiol. Biotechnol. 2014, 98, 9545–9560. [Google Scholar] [CrossRef]
- Ni, S.S.; Sheldrick, G.M.; Benning, M.M.; Kennedy, M.A. The 2 angstrom resolution crystal structure of HetL, a pentapeptide repeat protein involved in regulation of heterocyst differentiation in the cyanobacterium Nostoc sp. strain PCC 7120. J. Struct. Biol. 2009, 165, 47–52. [Google Scholar] [CrossRef]
- Vetting, M.W.; Hegde, S.S.; Hazleton, K.Z.; Blanchard, J.S. Structural characterization of the fusion of two pentapeptide repeat proteins, Np275 and Np276, from Nostoc punctiforme: Resurrection of an ancestral protein. Protein Sci. 2007, 16, 755–760. [Google Scholar] [CrossRef]
- Buchko, G.W.; Ni, S.S.; Robinson, H.; Welsh, E.A.; Pakrasi, H.B.; Kennedy, M.A. Characterization of two potentially universal turn motifs that shape the repeated five-residues fold-Crystal structure of a lumenal pentapeptide repeat protein from Cyanothece 51142. Protein Sci. 2006, 15, 2579–2595. [Google Scholar] [CrossRef]
- Buchko, G.W.; Robinson, H.; Pakrasi, H.B.; Kennedy, M.A. Insights into the structural variation between pentapeptide repeat proteins-Crystal structure of Rfr23 from Cyanothece 51142. J. Struct. Biol. 2008, 162, 184–192. [Google Scholar] [CrossRef]
- Ni, S.S.; McGookey, M.E.; Tinch, S.L.; Jones, A.N.; Jayaraman, S.; Tong, L.; Kennedy, M.A. The 1.7 A resolution structure of At2g44920, a pentapeptide-repeat protein in the thylakoid lumen of Arabidopsis thaliana. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2011, 67, 1480–1484. [Google Scholar] [CrossRef]
- Vetting, M.W.; Hegde, S.S.; Blanchard, J.S. Crystallization of a pentapeptide-repeat protein by reductive cyclic pentylation of free amines with glutaraldehyde. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 462–469. [Google Scholar] [CrossRef]
- Vetting, M.W.; Hegde, S.S.; Wang, M.H.; Jacoby, G.A.; Hooper, D.C.; Blanchard, J.S. Structure of QnrB1, a Plasmid-mediated Fluoroquinolone Resistance Factor. J. Biol. Chem. 2011, 286, 25265–25273. [Google Scholar] [CrossRef]
- Vetting, M.W.; Hegde, S.S.; Zhang, Y.; Blanchard, J.S. Pentapeptide-repeat proteins that act as topoisomerase poison resistance factors have a common dimer interface. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2011, 67, 296–302. [Google Scholar] [CrossRef]
- Xiong, X.L.; Bromley, E.H.C.; Oelschlaeger, P.; Woolfson, D.N.; Spencer, J. Structural insights into quinolone antibiotic resistance mediated by pentapeptide repeat proteins: Conserved surface loops direct the activity of a Qnr protein from a Gram-negative bacterium. Nucleic Acids Res. 2011, 39, 3917–3927. [Google Scholar] [CrossRef]
- Zhang, R.; Ni, S.; Kennedy, M.A. Type I beta turns make a new twist in pentapeptide repeat proteins: Crystal structure of Alr5209 from Nostoc sp. PCC 7120 determined at 1.7 angström resolution. J. Struct. Biol. X 2019, 3, 100010. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Kennedy, M.A. Structural dynamics of pentapeptide repeat proteins. Proteins 2020, 88, 1493–1512. [Google Scholar] [CrossRef] [PubMed]
- Lechno-Yossef, S.; Fan, Q.; Wojciuch, E.; Wolk, C.P. Identification of Ten Anabaena sp. Genes That under Aerobic Conditions Are Required for Growth on Dinitrogen but Not for Growth on Fixed Nitrogen. J. Bacteriol. 2011, 193, 3482–3489. [Google Scholar] [CrossRef] [PubMed]
- Buikema, W.J.; Haselkorn, R. Isolation and complementation of nitrogen fixation mutants of the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 1991, 173, 1879–1885. [Google Scholar] [PubMed]
- Arévalo, S.; Flores, E. Pentapeptide-repeat, cytoplasmic-membrane protein HglK influences the septal junctions in the heterocystous cyanobacterium Anabaena. Mol. Microbiol. 2020, 113, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Botello-Morte, L.; González, A.; Bes, M.T.; Peleato, M.L.; Fillat, M.F. Chapter Four-Functional Genomics of Metalloregulators in Cyanobacteria. In Advances in Botanical Research; Chauvat, F., Cassier-Chauvat, C., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 65, pp. 107–156. [Google Scholar]
- Vilchèze, C.; Jacobs, W.R., Jr. Resistance to Isoniazid and Ethionamide in Mycobacterium tuberculosis: Genes, Mutations, and Causalities. Microbiol. Spectr. 2014, 2, Mgm2-0014-2013. [Google Scholar] [CrossRef] [PubMed]
- Ray, N.; Cavin, X.; Paul, J.C.; Maigret, B. Intersurf: Dynamic interface between proteins. J. Mol. Graph. Model. 2005, 23, 347–354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Arsene, S.; Leclercq, R. Role of a qnr-like gene in the intrinsic resistance of Enterococcus faecalis to fluoroquinolones. Antimicrob. Agents Chemother. 2007, 51, 3254–3258. [Google Scholar] [CrossRef]
- Tran, J.H.; Jacoby, G.A. Mechanism of plasmid-mediated quinolone resistance. Proc. Natl. Acad. Sci. USA 2002, 99, 5638–5642. [Google Scholar] [CrossRef]
- Strahilevitz, J.; Jacoby, G.A.; Hooper, D.C.; Robicsek, A. Plasmid-mediated quinolone resistance: A multifaceted threat. Clin. Microbiol. Rev. 2009, 22, 664–689. [Google Scholar] [CrossRef]
- Li, X.J.; Zhang, Y.J.; Zhou, X.T.; Hu, X.L.; Zhou, Y.X.; Liu, D.; Maxwell, A.; Mi, K.X. The plasmid-borne quinolone resistance protein QnrB, a novel DnaA-binding protein, increases the bacterial mutation rate by triggering DNA replication stress. Mol. Microbiol. 2019, 111, 1529–1543. [Google Scholar] [CrossRef]
- Jacoby, G.A.; Walsh, K.E.; Mills, D.M.; Walker, V.J.; Oh, H.; Robicsek, A.; Hooper, D.C. qnrB, another plasmid-mediated gene for quinolone resistance. Antimicrob. Agents Chemother. 2006, 50, 1178–1182. [Google Scholar] [CrossRef]
- Wang, M.H.; Guo, Q.L.; Xu, X.G.; Wang, X.Y.; Ye, X.Y.; Wu, S.; Hooper, D.C.; Wang, M.G. New Plasmid-Mediated Quinolone Resistance Gene, qnrC, Found in a Clinical Isolate of Proteus mirabilis. Antimicrob. Agents Chemother. 2009, 53, 1892–1897. [Google Scholar] [CrossRef]
- Cavaco, L.M.; Frimodt-Moller, N.; Hasman, H.; Guardabassi, L.; Nielsen, L.; Aarestrup, F.M. Prevalence of quinolone resistance mechanisms and associations to minimum inhibitory concentrations in quinolone-resistant Escherichia coli isolated from humans and swine in Denmark. Microb. Drug Resist. 2008, 14, 163–169. [Google Scholar] [CrossRef]
- Albornoz, E.; Tijet, N.; De Belder, D.; Gomez, S.; Martino, F.; Corso, A.; Melano, R.G.; Petroni, A. qnrE1, a Member of a New Family of Plasmid-Located Quinolone Resistance Genes, Originated from the Chromosome of Enterobacter Species. Antimicrob. Agents Chemother. 2017, 61, e02555-16. [Google Scholar] [CrossRef]
- Hata, M.; Suzuki, M.; Matsumoto, M.; Takahashi, M.; Sato, K.; Ibe, S.; Sakae, K. Cloning of a novel gene for quinolone resistance from a transferable plasmid in Shigella flexneri 2b. Antimicrob. Agents Chemother. 2005, 49, 801–803. [Google Scholar] [CrossRef]
- Fonseca, E.L.; Dos Santos Freitas, F.; Vieira, V.V.; Vicente, A.C. New qnr gene cassettes associated with superintegron repeats in Vibrio cholerae O1. Emerg. Infect Dis. 2008, 14, 1129–1131. [Google Scholar] [CrossRef]
- Zhan, Y.Y.; Zheng, Z.W.; Chan, E.W.C.; Dong, N.; Xia, X.D.; Chen, S. Molecular Characterization of qnrVC Genes and Their Novel Alleles in Vibrio spp. Isolated from Food Products in China. Antimicrob. Agents Chemother. 2018, 62, e00529-18. [Google Scholar]
- Ruiz, J. Transferable Mechanisms of Quinolone Resistance from 1998 Onward. Clin. Microbiol. Rev. 2019, 32, e00007-19. [Google Scholar] [CrossRef]
- Hashimi, S.M.; Wall, M.K.; Smith, A.B.; Maxwell, A.; Birch, R.G. The phytotoxin albicidin is a novel inhibitor of DNA gyrase. Antimicrob. Agents Chemother. 2007, 51, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Notari, L.; Martinez-Carranza, M.; Farias-Rico, J.A.; Stenmark, P.; von Heijne, G. Cotranslational Folding of a Pentarepeat beta-Helix Protein. J. Mol. Biol. 2018, 430, 5196–5206. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Zhang, Y.; Huibregtse, J.M.; Zhou, D.; Chen, J. Crystal structure of SopA, a Salmonella effector protein mimicking a eukaryotic ubiquitin ligase. Nat. Struct. Mol. Biol. 2008, 15, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.S.; Aoki, S.K.; Low, D.A. Bacterial contact-dependent delivery systems. Annu. Rev. Genet. 2010, 44, 71–90. [Google Scholar] [CrossRef]
- Galan, J.E.; Wolf-Watz, H. Protein delivery into eukaryotic cells by type III secretion machines. Nature 2006, 444, 567–573. [Google Scholar] [CrossRef]
- Wang, Y.; Argiles-Castillo, D.; Kane, E.I.; Zhou, A.; Spratt, D.E. HECT E3 ubiquitin ligases—Emerging insights into their biological roles and disease relevance. J. Cell Sci. 2020, 133, jcs228072. [Google Scholar] [CrossRef]
- Zhang, Y.; Higashide, W.M.; McCormick, B.A.; Chen, J.; Zhou, D.G. The inflammation-associated Salmonella SopA is a HECT-like E3 ubiquitin ligase. Mol. Microbiol. 2006, 62, 786–793. [Google Scholar] [CrossRef]
- Fiskin, E.; Bhogaraju, S.; Herhaus, L.; Kalayil, S.; Hahn, M.; Dikic, I. Structural basis for the recognition and degradation of host TRIM proteins by Salmonella effector SopA. Nat. Commun. 2017, 8, 14004. [Google Scholar] [CrossRef]
- Deshaies, R.J.; Joazeiro, C.A. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 2009, 78, 399–434. [Google Scholar] [CrossRef]
- Kamanova, J.; Sun, H.; Lara-Tejero, M.; Galan, J.E. The Salmonella Effector Protein SopA Modulates Innate Immune Responses by Targeting TRIM E3 Ligase Family Members. PLoS Pathog. 2016, 12, e1005552. [Google Scholar] [CrossRef]
- Lin, D.Y.W.; Diao, J.B.; Zhou, D.G.; Chen, J. Biochemical and Structural Studies of a HECT-like Ubiquitin Ligase from Escherichia coli O157:H7. J. Biol. Chem. 2011, 286, 441–449. [Google Scholar] [CrossRef]
- Sheng, X.; You, Q.; Zhu, H.; Chang, Z.; Li, Q.; Wang, H.; Wang, C.; Wang, H.; Hui, L.; Du, C.; et al. Bacterial effector NleL promotes enterohemorrhagic E. coli-induced attaching and effacing lesions by ubiquitylating and inactivating JNK. PLoS Pathog. 2017, 13, e1006534. [Google Scholar] [CrossRef]
- Sheng, X.; You, Q.; Zhu, H.; Li, Q.; Gao, H.; Wang, H.; You, C.; Meng, Q.; Nie, Y.; Zhang, X.; et al. Enterohemorrhagic E. coli effector NleL disrupts host NF-kappaB signaling by targeting multiple host proteins. J. Mol. Cell Biol. 2020, 12, 318–321. [Google Scholar] [CrossRef]
- Lin, D.Y.W.; Diao, J.B.; Chen, J. Crystal structures of two bacterial HECT-like E3 ligases in complex with a human E2 reveal atomic details of pathogen-host interactions. Proc. Natl. Acad. Sci. USA 2012, 109, 1925–1930. [Google Scholar] [CrossRef]
- Janz, R.; Goda, Y.; Geppert, M.; Missler, M.; Südhof, T.C. SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron 1999, 24, 1003–1016. [Google Scholar] [CrossRef]
- Dong, M.; Yeh, F.; Tepp, W.H.; Dean, C.; Johnson, E.A.; Janz, R.; Chapman, E.R. SV2 Is the Protein Receptor for Botulinum Neurotoxin A. Science 2006, 312, 592–596. [Google Scholar] [CrossRef]
- Yao, G.R.; Zhang, S.C.; Mahrhold, S.; Lam, K.H.; Stern, D.; Bagramyan, K.; Perry, K.; Kalkum, M.; Rummel, A.; Dong, M.; et al. N-linked glycosylation of SV2 is required for binding and uptake of botulinum neurotoxin A. Nat. Struct. Mol. Biol. 2016, 23, 656–662. [Google Scholar] [CrossRef]
- Benoit, R.M.; Frey, D.; Hilbert, M.; Kevenaar, J.T.; Wieser, M.M.; Stirnimann, C.U.; McMillan, D.; Ceska, T.; Lebon, F.; Jaussi, R.; et al. Structural basis for recognition of synaptic vesicle protein 2C by botulinum neurotoxin A. Nature 2014, 505, 108–111. [Google Scholar] [CrossRef]
- Benoit, R.M.; Scharer, M.A.; Wieser, M.M.; Li, X.D.; Frey, D.; Kammerer, R.A. Crystal structure of the BoNT/A2 receptor-binding domain in complex with the luminal domain of its neuronal receptor SV2C. Sci. Rep. 2017, 7, 43588. [Google Scholar] [CrossRef]
- Gustafsson, R.; Zhang, S.C.; Masuyer, G.; Dong, M.; Stenmark, P. Crystal Structure of Botulinum Neurotoxin A2 in Complex with the Human Protein Receptor SV2C Reveals Plasticity in Receptor Binding. Toxins 2018, 10, 153. [Google Scholar] [CrossRef]
- Liu, D.; Golden, J.W. hetL overexpression stimulates heterocyst formation in Anabaena sp. strain PCC 7120. J Bacteriol. 2002, 184, 6873–6881. [Google Scholar] [CrossRef]
- Zhang, R.J.; Ni, S.S.; Kennedy, M.A. Crystal structure of Alr1298, a pentapeptide repeat protein from the cyanobacterium Nostoc sp. PCC 7120, determined at 2.1 angstrom resolution. Proteins 2020, 88, 1143–1153. [Google Scholar] [CrossRef]
- Jacob, F.; Monod, J. On Regulation of Gene Activity. Cold Spring Harb. Symp. 1961, 26, 193–211. [Google Scholar] [CrossRef]
- Lawrence, J.G. Shared strategies in gene organization among prokaryotes and eukaryotes. Cell 2002, 110, 407–413. [Google Scholar] [CrossRef]
- Ralston, A. Operons and prokaryotic gene regulation. Nat. Educ. 2008, 1, 216. [Google Scholar]
- Ehira, S.; Ohmori, M. NrrA, a nitrogen-responsive response regulator facilitates heterocyst development in the cyanobacterium Anabaena sp. strain PCC 7120. Mol. Microbiol. 2006, 59, 1692–1703. [Google Scholar] [CrossRef]
- Zybailov, B.; Rutschow, H.; Friso, G.; Rudella, A.; Emanuelsson, O.; Sun, Q.; Van Wijk, K.J. Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS ONE 2008, 3, e1994. [Google Scholar] [CrossRef]
- Agrawal, G.K.; Yonekura, M.; Iwahashi, Y.; Iwahashi, H.; Rakwal, R. System, trends and perspectives of proteomics in dicot plants Part I: Technologies in proteome establishment. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 815, 109–123. [Google Scholar] [CrossRef]
- Barbazuk, W.B.; Fu, Y.; McGinnis, K.M. Genome-wide analyses of alternative splicing in plants: Opportunities and challenges. Genome Res. 2008, 18, 1381–1392. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Kennedy, M.A. Current Understanding of the Structure and Function of Pentapeptide Repeat Proteins. Biomolecules 2021, 11, 638. https://doi.org/10.3390/biom11050638
Zhang R, Kennedy MA. Current Understanding of the Structure and Function of Pentapeptide Repeat Proteins. Biomolecules. 2021; 11(5):638. https://doi.org/10.3390/biom11050638
Chicago/Turabian StyleZhang, Ruojing, and Michael A. Kennedy. 2021. "Current Understanding of the Structure and Function of Pentapeptide Repeat Proteins" Biomolecules 11, no. 5: 638. https://doi.org/10.3390/biom11050638
APA StyleZhang, R., & Kennedy, M. A. (2021). Current Understanding of the Structure and Function of Pentapeptide Repeat Proteins. Biomolecules, 11(5), 638. https://doi.org/10.3390/biom11050638






