The Most Competent Plant-Derived Natural Products for Targeting Apoptosis in Cancer Therapy
Abstract
1. Introduction
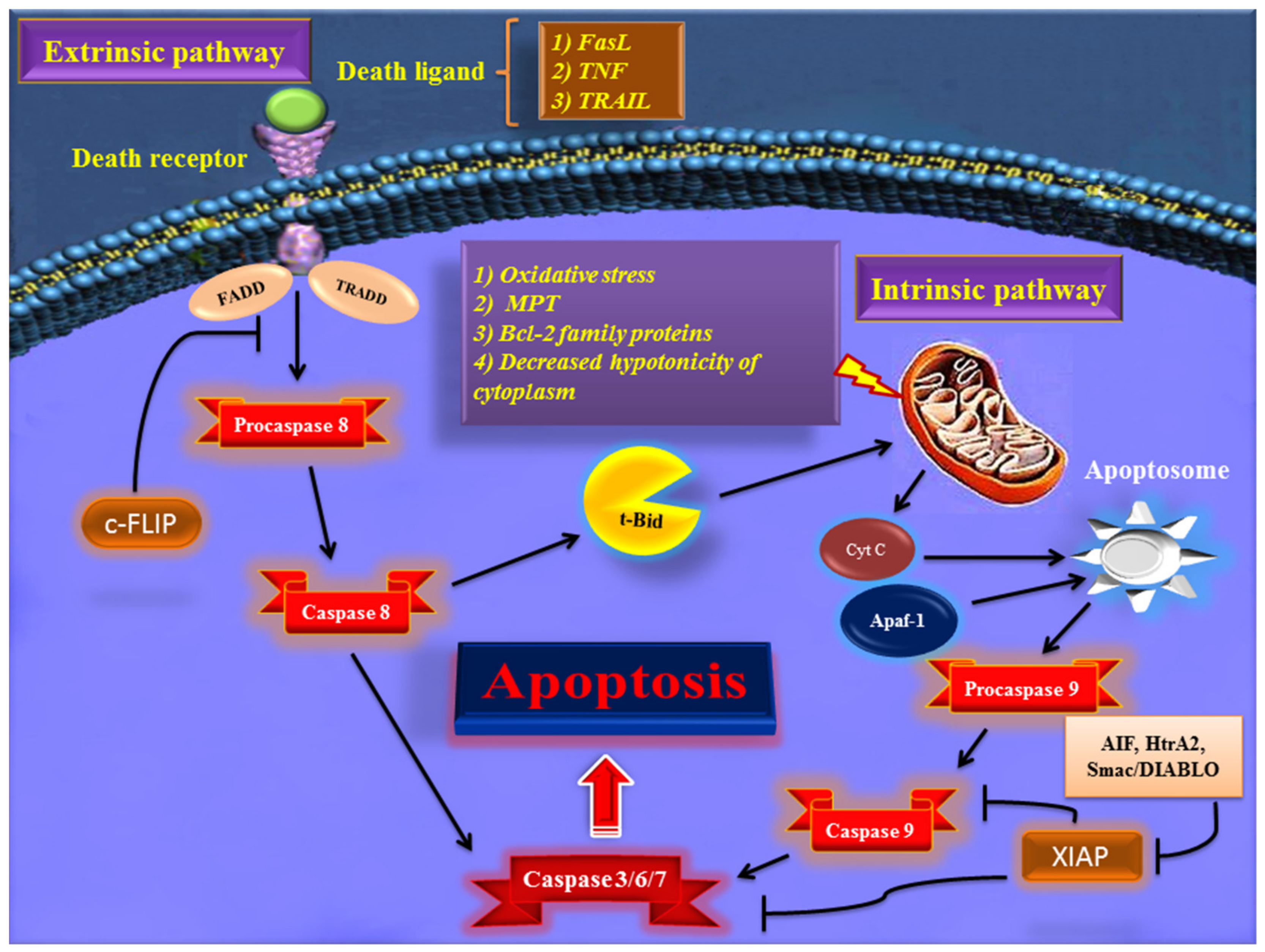
2. Major Apoptotic Pathways
2.1. Extrinsic Pathway of Apoptosis
2.2. Intrinsic Pathway of Apoptosis
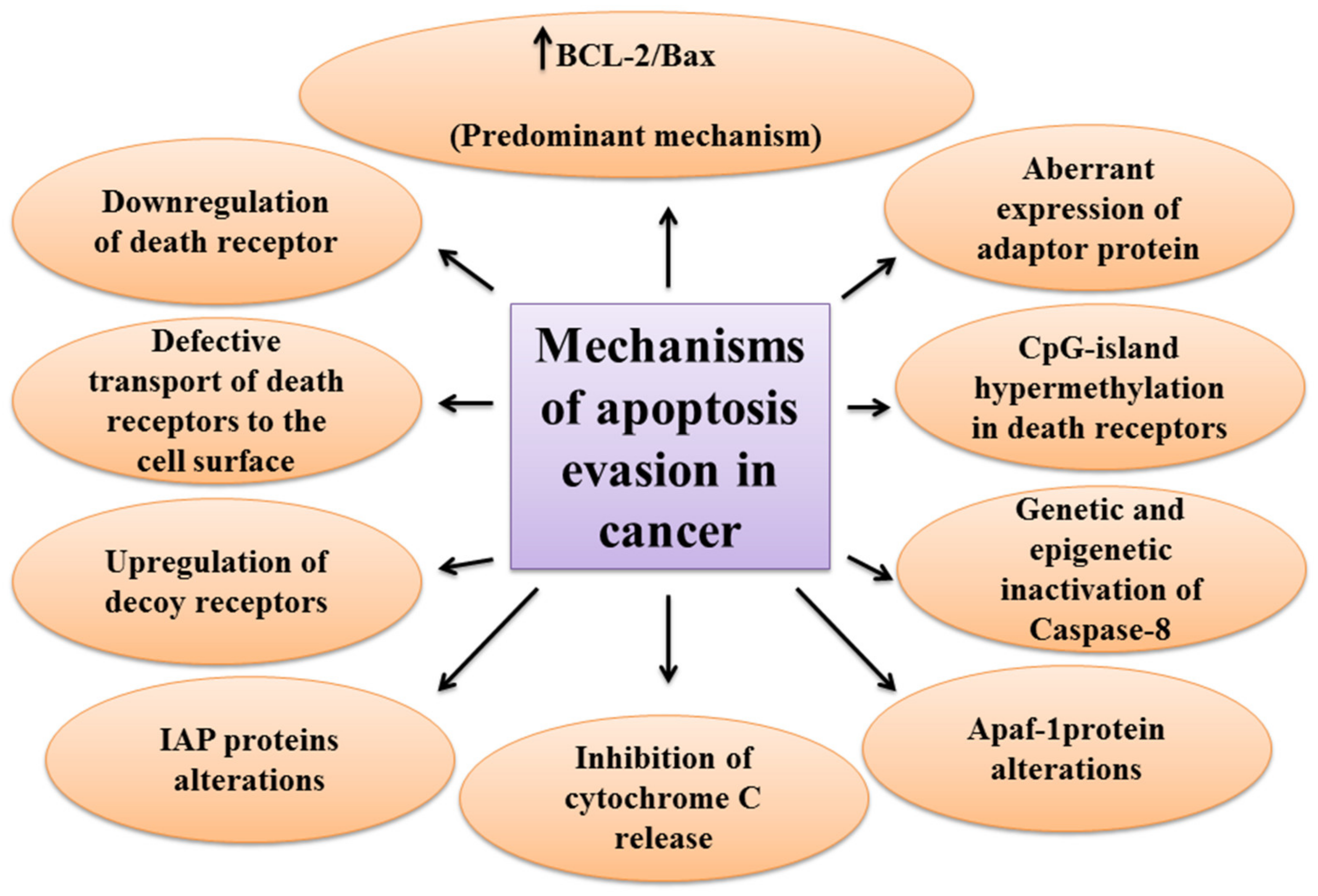
2.3. The Mechanisms of Apoptosis Evasion in Cancer
2.4. Plant Materials That Simultaneously Target Both Intrinsic and Extrinsic Pathways
2.4.1. Plant Extracts
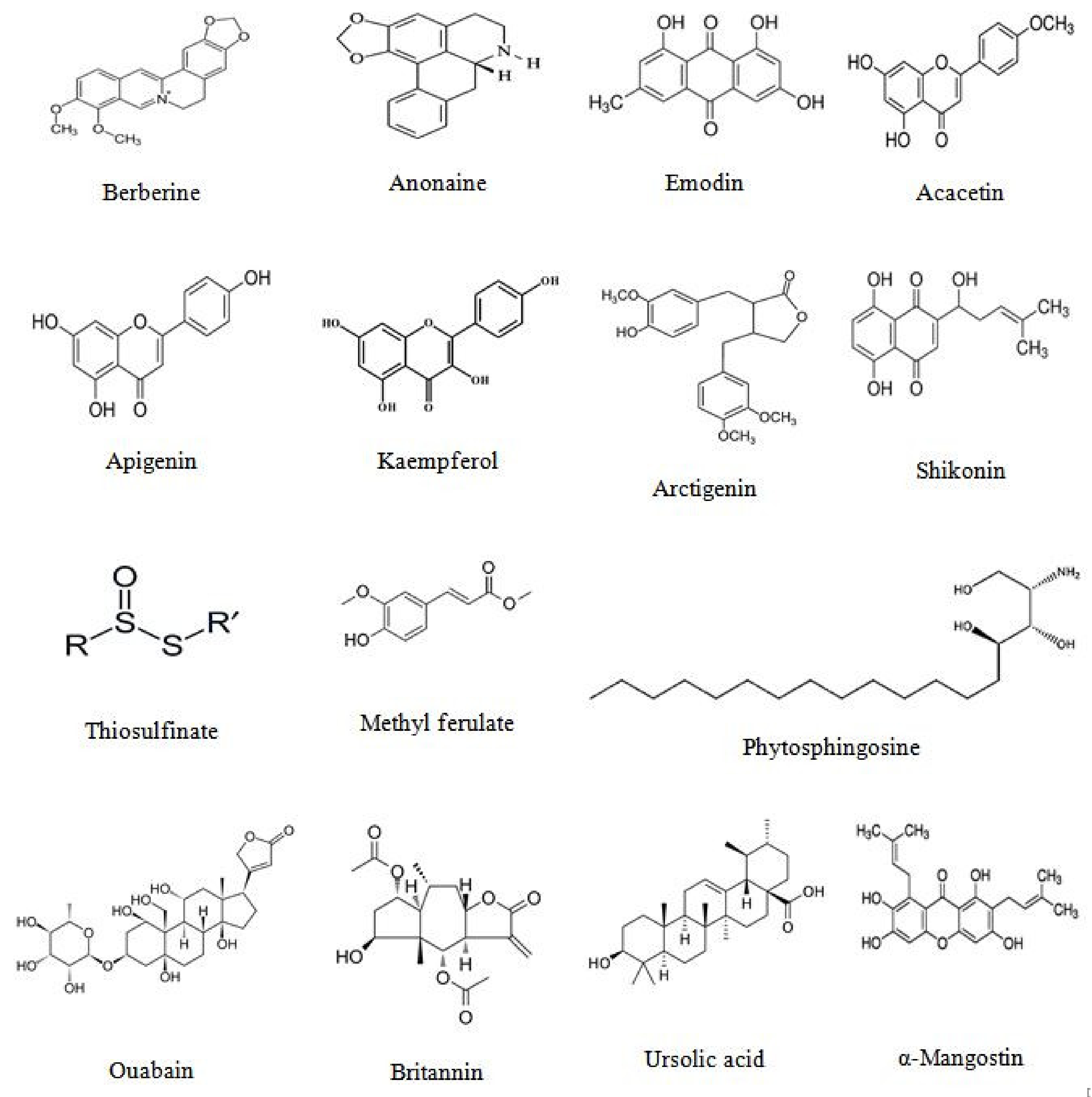
2.4.2. Isolated Phytoconstituents
2.5. Use of Plant Extracts and Plant Molecules in Human Clinical Trials
2.6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashraf, M.A. Phytochemicals as Potential Anticancer Drugs: Time to Ponder Nature’s Bounty. Biomed. Res. Int. 2020, 2020, 8602879. [Google Scholar] [CrossRef]
- The global challenge of cancer. Nat. Cancer 2020, 1, 1–2. [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Sonnenschein, C.; Soto, A.M. The aging of the 2000 and 2011 Hallmarks of Cancer reviews: A critique. J. Biosci. 2013, 38, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar] [PubMed]
- Koff, J.L.; Ramachandiran, S.; Bernal-Mizrachi, L. A time to kill: Targeting apoptosis in cancer. Int. J. Mol. Sci. 2015, 16, 2942–2955. [Google Scholar] [CrossRef] [PubMed]
- Omara, T.; Kiprop, A.K.; Ramkat, R.C.; Cherutoi, J.; Kagoya, S.; Moraa Nyangena, D.; Azeze Tebo, T.; Nteziyaremye, P.; Nyambura Karanja, L.; Jepchirchir, A.; et al. Medicinal Plants Used in Traditional Management of Cancer in Uganda: A Review of Ethnobotanical Surveys, Phytochemistry, and Anticancer Studies. Evid. Based Complement. Alternat. Med. 2020, 2020, 3529081. [Google Scholar] [CrossRef] [PubMed]
- Aye, M.M.; Aung, H.T.; Sein, M.M.; Armijos, C. A Review on the Phytochemistry, Medicinal Properties and Pharmacological Activities of 15 Selected Myanmar Medicinal Plants. Molecules 2019, 24, 293. [Google Scholar] [CrossRef] [PubMed]
- Girma, B.; Mulisa, E.; Tessema, S.; Amelo, W. Ethnomedicine Claim Directed in Silico Prediction of Anticancer Activity. Ethiop. J. Health Sci. 2018, 28, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer Plants: A Review of the Active Phytochemicals, Applications in Animal Models, and Regulatory Aspects. Biomolecules 2019, 10, 47. [Google Scholar] [CrossRef]
- Duque-Parra, J.E. Note on the origin and history of the term “apoptosis”. Anat. Rec. B New Anat. 2005, 283, 2–4. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; El-Deiry, W.S.; Golstein, P.; Peter, M.E.; Vaux, D.; Vandenabeele, P.; Zhivotovsky, B.; Blagosklonny, M.V.; Malorni, W.; Knight, R.A.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005, 12, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, U.; Groscurth, P. Morphological features of cell death. News Physiol. Sci. 2004, 19, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Segawa, K.; Nagata, S. An Apoptotic “Eat Me” Signal: Phosphatidylserine Exposure. Trends Cell Biol. 2015, 25, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Chaudhry, G.E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef]
- Jin, Z.; El-Deiry, W.S. Overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 139–163. [Google Scholar] [CrossRef]
- Itoh, N.; Yonehara, S.; Ishii, A.; Yonehara, M.; Mizushima, S.; Sameshima, M.; Hase, A.; Seto, Y.; Nagata, S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 1991, 66, 233–243. [Google Scholar] [CrossRef]
- Pan, G.; O’Rourke, K.; Chinnaiyan, A.M.; Gentz, R.; Ebner, R.; Ni, J.; Dixit, V.M. The receptor for the cytotoxic ligand TRAIL. Science 1997, 276, 111–113. [Google Scholar] [CrossRef]
- Schneider, P.; Thome, M.; Burns, K.; Bodmer, J.L.; Hofmann, K.; Kataoka, T.; Holler, N.; Tschopp, J. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity 1997, 7, 831–836. [Google Scholar] [CrossRef]
- Huang, B.; Eberstadt, M.; Olejniczak, E.T.; Meadows, R.P.; Fesik, S.W. NMR structure and mutagenesis of the Fas (APO-1/CD95) death domain. Nature 1996, 384, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Pobezinskaya, Y.L.; Morgan, M.J.; Liu, Z.G. The role of TRADD in TRAIL-induced apoptosis and signaling. FASEB J. 2011, 25, 1353–1358. [Google Scholar] [CrossRef]
- Lee, E.W.; Seo, J.; Jeong, M.; Lee, S.; Song, J. The roles of FADD in extrinsic apoptosis and necroptosis. BMB Rep. 2012, 45, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, L.; Espona-Fiedler, M.; Longley, D.B. FLIP as a therapeutic target in cancer. FEBS J. 2018, 285, 4104–4123. [Google Scholar] [CrossRef]
- Billen, L.P.; Shamas-Din, A.; Andrews, D.W. Bid: A Bax-like BH3 protein. Oncogene 2008, 27, S93–S104. [Google Scholar] [CrossRef] [PubMed]
- Ozören, N.; El-Deiry, W.S. Defining characteristics of Types I and II apoptotic cells in response to TRAIL. Neoplasia 2002, 4, 551–557. [Google Scholar] [CrossRef]
- Sheikh, M.S.; Fornace, A.J., Jr. Death and decoy receptors and p53-mediated apoptosis. Leukemia 2000, 14, 1509–1513. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef]
- Loreto, C.; La Rocca, G.; Anzalone, R.; Caltabiano, R.; Vespasiani, G.; Castorina, S.; Ralph, D.J.; Cellek, S.; Musumeci, G.; Giunta, S.; et al. The role of intrinsic pathway in apoptosis activation and progression in Peyronie’s disease. Biomed. Res. Int. 2014, 2014, 616149. [Google Scholar] [CrossRef]
- Muñoz-Pinedo, C. Signaling pathways that regulate life and cell death: Evolution of apoptosis in the context of self-defense. Adv. Exp. Med. Biol. 2012, 738, 124–143. [Google Scholar] [CrossRef]
- Mishra, N.C.; Kumar, S. Apoptosis: A mitochondrial perspective on cell death. Indian J. Exp. Biol. 2005, 43, 25–34. [Google Scholar] [PubMed]
- Zhang, J.; Yu, Q.; Han, L.; Chen, C.; Li, H.; Han, G. Study on the apoptosis mediated by cytochrome c and factors that affect the activation of bovine longissimus muscle during postmortem aging. Apoptosis 2017, 22, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim. Biophys. Acta 2006, 1757, 639–647. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Opferman, J.T.; Kothari, A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ. 2018, 25, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Sinha, S.; Kroemer, G. Bcl-2 family members: Dual regulators of apoptosis and autophagy. Autophagy 2008, 4, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; McCallum, J.E.; Varghese, E.; Florea, A.M.; Büsselberg, D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis 2017, 22, 898–919. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Tumor resistance to apoptosis. Int. J. Cancer 2009, 124, 511–515. [Google Scholar] [CrossRef]
- Friesen, C.; Fulda, S.; Debatin, K.M. Deficient activation of the CD95 (APO-1/Fas) system in drug-resistant cells. Leukemia 1997, 11, 1833–1841. [Google Scholar] [CrossRef]
- Tourneur, L.; Delluc, S.; Lévy, V.; Valensi, F.; Radford-Weiss, I.; Legrand, O.; Vargaftig, J.; Boix, C.; Macintyre, E.A.; Varet, B.; et al. Absence or low expression of fas-associated protein with death domain in acute myeloid leukemia cells predicts resistance to chemotherapy and poor outcome. Cancer Res. 2004, 64, 8101–8108. [Google Scholar] [CrossRef]
- Jin, Z.; McDonald, E.R., 3rd; Dicker, D.T.; El-Deiry, W.S. Deficient tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor transport to the cell surface in human colon cancer cells selected for resistance to TRAIL-induced apoptosis. J. Biol. Chem. 2004, 279, 35829–35839. [Google Scholar] [CrossRef] [PubMed]
- Manoochehri, M.; Borhani, N.; Karbasi, A.; Koochaki, A.; Kazemi, B. Promoter hypermethylation and downregulation of the FAS gene may be involved in colorectal carcinogenesis. Oncol. Lett. 2016, 12, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Pitti, R.M.; Marsters, S.A.; Lawrence, D.A.; Roy, M.; Kischkel, F.C.; Dowd, P.; Huang, A.; Donahue, C.J.; Sherwood, S.W.; Baldwin, D.T.; et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature 1998, 396, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Korkolopoulou, P.; Saetta, A.A.; Levidou, G.; Gigelou, F.; Lazaris, A.; Thymara, I.; Scliri, M.; Bousboukea, K.; Michalopoulos, N.V.; Apostolikas, N.; et al. c-FLIP expression in colorectal carcinomas: Association with Fas/FasL expression and prognostic implications. Histopathology 2007, 51, 150–156. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, T.G.; Yang, H.; DeWolf, W.C.; Khosravi-Far, R.; Olumi, A.F. Persistent c-FLIP(L) expression is necessary and sufficient to maintain resistance to tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in prostate cancer. Cancer Res. 2004, 64, 7086–7091. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Song, X.; Sima, N.; Xu, X.; Luo, A.; Chen, G.; Deng, D.; Xu, Q.; Meng, L.; et al. The relationship between c-FLIP expression and human papillomavirus E2 gene disruption in cervical carcinogenesis. Gynecol. Oncol. 2007, 105, 571–577. [Google Scholar] [CrossRef]
- Teitz, T.; Wei, T.; Valentine, M.B.; Vanin, E.F.; Grenet, J.; Valentine, V.A.; Behm, F.G.; Look, A.T.; Lahti, J.M.; Kidd, V.J. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat. Med. 2000, 6, 529–535. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, J.W.; Soung, Y.H.; Park, W.S.; Kim, S.Y.; Lee, J.H.; Park, J.Y.; Cho, Y.G.; Kim, C.J.; Jeong, S.W.; et al. Inactivating mutations of caspase-8 gene in colorectal carcinomas. Gastroenterology 2003, 125, 708–715. [Google Scholar] [CrossRef]
- Mandruzzato, S.; Brasseur, F.; Andry, G.; Boon, T.; van der Bruggen, P. A CASP-8 mutation recognized by cytolytic T lymphocytes on a human head and neck carcinoma. J. Exp. Med. 1997, 186, 785–793. [Google Scholar] [CrossRef]
- Shivapurkar, N.; Reddy, J.; Matta, H.; Sathyanarayana, U.G.; Huang, C.X.; Toyooka, S.; Minna, J.D.; Chaudhary, P.M.; Gazdar, A.F. Loss of expression of death-inducing signaling complex (DISC) components in lung cancer cell lines and the influence of MYC amplification. Oncogene 2002, 21, 8510–8514. [Google Scholar] [CrossRef][Green Version]
- Zuzak, T.J.; Steinhoff, D.F.; Sutton, L.N.; Phillips, P.C.; Eggert, A.; Grotzer, M.A. Loss of caspase-8 mRNA expression is common in childhood primitive neuroectodermal brain tumour/medulloblastoma. Eur. J. Cancer 2002, 38, 83–91. [Google Scholar] [CrossRef]
- Fernald, K.; Kurokawa, M. Evading apoptosis in cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef]
- Yip, K.W.; Reed, J.C. Bcl-2 family proteins and cancer. Oncogene 2008, 27, 6398–6406. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.B.; Chapagain, P.P.; Üren, A. Investigating molecular interactions between oxidized neuroglobin and cytochrome c. Sci. Rep. 2018, 8, 10557. [Google Scholar] [CrossRef] [PubMed]
- Fiocchetti, M.; Fernandez, V.S.; Montalesi, E.; Marino, M. Neuroglobin: A Novel Player in the Oxidative Stress Response of Cancer Cells. Oxidative Med. Cell Longev. 2019, 2019, 6315034. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Kwok, R.P.; Shukla, A.; Kshirsagar, M.; Zhao, L.; Opipari, A.W., Jr.; Liu, J.R. Trichostatin A restores Apaf-1 function in chemoresistant ovarian cancer cells. Cancer 2011, 117, 784–794. [Google Scholar] [CrossRef]
- Tian, J.; Shen, R.; Yan, Y.; Deng, L. miR-186 promotes tumor growth in cutaneous squamous cell carcinoma by inhibiting apoptotic protease activating factor-1. Exp. Ther. Med. 2018, 16, 4010–4018. [Google Scholar] [CrossRef]
- Obexer, P.; Ausserlechner, M.J. X-linked inhibitor of apoptosis protein—A critical death resistance regulator and therapeutic target for personalized cancer therapy. Front. Oncol. 2014, 4, 197. [Google Scholar] [CrossRef]
- Subapriya, R.; Bhuvaneswari, V.; Nagini, S. Ethanolic neem (Azadirachta indica) leaf extract induces apoptosis in the hamster buccal pouch carcinogenesis model by modulation of Bcl-2, Bim, caspase 8 and caspase 3. Asian Pac. J. Cancer Prev. 2005, 6, 515–520. [Google Scholar]
- Hajiaghaalipour, F.; Kanthimathi, M.S.; Sanusi, J.; Rajarajeswaran, J. White tea (Camellia sinensis) inhibits proliferation of the colon cancer cell line, HT-29, activates caspases and protects DNA of normal cells against oxidative damage. Food Chem. 2015, 169, 401–410. [Google Scholar] [CrossRef]
- Pal, H.C.; Sehar, I.; Bhushan, S.; Gupta, B.D.; Saxena, A.K. Activation of caspases and poly (ADP-ribose) polymerase cleavage to induce apoptosis in leukemia HL-60 cells by Inula racemosa. Toxicol. In Vitro 2010, 24, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.C.; Jian, C.B.; Huang, Y.T.; Lai, C.S.; Hsu, P.C.; Pan, M.H. Induction of apoptosis by Uncaria tomentosa through reactive oxygen species production, cytochrome c release, and caspases activation in human leukemia cells. Food Chem. Toxicol. 2007, 45, 2206–2218. [Google Scholar] [CrossRef]
- Liu, Y.K.; Chen, K.H.; Leu, Y.L.; Way, T.D.; Wang, L.W.; Chen, Y.J.; Liu, Y.M. Ethanol extracts of Cinnamomum kanehirai Hayata leaves induce apoptosis in human hepatoma cell through caspase-3 cascade. Onco Targets Ther. 2015, 8, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.; Yong, M.J.; Gyawali, R.; Mosaddik, A.; Ryu, Y.C.; Cho, S.K. Mango (Mangifera indica L.) peel extracts inhibit proliferation of HeLa human cervical carcinoma cell via induction of apoptosis. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 397–405. [Google Scholar] [CrossRef]
- Chiu, C.H.; Chou, Y.C.; Lin, J.P.; Kuo, C.L.; Lu, H.F.; Huang, Y.P.; Yu, C.C.; Lin, M.L.; Chung, J.G. Chloroform Extract of Solanum lyratum Induced G0/G1 Arrest via p21/p16 and Induced Apoptosis via Reactive Oxygen Species, Caspases and Mitochondrial Pathways in Human Oral Cancer Cell Lines. Am. J. Chin. Med. 2015, 43, 1453–1469. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiu, Z.; Jin, Q.; Chen, G.; Guo, M. Cell Cycle Arrest and Apoptosis in HT-29 Cells Induced by Dichloromethane Fraction From Toddalia asiatica (L.) Lam. Front. Pharmacol. 2018, 9, 629. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Shin, W.T.; Park, C.; Hong, S.H.; Kim, G.Y.; Kim, S.O.; Ryu, C.H.; Hong, S.H.; Choi, Y.H. Induction of apoptosis in MDA-MB-231 human breast carcinoma cells with an ethanol extract of Cyperus rotundus L. by activating caspases. Oncol. Rep. 2014, 32, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Kwan, Y.P.; Saito, T.; Ibrahim, D.; Al-Hassan, F.M.; Ein Oon, C.; Chen, Y.; Jothy, S.L.; Kanwar, J.R.; Sasidharan, S. Evaluation of the cytotoxicity, cell-cycle arrest, and apoptotic induction by Euphorbia hirta in MCF-7 breast cancer cells. Pharm. Biol. 2016, 54, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Han, X.; Du, J.; Han, X.; Liu, W.; Shao, S.; Liu, X. Euphorbia lunulata extract acts on multidrug resistant gastric cancer cells to inhibit cell proliferation, migration and invasion, arrest cell cycle progression, and induce apoptosis. J. Ethnopharmacol. 2018, 212, 8–17. [Google Scholar] [CrossRef]
- Lin, H.-H.; Chan, K.-C.; Sheu, J.-Y.; Hsuan, S.-W.; Wang, C.-J.; Chen, J.-H. Hibiscus sabdariffa leaf induces apoptosis of human prostate cancer cells in vitro and in vivo. Food Chem. 2012, 132, 880–891. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Ren, W.; Hu, W.X. Apoptosis of HL-60 cells induced by extracts from Narcissus tazetta var. chinensis. Cancer Lett. 2006, 242, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Sitarek, P.; Skała, E.; Toma, M.; Wielanek, M.; Szemraj, J.; Skorski, T.; Białas, A.J.; Sakowicz, T.; Kowalczyk, T.; Radek, M.; et al. Transformed Root Extract of Leonurus sibiricus Induces Apoptosis through Intrinsic and Extrinsic Pathways in Various Grades of Human Glioma Cells. Pathol. Oncol. Res. 2017, 23, 679–687. [Google Scholar] [CrossRef]
- Zhong, P.; Yang, H.; Lin, S.; Peng, J.; Lin, J. A Traditional Chinese Medicine Herb Mixture Qingjie Fuzheng Granules Inhibits Hepatocellular Carcinoma Cells Growth by Inducing Apoptosis. J. Evid. Based Integr. Med. 2018, 23, 2515690x18789632. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, J.X.; Shang, H.X.; Lin, S.; Zhao, J.Y.; Lin, J.M. Qingjie Fuzheng granules inhibit colorectal cancer cell growth by the PI3K/AKT and ERK pathways. World J. Gastrointest. Oncol. 2019, 11, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Choi, H.; Lee, J.M.; Ha, S.H.; Kwak, C.H.; Abekura, F.; Park, J.Y.; Chang, Y.C.; Ha, K.T.; Cho, S.H.; et al. Oldenlandia diffusa suppresses metastatic potential through inhibiting matrix metalloproteinase-9 and intercellular adhesion molecule-1 expression via p38 and ERK1/2 MAPK pathways and induces apoptosis in human breast cancer MCF-7 cells. J. Ethnopharmacol. 2017, 195, 309–317. [Google Scholar] [CrossRef]
- Lee, M.H.; Hong, S.H.; Park, C.; Kim, G.Y.; Leem, S.H.; Choi, S.H.; Keum, Y.S.; Hyun, J.W.; Kwon, T.K.; Hong, S.H.; et al. Hwang-Heuk-San induces apoptosis in HCT116 human colorectal cancer cells through the ROS-mediated activation of caspases and the inactivation of the PI3K/Akt signaling pathway. Oncol. Rep. 2016, 36, 205–214. [Google Scholar] [CrossRef]
- Yim, N.H.; Kim, A.; Jung, Y.P.; Kim, T.; Ma, C.J.; Ma, J.Y. Fermented So-Cheong-Ryong-Tang (FCY) induces apoptosis via the activation of caspases and the regulation of MAPK signaling pathways in cancer cells. BMC Complement. Altern. Med. 2015, 15, 336. [Google Scholar] [CrossRef]
- Moon, J.Y.; Mosaddik, A.; Kim, H.; Cho, M.; Choi, H.-K.; Kim, Y.S.; Cho, S.K. The chloroform fraction of guava (Psidium cattleianum sabine) leaf extract inhibits human gastric cancer cell proliferation via induction of apoptosis. Food Chem. 2011, 125, 369–375. [Google Scholar] [CrossRef]
- Alshammari, G.M.; Balakrishnan, A.; Alshatwi, A.A.; Al-Khalifa, A. Cucurbita ficifolia Fruit Extract Induces Tp53/Caspase-Mediated Apoptosis in MCF-7 Breast Cancer Cells. Biomed. Res. Int. 2020, 2020, 3712536. [Google Scholar] [CrossRef]
- Liao, C.L.; Hsu, S.C.; Yu, C.C.; Yang, J.S.; Tang, N.Y.; Wood, W.G.; Lin, J.G.; Chung, J.G. The crude extract of Corni Fructus induces apoptotic cell death through reactive oxygen species-modulated pathways in U-2 OS human osteosarcoma cells. Environ. Toxicol. 2014, 29, 1020–1031. [Google Scholar] [CrossRef]
- Bagheri, E.; Hajiaghaalipour, F.; Nyamathulla, S.; Salehen, N. The apoptotic effects of Brucea javanica fruit extract against HT29 cells associated with p53 upregulation and inhibition of NF-κB translocation. Drug Des. Devel. Ther. 2018, 12, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.; Hwang, Y.; Kim, W.J.; Kim, G. In-vitro Morphological Assessment of Apoptosis Induced by Nimbolide—A Limonoid from Azadirachta Indica (Neem Tree). Iran. J. Pharm. Res. 2019, 18, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, S.M.; Park, S.M.; Park, J.H.; Shin, D.Y.; Kim, G.Y.; Ryu, C.H.; Shin, S.C.; Jung, J.M.; Kang, H.S.; et al. Induction of apoptosis in human leukemia U937 cells by anthocyanins through down-regulation of Bcl-2 and activation of caspases. Int. J. Oncol. 2009, 34, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Park, M.T.; Kang, J.A.; Choi, J.A.; Kang, C.M.; Kim, T.H.; Bae, S.; Kang, S.; Kim, S.; Choi, W.I.; Cho, C.K.; et al. Phytosphingosine induces apoptotic cell death via caspase 8 activation and Bax translocation in human cancer cells. Clin. Cancer Res. 2003, 9, 878–885. [Google Scholar] [PubMed]
- Kim, B.M.; Choi, Y.J.; Han, Y.; Yun, Y.S.; Hong, S.H. N,N-dimethyl phytosphingosine induces caspase-8-dependent cytochrome c release and apoptosis through ROS generation in human leukemia cells. Toxicol. Appl. Pharmacol. 2009, 239, 87–97. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, W.; Tang, C.; Ding, H.; Jang, J.; Weng, M.; Cai, Y.; Zou, G. NF-κB, JNK and p53 pathways are involved in tubeimoside-1-induced apoptosis in HepG2 cells with oxidative stress and G₂/M cell cycle arrest. Food Chem. Toxicol. 2011, 49, 3046–3054. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Tu, Y.; Masaki, H.; Tanaka, S.; Onda, K.; Sugiyama, K.; Yamada, H.; Hirano, T. Tetrandrine and cepharanthine induce apoptosis through caspase cascade regulation, cell cycle arrest, MAPK activation and PI3K/Akt/mTOR signal modification in glucocorticoid resistant human leukemia Jurkat T cells. Chem. Biol. Interact. 2019, 310, 108726. [Google Scholar] [CrossRef]
- Ho, Y.T.; Lu, C.C.; Yang, J.S.; Chiang, J.H.; Li, T.C.; Ip, S.W.; Hsia, T.C.; Liao, C.L.; Lin, J.G.; Wood, W.G.; et al. Berberine induced apoptosis via promoting the expression of caspase-8, -9 and -3, apoptosis-inducing factor and endonuclease G in SCC-4 human tongue squamous carcinoma cancer cells. Anticancer Res. 2009, 29, 4063–4070. [Google Scholar]
- Lin, C.C.; Kuo, C.L.; Lee, M.H.; Lai, K.C.; Lin, J.P.; Yang, J.S.; Yu, C.S.; Lu, C.C.; Chiang, J.H.; Chueh, F.S.; et al. Wogonin triggers apoptosis in human osteosarcoma U-2 OS cells through the endoplasmic reticulum stress, mitochondrial dysfunction and caspase-3-dependent signaling pathways. Int. J. Oncol. 2011, 39, 217–224. [Google Scholar] [CrossRef][Green Version]
- Chung, H.; Jung, Y.M.; Shin, D.H.; Lee, J.Y.; Oh, M.Y.; Kim, H.J.; Jang, K.S.; Jeon, S.J.; Son, K.H.; Kong, G. Anticancer effects of wogonin in both estrogen receptor-positive and -negative human breast cancer cell lines in vitro and in nude mice xenografts. Int. J. Cancer 2008, 122, 816–822. [Google Scholar] [CrossRef]
- Chou, W.H.; Liu, K.L.; Shih, Y.L.; Chuang, Y.Y.; Chou, J.; Lu, H.F.; Jair, H.W.; Lee, M.Z.; Au, M.K.; Chung, J.G. Ouabain Induces Apoptotic Cell Death Through Caspase- and Mitochondria-dependent Pathways in Human Osteosarcoma U-2 OS Cells. Anticancer Res. 2018, 38, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Hiyoshi, H.; Abdelhady, S.; Segerström, L.; Sveinbjörnsson, B.; Nuriya, M.; Lundgren, T.K.; Desfrere, L.; Miyakawa, A.; Yasui, M.; Kogner, P.; et al. Quiescence and γH2AX in neuroblastoma are regulated by ouabain/Na,K-ATPase. Br. J. Cancer 2012, 106, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Hong, S.H. Sequential caspase-2 and caspase-8 activation is essential for saikosaponin a-induced apoptosis of human colon carcinoma cell lines. Apoptosis 2011, 16, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Su, X.; Liang, Y.; Zhang, J.; Shi, C.; Lu, Y.; Gu, L.; Fu, L. Emodin azide methyl anthraquinone derivative triggers mitochondrial-dependent cell apoptosis involving in caspase-8-mediated Bid cleavage. Mol. Cancer Ther. 2008, 7, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.S.; Weng, S.W.; Lin, M.W.; Lu, C.C.; Chiang, J.H.; Yang, J.S.; Lai, K.C.; Lin, J.P.; Tang, N.Y.; Lin, J.G.; et al. Antitumor effects of emodin on LS1034 human colon cancer cells in vitro and in vivo: Roles of apoptotic cell death and LS1034 tumor xenografts model. Food Chem. Toxicol. 2012, 50, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Zou, X.; Zhou, J.Y.; Sun, W.; Wu, J.; Xu, J.L.; Wang, R.P. Raddeanin A induces human gastric cancer cells apoptosis and inhibits their invasion in vitro. Biochem. Biophys. Res. Commun. 2013, 439, 196–202. [Google Scholar] [CrossRef][Green Version]
- Wang, M.K.; Ding, L.S.; Wu, F.E. Antitumor effects of raddeanin A on S180, H22 and U14 cell xenografts in mice. Ai Zheng 2008, 27, 910–913. [Google Scholar]
- Mou, H.; Zheng, Y.; Zhao, P.; Bao, H.; Fang, W.; Xu, N. Celastrol induces apoptosis in non-small-cell lung cancer A549 cells through activation of mitochondria- and Fas/FasL-mediated pathways. Toxicol. In Vitro 2011, 25, 1027–1032. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhang, J.; Sun, L.L.; Li, B.H.; Gao, H.L.; Xie, T.; Zhang, N.; Ye, Z.M. Celastrol induces apoptosis and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells: An in vitro and in vivo study. Cell Death Dis. 2015, 6, e1604. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, Y.; Fan, Y.; Zhou, D. Celastrol inhibits the growth of human glioma xenografts in nude mice through suppressing VEGFR expression. Cancer Lett. 2008, 264, 101–106. [Google Scholar] [CrossRef]
- Lu, Z.; Cao, S.; Zhou, H.; Hua, L.; Zhang, S.; Cao, J. Mechanism of Arctigenin-Induced Specific Cytotoxicity against Human Hepatocellular Carcinoma Cell Lines: Hep G2 and SMMC7721. PLoS ONE 2015, 10, e0125727. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhu, B.; Yong, L.; Song, C.; Liu, X.; Yu, H.; Wang, P.; Liu, Z.; Liu, X. Regulation of Intrinsic and Extrinsic Apoptotic Pathways in Osteosarcoma Cells Following Oleandrin Treatment. Int. J. Mol. Sci. 2016, 17, 1950. [Google Scholar] [CrossRef]
- Tang, S.Y.; Zhong, M.Z.; Yuan, G.J.; Hou, S.P.; Yin, L.L.; Jiang, H.; Yu, Z. Casticin, a flavonoid, potentiates TRAIL-induced apoptosis through modulation of anti-apoptotic proteins and death receptor 5 in colon cancer cells. Oncol. Rep. 2013, 29, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.S.; Lee, H.J.; Hwang, J.; Han, H.; Kim, B.; Shim, B.; Kim, S.H. Lambertianic Acid Sensitizes Non-Small Cell Lung Cancers to TRAIL-Induced Apoptosis via Inhibition of XIAP/NF-κB and Activation of Caspases and Death Receptor 4. Int. J. Mol. Sci. 2018, 19, 1476. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Shin, E.A.; Jung, J.H.; Park, J.E.; Koo, J.; Koo, J.I.; Shim, B.S.; Kim, S.H. Galbanic acid potentiates TRAIL induced apoptosis in resistant non-small cell lung cancer cells via inhibition of MDR1 and activation of caspases and DR5. Eur. J. Pharmacol. 2019, 847, 91–96. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Zha, D.; Cai, F.; Zhang, W.; He, Y.; Huang, Q.; Zhuang, H.; Hua, Z.C. Apigenin potentiates TRAIL therapy of non-small cell lung cancer via upregulating DR4/DR5 expression in a p53-dependent manner. Sci. Rep. 2016, 6, 35468. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, B.; Wang, Y.; Ding, H. Kaempferol Sensitizes Human Ovarian Cancer Cells-OVCAR-3 and SKOV-3 to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)-Induced Apoptosis via JNK/ERK-CHOP Pathway and Up-Regulation of Death Receptors 4 and 5. Med. Sci. Monit. 2017, 23, 5096–5105. [Google Scholar] [CrossRef]
- Sui, C.G.; Meng, F.D.; Li, Y.; Jiang, Y.H. Antiproliferative activity of rosamultic acid is associated with induction of apoptosis, cell cycle arrest, inhibition of cell migration and caspase activation in human gastric cancer (SGC-7901) cells. Phytomedicine 2015, 22, 796–806. [Google Scholar] [CrossRef]
- Pan, M.H.; Lai, C.S.; Hsu, P.C.; Wang, Y.J. Acacetin induces apoptosis in human gastric carcinoma cells accompanied by activation of caspase cascades and production of reactive oxygen species. J. Agric. Food Chem. 2005, 53, 620–630. [Google Scholar] [CrossRef]
- Liu, J.; Hu, W.X.; He, L.F.; Ye, M.; Li, Y. Effects of lycorine on HL-60 cells via arresting cell cycle and inducing apoptosis. FEBS Lett. 2004, 578, 245–250. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Tang, L.J.; Shi, Y.W.; Ren, W.; Hu, W.X. Apoptosis induced by lycorine in KM3 cells is associated with the G0/G1 cell cycle arrest. Oncol. Rep. 2007, 17, 377–384. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Zhu, X.F.; Xiao, Z.J.; Wang, H.H.; Zhou, J.M.; Mei, Y.P.; Deng, R.; Jiang, W.Q.; Liu, Z.C. Inducement effect of Meisoindigo on apoptosis of leukemia cell line HL-60 and its mechanism. Ai Zheng 2005, 24, 1464–1468. [Google Scholar] [PubMed]
- Abaza, M.S.; Afzal, M.; Al-Attiyah, R.J.; Guleri, R. Methylferulate from Tamarix aucheriana inhibits growth and enhances chemosensitivity of human colorectal cancer cells: Possible mechanism of action. BMC Complement. Altern. Med. 2016, 16, 384. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, K.W.; Kim, J.Y.; Jeong, I.Y.; Byun, M.W.; Park, J.E.; Yee, S.T.; Kim, K.H.; Rhim, J.S.; Yamada, K.; et al. Thiosulfinates from Allium tuberosum L. induce apoptosis via caspase-dependent and -independent pathways in PC-3 human prostate cancer cells. Bioorg. Med. Chem. Lett. 2008, 18, 199–204. [Google Scholar] [CrossRef]
- Lee, J.H.; Yang, H.S.; Park, K.W.; Kim, J.Y.; Lee, M.K.; Jeong, I.Y.; Shim, K.H.; Kim, Y.S.; Yamada, K.; Seo, K.I. Mechanisms of thiosulfinates from Allium tuberosum L.-induced apoptosis in HT-29 human colon cancer cells. Toxicol. Lett. 2009, 188, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Park, H.Y.; Kim, J.Y.; Jeong, I.Y.; Lee, M.K.; Seo, K.I. Apoptotic action of ursolic acid isolated from Corni fructus in RC-58T/h/SA#4 primary human prostate cancer cells. Bioorg. Med. Chem. Lett. 2010, 20, 6435–6438. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, K.W.; Moon, K.D.; Lee, M.K.; Choi, J.; Yee, S.T.; Shim, K.H.; Seo, K.I. Induction of apoptosis in HT-29 colon cancer cells by crude saponin from Platycodi Radix. Food Chem. Toxicol. 2008, 46, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.M.; Tseng, H.H.; Peng, C.W.; Chen, W.S.; Chiu, S.J. Dietary flavonoid fisetin targets caspase-3-deficient human breast cancer MCF-7 cells by induction of caspase-7-associated apoptosis and inhibition of autophagy. Int. J. Oncol. 2012, 40, 469–478. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, M.L.; Murphy, K.; Doucette, C.D.; Greenshields, A.L.; Hoskin, D.W. The Dietary Flavonoid Fisetin Causes Cell Cycle Arrest, Caspase-Dependent Apoptosis, and Enhanced Cytotoxicity of Chemotherapeutic Drugs in Triple-Negative Breast Cancer Cells. J. Cell Biochem. 2016, 117, 1913–1925. [Google Scholar] [CrossRef]
- Khan, N.; Afaq, F.; Syed, D.N.; Mukhtar, H. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis 2008, 29, 1049–1056. [Google Scholar] [CrossRef]
- Touil, Y.S.; Seguin, J.; Scherman, D.; Chabot, G.G. Improved antiangiogenic and antitumour activity of the combination of the natural flavonoid fisetin and cyclophosphamide in Lewis lung carcinoma-bearing mice. Cancer Chemother. Pharmacol. 2011, 68, 445–455. [Google Scholar] [CrossRef]
- Chen, C.Y.; Liu, T.Z.; Tseng, W.C.; Lu, F.J.; Hung, R.P.; Chen, C.H.; Chen, C.H. (-)-Anonaine induces apoptosis through Bax- and caspase-dependent pathways in human cervical cancer (HeLa) cells. Food Chem. Toxicol. 2008, 46, 2694–2702. [Google Scholar] [CrossRef]
- Guo, Z.; Guozhang, H.; Wang, H.; Li, Z.; Liu, N. Ampelopsin inhibits human glioma through inducing apoptosis and autophagy dependent on ROS generation and JNK pathway. Biomed. Pharmacother. 2019, 116, 108524. [Google Scholar] [CrossRef]
- Cui, Y.Q.; Liu, Y.J.; Zhang, F. The suppressive effects of Britannin (Bri) on human liver cancer through inducing apoptosis and autophagy via AMPK activation regulated by ROS. Biochem. Biophys. Res. Commun. 2018, 497, 916–923. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, R.; Du, J.; Xin, H.; Yi, T.; Sheng, J.; Wang, Y.; Ling, C. Corosolic acid induces apoptosis through mitochondrial pathway and caspase activation in human cervix adenocarcinoma HeLa cells. Cancer Lett. 2009, 284, 229–237. [Google Scholar] [CrossRef]
- Chung, K.S.; Choi, J.H.; Back, N.I.; Choi, M.S.; Kang, E.K.; Chung, H.G.; Jeong, T.S.; Lee, K.T. Eupafolin, a flavonoid isolated from Artemisia princeps, induced apoptosis in human cervical adenocarcinoma HeLa cells. Mol. Nutr. Food Res. 2010, 54, 1318–1328. [Google Scholar] [CrossRef]
- Kim, E.J.; Lim, S.S.; Park, S.Y.; Shin, H.K.; Kim, J.S.; Park, J.H. Apoptosis of DU145 human prostate cancer cells induced by dehydrocostus lactone isolated from the root of Saussurea lappa. Food Chem. Toxicol. 2008, 46, 3651–3658. [Google Scholar] [CrossRef] [PubMed]
- Seon, M.R.; Lim, S.S.; Choi, H.J.; Park, S.Y.; Cho, H.J.; Kim, J.K.; Kim, J.; Kwon, D.Y.; Park, J.H. Isoangustone A present in hexane/ethanol extract of Glycyrrhiza uralensis induces apoptosis in DU145 human prostate cancer cells via the activation of DR4 and intrinsic apoptosis pathway. Mol. Nutr. Food Res. 2010, 54, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Havelek, R.; Seifrtova, M.; Kralovec, K.; Bruckova, L.; Cahlikova, L.; Dalecka, M.; Vavrova, J.; Rezacova, M.; Opletal, L.; Bilkova, Z. The effect of Amaryllidaceae alkaloids haemanthamine and haemanthidine on cell cycle progression and apoptosis in p53-negative human leukemic Jurkat cells. Phytomedicine 2014, 21, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.Q.; Kim, Y.H.; Moon, C.K.; Lee, B.H. Reactive oxygen species-mediated induction of apoptosis by a plant alkaloid 6-methoxydihydrosanguinarine in HepG2 cells. Biochem. Pharmacol. 2005, 70, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.R.; Al-Jomah, N.A.; Siraj, A.K.; Manogaran, P.; Al-Hussein, K.; Abubaker, J.; Platanias, L.C.; Al-Kuraya, K.S.; Uddin, S. Sanguinarine-dependent induction of apoptosis in primary effusion lymphoma cells. Cancer Res. 2007, 67, 3888–3897. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Won, S.J.; Chao, C.L.; Wu, F.L.; Liu, H.S.; Ling, P.; Lin, C.N.; Su, C.L. Morusin induces apoptosis and suppresses NF-kappaB activity in human colorectal cancer HT-29 cells. Biochem. Biophys. Res. Commun. 2008, 372, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Souza-Fagundes, E.M.; Brumatti, G.; Martins-Filho, O.A.; Corrêa-Oliveira, R.; Zani, C.L.; Amarante-Mendes, G.P. Myriadenolide, a labdane diterpene isolated from Alomia myriadenia (asteraceae) induces depolarization of mitochondrial membranes and apoptosis associated with activation of caspases-8, -9, and -3 in Jurkat and THP-1 cells. Exp. Cell Res. 2003, 290, 420–426. [Google Scholar] [CrossRef]
- Xu, K.H.; Lu, D.P. Plumbagin induces ROS-mediated apoptosis in human promyelocytic leukemia cells in vivo. Leuk. Res. 2010, 34, 658–665. [Google Scholar] [CrossRef]
- Mohan, S.; Abdelwahab, S.I.; Kamalidehghan, B.; Syam, S.; May, K.S.; Harmal, N.S.; Shafifiyaz, N.; Hadi, A.H.; Hashim, N.M.; Rahmani, M.; et al. Involvement of NF-κB and Bcl2/Bax signaling pathways in the apoptosis of MCF7 cells induced by a xanthone compound Pyranocycloartobiloxanthone A. Phytomedicine 2012, 19, 1007–1015. [Google Scholar] [CrossRef]
- Ibrahim, M.Y.; Hashim, N.M.; Mohan, S.; Abdulla, M.A.; Kamalidehghan, B.; Ghaderian, M.; Dehghan, F.; Ali, L.Z.; Arbab, I.A.; Yahayu, M.; et al. α-Mangostin from Cratoxylum arborescens demonstrates apoptogenesis in MCF-7 with regulation of NF-κB and Hsp70 protein modulation in vitro, and tumor reduction in vivo. Drug Des. Devel. Ther. 2014, 8, 1629–1647. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour Babaei, M.; Zaman Huri, H.; Kamalidehghan, B.; Yeap, S.K.; Ahmadipour, F. Apoptotic induction and inhibition of NF-κB signaling pathway in human prostatic cancer PC3 cells by natural compound 2,2′-oxybis (4-allyl-1-methoxybenzene), biseugenol B, from Litsea costalis: An in vitro study. OncoTargets Ther. 2017, 10, 277–294. [Google Scholar] [CrossRef]
- Gong, K.; Li, W. Shikonin, a Chinese plant-derived naphthoquinone, induces apoptosis in hepatocellular carcinoma cells through reactive oxygen species: A potential new treatment for hepatocellular carcinoma. Free Radic. Biol. Med. 2011, 51, 2259–2271. [Google Scholar] [CrossRef]
- Min, R.; Tong, J.; Wenjun, Y.; Wenhu, D.; Xiaojian, Z.; Jiacai, H.; Jian, Z.; Wantao, C.; Chenping, Z. Growth inhibition and induction of apoptosis in human oral squamous cell carcinoma Tca-8113 cell lines by Shikonin was partly through the inactivation of NF-kappaB pathway. Phytother. Res. 2008, 22, 407–415. [Google Scholar] [CrossRef]
- Nakachi, K.; Matsuyama, S.; Miyake, S.; Suganuma, M.; Imai, K. Preventive effects of drinking green tea on cancer and cardiovascular disease: Epidemiological evidence for multiple targeting prevention. Biofactors 2000, 13, 49–54. [Google Scholar] [CrossRef]
- Shimizu, M.; Fukutomi, Y.; Ninomiya, M.; Nagura, K.; Kato, T.; Araki, H.; Suganuma, M.; Fujiki, H.; Moriwaki, H. Green tea extracts for the prevention of metachronous colorectal adenomas: A pilot study. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3020–3025. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.M.; Lee, D.H.; Seo, A.Y.; Lee, H.J.; Kim, S.B.; Son, W.C.; Kim, Y.K.; Lee, S.J.; Park, S.H.; Kim, N.; et al. Green tea extracts for the prevention of metachronous colorectal polyps among patients who underwent endoscopic removal of colorectal adenomas: A randomized clinical trial. Clin. Nutr. 2018, 37, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Kim, C.S.; Maeng, S. Effects of pumpkin seed oil and saw palmetto oil in Korean men with symptomatic benign prostatic hyperplasia. Nutr. Res. Pract. 2009, 3, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Santos Araújo Mdo, C.; Farias, I.L.; Gutierres, J.; Dalmora, S.L.; Flores, N.; Farias, J.; de Cruz, I.; Chiesa, J.; Morsch, V.M.; Chitolina Schetinger, M.R. Uncaria tomentosa-Adjuvant Treatment for Breast Cancer: Clinical Trial. Evid. Based Complement. Alternat. Med. 2012, 2012, 676984. [Google Scholar] [CrossRef]
- Li, X. A Research of Berberine Hydrochloride to Prevent Colorectal Adenomas in Patients with Previous Colorectal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03281096 (accessed on 13 September 2017).
- Wu, K. Berberine Chloride in Preventing Colorectal Cancer in Patients with Ulcerative Colitis in Remission. Available online: https://prevention.cancer.gov/clinical-trials/clinical-trials-search/nct02365480 (accessed on 14 April 2020).
- Zhang, J.; Cao, H.; Zhang, B.; Cao, H.; Xu, X.; Ruan, H.; Yi, T.; Tan, L.; Qu, R.; Song, G.; et al. Berberine potently attenuates intestinal polyps growth in ApcMin mice and familial adenomatous polyposis patients through inhibition of Wnt signalling. J. Cell Mol. Med. 2013, 17, 1484–1493. [Google Scholar] [CrossRef]
- Co-Operative Group of the Treatment of CML with Meisoindigo. Clinical studies of 134 cases of CML treated with meisoindigo. Chin. J. Haematol. 1988, 9, 135–137. [Google Scholar]
- Phase III clinical trial on meisoindico in the treatment of chronic myelogenous leukemia. Zhonghua Xue Ye Xue Za Zhi 1997, 18, 69–72.
- Bettuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peracchia, G.; Corti, A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: A preliminary report from a one-year proof-of-principle study. Cancer Res. 2006, 66, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Micali, S.; Territo, A.; Pirola, G.M.; Ferrari, N.; Sighinolfi, M.C.; Martorana, E.; Navarra, M.; Bianchi, G. Effect of green tea catechins in patients with high-grade prostatic intraepithelial neoplasia: Results of a short-term double-blind placebo controlled phase II clinical trial. Arch. Ital. Urol. Androl. 2017, 89, 197–202. [Google Scholar] [CrossRef]
- Stingl, J.C.; Ettrich, T.; Muche, R.; Wiedom, M.; Brockmöller, J.; Seeringer, A.; Seufferlein, T. Protocol for minimizing the risk of metachronous adenomas of the colorectum with green tea extract (MIRACLE): A randomised controlled trial of green tea extract versus placebo for nutriprevention of metachronous colon adenomas in the elderly population. BMC Cancer. 2011, 11, 360. [Google Scholar] [CrossRef]
- Guo, X.P.; Zhang, X.Y.; Zhang, S.D. Clinical trial on the effects of shikonin mixture on later stage lung cancer. Zhong Xi Yi Jie He Za Zhi 1991, 11, 598–599. [Google Scholar] [PubMed]
- Qian, Z.; Wang, X.; Song, Z.; Zhang, H.; Zhou, S.; Zhao, J.; Wang, H. A phase I trial to evaluate the multiple-dose safety and antitumor activity of ursolic acid liposomes in subjects with advanced solid tumors. Biomed. Res. Int. 2015, 2015, 809714. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]

| Plant Botanicalname | Extraction Solvent | Plant Part Used | Concentration | Cell lines or Animal Model Used | Altered Factors |
|---|---|---|---|---|---|
| Azadirachta indica | Ethanol | Leaf | 200 mg/kg BW | squamous cell carcinoma in a hamster model | Increased: Bim; activation of caspase-3 and -8 Decreased: Bcl-2 |
| Brucea javanica | Ethanol | Fruit | Different concentrations for each assay (25, 50, and 100 µg/mL) | HT29 | Increased: Fas; TNFR1; TNF2; DR6; CD40; Bid; caspase-8; caspse-9; TRAIL-4; Bax; Bad; cytochrome c release Decreased: Bcl-2 |
| Camellia sinensis | Water | Leaf | IC50 = 86.68 ± 0.73 μg/mL | HT-29 | Increased: activation of caspase-3, -9, and -8 Decreased: NR |
| Camellia sinensis | Water | Leaf | 15 × 105 μg/day | Clinical trial, patients with colorectal cancer | Increased: NR Decreased: incidence of metachronous adenomas; size of relapsed adenomas |
| Camellia sinensis | Water | Leaf | 9 × 105 μg/day | Clinical trial, patients with metachronous colorectal adenoma and cancer | Increased: NR Decreased: incidence of metachronous adenomas; the number of relapsed adenomas |
| Cinnamomum kanehirai Hayata | Ethanol | Leaf | Different concentrations for each assay (0.25–1.0 mg/mL) | HepG2 and HA22T/VGH | Increased: activation of caspase-3, -9, and -8; Bax Decreased: Bcl-2 |
| Corni Fructus | Water | Whole plant | 2500 μg/mL | U-2OS | Increased: Bax; cytochrome c release; AIF, Fas, TRAIL; activity and protein level of caspase-3, -9, and -8 Decreased: MMP |
| Cucurbita ficifolia | chloroform | Fruit | IC50 = 90 μg/mL | MCF-7 | Increased: FADD; BAK; BAX; caspase-3, -9, and -8 Decreased: NR |
| Cucurbita ficifolia | Ethanol | Seed | 32 × 104 μg/day | Clinical trial, patients with symptomatic benign prostatic hyperplasia | Increased: quality of life score; maximal urinary flow rate Decreased: international prostate symptom score; Serum prostate-specific antigen |
| Cyperus rotundus L. | Ethanol | Rhizome | 200 μg/mL | MDA-MB-231 | Increased: Bax; DR5; activation of Bid; activation of caspase-3, -9, and -8 Decreased: Bcl-2; survivin; MMP |
| Euphorbia hirta L. | Methanol | Whole plant | IC50 = 25.26 µg/mL | MCF-7 | Increased: activation of caspase-2, -6, -8, -9, and -3 Decreased: NR |
| Euphorbia lunulata | n-hexane | Aerial parts | IC50 = 20 μg/mL | SGC7901/ADR | Increased: Bax; activation of caspase-3, -9, and -8; cytochrome c release Decreased: Bcl-2 |
| Hibiscus sabdariffa | Water | Leaf | 50 and 100 μg/mL | LNCaP and LNCaP xenograft nude mice | Increased: Bax; cytochrome c release; activation of caspase-3, -9, and -8; activation of Bid; FasL Decreased: Bcl-2; MMP |
| Hwang-Heuk-San (HHS) | Water | Polyplant formula | Different concentrations for each assay (0–6.1 mg/mL) | HCT116 | Increased: Bax; cytochrome c release; activation of caspase-3, -9, and -8; activation of Bid; FasL; DR4; DR5 Decreased: Bcl-2; MMP |
| Inula racemosa Hook.f. | Ethanol | Root | IC50 = 16.70 mg/mL for n-hexane fraction | HL-60 | Increased: activation of caspase-3, -9, and -8; cytochrome c release; Bax translocation Decreased: MMP |
| Leonurus sibiricus | Methanol | Root | IC50 = 1 mg/mL | Grades (I-III) of human glioma cells derived from patients | Increased: Bax; p53; caspase-3, -8, and -9 Decreased: Bcl-2; MMP |
| Mangifera indica | Ethanol | Fruit peel | Different concentrations for each assay (0–400 µg/mL) | HeLa | Increased: activation of caspase-3, -9, and -8 Decreased: Bcl-2 |
| Narcissus tazetta var. chinensis | Chloroform | Stem and leaf | 5.0 μg/mL | HL-60 | Increased: Bax; cytochrome c release; activation of caspase-3, -9, and -8 Decreased: Bcl-2 |
| Oldenlandia diffusa | Methanol and butanol | Whole plant | Different concentrations for each assay (0–20 µg/mL) Oldenlandia diffusa | MCF-7 | Increased: Bax; activation of caspase-8 and -7 Decreased: Bcl-2 |
| Psidium cattleianum Sabine | Chloroform | Leaf | Different concentrations for each assay (0–200 µg/mL) Oldenlandia diffusa | SNU-16 | Increased: Bax; PARP; caspase-3 and -8 Decreased: Bcl-2 |
| Qingjie Fuzheng granule (QFG) | Water | Polyplant formula | Different concentrations for each assay (0–1500 µg/mL) for cell lines; 0.75 g/kg and 1.5 g/kg for mice | SK-Hep-1, Bel-7402, HCT-116, and HCT-8; mouse xenograft model | Increased: Fas; FasL; Bax; activation of caspase-3, -9, and -8 Decreased: Bcl-2; tumor weight in mice |
| Solanum lyratum | Chloroform | Whole plant | 40 μg/mL | HSC-3, SAS, and CAL-27 | Increased: Bax and Bad; activation of caspase-3, -9, and -8 Decreased: Bcl-2 and Bcl-xl; MMP |
| So-Cheong-Ryong-Tang | Water | Polyplant formula | 500 and 1000 μg/mL for cell line; 157.5 mg/kg/day for mice | AGS; mouse xenograft model | Increased: activation of caspase-3, -9, and -8 Decreased: tumor weight in mice |
| Toddalia asiatica (L.) Lam. | Dichloromethane | Root | IC50 = 18 μg/mL | HT-29 | Increased: activation of caspase-3, -9, and -8 Decreased: NR |
| Uncaria tomentosa (Wild.) DC. | Ethyl acetate | Whole plant | 100 μg/mL | HL-60 | Increased: Fas, activation of caspase-3, -9, and -8; Bax; cytochrome c release Decreased: MMP; Bcl-XL |
| Uncaria tomentosa (Wild.) DC. | Ethanol | Bark | 30 × 104 μg/day | Clinical trial, patients with breast cancer | Increased: Neutrophil count; Superoxide dismutase activity Decreased: DNA damage |
| Chemical Family | Molecule Name | Concentration (µM) | Cell Line | Altered Factors |
|---|---|---|---|---|
| Alkaloid | (-)-Anonaine | 100 μM | HeLa | Increased: Bax; cytochrome C release; activation of caspase-3, -7, -9, and -8 Decreased: MMP |
| Berberine | IC50 = 75 μM | SCC-4 | Increased: Bax; cytochrome C release; activation of caspase-3, -9, and -8; AIF; Endo G Decreased: MMP Bcl-2 | |
| 30 × 104 μg/day | Clinical trial, patients with familial adenomatous polyposis | Increased: NR Decreased: polyp size and number | ||
| Hemanthamine and hemanthidine | Various concentrations (5–20 μM) | Jurkat | Increased: activation of caspase-3, -7, -9, and -8 Decreased: MMP | |
| Lycorine | IC50 = 1 µM | HL-60 | Increased: Bax; activation of caspase-3, -9, and -8 Decreased: Bcl-2 | |
| IC50 = 1.25 μM | KM3 | Increased: Bax; activation of caspase-3, -9, and -8 Decreased: Bcl-2 | ||
| Meisoindigo | 20 µM | HL-60 | Increased: Bax; cytochrome C release; activation of caspase-3, -9, and -8; FasL Decreased: Bcl-2 | |
| 75–150 × 103 µg/day | Clinical trial phase II, patients with chronic myelogenous leukemia | Increased: Hematological complete response (CR) and partial response (PR) rates of 32.1% and 48.5%, respectively Decreased: NR | ||
| 100–150 × 103 µg/day | Clinical trial phase III, patients with chronic myelogenous leukemia | Increased: hematological CR and PR rates of 45.0% and 39.3% for newly diagnosed patients and 35.9% and 41.4% for pretreated patients Decreased: NR | ||
| 6-methoxydihydrosanguinarine | IC50 = 3.8 µM | HepG2 | Increased: Bax; cytochrome C release; activation of caspase-3, -9, and -8 Decreased: Bcl-2 | |
| Sanguinarine | Various concentrations (0.25–4 μM) | BC1, BC3, BCBL1, and HBL6 | Increased: Bax; cytochrome C release; activation of caspase-3, -9, and -8; activation of Bid; DR4 Decreased: MMP | |
| Tetrandrine and Cepharanthine | Various concentrations (3–15 μM) | Jurkat | Increased: Bax; activation of caspase-3, -6, -9, and -8 Decreased: Bcl-2 | |
| Anthraquinone | Emodin | IC50 = 9.06 μM for MDA-MB-453 cellsIC50 = 0.83 μM for Calu-3 cells | MDA-MB-453 and Calu-3 | Increased: cytochrome C release; activation of caspase-3, -9, and -8; activation of Bid Decreased: MMP |
| 40 mg/kg/once every 3 days | LS1034 colon cancer cells xenografts into male athymic BALB/c nu/nu mice | Increased: NR Decreased: tumor volume | ||
| Flavonoid | Acacetin | IC50 = 60 µM | AGS | Increased: Bax; cytochrome C release; activation of caspase-3, -9, and -8; activation of Bid and Bad; FasL; Fas Decreased: Bcl-2; MMP |
| Ampelopsin | IC50 = 39.6 µM for U251IC50 = 35.8 µM for A172 | U251 and A172 | Increased: activation of caspase-3, -9, and -8 Decreased: NR | |
| 50 and 100 mg/kg/day for 30 days | U251 bearing BALB/c-nu mice | Increased: activation of caspase-3, -9, and -8; PARP Decreased: tumor volume and progression | ||
| Anthocyanins | Various concentrations 0–265.4 µM | U937 | Increased: Bax; activation of caspase-3, -9, and -8; activation of Bid Decreased: Bcl-2; XIAP; cIAP-1; cIAP-2; MMP | |
| Apigenin in combination with TRAIL | IC50 = 20 μM | A549 and H1299 | Increased: Bax; Bad; DR4; DR5 Decreased: Bcl-2; Bcl-xL | |
| 10 μg/mouse | Tumor xenografts A549 | Increased: DR4; DR5; apoptotic and necrotic cell death Decreased: tumor volume | ||
| Casticin | IC50 = 0.85 µM | HT-29, HCT-116, and SW480 | Increased: Bax; activation of caspase-3; DR5; activation of Bid Decreased: Bcl-2; Bcl-xL; XIAP; cFLIP | |
| Catechins in green tea | 6 × 105 μg/day | Clinical trial, patients with high-grade prostate intraepithelial neoplasia | Increased: NR Decreased: incidence of the tumor, international prostate symptom score, and quality of life scores | |
| Catechins in green tea | 6 × 105 μg/day | Clinical trial, patients with high-grade prostate intraepithelial neoplasia | Increased: NR Decreased: prostate-specific antigen (PSA) | |
| Epigallocatechin gallate | 3 × 105 μg/day | Clinical trial, patients with metachronous colon adenomas | Increased: NR Decreased: NR | |
| Eupafolin | IC50 = 26.75 μM | HeLa | Increased: cytochrome C release; activation of caspase-3, -6, -7, -9, and -8 Decreased: Bcl-2; MMP | |
| Fisetin | Various concentrations (0–100 μM) | MCF-7 | Increased: activation of caspase-7, -9, and -8 Decreased: MMP | |
| Various concentrations (0–100 μM) | MDA-MB-468 and MDA-MB-231 | Increased: activation of caspase -9 and -8 Decreased: NR | ||
| Various concentrations (10–60 μM) | LNCaP | Increased: cytochrome C release; activation of caspase-3, -9, and -8 Decreased: Bcl-2; XIAP | ||
| 223 mg/kg/day for two weeks | LLC bearing C57BL/6 J female mice | Increased: NR Decreased: tumor volume and angiogenesis | ||
| Isoangustone A | Various concentrations (2.4–17.7 μM) | DU145 | Increased: cytochrome C release; activation of caspase-3, -7, -9, and -8; activation of Bid; Fas; DR4 Decreased: MMP | |
| Kaempferol | Various concentrations (20–100 μM) | OVCAR-3 and SKOV-3 | Increased: Bax; activation of caspase-3, -9, and -8 Decreased: Bcl-2; Bcl-xL; XIAP; cFLIP | |
| Morusin | IC50 = 6.1 µM | HT-29 | Increased: Smac/DIABLO; cytochrome C release; activation of caspase-3, -9, and -8 Decreased: XIAP; MMP | |
| Wogonin | IC50 = 75 µM | U-2OS | Increased: Bax; Bad; cytochrome C release; activation of caspase-3, -4, -9, and -8; AIF; Endo G; Fas Decreased: NR | |
| Lignin | Arctigenin | IC50 = 0.24 μM | Hep G2 and SMMC7721 | Increased: Bax; cytochrome C release; activation of caspase-3, -9, and -8; FasL; Fas Decreased: Bcl-2; MMP |
| Naphthoquinone | Plumbagin | IC50 = 9 μM | NB4 | Increased: Bax; Bak; activation of caspase-3, -9, and -8 Decreased: Bcl-xL; MMP |
| 2 mg/kg | NB4 cell bearing male NOD/SCID mice | Increased: NR Decreased: tumor volume | ||
| Shikonin | IC50 = 4 µM for Huh7 IC50 = 5.3 µM for BEL7402 | BEL7402 and Huh7 | Increased: activation of caspase-9 and -8; activation of Bid Decreased: Bcl-2; c-FLIP | |
| 5 or 10 mg/kg for 30 days | Huh7 cell bearing male BALB/c nude mice | Increased: activation of caspase-9 and -8, and PARP Decreased: tumor volume | ||
| IC50 = 32.5 μM | Tca-8113 | Increased: activation of caspase-3, -9, and -8 Decreased: Bcl-2 | ||
| 5–10 (mg/kg/day) | Clinical trial, patients with later-stage lung cancer | Increased: immune system; survival rate Decreased: tumor growth; remission rate | ||
| Organosulfur derivative | Thiosulfinates | IC50 = 10.07 μM | PC-3 | Increased: Bax; AIF; activation of caspase-3, -9, and -8; activation of Bid; Decreased: Bcl-2 |
| 40 and 80 μM | HT-29 | Increased: Bax; AIF; activation of caspase-3, -9, and -8; activation of Bid; Decreased: Bcl-2 | ||
| Eugenol ortho dimer | Biseugenol B | IC50 = 4 μM | PC3 | Increased: Bax; cytochrome C release; activation of caspase-3, -7, -9, and -8 Decreased: Bcl-2; MMP |
| Hydroxycinnamic acids derivative | Methyl ferulate | IC50 = 1.73–1.9 μM | SW1116 and SW837 | Increased: Bax; Bad; Apaf1; Bid; Bim; Smac; caspase-2, -3, -6, -7, -8, and -9 Decreased: Bcl-2; c-IAP-1; c-IAP-2; FLIP |
| Phospholipid | N, N-dimethyl Phytosphingosine | Various concentrations (0–7.5 μM) | HL-60 | Increased: activation of caspase-3, -9, and -8; cytochrome C release Decreased: Bcl-2; MMP |
| Phytosphingosine | 15.8 or 31.5 μM | Jurkat and NCI-H460 | Increased: Bax translocation to mitochondria; cytochrome C release; activation of caspase-3, -9, and -8 Decreased: MMP | |
| Steroid | Oleandrin | Various concentrations (0–0.05 μM) | U-2OS and SaOS-2 | Increased: Bax; cytochrome C release; activation of caspase-3, -9, and -8; FasL; Fas Decreased: Bcl-2; MMP |
| Ouabain | IC50 = 5 μM | U-2OS | Increased: Bax; cytochrome C release; activation of caspase-3, -9, and -8; AIF; Endo G Decreased: Bcl-2; MMP | |
| 2 mg/kg/day for 13 days | Mouse model of xenografted SH-SY5Yneuroblastoma cells | Increased: activation of caspase-3 Decreased: tumor volume | ||
| Terpene | Britannin | Various concentrations (0–80 μM) | SMMC-7721 and HepG2 | Increased: activation of caspase-3, -9, and -8 Decreased: Bcl-2 |
| Various concentrations (0–30 mg/kg/day for 21 days) | HepG2 bearing male BALB/c nu/nu nude mice | Increased: p-AMPK, cleaved caspase-3 and LC3 II Decreased: p-mTOR; Ki-67; tumor volume | ||
| Celastrol | IC50 = 2.12 μM | A549 | Increased: Bax; cytochrome C release; activation of caspase-3, -9, and -8; FasL; Fas Decreased: Bcl-2 | |
| IC50 = 2.55 μM for HOS IC50 = 1.97 μM for MG-63 | HOS and MG-63 | Increased: activation of caspase-3, -9, and -8; activation of Bid; DR5 Decreased: MMP | ||
| 4.5 mg/kg/day for 28 days | Xenografts of glioma SHG44 cells in female BALB/c mice | Increased: NR Decreased: tumor growth | ||
| Corosolic acid | IC50 = 28 μM | HeLa | Increased: Bax; cytochrome C release; activation of caspase-3, -9, and -8 Decreased: Bcl-2; MMP | |
| Dehydrocostus lactone | 8.7 μM | DU145 | Increased: Bax; Bak; Bok; Bik; Bmf; t-Bid; activation of caspase-3, -9, and -8 Decreased: Bcl-xL | |
| Galbanic acid in combination with TRAIL | Various concentrations (0–50 μM) | H460/R | Increased: activation of caspase-9 and -8; DR5; activation of Bid Decreased: Bcl-2; Bcl-xL; XIAP | |
| Lambertianic acid in combination with TRAIL | IC50 = 20 μM | A549 and H1299 | Increased: activation of caspase-3, -9, and -8; DR4; activation of Bid Decreased: Bcl-2; XIAP; cFLIP | |
| Myriadenolide | IC50 = 30 μM | Jurkat and THP-1 | Increased: activation of caspase-3, -9, and -8; activation of Bid Decreased: MMP | |
| Nimbolide | IC50 = 5 µM | DU-145, PC-3, A-549 | Increased: activation of caspase-3, -9, and -8 Decreased: NR | |
| Raddeanin A | IC50 = 5.34 µM for BGC-823, IC50 = 6.61 µM for SGC-7901, and IC50 = 4.98 μM for MKN-28 | BGC-823, SGC-7901, and MKN-28 | Increased: Bax; activation of caspase-3, -9, and -8 Decreased: Bcl-2; Bcl-xL | |
| Different concentrations of raddeanin A (0.5, 1.5, and 4.5 mg/kg) | Granuloma cell line S180, hepatic carcinoma cell line H22, and cervical cancer cell line U14 mice models | Increased: NR Decreased: tumor volume of granuloma cell line S180, hepatic carcinoma cell line H22, and cervical cancer cell line U14 models | ||
| Rosamultic acid | Various concentrations (0–100 μM) | SGC-7901 | Increased: activation of caspase-3, -9, and -8 Decreased: NR | |
| Saikosaponin A | IC50 = 20 μM | LoVo, SW48 | Increased: Bax; activation of caspase-3, -2, -9, and -8; activation of Bid Decreased: Bcl-2; MMP | |
| Saponins | 30.3 μM | HT-29 | Increased: Bax; activation of caspase-3, -9, and -8; activation of BidDecreased: Bcl-2 | |
| Tubeimoside-1 | Various concentrations (0–40 μM) | HepG2 | Increased: Bak; activation of caspase-3, -9, and -8; Fas; FasL Decreased: Bcl-2; MMP | |
| Ursolic acid | 40 μM | RC-58 T/h/SA#4 | Increased: Bax; activation of caspase-3, -9, and -8; activation of Bid Decreased: Bcl-2 | |
| 56, 74, and 98 mg/m2 | Clinical trial; patients with advanced solid tumors | Increased: 60% of patients had stable disease; 1 lung cancer patient showed significant improvement Decreased: The lesion size | ||
| Xanthone | α-Mangostin | IC50 = 24.9 µM | MCF7 | Increased: Bax; cytochrome C release; activation of caspase-3, -7, -9, and -8 Decreased: Bcl-2; MMP |
| 30 and 60 mg/kg | LA7 cells bearing female Sprague-Dawley rats | Increased: NR Decreased: tumor volume | ||
| Pyranocycloartobiloxanthone A | IC50 = 1.4 µM | MCF7 | Increased: Bax; cytochrome C release; activation of caspase-3, -7, -9, and -8 Decreased: Bcl-2; MMP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajabi, S.; Maresca, M.; Yumashev, A.V.; Choopani, R.; Hajimehdipoor, H. The Most Competent Plant-Derived Natural Products for Targeting Apoptosis in Cancer Therapy. Biomolecules 2021, 11, 534. https://doi.org/10.3390/biom11040534
Rajabi S, Maresca M, Yumashev AV, Choopani R, Hajimehdipoor H. The Most Competent Plant-Derived Natural Products for Targeting Apoptosis in Cancer Therapy. Biomolecules. 2021; 11(4):534. https://doi.org/10.3390/biom11040534
Chicago/Turabian StyleRajabi, Sadegh, Marc Maresca, Alexei Valerievich Yumashev, Rasool Choopani, and Homa Hajimehdipoor. 2021. "The Most Competent Plant-Derived Natural Products for Targeting Apoptosis in Cancer Therapy" Biomolecules 11, no. 4: 534. https://doi.org/10.3390/biom11040534
APA StyleRajabi, S., Maresca, M., Yumashev, A. V., Choopani, R., & Hajimehdipoor, H. (2021). The Most Competent Plant-Derived Natural Products for Targeting Apoptosis in Cancer Therapy. Biomolecules, 11(4), 534. https://doi.org/10.3390/biom11040534







