Endurance Training Regulates Expression of Some Angiogenesis-Related Genes in Cardiac Tissue of Experimentally Induced Diabetic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Models and Ethical Statement
2.2. ET Protocol
2.3. Biochemical Assays
2.4. qRT-PCR Analysis
2.5. Statistical Analysis
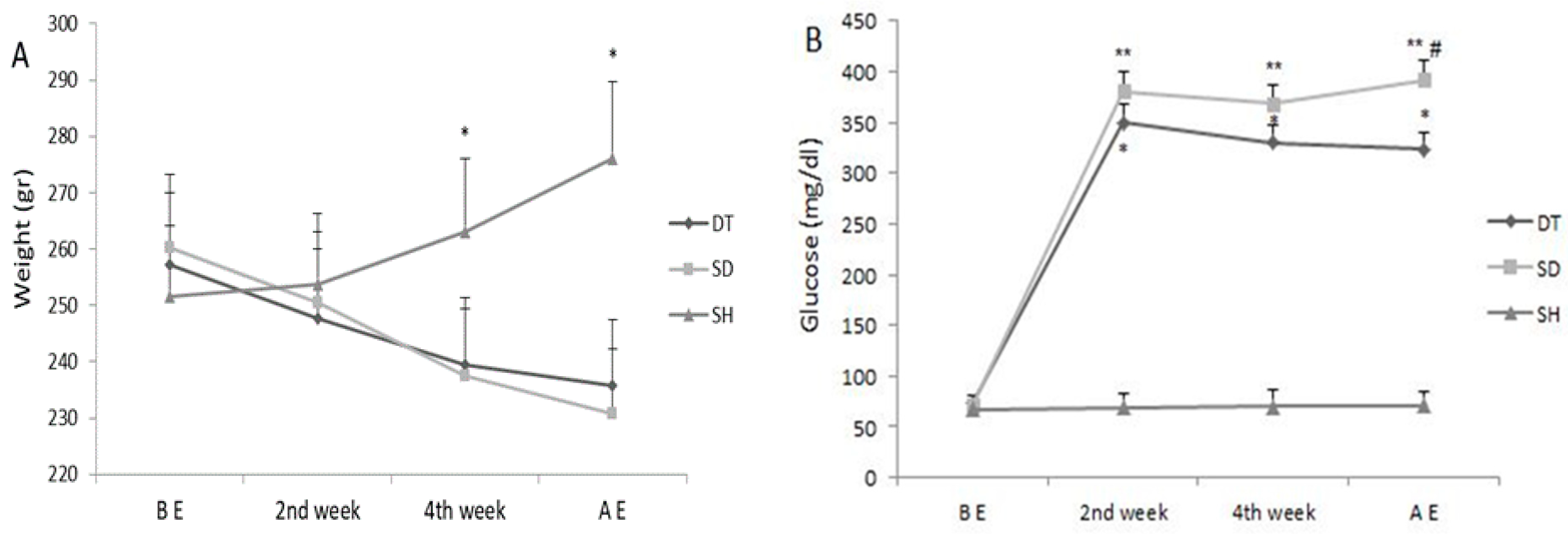
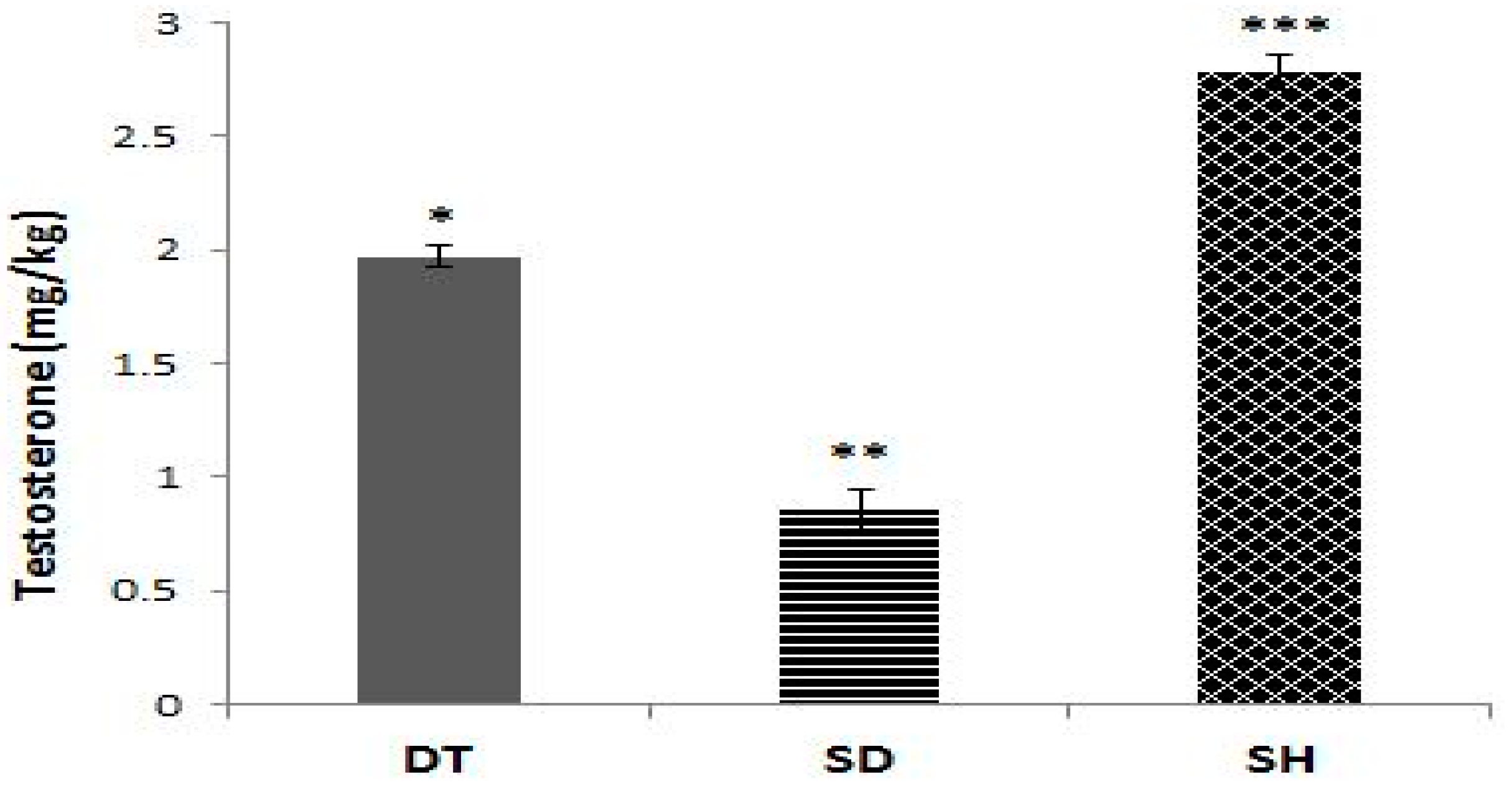
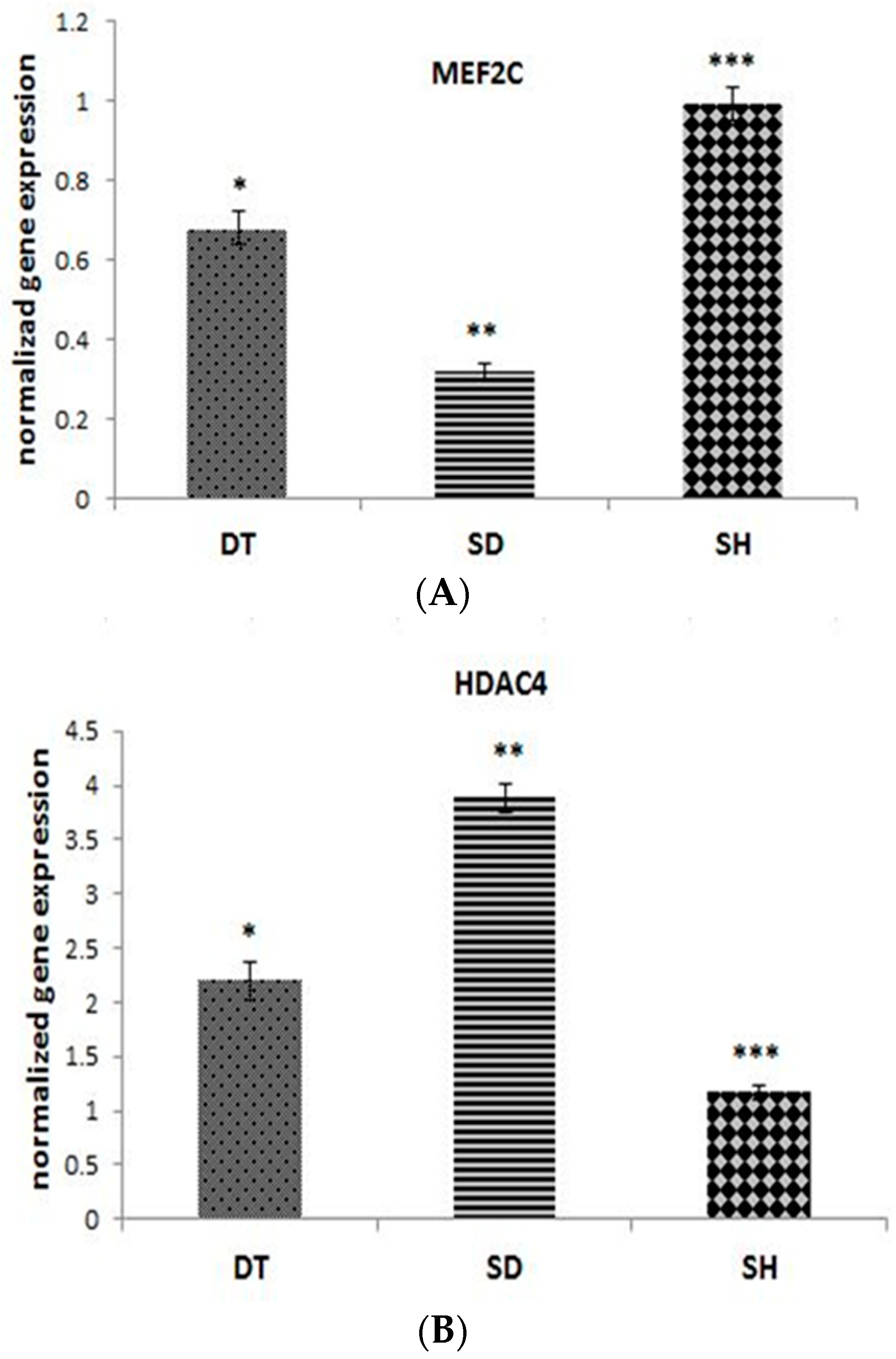
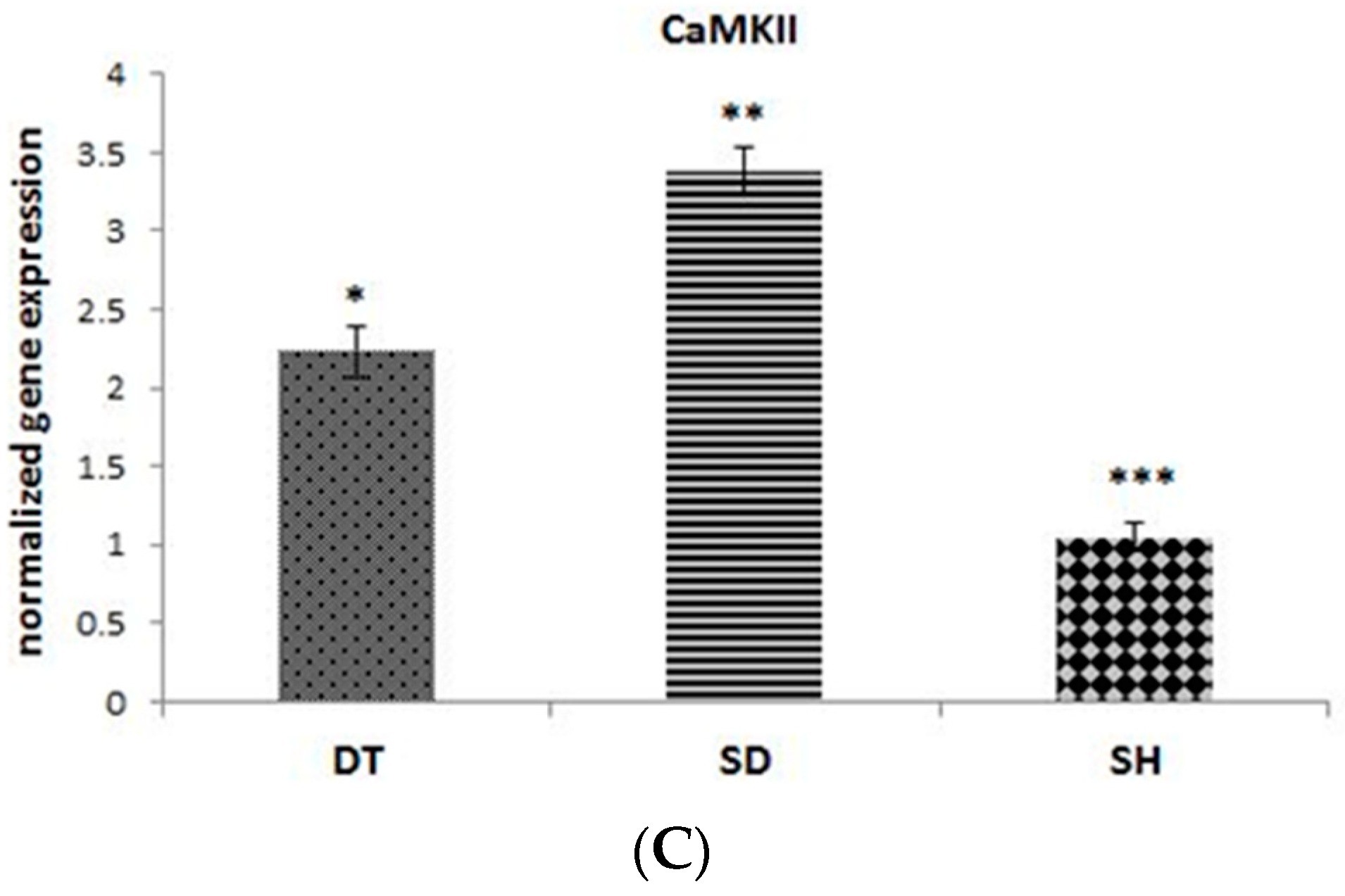
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaw, J.; Sicree, R.; Zimmet, P. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Prac. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Mendoza-Marí, Y.; Rodríguez-Rodríguez, N.; Herrera, D.G.D.B.; García-Ojalvo, A.; Fernández-Mayola, M.; Guillén-Nieto, G.; Valdés-Sosa, P.A. Burn injury insulin resistance and central nervous system complications: A review. Burn. Open 2020, 4, 41–52. [Google Scholar] [CrossRef]
- Zarich, S.W. Treating the diabetic patient: Appropriate care for glycemic control and cardiovascular disease risk factors. Rev. Cardiovasc. Med. 2003, 4, 19–28. [Google Scholar]
- Costa, P.Z.; Soares, R. Neovascularization in diabetes and its complications. Unraveling the angiogenic paradox. Life Sci. 2013, 92, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.Z.; Yeasmin, F.; Rabi, D.M.; Ronksley, P.E.; Turin, T.C. Prognostic tools for cardiovascular disease in patients with type 2 diabetes: A systematic review and meta-analysis of C-statistics. J. Diabetes Complicat. 2019, 33, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Shen, M.; Wang, R.; Liu, H.; Gao, C.; Xu, J.; Tao, L.; Yin, Z.; Yin, T. Thioredoxin1 Inactivation Mediates the Impairment of Ischemia-Induced Angiogenesis and Further Injury in Diabetic Myocardium. J. Vasc. Res. 2020, 57, 76–85. [Google Scholar] [CrossRef]
- Abacı, A.; Oguzhan, A.; Kahraman, S.; Eryol, N.K.; Unal, S.; Arınc, H.; Ergin, A. Effect of Diabetes Mellitus on Formation of Coronary Collateral Vessels. Circulation 1999, 99, 2239–2242. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Jośko, J.; Mazurek, M. Transcription factors having impact on vascular endothelial growth factor (VEGF) gene expression in angiogenesis. Med Sci. Monit. 2004, 10, 89. [Google Scholar]
- Hamik, A.; Wang, B.; Jain, M.K. Transcriptional Regulators of Angiogenesis. Arter. Thromb. Vasc. Biol. 2006, 26, 1936–1947. [Google Scholar] [CrossRef] [PubMed]
- Maiti, D.; Xu, Z.; Duh, E.J. Vascular endothelial growth factor induces MEF2C and MEF2-dependent activity in endothe-lial cells. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3640–3648. [Google Scholar] [CrossRef] [PubMed]
- Razeghi, P.; Young, M.E.; Cockrill, T.C.; Frazier, O.H.; Taegtmeyer, H. Downregulation of myocardial myocyte enhancer factor 2C and myocyte enhancer factor 2C-regulated gene expression in diabetic patients with nonischemic heart failure. Circulation 2002, 106, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Boudina, S.; Abel, E.D. Diabetic cardiomyopathy, causes and effects. Rev. Endocr. Metab. Disord. 2010, 11, 31–39. [Google Scholar] [CrossRef]
- Ruijter, A.J.D.; Gennip, A.H.V.; Caron, H.N.; Kemp, S.; Kuilenburg, A.B.V. Histone deacetylases (HDACs): Characterization of the classical HDAC family. Biochem. J. 2003, 370, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Xiao, C.; Long, F.; Su, Z.; Jia, W.; Qin, M.; Huang, M.; Wu, W.; Suguro, R.; Liu, X.; et al. HDAC4 regulates vascular inflammation via activation of autophagy. Cardiovasc. Res. 2018, 114, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Cuello, F.; Lorenz, K. Inhibition of cardiac CaMKII to cure heart failure: Step by step towards translation? Basic Res. Cardiol. 2016, 111, 1–4. [Google Scholar] [CrossRef]
- Backs, J.; Backs, T.; Bezprozvannaya, S.; McKinsey, T.A.; Olson, E.N. Histone Deacetylase 5 Acquires Calcium/Calmodulin-Dependent Kinase II Responsiveness by Oligomerization with Histone Deacetylase 4. Mol. Cell. Biol. 2008, 28, 3437–3445. [Google Scholar] [CrossRef]
- Chen, Y.; Du, J.; Zhao, Y.T.; Zhang, L.; Lv, G.; Zhuang, S.; Qin, G.; Zhao, T.C. Histone deacetylase (HDAC) inhibition improves myocardial function and prevents cardiac remodeling in diabetic mice. Cardiovasc. Diabetol. 2015, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Mollova, M.Y.; Katus, H.A.; Ebacks, J. Regulation of CaMKII signaling in cardiovascular disease. Front. Pharmacol. 2015, 6, 178. [Google Scholar] [CrossRef]
- Hirata, Y.; Nomura, K.; Senga, Y.; Okada, Y.; Kobayashi, K.; Okamoto, S.; Minokoshi, Y.; Imamura, M.; Takeda, S.; Hosooka, T.; et al. Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. JCI Insight 2019, 4, 1–12. [Google Scholar] [CrossRef]
- Death, A.K.; McGrath, K.C.; Sader, M.A.; Nakhla, S.; Jessup, W.; Handelsman, D.J.; Celermajer, D.S. Dihydrotestosterone promotes vascular cell adhesion molecule-1 expression in male human endothelial cells via a nuclear factor-κB-dependent pathway. Endocrinology 2004, 145, 1889–1897. [Google Scholar] [CrossRef]
- Ng, M.K.; Quinn, C.M.; McCrohon, J.A.; Nakhla, S.; Jessup, W.; Handelsman, D.J.; Celermajer, D.S.; Death, A.K. Androgens up-regulate atherosclerosis-related genes in macrophages from males but not females: Molecular insights into gender differences in atherosclerosis. J. Am. Coll. Cardiol. 2003, 42, 1306–1313. [Google Scholar] [CrossRef]
- Kapoor, D.; Goodwin, E.; Channer, K.S.; Jones, T.H. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolemia in hypogonadal men with type 2 diabetes. Eur. J. Endocrinol. 2006, 154, 899–906. [Google Scholar] [CrossRef]
- Sami, W.; Ansari, T.; Butt, N.S.; Ab Hamid, M.R. Effect of diet on type 2 diabetes mellitus: A review. Int. J. Health Sci. 2017, 11, 65–71. [Google Scholar]
- Booth, F.W.; Roberts, C.K.; Thyfault, J.P.; Ruegsegger, G.N.; Toedebusch, R.G. Role of Inactivity in Chronic Diseases: Evolutionary Insight and Pathophysiological Mechanisms. Physiol. Rev. 2017, 97, 1351–1402. [Google Scholar] [CrossRef] [PubMed]
- Hambrecht, R.; Wolf, A.; Gielen, S.; Linke, A.; Hofer, J.; Erbs, S.; Schoene, N.; Schuler, G. Effect of Exercise on Coronary Endothelial Function in Patients with Coronary Artery Disease. N. Engl. J. Med. 2000, 342, 454–460. [Google Scholar] [CrossRef]
- Pedersen, L.R.; Olsen, R.H.; Anholm, C.; Astrup, A.; Eugen-Olsen, J.; Fenger, M.; Simonsen, L.; Walzem, R.L.; Haugaard, S.B.; Prescott, E. Effects of 1 year of exercise training versus combined exercise training and weight loss on body compo-sition, low-grade inflammation and lipids in overweight patients with coronary artery disease: A randomized trial. Cardio-Vasc. Diabetol. 2019, 18, 127. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tan, S.; Wang, Z.; Guo, Z.; Li, Q.; Wang, J. Aerobic exercise training at maximal fat oxidation intensity improves body composition, glycemic control, and physical capacity in older people with type 2 diabetes. J. Exerc. Sci. Fit. 2020, 18, 7–13. [Google Scholar] [CrossRef]
- Yakasai, A.M.; Maharaj, S.S.; Nuhu, J.M. Moderate intensity endurance exercise on responses of relative cardiovascular parameters of primary and secondary hypertensive patients: Protocol for a randomized controlled trials. Eur. J. Physiother. 2019, 21, 1–6. [Google Scholar] [CrossRef]
- Venables, M.C.; Jeukendrup, A.E. Endurance training and obesity: Effect on substrate metabolism and insulin sensitivity. Med. Sci. Sports Exerc. 2008, 40, 495–502. [Google Scholar] [CrossRef]
- Colberg, S.R.; Niederseer, D. Impact of Exercise on Cardiovascular Risk Factors: Diabetes Mellitus. In Textbook of Sports and Exercise Cardiology; Metzler, J.B., Ed.; Springer: New York, NY, USA, 2020; pp. 769–792. [Google Scholar]
- Crisafulli, A.; Pagliaro, P.; Roberto, S.; Cugusi, L.; Mercuro, G.; Lazou, A.; Beauloye, C.; Bertrand, L.; Hausenloy, D.J.; Aragno, M.; et al. Diabetic cardiomyopathy and ischemic heart disease: Prevention and therapy by exercise and condition-ing. Int. J. Mol. Sci. 2020, 21, 2896. [Google Scholar] [CrossRef]
- Després, J.-P. Physical Activity, Sedentary Behaviours, and Cardiovascular Health: When Will Cardiorespiratory Fitness Become a Vital Sign? Can. J. Cardiol. 2016, 32, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Erekat, N.S.; Al-Jarrah, M.D.; Al Khatib, A.J. Treadmill Exercise Training Improves Vascular Endothelial Growth Factor Expression in the Cardiac Muscle of Type I Diabetic Rats. Cardiol. Res. 2014, 5, 23–29. [Google Scholar] [CrossRef][Green Version]
- Graham, K.; Yarar-Fisher, C.; Li, J.; McCully, K.M.; Rimmer, J.H.; Powell, D.; Bickel, C.S.; Fisher, G. Effects of High-Intensity Interval Training Versus Moderate-Intensity Training on Cardiometabolic Health Markers in Individuals With Spinal Cord Injury: A Pilot Study. Top. Spinal Cord Inj. Rehabil. 2019, 25, 248–259. [Google Scholar] [CrossRef]
- Ardakanizade, M. The effects of mid- and long-term endurance exercise on heart angiogenesis and oxidative stress. Iran. J. Basic. Med. Sci. 2018, 21, 800–805. [Google Scholar] [PubMed]
- Ne’Eman, Z.; Barash, V.; Rosenmann, E.; Shafrir, E. Localization of glycogen in the placenta of diabetic rats: A Light and electron microscopic study. Placenta 1987, 8, 201–208. [Google Scholar] [CrossRef]
- Sugimoto, K.; Rashid, I.B.; Shoji, M.; Suda, T.; Yasujima, M. Early changes in insulin receptor signaling and pain sensation in streptozotocin-induced diabetic neu-ropathy in rats. J. Pain 2008, 9, 237–245. [Google Scholar] [CrossRef]
- Chae, C.H.; Jung, S.L.; An, S.H.; Jung, C.K.; Nam, S.N.; Kim, H.T. Treadmill exercise suppresses muscle cell apoptosis by increasing nerve growth factor levels and stimulat-ing p-phosphatidylinositol 3-kinase activation in the soleus of diabetic rats. J. Physiol. Biochem. 2011, 67, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Seguro, C.; Viana, R.; Lima, G.; Galvão, L.; Silva, L.; Jardim, T.; Jardim, P.; Gentil, P. Improvements in health parameters of a diabetic and hypertensive patient with only 40 min of exercise per week: A case study. Disabil. Rehabil. 2020, 42, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.; Thomas, M.C.; Panagiotopoulos, S.; Sharpe, K.; MacIsaac, R.J.; Clarke, S.; Zajac, J.D.; Jerums, G. Low Testosterone Levels Are Common and Associated with Insulin Resistance in Men with Diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1834–1840. [Google Scholar] [CrossRef]
- Khaneshi, F.; Nasrolahi, O.; Azizi, S.; Nejati, V. Sesame effects on testicular damage in streptozotocin-induced diabetes rats. Avicenna J. Phytomedicine 2013, 3, 347–355. [Google Scholar]
- Zumoff, B.; Strain, G.W.; Miller, L.K.; Rosner, W.; Senie, R.; Seres, D.S.; Rosenfeld, R.S. Plasma Free and Non-Sex-Hormone-Binding-Globulin Bound Testosterone Are Decreased in Obese Men in Proportion to Their Degree of Obesity. J. Clin. Endocrinol. Metab. 1990, 71, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Akerstrom, T.; Laub, L.; Vedel, K.; Brand, C.L.; Pedersen, B.K.; Lindqvist, A.K.; Wojtaszewski, J.F.P.; Hellsten, Y. Increased skeletal muscle capillarization enhances insulin sensitivity. Am. J. Physiol. Metab. 2014, 307, E1105–E1116. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.M.; Kelly, D.M.; Jones, T.H. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat. Rev. Endocrinol. 2013, 9, 479–493. [Google Scholar] [CrossRef]
- Sacilotto, N.; Chouliaras, K.M.; Nikitenko, L.L.; Lu, Y.W.; Fritzsche, M.; Wallace, M.D.; Nornes, S.; García-Moreno, F.; Payne, S.; Bridges, E.; et al. MEF2 transcription factors are key regulators of sprouting angiogenesis. Genes Dev. 2016, 30, 2297–2309. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Shi, A.; Zhao, J. Epidemiological Perspectives of Diabetes. Cell Biophys. 2015, 73, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Ago, T.; Liu, T.; Zhai, P.; Chen, W.; Li, H.; Molkentin, J.D.; Vatner, S.F.; Sadoshima, J. A Redox-Dependent Pathway for Regulating Class II HDACs and Cardiac Hypertrophy. Cell 2008, 133, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wei, L.; Chen, Q.; Terek, R.M. HDAC4 Represses Vascular Endothelial Growth Factor Expression in Chondrosarcoma by Modulating RUNX2 Activity. J. Biol. Chem. 2009, 284, 21881–21890. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, X.; Li, Q.; Zhou, S.-M.; Hu, B.; Hu, G.-W.; Niu, X.; Guo, S.-C.; Wang, Y.; Deng, Z.-F. Role of Phosphorylated HDAC4 in Stroke-Induced Angiogenesis. BioMed Res. Int. 2017, 2017, 2957538 . [Google Scholar] [CrossRef] [PubMed]
- Kaurstad, G.; Alves, M.N.; Kemi, O.J.; Rolim, N.; Høydal, M.A.; Wisløff, H.; Stølen, T.O.; Wisløff, U. Chronic CaMKII inhibition blunts the cardiac contractile response to exercise training. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 112, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Stølen, T.O.; Høydal, M.A.; Kemi, O.J.; Catalucci, D.; Ceci, M.; Aasum, E.; Larsen, T.; Rolim, N.; Condorelli, G.; Smith, G.L.; et al. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release syn-chronicity in a mouse model of diabetic cardiomyopathy. Circ. Res. 2009, 105, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Turtle, C. Altered Contractile Mechanics and Ca2+ Handling Contribute to Cardiomyopathy Pathogenesis; Oxford University: Oxford, UK, 2015. [Google Scholar]
- Hegyi, B.; Bers, D.M.; Bossuyt, J. CaMKII signaling in heart diseases: Emerging role in diabetic cardiomyopathy. J. Mol. Cell. Cardiol. 2019, 127, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.; Kunhi, M.; Little, G.H.; Bai, Y.; An, W.; Bers, D.; Kedes, L.; Poizat, C. Nuclear CaMKII enhances histone H3 phosphorylation and remodels chromatin during cardiac hypertro-phy. Nucleic Acids Res. 2013, 41, 7656–7672. [Google Scholar] [CrossRef] [PubMed]
| Weeks | First Week | Second Week | Third Week | Forth Week | Fifth Week | Sixth Week |
|---|---|---|---|---|---|---|
| Duration (minutes) | 10 | 20 | 20 | 30 | 30 | 30 |
| Speed (m/minutes) Slope | 10 0 | 10 0 | 15 0 | 15 0 | 17–18 0 | 17–18 0 |
| Genes | Forward | Reverse | Amplicon Size (bp) |
|---|---|---|---|
| GAPDH | AGTTCAACGGCACAGTCAAG | TACTCAGCACCAGCATCACC | 119 |
| MEF-2C | CTTCAACAGCACCAACAAGC | TCAATGCCTCCACAATGTCC | 125 |
| HDAC4 | CTCTGCCAAATGTTTCGGGT | CAAGCTCATTTCCCAGCAGA | 149 |
| CaMKII | AGTGACACCTGAAGCCAAAG | GTCAAGATGGCACCCTTCAA | 198 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khajehlandi, M.; Bolboli, L.; Siahkuhian, M.; Rami, M.; Tabandeh, M.; Khoramipour, K.; Suzuki, K. Endurance Training Regulates Expression of Some Angiogenesis-Related Genes in Cardiac Tissue of Experimentally Induced Diabetic Rats. Biomolecules 2021, 11, 498. https://doi.org/10.3390/biom11040498
Khajehlandi M, Bolboli L, Siahkuhian M, Rami M, Tabandeh M, Khoramipour K, Suzuki K. Endurance Training Regulates Expression of Some Angiogenesis-Related Genes in Cardiac Tissue of Experimentally Induced Diabetic Rats. Biomolecules. 2021; 11(4):498. https://doi.org/10.3390/biom11040498
Chicago/Turabian StyleKhajehlandi, Mojdeh, Lotfali Bolboli, Marefat Siahkuhian, Mohammad Rami, Mohammadreza Tabandeh, Kayvan Khoramipour, and Katsuhiko Suzuki. 2021. "Endurance Training Regulates Expression of Some Angiogenesis-Related Genes in Cardiac Tissue of Experimentally Induced Diabetic Rats" Biomolecules 11, no. 4: 498. https://doi.org/10.3390/biom11040498
APA StyleKhajehlandi, M., Bolboli, L., Siahkuhian, M., Rami, M., Tabandeh, M., Khoramipour, K., & Suzuki, K. (2021). Endurance Training Regulates Expression of Some Angiogenesis-Related Genes in Cardiac Tissue of Experimentally Induced Diabetic Rats. Biomolecules, 11(4), 498. https://doi.org/10.3390/biom11040498







