Characterization of the Lipidome and Biophysical Properties of Membranes from High Five Insect Cells Expressing Mouse P-Glycoprotein
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Lipid Composition of the Membranes Isolated from High Five Insect Cells
3.1.1. Relative Abundance of Lipids and Proteins
3.1.2. Relative Abundance of the Different Phospholipid Classes
3.1.3. Quantification of the Fatty Acid Composition in the Phospholipid Pool
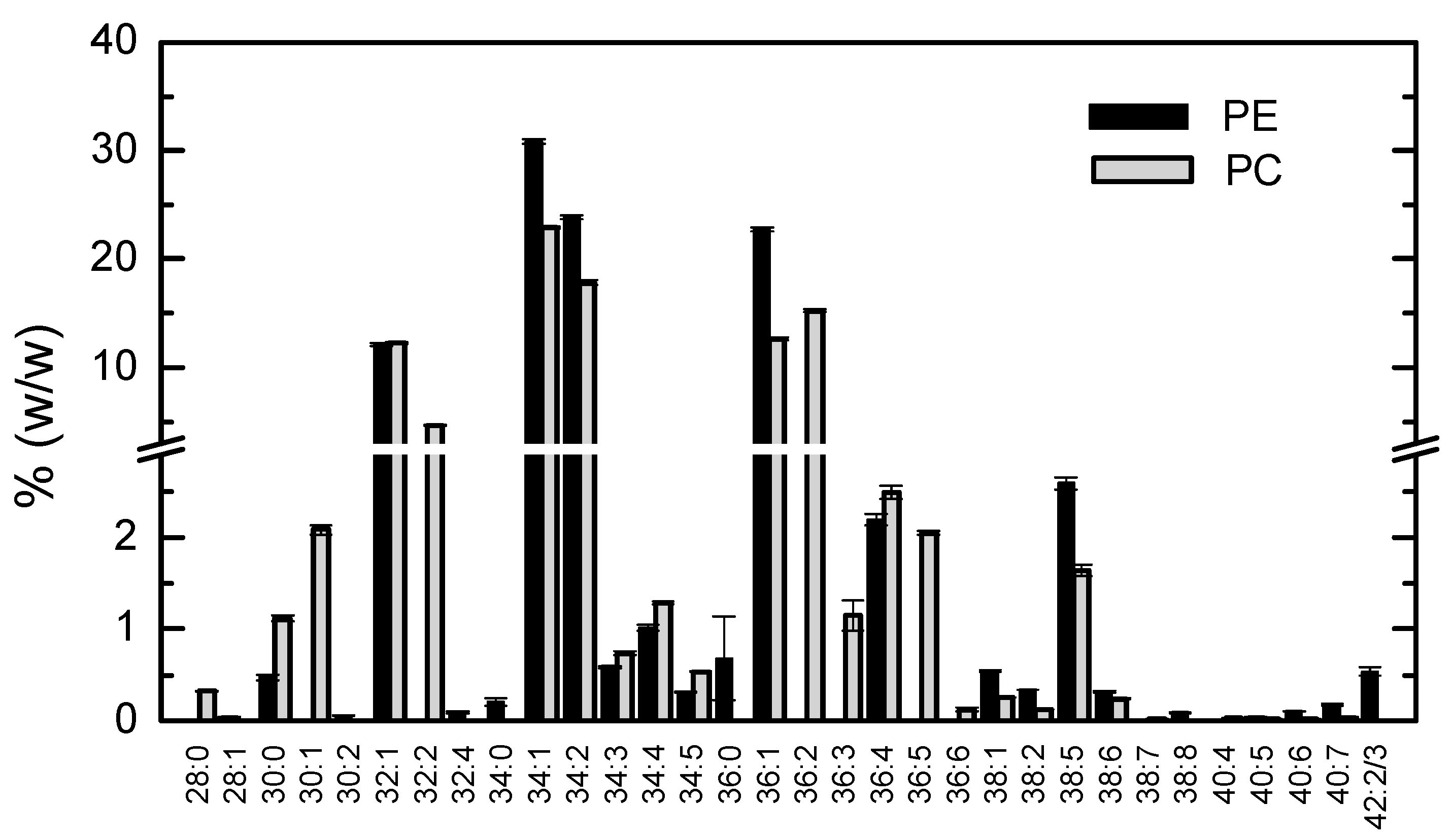
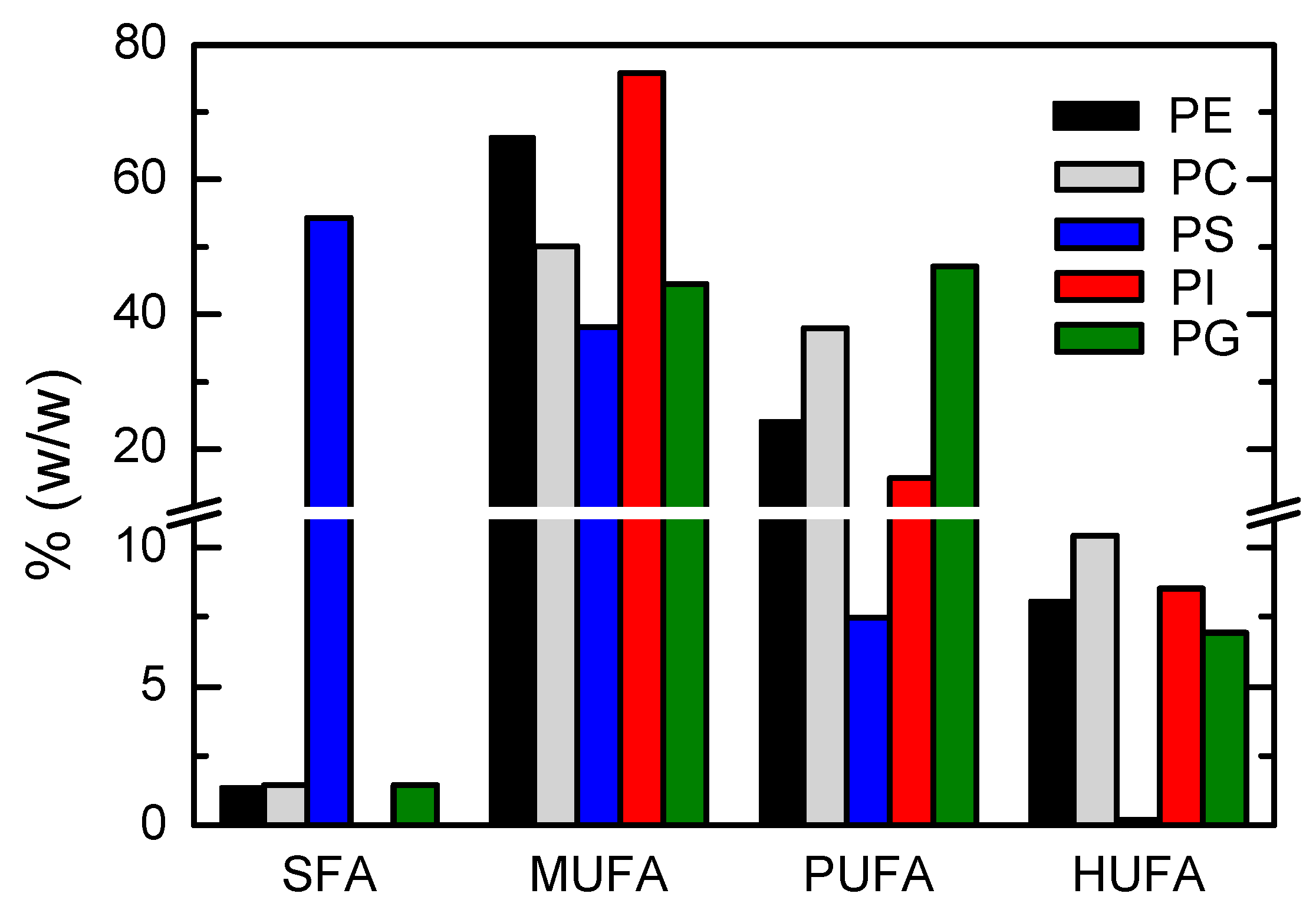
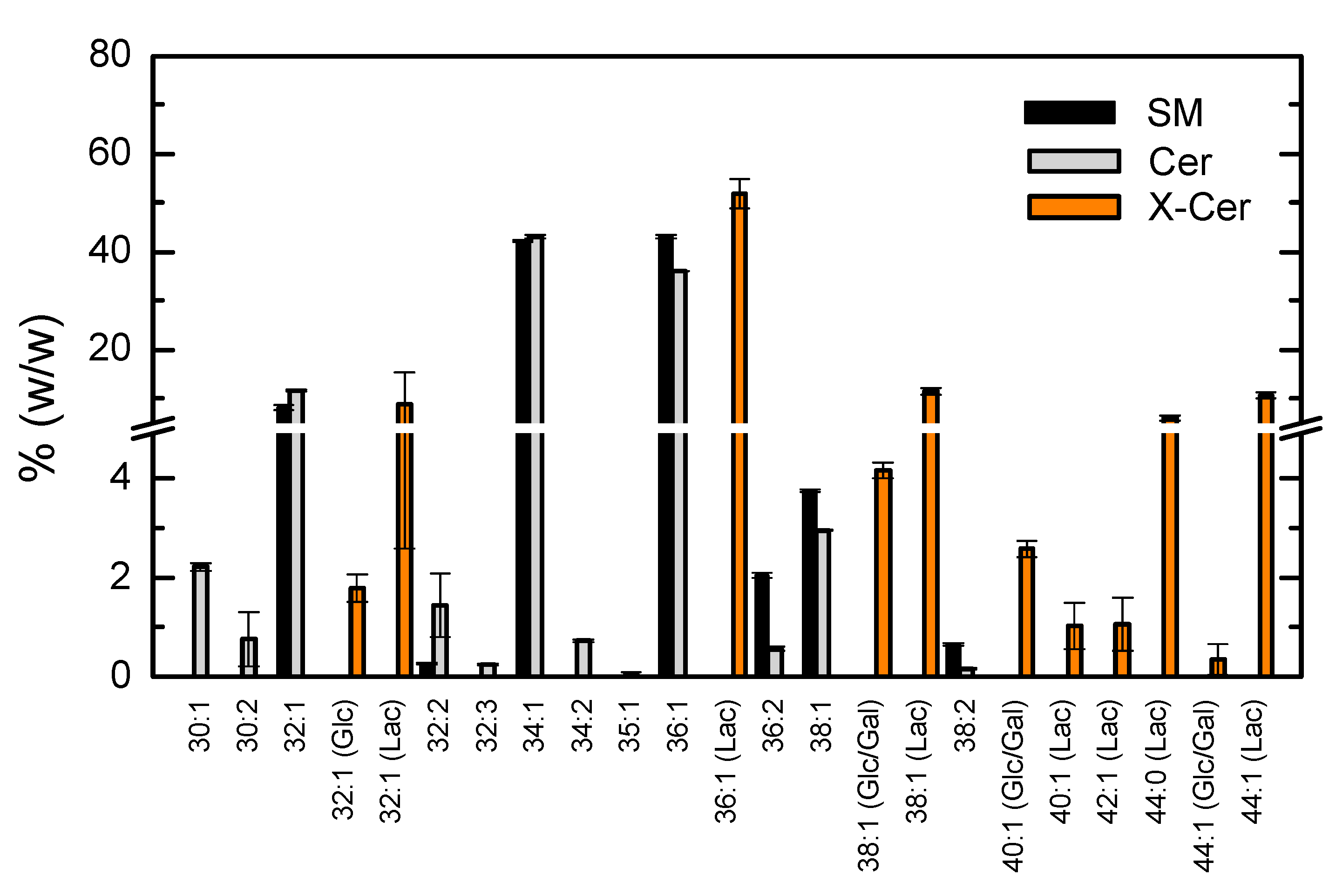
3.1.4. Lipidome Characterization of the Most Abundant Phospholipid Classes
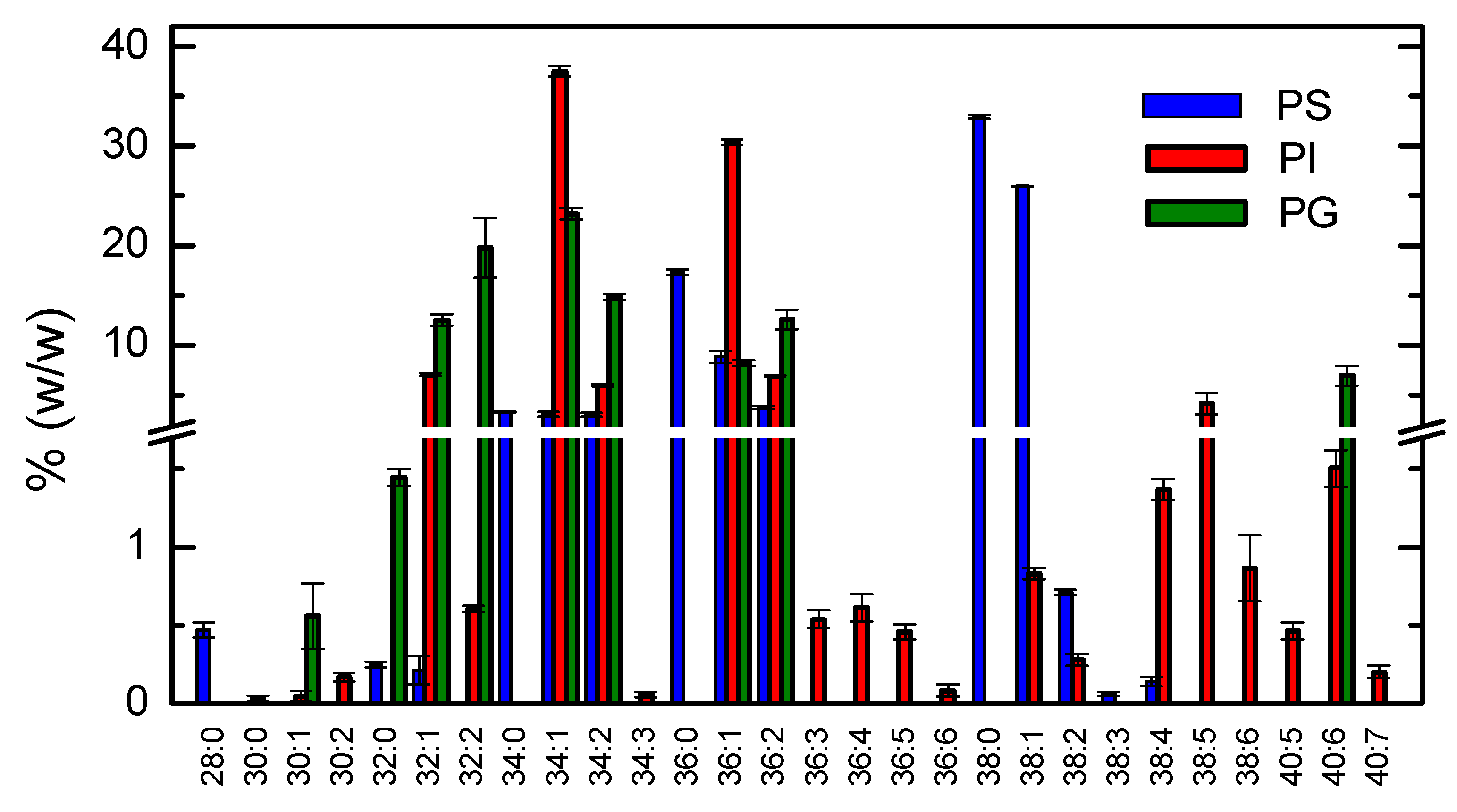
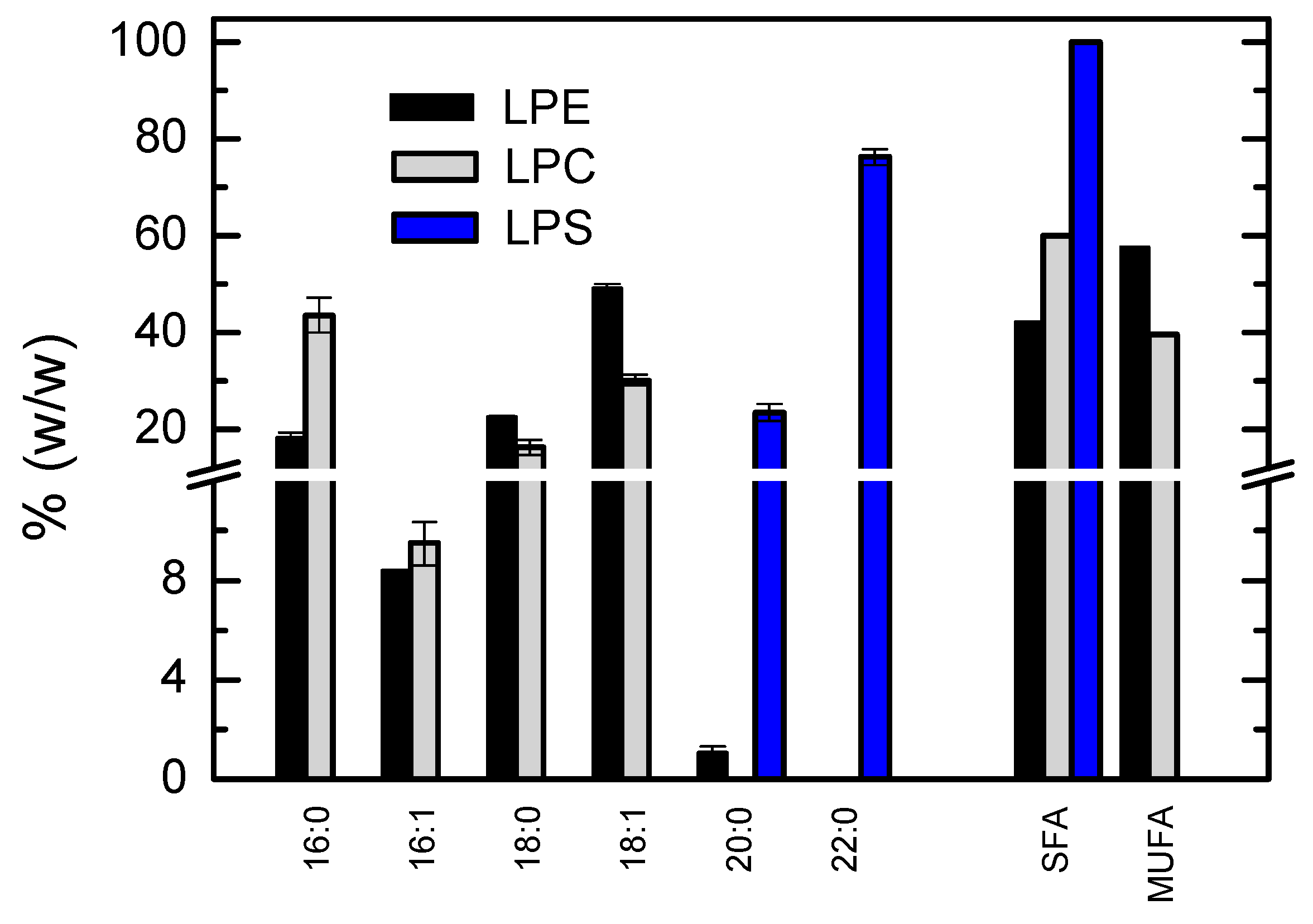
3.1.5. Lipidome Characterization for the Additional Phospholipid Classes
3.2. Biophysical Properties of the Membranes Obtained from High Five Insect Cells
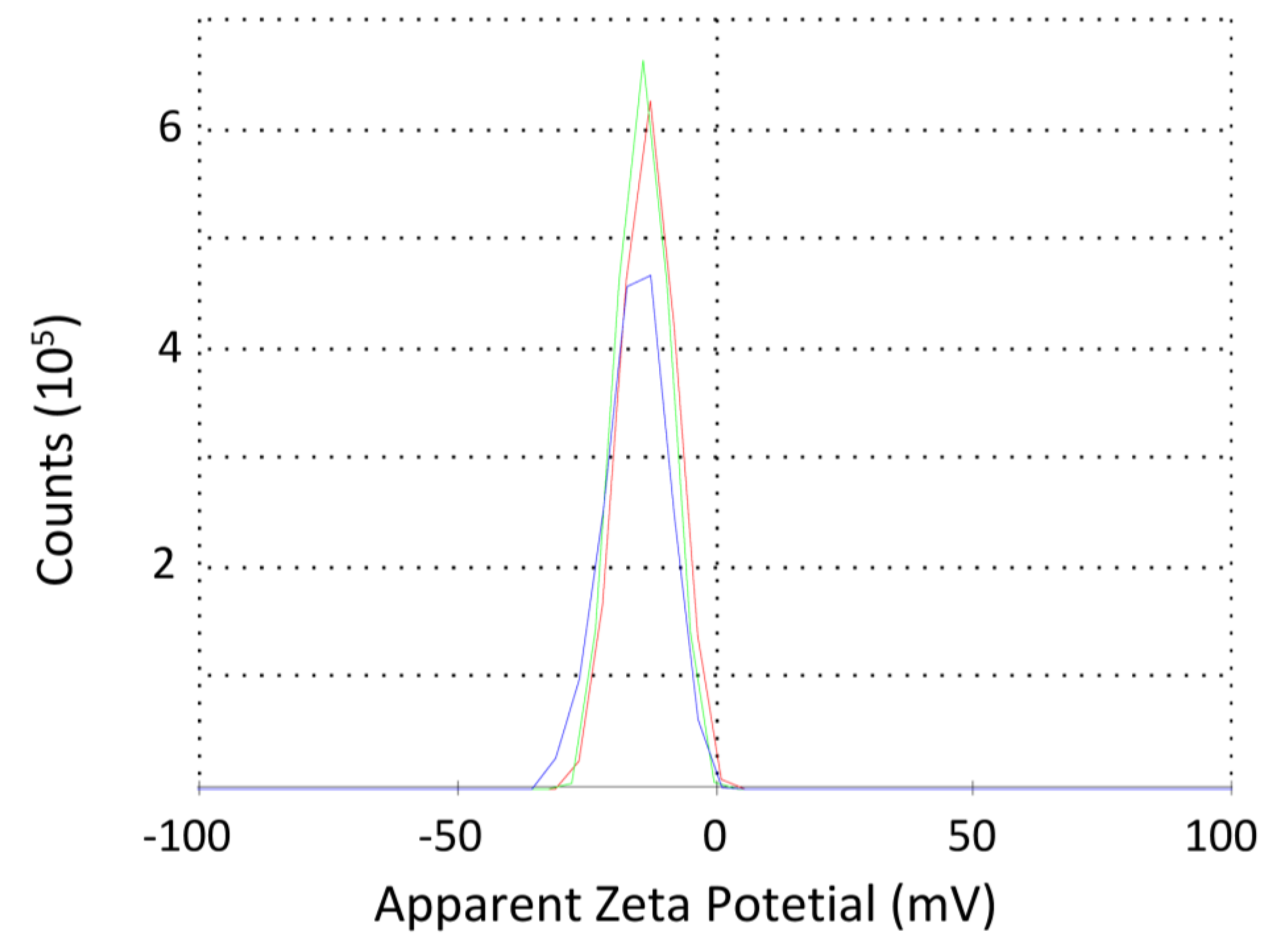
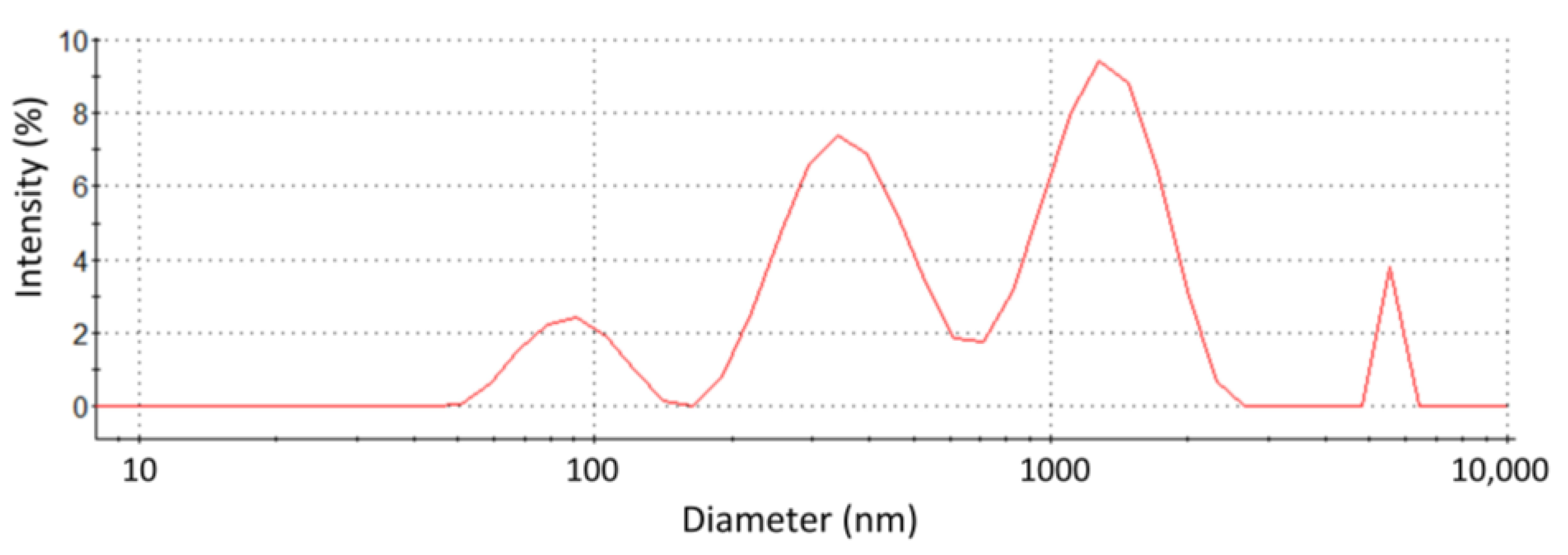
3.2.1. Surface Charge and Size of the Vesicles
3.2.2. Membrane Polarity and Fluidity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Ramachandra, M.; Ambudkar, S.V.; Chen, D.; Hrycyna, C.A.; Dey, S.; Gottesman, M.M.; Pastan, I. Human P-glycoprotein exhibits reduced affinity for substrates during a catalytic transition state. Biochemistry 1998, 37, 5010–5019. [Google Scholar] [CrossRef] [PubMed]
- Gribar, J.J.; Ramachandra, M.; Hrycyna, C.A.; Dey, S.; Ambudkar, S.V. Functional characterization of glycosylation-deficient human P-glycoprotein using a vaccinia virus expression system. J. Membr. Biol. 2000, 173, 203–214. [Google Scholar] [CrossRef]
- Evans, G.L.; Ni, B.F.; Hrycyna, C.A.; Chen, D.; Ambudkar, S.V.; Pastan, I.; Germann, U.A.; Gottesman, M.M. Heterologous expression systems for P-glycoprotein:E. coli, yeast, and baculovirus. J. Bioenerg. Biomembr. 1995, 27, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.A.; Shukla, S.; Rao, P.; Borgnia, M.J.; Bartesaghi, A.; Merk, A.; Mobin, A.; Esser, L.; Earl, L.A.; Gottesman, M.M.; et al. Cryo-EM Analysis of the Conformational Landscape of Human P-glycoprotein (ABCB1) During its Catalytic Cycle. Mol. Pharmacol. 2016, 90, 35–41. [Google Scholar] [CrossRef]
- Shukla, S.; Abel, B.; Chufan, E.E.; Ambudkar, S.V. Effects of a detergent micelle environment on P-glycoprotein (ABCB1)-ligand interactions. J. Biol. Chem. 2017, 292, 7066–7076. [Google Scholar] [CrossRef] [PubMed]
- Corradi, V.; Mendez-Villuendas, E.; Ingólfsson, H.I.; Gu, R.-X.; Siuda, I.; Melo, M.N.; Moussatova, A.; DeGagné, L.J.; Sejdiu, B.I.; Singh, G.; et al. Lipid-Protein Interactions Are Unique Fingerprints for Membrane Proteins. ACS Cent. Sci. 2018, 4, 709–717. [Google Scholar] [CrossRef]
- Sharom, F. Complex Interplay between the P-Glycoprotein Multidrug Efflux Pump and the Membrane: Its Role in Modulating Protein Function. Front. Oncol. 2014, 4, 41. [Google Scholar] [CrossRef]
- Clay, A.T.; Sharom, F.J. Lipid Bilayer Properties Control Membrane Partitioning, Binding, and Transport of P-Glycoprotein Substrates. Biochemistry 2013, 52, 343–354. [Google Scholar] [CrossRef]
- Belli, S.; Elsener, P.M.; Wunderli-Allenspach, H.; Kramer, S.D. Cholesterol-Mediated Activation of P-Glycoprotein: Distinct Effects on Basal and Drug-Induced ATPase Activities. J. Pharm. Sci. 2009, 98, 1905–1918. [Google Scholar] [CrossRef] [PubMed]
- Bucher, K.; Belli, S.; Wunderli-Allenspach, H.; Kramer, S.D. P-glycoprotein in proteoliposomes with low residual detergent: The effects of cholesterol. Pharm. Res. 2007, 24, 1993–2004. [Google Scholar] [CrossRef]
- Sharom, F.J. The P-glycoprotein efflux pump: How does it transport drugs? J. Membr. Biol. 1997, 160, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Wadkins, R.M.; Houghton, P.J. The Role of Drug Lipid Interactions in the Biological-Activity of Modulators of Multidrug-Resistance. Biochim. Biophys. Acta 1993, 1153, 225–236. [Google Scholar] [CrossRef]
- Seddon, A.M.; Casey, D.; Law, R.V.; Gee, A.; Templer, R.H.; Ces, O. Drug interactions with lipid membranes. Chem. Soc. Rev. 2009, 38, 2509–2519. [Google Scholar] [CrossRef] [PubMed]
- Escriba, P.V.; Gonzalez-Ros, J.M.; Goni, F.M.; Kinnunen, P.K.J.; Vigh, L.; Sanchez-Magraner, L.; Fernandez, A.M.; Busquets, X.; Horvath, I.; Barcelo-Coblijn, G. Membranes: A meeting point for lipids, proteins and therapies. J. Cell. Mol. Med. 2008, 12, 829–875. [Google Scholar] [CrossRef]
- Cheng, X.L.; Smith, J.C. Biological Membrane Organization and Cellular Signaling. Chem. Rev. 2019, 119, 5849–5880. [Google Scholar] [CrossRef]
- Chwastek, G.; Surma, M.A.; Rizk, S.; Grosser, D.; Lavrynenko, O.; Rucinska, M.; Jambor, H.; Saenz, J. Principles of Membrane Adaptation Revealed through Environmentally Induced Bacterial Lipidome Remodeling. Cell Rep. 2020, 32, 108165. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.; Schmidt, C. Mass spectrometry of membrane protein complexes. Biol. Chem. 2019, 400, 813–829. [Google Scholar] [CrossRef]
- Santos, A.L.; Preta, G. Lipids in the cell: Organisation regulates function. Cell. Mol. Life Sci. 2018, 75, 1909–1927. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.; Surma, M.A.; Simons, K. Organellar lipidomics—background and perspectives. Curr. Opin. Cell Biol. 2013, 25, 406–413. [Google Scholar] [CrossRef]
- Coskun, U.; Simons, K. Cell Membranes: The Lipid Perspective. Structure 2011, 19, 1543–1548. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Gerl, M.J.; Sampaio, J.L.; Urban, S.; Kalvodova, L.; Verbavatz, J.M.; Binnington, B.; Lindemann, D.; Lingwood, C.A.; Shevchenko, A.; Schroeder, C.; et al. Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane. J. Cell Biol. 2012, 196, 213–221. [Google Scholar] [CrossRef]
- Sampaio, J.L.; Gerl, M.J.; Klose, C.; Ejsing, C.S.; Beug, H.; Simons, K.; Shevchenko, A. Membrane lipidome of an epithelial cell line. Proc. Natl. Acad. Sci. USA 2011, 108, 1903–1907. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.Y.; Han, R.H.; Han, X.L. Novel advances in shotgun lipidomics for biology and medicine. Prog. Lipid Res. 2016, 61, 83–108. [Google Scholar] [CrossRef]
- Shevchenko, A.; Simons, K. Lipidomics: Coming to grips with lipid diversity. Nat. Rev. Mol. Cell Biol. 2010, 11, 593–598. [Google Scholar] [CrossRef]
- Kalvodova, L.; Sampaio, J.L.; Cordo, S.; Ejsing, C.S.; Shevchenko, A.; Simons, K. The Lipidomes of Vesicular Stomatitis Virus, Semliki Forest Virus, and the Host Plasma Membrane Analyzed by Quantitative Shotgun Mass Spectrometry. J. Virol. 2009, 83, 7996–8003. [Google Scholar] [CrossRef] [PubMed]
- Ejsing, C.S.; Sampaio, J.L.; Surendranath, V.; Duchoslav, E.; Ekroos, K.; Klemm, R.W.; Simons, K.; Shevchenko, A. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc. Natl. Acad. Sci. USA 2009, 106, 2136–2141. [Google Scholar] [CrossRef]
- Hammad, L.A.; Cooper, B.S.; Fisher, N.P.; Montooth, K.L.; Karty, J.A. Profiling and quantification of Drosophila melanogaster lipids using liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 2959–2968. [Google Scholar] [CrossRef] [PubMed]
- Gerbal, M.; Fournier, P.; Barry, P.; Mariller, M.; Odier, F.; Devauchelle, G.; Duonor-Cerutti, M. Adaptation of an insect cell line of Spodoptera frugiperda to grow at 37 degrees C: Characterization of an endodiploid clone. Vitr. Cell. Dev. Biol. Anim. 2000, 36, 117–124. [Google Scholar] [CrossRef]
- Marheineke, K.; Grunewald, S.; Christie, W.; Reilander, H. Lipid composition of Spodoptera frugiperda (Sf9) and Trichoplusia ni (Tn) insect cells used for baculovirus infection. FEBS Lett. 1998, 441, 49–52. [Google Scholar] [CrossRef]
- Ghioni, C.; Bell, J.G.; Sargent, J.R. Polyunsaturated fatty acids in neutral lipids and phospholipids of some freshwater insects. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 114, 161–170. [Google Scholar] [CrossRef]
- Zinser, E.; Daum, G. Isolation and biochemical characterization of organelles from the yeast, Saccharomyces cerevisiae. Yeast 1995, 11, 493–536. [Google Scholar] [CrossRef] [PubMed]
- Fournier, B.R.; Wolff, R.L.; Nogaro, M.; Radallah, D.; Darret, D.; Larrue, J.; Girardie, A. Fatty-Acid Composition of Phospholipids and Metabolism in Rectal Tissues of The African Locust. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1995, 111, 361–370. [Google Scholar] [CrossRef]
- Petzel, D.H.; Parrish, A.K.; Ogg, C.L.; Witters, N.A.; Howard, R.W.; Stanleysamuelson, D.W. Arachidonic-Acid and Prostaglandin E(2) in Malpighian Tubules of Female Yellow-Fever Mosquitos. Insect Biochem. Mol. Biol. 1993, 23, 431–437. [Google Scholar] [CrossRef]
- Yoshida, S.; Uemura, M. LIPID-COMPOSITION OF PLASMA-MEMBRANES AND TONOPLASTS ISOLATED FROM ETIOLATED SEEDLINGS OF MUNG BEAN (VIGNA-RADIATA L). Plant Physiol. 1986, 82, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.M.S.; Filipe, H.A.L.; Gomes, F.; Moreira, N.D.; Vaz, W.L.C.; Moreno, M.J. Chain Length Effect on the Binding of Amphiphiles to Serum Albumin and to POPC Bilayers. J. Phys. Chem. B 2010, 114, 16337–16346. [Google Scholar] [CrossRef]
- Schaffner, W.; Weissmann, C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal. Biochem. 1973, 56, 502–514. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Bartlett, E.M.; Lewis, D.H. Spectrophotometric Determination of Phosphate Esters in Presence and Absence of Orthophosphate. Anal. Biochem. 1970, 36, 159–167. [Google Scholar] [CrossRef]
- Melo, T.; Alves, E.; Azevedo, V.; Martins, A.S.; Neves, B.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Lipidomics as a new approach for the bioprospecting of marine macroalgae—Unraveling the polar lipid and fatty acid composition of Chondrus crispus. Algal Res. 2015, 8, 181–191. [Google Scholar] [CrossRef]
- Aued-Pimentel, S.; Lago, J.H.G.; Chaves, M.H.; Kumagai, E.E. Evaluation of a methylation procedure to determine cyclopropenoids fatty acids from Sterculia striata St. Hil. Et Nauds seed oil. J. Chromatogr. A 2004, 1054, 235–239. [Google Scholar] [CrossRef]
- Parathath, S.; Connelly, M.A.; Rieger, R.A.; Klein, S.M.; Abumrad, N.A.; de la Llera-Moya, M.; Iden, C.R.; Rothblat, G.H.; Williams, D.L. Changes in plasma membrane properties and phosphatidylcholine subspecies of insect Sf9 cells due to expression of scavenger receptor class B, type I, and CD36. J. Biol. Chem. 2004, 279, 41310–41318. [Google Scholar] [CrossRef]
- Tattrie, N.H. Positional Distribution of Saturated and Unsaturated Fatty Acids on Egg Lecithin. J. Lipid Res. 1959, 1, 60–65. [Google Scholar] [CrossRef]
- Yabuuchi, H.; Obrien, J.S. Positional Distribution of Fatty Acids in Glycerophosphatides of Bovine Gray Matter. J. Lipid Res. 1968, 9, 65–67. [Google Scholar] [CrossRef]
- Mayer, R.J.; Marshall, L.A. New Insights on Mammalian Phospholipase-A2(S)—Comparison of Arachidonoyl-Selective and Arachidonoyl-Nonselective Enzymes. FASEB J. 1993, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.J.; Bastos, M.; Velazquez-Campoy, A. Partition of amphiphilic molecules to lipid bilayers by isothermal titration calorimetry. Anal. Biochem. 2010, 399, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Matos, C.; de Castro, B.; Gameiro, P.; Lima, J.; Reis, S. Zeta-Potential Measurements as a Tool to Quantify the Effect of Charged Drugs on the Surface Potential of Egg Phosphatidylcholine Liposomes. Langmuir 2004, 20, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G. Membrane Electrostatics. Biochim. Biophys. Acta 1990, 1031, 311–382. [Google Scholar] [CrossRef]
- Eisenberg, M.; Gresalfi, T.; Riccio, T.; McLaughlin, S. Adsorption of Mono-Valent Cations to Bilayer Membranes Containing Negative Phospholipids. Biochemistry 1979, 18, 5213–5223. [Google Scholar] [CrossRef]
- Kucerka, N.; Nieh, M.P.; Katsaras, J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2761–2771. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.D.; London, E. Location of diphenylhexatriene (DPH) and its derivatives within membranes: Comparison of different fluorescence quenching analyses of membrane depth. Biochemistry 1998, 37, 8180–8190. [Google Scholar] [CrossRef] [PubMed]
- Do Canto, A.; Robalo, J.R.; Santos, P.D.; Carvalho, A.J.P.; Ramalho, J.P.P.; Loura, L.M.S. Diphenylhexatriene membrane probes DPH and TMA-DPH: A comparative molecular dynamics simulation study. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 2647–2661. [Google Scholar] [CrossRef]
- Trotter, P.J.; Storch, J. 3-[P-(6-Phenyl)-1,3,5-Hexatrienyl]Phenylpropionic Acid (PA-DPH)—Characterization as a Fluorescent Membrane Probe and Binding to Fatty-Acid Binding-Proteins. Biochim. Biophys. Acta 1989, 982, 131–139. [Google Scholar] [CrossRef]
- Prendergast, F.G.; Haugland, R.P.; Callahan, P.J. 1-[4-(Trimethylamino)Phenyl]-6-Phenylhexa-1,3,5-Triene—Synthesis, Fluorescence Properties, and use as a Fluorescence Probe of Lipid Bilayers. Biochemistry 1981, 20, 7333–7338. [Google Scholar] [CrossRef] [PubMed]
- Filipe, H.A.L.; Bowman, D.; Palmeira, T.; Cardoso, R.M.S.; Loura, L.M.S.; Moreno, M.J. Interaction of NBD-labelled fatty amines with liquid-ordered membranes: A combined molecular dynamics simulation and fluorescence spectroscopy study. Phys. Chem. Chem. Phys. 2015, 17, 27534–27547. [Google Scholar] [CrossRef]
- Filipe, H.A.L.; Moreno, M.J.; Loura, L.M.S. Interaction of 7-Nitrobenz-2-oxa-1,3-diazol-4-yl-Labeled Fatty Amines with 1-Palmitoyl, 2-Oleoyl-sn-glycero-3-phosphocholine Bilayers: A Molecular Dynamics Study. J. Phys. Chem. B 2011, 115, 10109–10119. [Google Scholar] [CrossRef] [PubMed]
- Slater, S.J.; Ho, C.; Taddeo, F.J.; Kelly, M.B.; Stubbs, C.D. Contribution of Hydrogen-Bonding to Lipid Lipid Interactions in Membranes and the Role of Lipid Order—Effects of Cholesterol, Increased Phospholipid Unsaturation, and Ethanol. Biochemistry 1993, 32, 3714–3721. [Google Scholar] [CrossRef] [PubMed]
- Lipids, A.P. Phase Transition Temperatures for Glycerophospholipids. Available online: https://avantilipids.com/tech-support/physical-properties/phase-transition-temps (accessed on 8 January 2021).
- Amaro, M.; Filipe, H.A.L.; Ramalho, J.P.P.; Hof, M.; Loura, L.M.S. Fluorescence of nitrobenzoxadiazole (NBD)-labeled lipids in model membranes is connected not to lipid mobility but to probe location. Phys. Chem. Chem. Phys. 2016, 18, 7042–7054. [Google Scholar] [CrossRef]
- Guidotti, G. Membrane Proteins. Annu. Rev. Biochem. 1972, 41, 731–752. [Google Scholar] [CrossRef]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef]
- Domicevica, L.; Koldso, H.; Biggin, P.C. Multiscale molecular dynamics simulations of lipid interactions with P-glycoprotein in a complex membrane. J. Mol. Graph. Model. 2018, 80, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.N.; Silva, L.C.; Futerman, A.H.; Prieto, M. Effect of ceramide structure on membrane biophysical properties: The role of acyl chain length and unsaturation. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Sonnino, S.; Prinetti, A.; Nakayama, H.; Yangida, M.; Ogawa, H.; Iwabuchi, K. Role of very long fatty acid-containing glycosphingolipids in membrane organization and cell signaling: The model of lactosylceramide in neutrophils. Glycoconj. J. 2009, 26, 615–621. [Google Scholar] [CrossRef]
- Kopitz, J. Lipid glycosylation: A primer for histochemists and cell biologists. Histochem. Cell Biol. 2017, 147, 175–198. [Google Scholar] [CrossRef] [PubMed]
- Wimalachandra, D.; Yang, J.X.; Zhu, L.N.; Tan, E.; Asada, H.; Chan, J.Y.K.; Lee, Y.H. Long-chain glucosylceramides crosstalk with LYN mediates endometrial cell migration. Biochim. Biophys. Acta 2018, 1863, 71–80. [Google Scholar] [CrossRef]
- Lavie, Y.; Fiucci, G.; Czarny, M.; Liscovitch, M. Changes in membrane microdomains and caveolae constituents in multidrug-resistant cancer cells. Lipids 1999, 34, S57–S63. [Google Scholar] [CrossRef]
- Inokuchi, J.; Uemura, S.; Kabayama, K.; Igarashi, Y. Glycosphingolipid deficiency affects functional microdomain formation in Lewis lung carcinoma cells. Glycoconj. J. 2000, 17, 239–245. [Google Scholar] [CrossRef]
- Bleicher, R.J.; Cabot, M.C. Glucosylceramide synthase and apoptosis. Biochim. Biophys. Acta 2002, 1585, 172–178. [Google Scholar] [CrossRef]
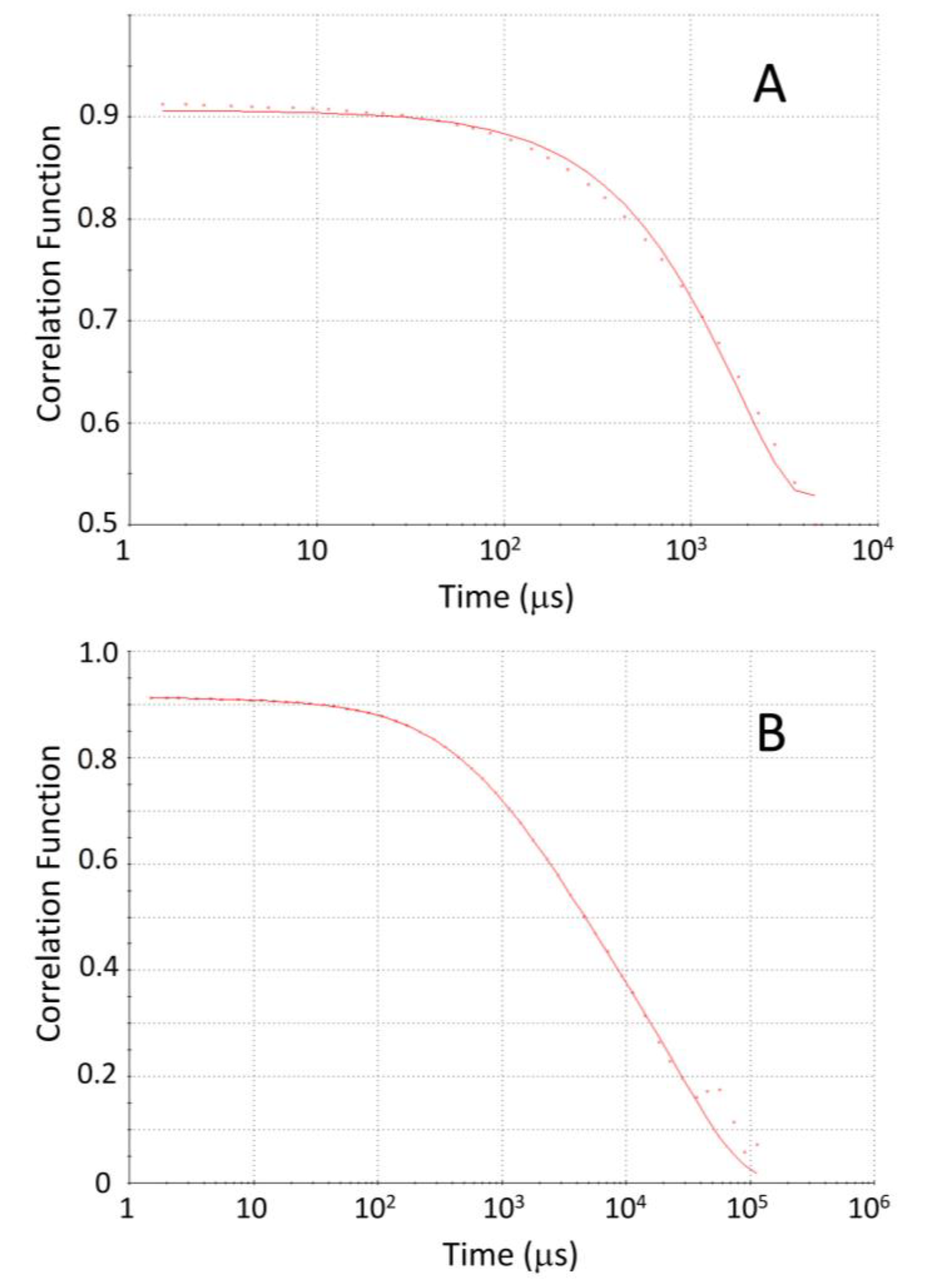
 ), POPE:POPC:POPS in the ratio 45:35:20 (
), POPE:POPC:POPS in the ratio 45:35:20 ( ), and in the membranes from High Five insect cells (
), and in the membranes from High Five insect cells ( ). The fluorescence intensity was normalized at the wavelength of maximum emission.
). The fluorescence intensity was normalized at the wavelength of maximum emission.
 ), POPE:POPC:POPS in the ratio 45:35:20 (
), POPE:POPC:POPS in the ratio 45:35:20 ( ), and in the membranes from High Five insect cells (
), and in the membranes from High Five insect cells ( ). The fluorescence intensity was normalized at the wavelength of maximum emission.
). The fluorescence intensity was normalized at the wavelength of maximum emission.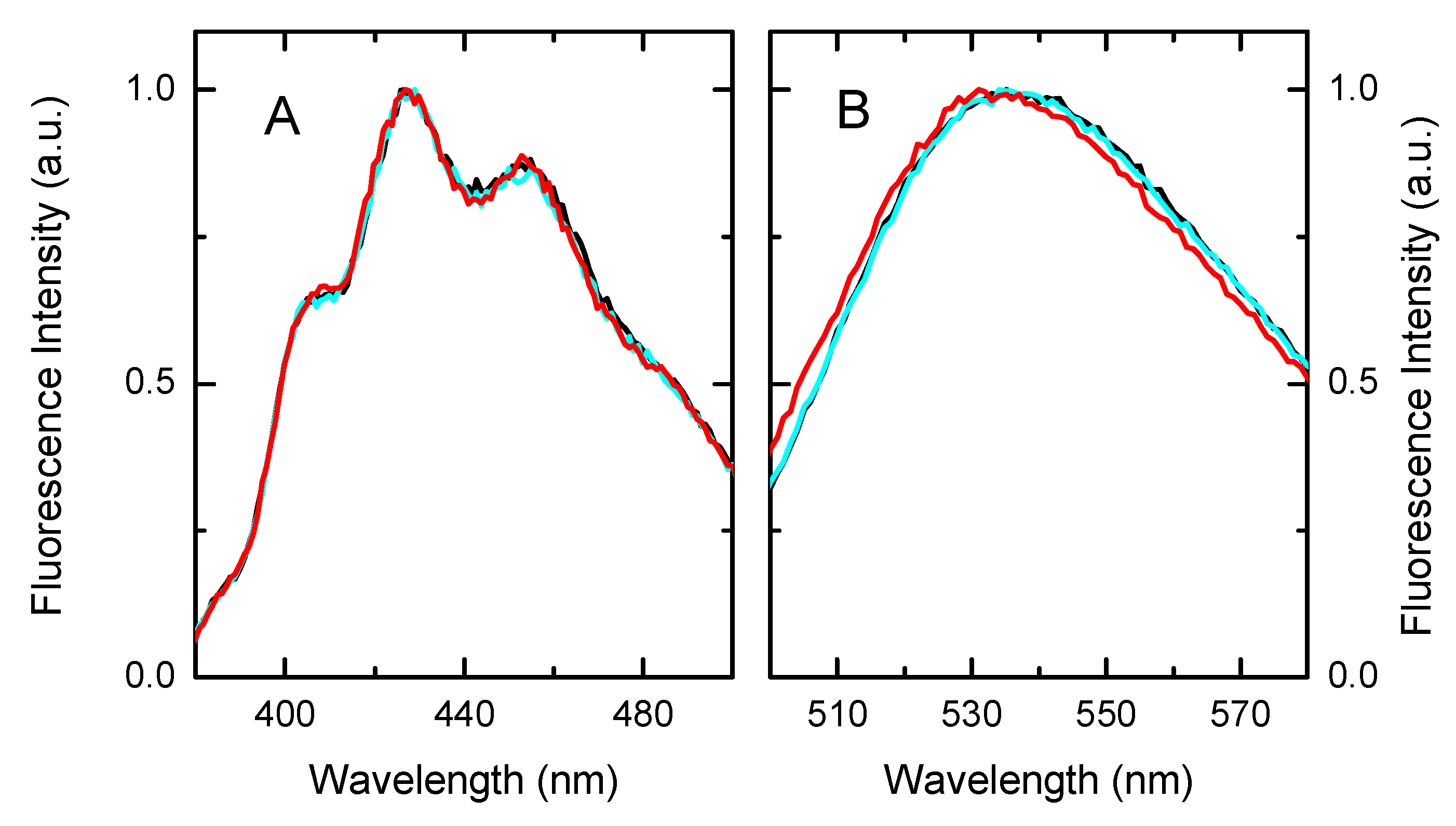
| Lipid Class | % (w/w) 1 |
|---|---|
| CL | 4.8 |
| LysoPL | 1.4 |
| PC | 24 |
| PE | 46 |
| PG | 5.1 |
| PI | 4.8 |
| PS | 8.7 |
| SM | 5.0 |
| Acyl Chain | % (w/w) 1 |
|---|---|
| C14:0 | 2.24 ± 0.27 |
| C16:0 | 14.2 ± 0.16 |
| C16:1n7 | 18.5 ± 0.14 |
| C18:0 | 15.7 ± 0.33 |
| C18:1n9 | 45.5 ± 1.4 |
| C18:1 | 2.23 ± 0.34 |
| C18:2 | 0.87 ± 0.25 |
| C20:0 | 0.88 ± 0.18 |
| C20:5 | 0.55 ± 0.06 |
| SFA | 33 |
| MUFA | 66 |
| PUFA | 1.4 |
| Fluorescent Probe | Membrane | Anisotropy |
|---|---|---|
| DPH | Hi5 membranes | 0.17 ± 0.01 |
| PE:PC:PS 45:35:20 | 0.12 ± 0.01 | |
| POPC | 0.10 ± 0.01 | |
| NBD-C16 | Hi5 membranes | 0.25 ± 0.05 |
| PE:PC:PS 45:35:20 | 0.20 ± 0.04 | |
| POPC | 0.18 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, M.J.; Teles Martins, P.A.; Bernardino, E.F.; Abel, B.; Ambudkar, S.V. Characterization of the Lipidome and Biophysical Properties of Membranes from High Five Insect Cells Expressing Mouse P-Glycoprotein. Biomolecules 2021, 11, 426. https://doi.org/10.3390/biom11030426
Moreno MJ, Teles Martins PA, Bernardino EF, Abel B, Ambudkar SV. Characterization of the Lipidome and Biophysical Properties of Membranes from High Five Insect Cells Expressing Mouse P-Glycoprotein. Biomolecules. 2021; 11(3):426. https://doi.org/10.3390/biom11030426
Chicago/Turabian StyleMoreno, Maria João, Patrícia Alexandra Teles Martins, Eva F. Bernardino, Biebele Abel, and Suresh V. Ambudkar. 2021. "Characterization of the Lipidome and Biophysical Properties of Membranes from High Five Insect Cells Expressing Mouse P-Glycoprotein" Biomolecules 11, no. 3: 426. https://doi.org/10.3390/biom11030426
APA StyleMoreno, M. J., Teles Martins, P. A., Bernardino, E. F., Abel, B., & Ambudkar, S. V. (2021). Characterization of the Lipidome and Biophysical Properties of Membranes from High Five Insect Cells Expressing Mouse P-Glycoprotein. Biomolecules, 11(3), 426. https://doi.org/10.3390/biom11030426








