Potential Effect of Pseudevernia furfuracea (L.) Zopf Extract and Metabolite Physodic Acid on Tumour Microenvironment Modulation in MCF-10A Cells
Abstract
1. Introduction
2. Material and Methods
2.1. Lichen Material and Isolation of Tested Compounds
2.2. High-Performance Liquid Chromatography (HLPC)
2.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.4. Cell Culture
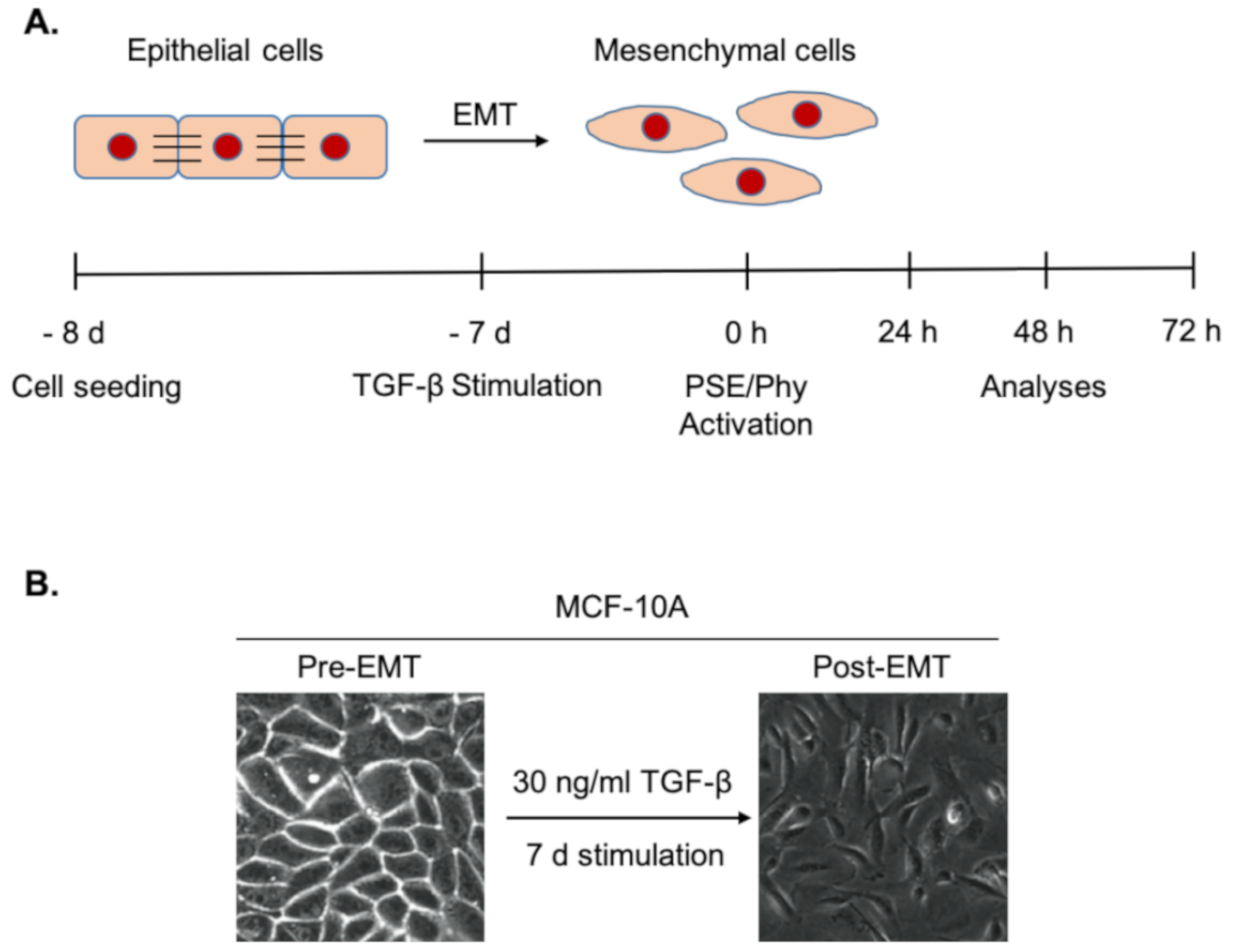
2.5. Experimental Design
2.6. MTS (Methyl Tetrazolium Salt) Cell Viability Assay
2.7. 5-Bromo-2′-deoxyuridine (BrdU) Cell Proliferation Assay
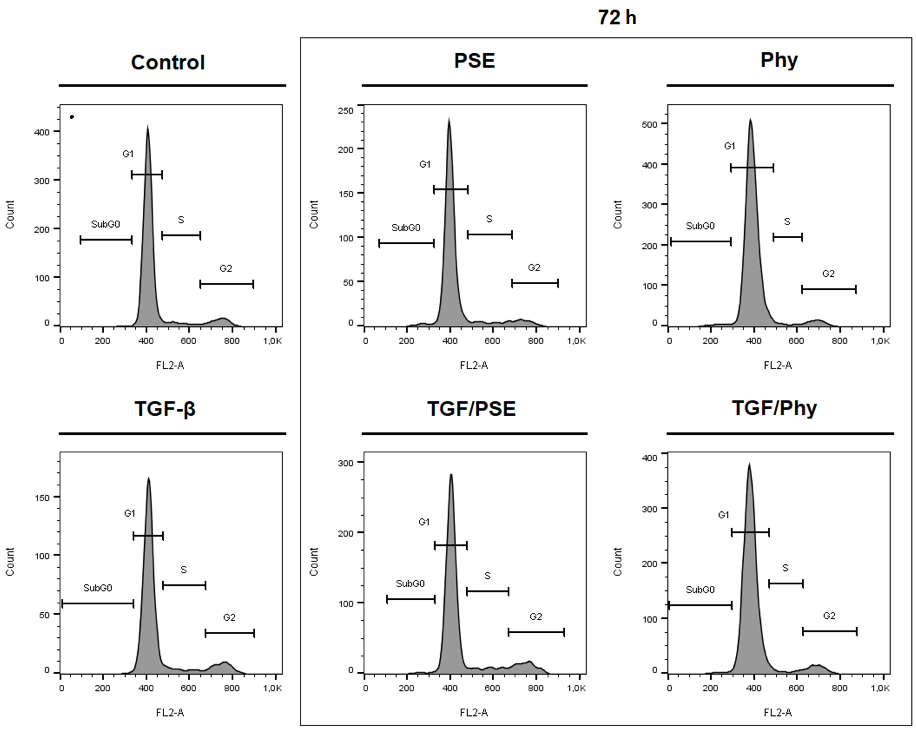
2.8. Flow Cytometry Protein Analyses and Cell Cycle
2.9. Western Blot
2.10. Immunofluorescence Microscopy
2.11. The Chorioallantoic Membrane (CAM) Assay
2.12. Statistical Analyses
3. Results
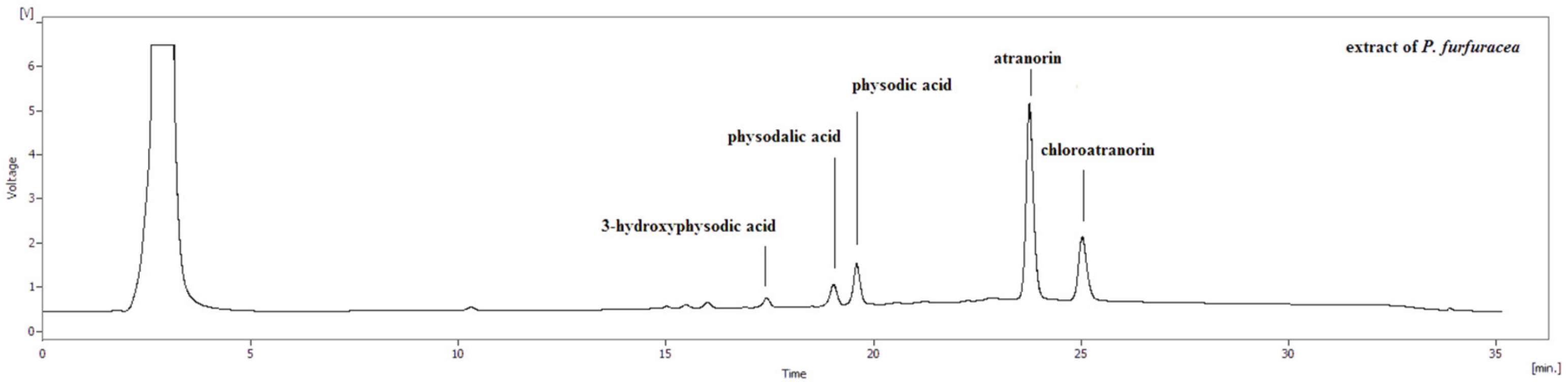
3.1. HPLC
3.2. NMR
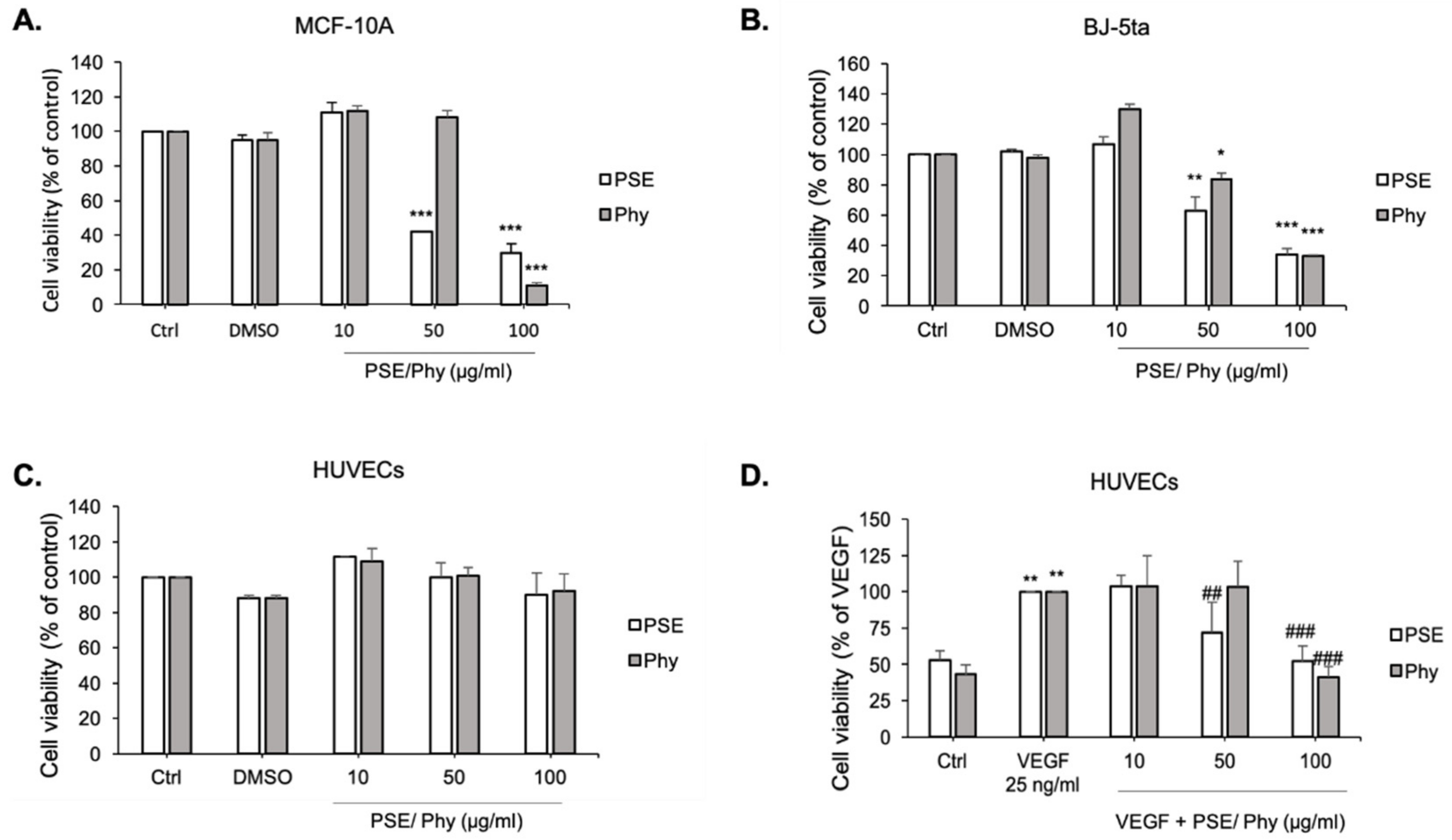
3.3. MTS Cytotoxic Assay
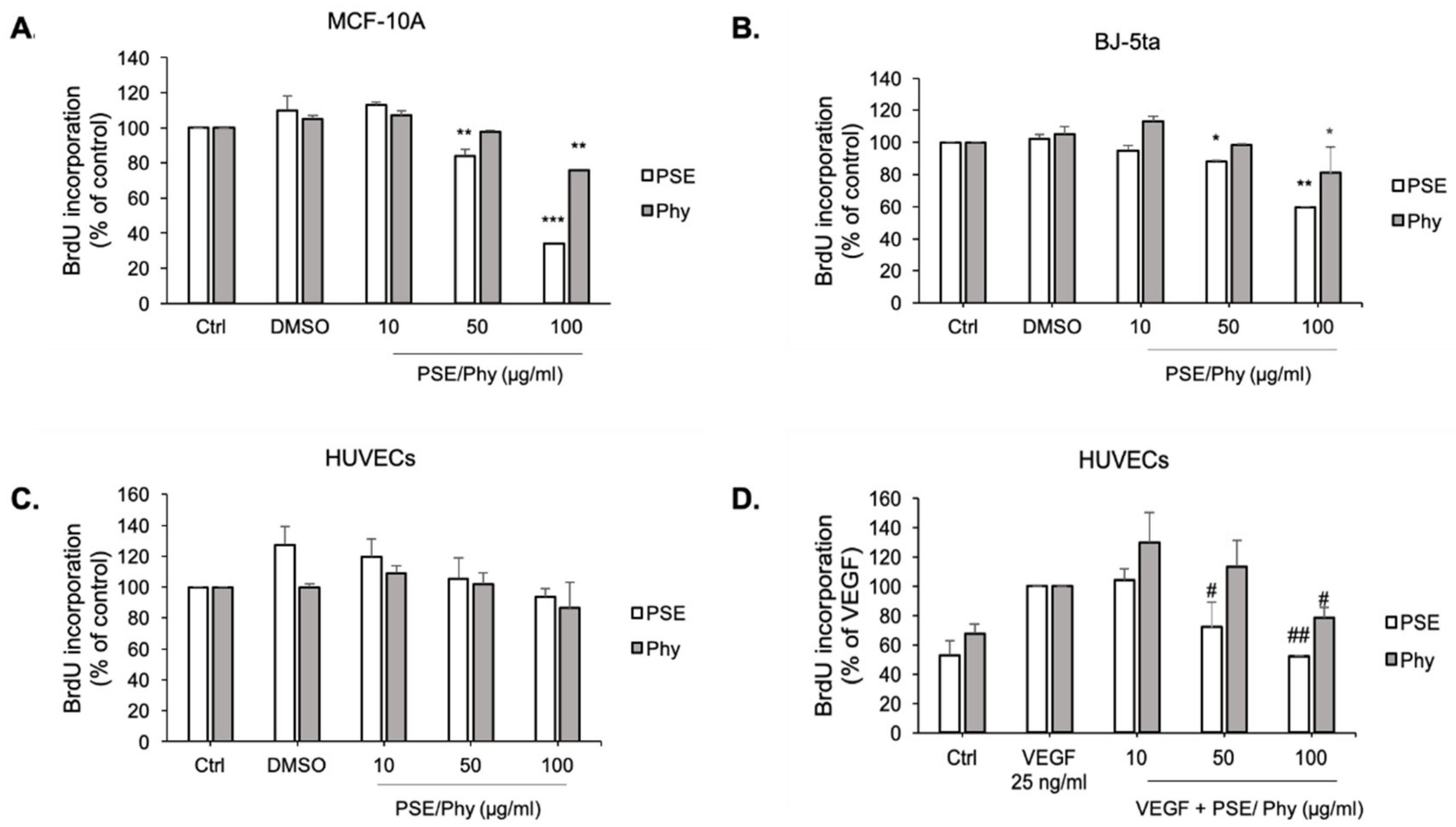
3.4. Bromdeoxyuridine (BrdU) Incorporation
3.5. Effect of IC10 Concentration of PSE and Phy on MCF-10A Cell Proliferation
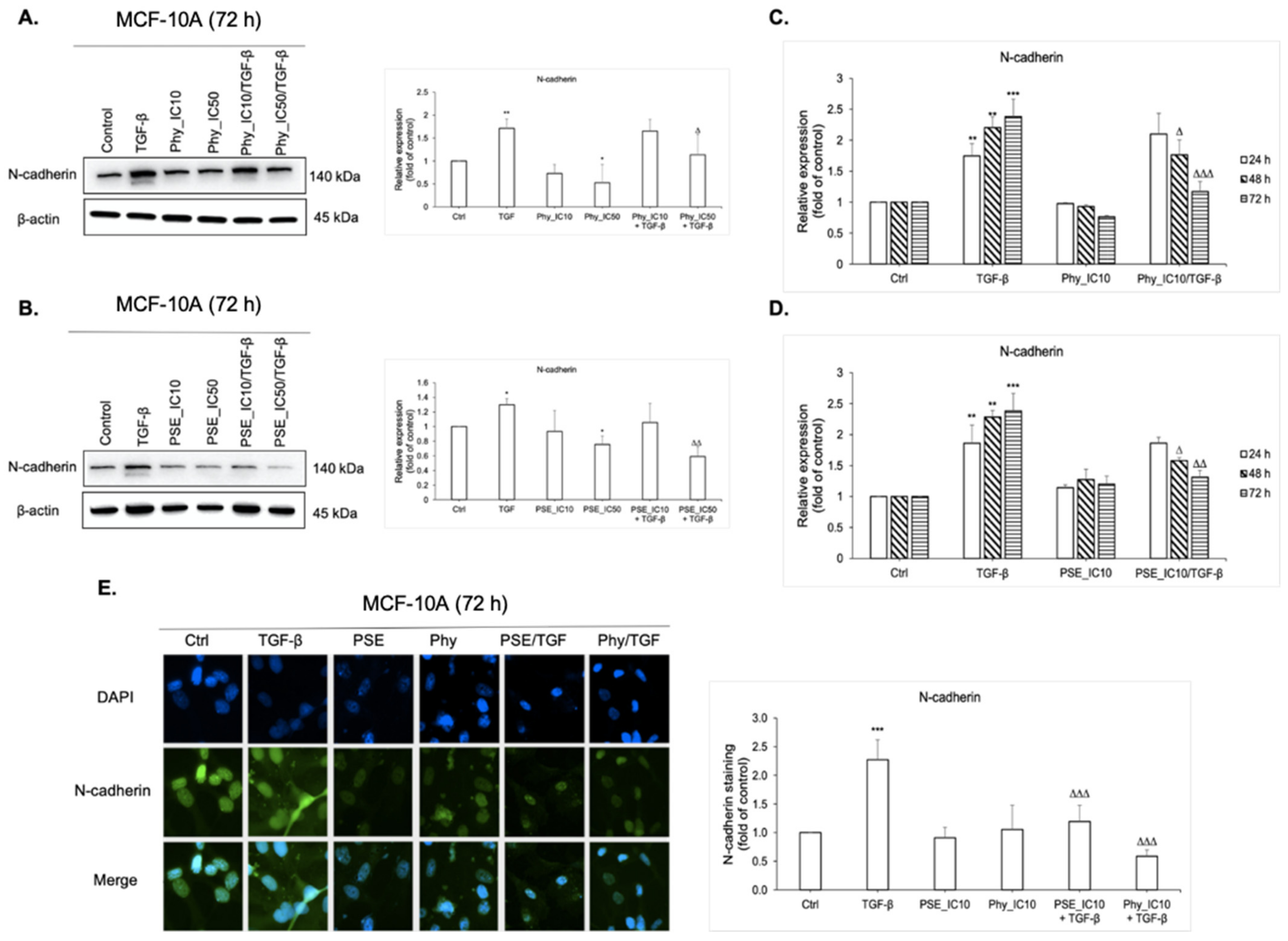
3.6. N-Cadherin Regulation Analyses after PSE and Phy Treatment
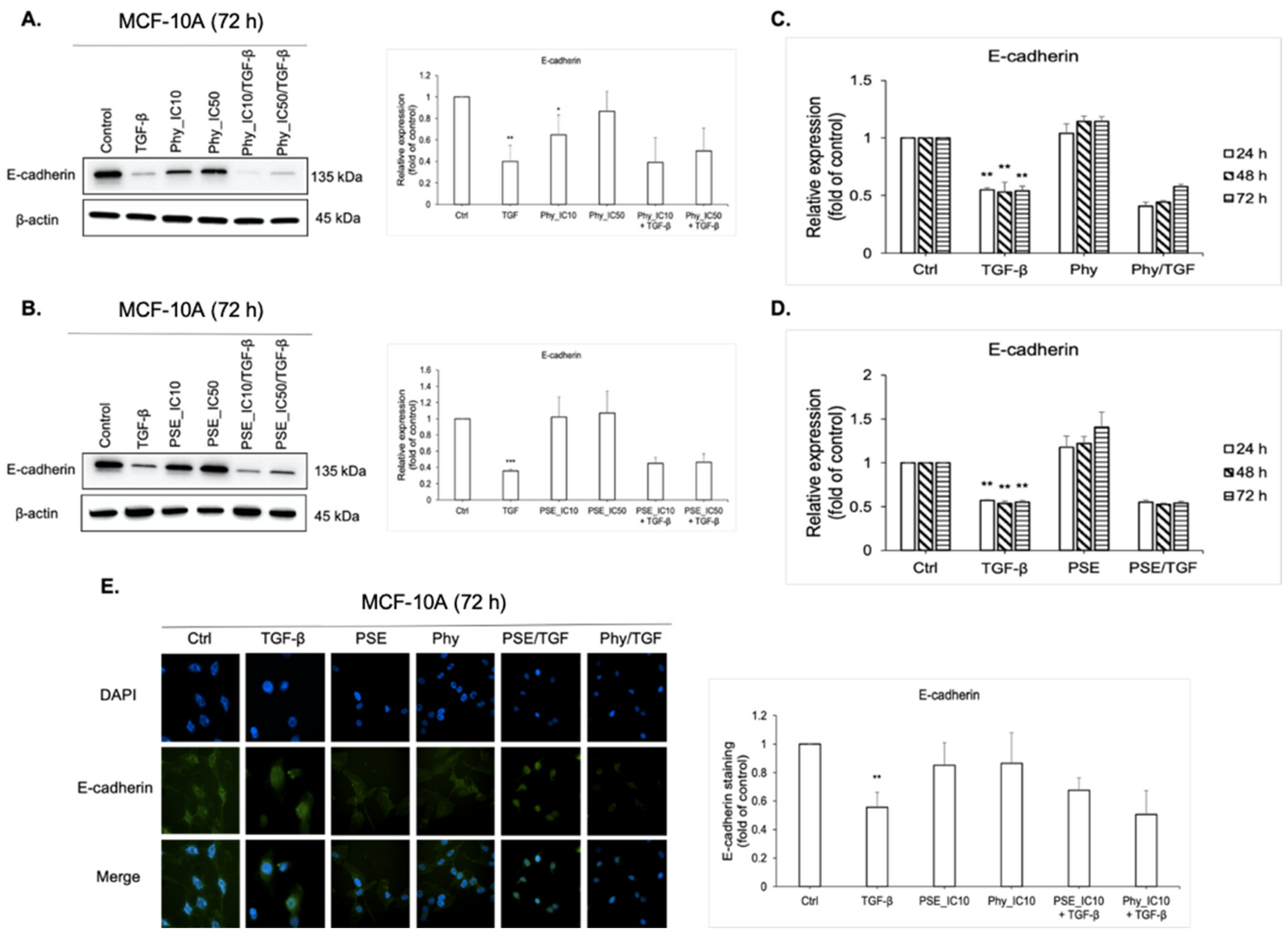
3.7. E-cadherin Regulation Analyses after PSE and Phy Treatment
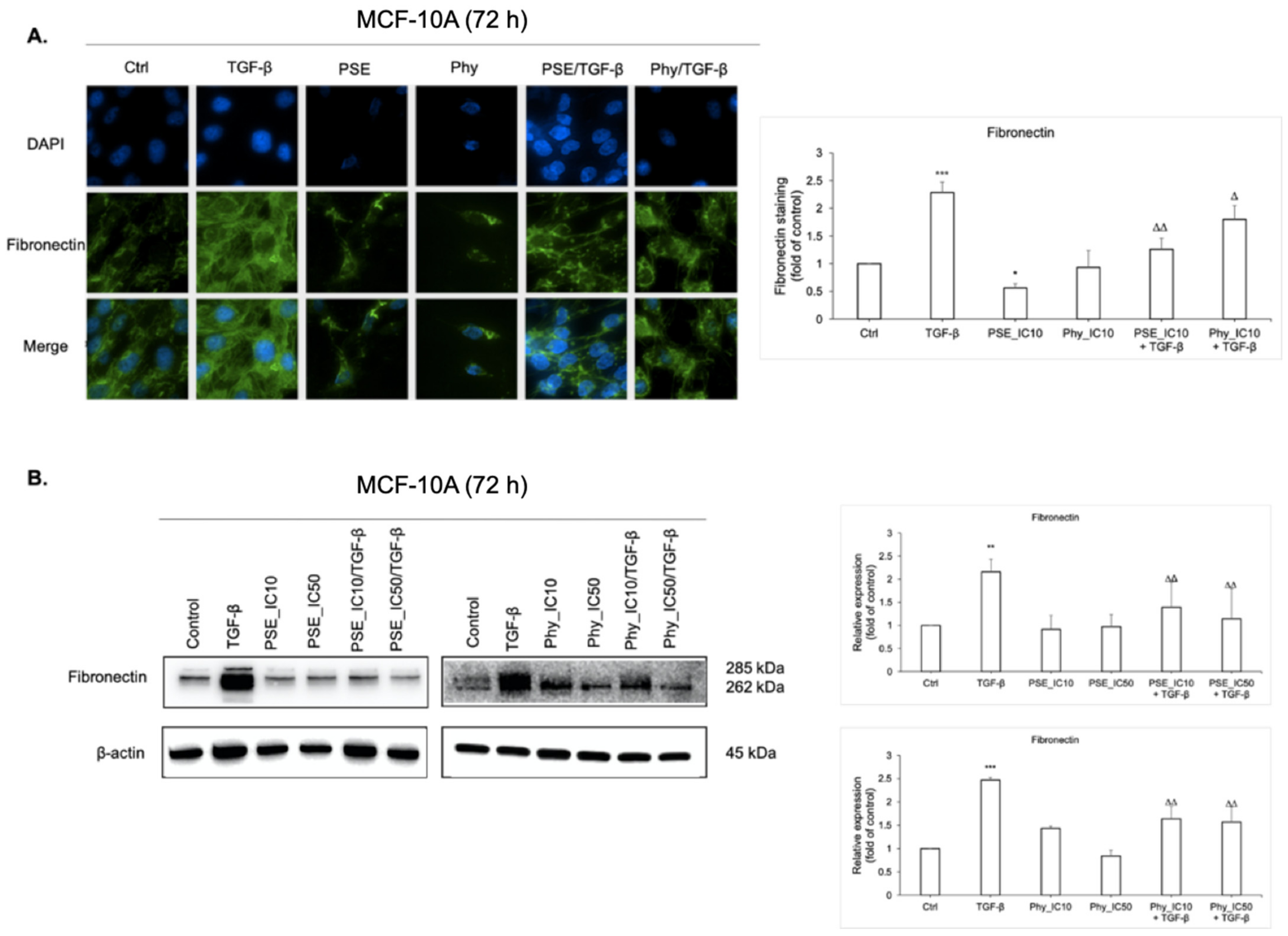
3.8. Fibronectin Expression Analyses after PSE and Phy Treatment
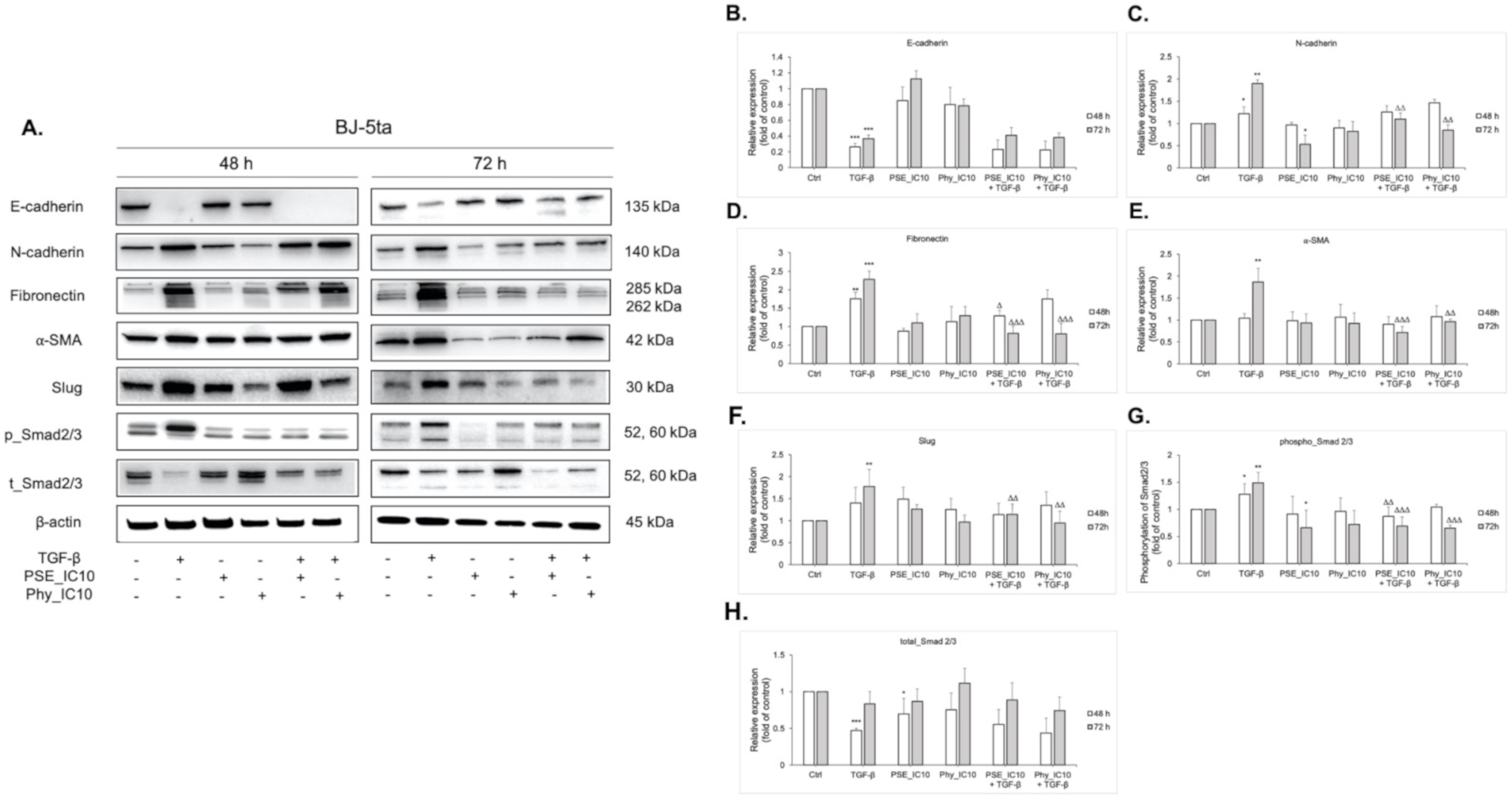
3.9. EMT-Associated Proteins’ Expression Analyses after PSE and Phy Treatment in TGF-β-Stimulated BJ-5ta Fibroblasts
3.10. Angiogenesis Analyses after PSE and Phy Treatment in a Quail Embryo CAM Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bahreyni, A.; Samani, S.S.; Rahmani, F.; Behnam-Rassouli, R.; Khazaei, M.; Ryzhikov, M.; Parizadeh, M.R.; Avan, A.; Hassanian, S.M. Role of Adenosine Signaling in the Pathogenesis of Breast Cancer. J. Cell Physiol. 2018, 233, 1836–1843. [Google Scholar] [CrossRef]
- Bahrami, A.; Hasanzadeh, M.; ShahidSales, S.; Yousefi, Z.; Kadkhodayan, S.; Farazestanian, M.; Joudi, M.M.; Gharib, M.; Mahdi, H.S.; Avan, A. Clinical Significance and Prognosis Value of Wnt Signaling Pathway in Cervical Cancer. J. Cell. Biochem. 2017, 118, 3028–3033. [Google Scholar] [CrossRef]
- Binabaj, M.M.; Bahrami, A.; ShahidSales, S.; Joodi, M.; Joudi, M.M.; Hassanian, S.M.; Anvari, K.; Avan, A. The Prognostic Value of MGMT Promoter Methylation in Glioblastoma: A Meta-Analysis of Clinical Trials. J. Cell Physiol. 2018, 233, 378–386. [Google Scholar] [CrossRef]
- Tajbakhsh, A.; Mokhtari-Zaer, A.; Rezaee, M.; Afzaljavan, F.; Rivandi, M.; Hassanian, S.M.; Ferns, G.A.; Pasdar, A.; Avan, A. Therapeutic Potentials of BDNF/TrkB in Breast Cancer; Current Status and Perspectives. J. Cell. Biochem. 2017, 118, 2502–2515. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.; Sharma, D.; Kalia, K.; Tekade, R.K. Tumor Microenvironment Targeted Nanotherapeutics for Cancer Therapy and Diagnosis: A Review. Acta Biomater. 2020, 101, 43–68. [Google Scholar] [CrossRef]
- Bissell, M.J.; Radisky, D.C.; Rizki, A.; Weaver, V.M.; Petersen, O.W. The Organizing Principle: Microenvironmental Influences in the Normal and Malignant Breast. Differentiation 2002, 70, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Hinohara, K.; Polyak, K. Intratumoral Heterogeneity: More Than Just Mutations. Trends Cell Biol. 2019, 29, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, G.; Chen, Y.; Wang, H.; Hua, Y.; Cai, Z. Immunogenic Cell Death in Cancer Therapy: Present and Emerging Inducers. J. Cell Mol. Med. 2019, 23, 4854–4865. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Kim, D.-H.; Surh, Y.-J. Resveratrol Suppresses Migration, Invasion and Stemness of Human Breast Cancer Cells by Interfering with Tumor-Stromal Cross-Talk. Arch. Biochem. Biophys. 2018, 643, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Katsuno, Y.; Lamouille, S.; Derynck, R. TGF-β Signaling and Epithelial–Mesenchymal Transition in Cancer Progression. Curr. Opin. Oncol. 2013, 25, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A. Epithelial Plasticity: A Common Theme in Embryonic and Cancer Cells. Science 2013, 342, 1234850. [Google Scholar] [CrossRef]
- Acloque, H.; Adams, M.S.; Fishwick, K.; Bronner-Fraser, M.; Nieto, M.A. Epithelial-Mesenchymal Transitions: The Importance of Changing Cell State in Development and Disease. J. Clin. Investig. 2009, 119, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Craene, B.D.; Berx, G. Regulatory Networks Defining EMT During Cancer Initiation and Progression. Nat. Rev. Cancer 2013, 13, 97–110. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial–Mesenchymal Transitions in Tumour Progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P. Prespecification and Plasticity: Shifting Mechanisms of Cell Migration. Curr. Opin. Cell Biol. 2004, 16, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, C.S.; Van Tubergen, E.A.; Inglehart, R.C.; D’Silva, N.J. Biomarkers of Epithelial-Mesenchymal Transition in Squamous Cell Carcinoma. J. Dent. Res. 2013, 92, 114–121. [Google Scholar] [CrossRef]
- Chen, T.; You, Y.; Jiang, H.; Wang, Z.Z. Epithelial-Mesenchymal Transition (EMT): A Biological Process in the Development, Stem Cell Differentiation, and Tumorigenesis. J. Cell Physiol 2017, 232, 3261–3272. [Google Scholar] [CrossRef] [PubMed]
- Russano, M.; Napolitano, A.; Ribelli, G.; Iuliani, M.; Simonetti, S.; Citarella, F.; Pantano, F.; Dell’Aquila, E.; Anesi, C.; Silvestris, N.; et al. Liquid Biopsy and Tumor Heterogeneity in Metastatic Solid Tumors: The Potentiality of Blood Samples. J. Exp. Clin. Cancer Res. 2020, 39, 95. [Google Scholar] [CrossRef] [PubMed]
- Babayan, A.; Hannemann, J.; Spötter, J.; Müller, V.; Pantel, K.; Joosse, S.A. Heterogeneity of Estrogen Receptor Expression in Circulating Tumor Cells from Metastatic Breast Cancer Patients. PLoS ONE 2013, 8, 75038. [Google Scholar] [CrossRef]
- Fehm, T.; Müller, V.; Aktas, B.; Janni, W.; Schneeweiss, A.; Stickeler, E.; Lattrich, C.; Löhberg, C.R.; Solomayer, E.; Rack, B.; et al. HER2 Status of Circulating Tumor Cells in Patients with Metastatic Breast Cancer: A Prospective, Multicenter Trial. Breast Cancer Res. Treat. 2010, 124, 403–412. [Google Scholar] [CrossRef]
- Moustakas, A.; Heldin, C.-H. The Regulation of TGF Signal Transduction. Development 2009, 136, 3699–3714. [Google Scholar] [CrossRef]
- Massagué, J. TGFβ in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef]
- Massagué, J. How Cells Read TGF-β Signals. Nat. Rev. Mol. Cell Biol 2000, 1, 169–178. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Gomis, R.R. The Logic of TGFβ Signaling. FEBS Letters 2006, 580, 2811–2820. [Google Scholar] [CrossRef]
- Feng, X.-H.; Derynck, R. Specificity and Versatility in TGF-β Signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005, 21, 659–693. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massagué, J. Mechanisms of TGF-β Signaling from Cell Membrane to the Nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Liao, T.-T.; Yang, M.-H. Revisiting Epithelial-Mesenchymal Transition in Cancer Metastasis: The Connection between Epithelial Plasticity and Stemness. Mol. Oncol 2017, 11, 792–804. [Google Scholar] [CrossRef]
- Folgueira, M.A.A.K.; Maistro, S.; Katayama, M.L.H.; Roela, R.A.; Mundim, F.G.L.; Nanogaki, S.; de Bock, G.H.; Brentani, M.M. Markers of Breast Cancer Stromal Fibroblasts in the Primary Tumour Site Associated with Lymph Node Metastasis: A Systematic Review Including Our Case Series. Biosci. Rep. 2013, 33, 85. [Google Scholar] [CrossRef]
- Ziani, L.; Chouaib, S.; Thiery, J. Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts. Front. Immunol. 2018, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Determinants of Tumor Blood Flow: A Review. Cancer Res. 1988, 48, 2641–2658. [Google Scholar]
- Chen, M.-C.; Lee, C.-F.; Huang, W.-H.; Chou, T.-C. Magnolol Suppresses Hypoxia-Induced Angiogenesis via Inhibition of HIF-1α/VEGF Signaling Pathway in Human Bladder Cancer Cells. Biochem. Pharmacol. 2013, 85, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Vascular Permeability Factor/Vascular Endothelial Growth Factor: A Critical Cytokine in Tumor Angiogenesis and a Potential Target for Diagnosis and Therapy. JCO 2002, 20, 4368–4380. [Google Scholar] [CrossRef] [PubMed]
- Nash, T.H. (Ed.) Lichen Biology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008; ISBN 978-0-521-69216-8. [Google Scholar]
- Ranković, B.; (Ed.) Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential, 1st ed.; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-13374-4. [Google Scholar]
- Thell, A.; Ahti, T. (Eds.) Parmeliaceae; Nordic Lichen Flora; Naturcentrum AB: Stenungsund, 2011; ISBN 978-91-85221-24-0. [Google Scholar]
- Gauslaa, Y.; Solhaug, K.A. Fungal Melanins as a Sun Screen for Symbiotic Green Algae in the Lichen Lobaria Pulmonaria. Oecologia 2001, 126, 462–471. [Google Scholar] [CrossRef]
- Feige, G.B.; Lumbsch, H.T.; Huneck, S.; Elix, J.A. Identification of Lichen Substances by a Standardized High-Performance Liquid Chromatographic Method. J. Chromatogr. A 1993, 646, 417–427. [Google Scholar] [CrossRef]
- Mrozik, K.M.; Blaschuk, O.W.; Cheong, C.M.; Zannettino, A.C.W.; Vandyke, K. N-Cadherin in Cancer Metastasis, Its Emerging Role in Haematological Malignancies and Potential as a Therapeutic Target in Cancer. BMC Cancer 2018, 18, 939. [Google Scholar] [CrossRef]
- Hugo, H.J.; Kokkinos, M.I.; Blick, T.; Ackland, M.L.; Thompson, E.W.; Newgreen, D.F. Defining the E-Cadherin Repressor Interactome in Epithelial-Mesenchymal Transition: The PMC42 Model as a Case Study. Cells Tissues Organs 2011, 193, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E. Fibronectin and Its Receptors. Annu Rev. Biochem. 1988, 57, 375–413. [Google Scholar] [CrossRef]
- Jeon, M.; Lee, J.; Nam, S.J.; Shin, I.; Lee, J.E.; Kim, S. Induction of Fibronectin by HER2 Overexpression Triggers Adhesion and Invasion of Breast Cancer Cells. Exp. Cell Res. 2015, 333, 116–126. [Google Scholar] [CrossRef]
- Slany, A.; Bileck, A.; Muqaku, B.; Gerner, C. Targeting Breast Cancer-Associated Fibroblasts to Improve Anti-Cancer Therapy. Breast 2015, 24, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Cirri, P.; Chiarugi, P. Cancer-Associated-Fibroblasts and Tumour Cells: A Diabolic Liaison Driving Cancer Progression. Cancer Metastasis Rev. 2012, 31, 195–208. [Google Scholar] [CrossRef]
- Franco, O.E.; Shaw, A.K.; Strand, D.W.; Hayward, S.W. Cancer Associated Fibroblasts in Cancer Pathogenesis. Semin. Cell Dev. Biol. 2010, 21, 33–39. [Google Scholar] [CrossRef]
- Räsänen, K.; Vaheri, A. Activation of Fibroblasts in Cancer Stroma. Exp. Cell Res. 2010, 316, 2713–2722. [Google Scholar] [CrossRef]
- Ringuette Goulet, C.; Bernard, G.; Tremblay, S.; Chabaud, S.; Bolduc, S.; Pouliot, F. Exosomes Induce Fibroblast Differentiation into Cancer-Associated Fibroblasts through TGFβ Signaling. Mol. Cancer Res. 2018, 16, 1196–1204. [Google Scholar] [CrossRef]
- Bačkorová, M.; Jendželovský, R.; Kello, M.; Bačkor, M.; Mikeš, J.; Fedoročko, P. Lichen Secondary Metabolites Are Responsible for Induction of Apoptosis in HT-29 and A2780 Human Cancer Cell Lines. Toxicol. In Vitro 2012, 26, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Bačkorová, M.; Bačkor, M.; Mikeš, J.; Jendželovský, R.; Fedoročko, P. Variable Responses of Different Human Cancer Cells to the Lichen Compounds Parietin, Atranorin, Usnic Acid and Gyrophoric Acid. Toxicol. In Vitro 2011, 25, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Paluszczak, J.; Kleszcz, R.; Studzińska-Sroka, E.; Krajka-Kuźniak, V. Lichen-Derived Caperatic Acid and Physodic Acid Inhibit Wnt Signaling in Colorectal Cancer Cells. Mol. Cell Biochem. 2018, 441, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Piovano, M.; Lombardo, L.; Vanella, L.; Cardile, V.; Garbarino, J. Pannarin Inhibits Cell Growth and Induces Cell Death in Human Prostate Carcinoma DU-145 Cells. Anti-Cancer Drugs 2006, 17, 1163–1169. [Google Scholar] [CrossRef]
- de Barros Alves, G.M.; de Sousa Maia, M.B.; de Souza Franco, E.; Galvão, A.M.; da Silva, T.G.; Gomes, R.M.; Martins, M.B.; da Silva Falcão, E.P.; de Castro, C.M.M.B.; da Silva, N.H. Expectorant and Antioxidant Activities of Purified Fumarprotocetraric Acid from Cladonia Verticillaris Lichen in Mice. Pulm. Pharmacol. Ther. 2014, 27, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Brisdelli, F.; Perilli, M.; Sellitri, D.; Piovano, M.; Garbarino, J.A.; Nicoletti, M.; Bozzi, A.; Amicosante, G.; Celenza, G. Cytotoxic Activity and Antioxidant Capacity of Purified Lichen Metabolites: An In Vitro Study: Cytotoxicity of Lichen Metabolites. Phytother. Res. 2013, 27, 431–437. [Google Scholar] [CrossRef]
- Esimone, C.O.; Ofokansi, K.C.; Adikwu, M.U.; Ibezim, E.C.; Abonyi, D.O.; Odaibo, G.N.; Olaleye, D.O. In Vitro Evaluation of the Antiviral Activity of Extracts from the Lichen Parmelia Perlata (L.) Ach. against Three RNA Viruses. J. Infect. Dev. Ctries. 2007, 1, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Goga, M.; Kello, M.; Vilkova, M.; Petrova, K.; Backor, M.; Adlassnig, W.; Lang, I. Oxidative Stress Mediated by Gyrophoric Acid from the Lichen Umbilicaria Hirsuta Affected Apoptosis and Stress/Survival Pathways in HeLa Cells. BMC Complement. Altern Med. 2019, 19, 221. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.H.; Dinh, M.H.; Chi, H.T.; Wang, S.-L.; Nguyen, Q.; Tran, T.D.; Nguyen, A.D. Antioxidant and Cytotoxic Activity of Lichens Collected from Bidoup Nui Ba National Park, Vietnam. Res. Che. Interm. 2019, 45, 33–49. [Google Scholar] [CrossRef]
- Taş, İ.; Han, J.; Park, S.-Y.; Yang, Y.; Zhou, R.; Gamage, C.D.B.; Van Nguyen, T.; Lee, J.-Y.; Choi, Y.J.; Yu, Y.H.; et al. Physciosporin Suppresses the Proliferation, Motility and Tumourigenesis of Colorectal Cancer Cells. Phytomedicine 2019, 56, 10–20. [Google Scholar] [CrossRef]
- Kosanić, M.; Manojlović, N.; Janković, S.; Stanojković, T.; Ranković, B. Evernia Prunastri and Pseudoevernia Furfuraceae Lichens and Their Major Metabolites as Antioxidant, Antimicrobial and Anticancer Agents. Food Chem. Toxicol. 2013, 53, 112–118. [Google Scholar] [CrossRef]
- Emsen, B.; Turkez, H.; Togar, B.; Aslan, A. Evaluation of Antioxidant and Cytotoxic Effects of Olivetoric and Physodic Acid in Cultured Human Amnion Fibroblasts. Hum. Exp. Toxic. 2017, 36, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Talapatra, S.K.; Rath, O.; Clayton, E.; Tomasi, S.; Kozielski, F. Depsidones from Lichens as Natural Product Inhibitors of M-Phase Phosphoprotein 1, a Human Kinesin Required for Cytokinesis. J. Nat. Prod. 2016, 79, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Cardile, V.; Graziano, A.C.E.; Avola, R.; Piovano, M.; Russo, A. Potential Anticancer Activity of Lichen Secondary Metabolite Physodic Acid. Chem. Biol. Interact. 2017, 263, 36–45. [Google Scholar] [CrossRef]
- Bareche, Y.; Venet, D.; Ignatiadis, M.; Aftimos, P.; Piccart, M.; Rothe, F.; Sotiriou, C. Unravelling Triple-Negative Breast Cancer Molecular Heterogeneity Using an Integrative Multiomic Analysis. Ann. Oncol. 2018, 29, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Burstein, M.D.; Tsimelzon, A.; Poage, G.M.; Covington, K.R.; Contreras, A.; Fuqua, S.A.W.; Savage, M.I.; Osborne, C.K.; Hilsenbeck, S.G.; Chang, J.C.; et al. Comprehensive Genomic Analysis Identifies Novel Subtypes and Targets of Triple-Negative Breast Cancer. Clin. Cancer Res. 2015, 21, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-Z.; Ma, D.; Suo, C.; Shi, J.; Xue, M.; Hu, X.; Xiao, Y.; Yu, K.-D.; Liu, Y.-R.; Yu, Y.; et al. Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers: Subtypes and Treatment Strategies. Cancer Cell 2019, 35, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Bareche, Y.; Buisseret, L.; Gruosso, T.; Girard, E.; Venet, D.; Dupont, F.; Desmedt, C.; Larsimont, D.; Park, M.; Rothé, F.; et al. Unraveling Triple-Negative Breast Cancer Tumor Microenvironment Heterogeneity: Towards an Optimized Treatment Approach. JNCI J. Natl. Cancer Inst. 2020, 112, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Oft, M.; Heider, K.-H.; Beug, H. TGFβ Signaling Is Necessary for Carcinoma Cell Invasiveness and Metastasis. Curr. Biol. 1998, 8, 1243–1252. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, X.-J.; Zhang, H.; Teng, Y.; Li, R.; Bai, F.; Elankumaran, S.; Xing, J. TGF- -Induced Epithelial-to-Mesenchymal Transition Proceeds through Stepwise Activation of Multiple Feedback Loops. Sci. Signal. 2014, 7, 91. [Google Scholar] [CrossRef]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Yang, Y.; Park, S.-Y.; Nguyen, T.T.; Yu, Y.H.; Nguyen, T.V.; Sun, E.G.; Udeni, J.; Jeong, M.-H.; Pereira, I.; Moon, C.; et al. Lichen Secondary Metabolite, Physciosporin, Inhibits Lung Cancer Cell Motility. PLoS ONE 2015, 10, 0137889. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Yoon, S.; Yang, Y.; Lee, H.-B.; Oh, S.; Jeong, M.-H.; Kim, J.-J.; Yee, S.-T.; Crişan, F.; Moon, C.; et al. Lichen Secondary Metabolites in Flavocetraria Cucullata Exhibit Anti-Cancer Effects on Human Cancer Cells through the Induction of Apoptosis and Suppression of Tumorigenic Potentials. PLoS ONE 2014, 9, 111575. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, H.; Remmers, N.; Hollingsworth, M.A. Loss of E-Cadherin and Epithelial to Mesenchymal Transition Is Not Required for Cell Motility in Tissues or for Metastasis. Tissue Barriers 2014, 2, 969112. [Google Scholar] [CrossRef]
- Nilsson, G.M.A.; Akhtar, N.; Kannius-Janson, M.; Baeckström, D. Loss of E-Cadherin Expression Is Not a Prerequisite for c-ErbB2-Induced Epithelial-Mesenchymal Transition. Int. J. Oncol. 2014, 45, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.E.; Kothari, A.N.; Wai, P.Y.; Li, N.Y.; Driver, J.; Zapf, M.A.C.; Franzen, C.A.; Gupta, G.N.; Osipo, C.; Zlobin, A.; et al. Osteopontin Mediates an MZF1–TGF-Β1-Dependent Transformation of Mesenchymal Stem Cells into Cancer-Associated Fibroblasts in Breast Cancer. Oncogene 2015, 34, 4821–4833. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Zeisberg, M. Fibroblasts in Cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Oshima, H.; Kitmura, T.; Taketo, M.M.; Oshima, M. Stromal Fibroblasts Activated by Tumor Cells Promote Angiogenesis in Mouse Gastric Cancer. J. Biol. Chem. 2008, 283, 19864–19871. [Google Scholar] [CrossRef] [PubMed]
- Noma, K.; Smalley, K.S.M.; Lioni, M.; Naomoto, Y.; Tanaka, N.; El–Deiry, W.; King, A.J.; Nakagawa, H.; Herlyn, M. The Essential Role of Fibroblasts in Esophageal Squamous Cell Carcinoma–Induced Angiogenesis. Gastroenterology 2008, 134, 1981–1993. [Google Scholar] [CrossRef]
- Shimoda, M.; Mellody, K.T.; Orimo, A. Carcinoma-Associated Fibroblasts Are a Rate-Limiting Determinant for Tumour Progression. Semin. Cell Dev. Biol. 2010, 21, 19–25. [Google Scholar] [CrossRef]
- Yashiro, M.; Hirakawa, K. Cancer–Stromal Interactions in Scirrhous Gastric Carcinoma. Cancer Microenviron. 2010, 3, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Houthuijzen, J.M.; Jonkers, J. Cancer-Associated Fibroblasts as Key Regulators of the Breast Cancer Tumor Microenvironment. Cancer Metastasis Rev. 2018, 37, 577–597. [Google Scholar] [CrossRef]
- De Wever, O.; Demetter, P.; Mareel, M.; Bracke, M. Stromal Myofibroblasts Are Drivers of Invasive Cancer Growth. Int. J. Cancer 2008, 123, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Desmoulière, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming Growth Factor-Beta 1 Induces Alpha-Smooth Muscle Actin Expression in Granulation Tissue Myofibroblasts and in Quiescent and Growing Cultured Fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Singha, P.K.; Pandeswara, S.; Geng, H.; Lan, R.; Venkatachalam, M.A.; Dobi, A.; Srivastava, S.; Saikumar, P. Increased Smad3 and Reduced Smad2 Levels Mediate the Functional Switch of TGF-β from Growth Suppressor to Growth and Metastasis Promoter through TMEPAI/PMEPA1 in Triple Negative Breast Cancer. Genes Cancer 2019, 10, 134–149. [Google Scholar] [CrossRef]
- Su, Y.; Cai, H.; Zheng, Y.; Qiu, Q.; Lu, W.; Shu, X.O.; Cai, Q. Associations of the Transforming Growth Factor β/Smad Pathway, Body Mass Index, and Physical Activity With Breast Cancer Outcomes: Results From the Shanghai Breast Cancer Study. Am. J. Epidemiol 2016, 184, 501–509. [Google Scholar] [CrossRef]
- Tian, F.; DaCosta Byfield, S.; Parks, W.T.; Yoo, S.; Felici, A.; Tang, B.; Piek, E.; Wakefield, L.M.; Roberts, A.B. Reduction in Smad2/3 Signaling Enhances Tumorigenesis but Suppresses Metastasis of Breast Cancer Cell Lines. Cancer Res. 2003, 63, 8284–8292. [Google Scholar] [PubMed]
- Tian, F.; Byfield, S.D.; Parks, W.T.; Stuelten, C.H.; Nemani, D.; Zhang, Y.E.; Roberts, A.B. Smad-Binding Defective Mutant of Transforming Growth Factor β Type I Receptor Enhances Tumorigenesis but Suppresses Metastasis of Breast Cancer Cell Lines. Cancer Res. 2004, 64, 4523–4530. [Google Scholar] [CrossRef]
- Naber, H.P.H.; Drabsch, Y.; Snaar-Jagalska, B.E.; ten Dijke, P.; van Laar, T. Snail and Slug, Key Regulators of TGF-β-Induced EMT, Are Sufficient for the Induction of Single-Cell Invasion. Biochem. Biophys. Res. Commun. 2013, 435, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Koparal, A.T. Anti-Angiogenic and Antiproliferative Properties of the Lichen Substances (-)-Usnic Acid and Vulpinic Acid. Z. Nat. C 2015, 70, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dai, F.; Zhai, D.; Dong, Y.; Zhang, J.; Lu, B.; Luo, J.; Liu, M.; Yi, Z. Usnic Acid Inhibits Breast Tumor Angiogenesis and Growth by Suppressing VEGFR2-Mediated AKT and ERK1/2 Signaling Pathways. Angiogenesis 2012, 15, 421–432. [Google Scholar] [CrossRef]
- Koparal, A.T.; Ulus, G.; Zeytinoğlu, M.; Tay, T.; Türk, A.Ã. Angiogenesis Inhibition by a Lichen Compound Olivetoric Acid. Phytother. Res. 2009, 24, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, L.M.; Parris, E.E.; Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The Chicken Chorioallantoic Membrane Model in Biology, Medicine and Bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane (CAM). A Multifaceted Experimental Model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef] [PubMed]

| Primary Antibodies | Mr (kDa) | Origin | Dilution | Source |
|---|---|---|---|---|
| Anti-Fibronectin antibody (ab2413) | 285, 262 | Rabbit | 1:1000 | Abcam |
| N-Cadherin (D4R1H) XP Rabbit mAb | 140 | Rabbit | 1:1000 | Cell Signaling Technology, Danvers, Massachusetts, United States |
| E-Cadherin (4A2) Mouse mAb | 135 | Mouse | 1:1000 | |
| α-Smooth Muscle Actin (D4K9N) XP Rabbit mAb | 42 | Rabbit | 1:1000 | |
| Slug (C19G7) Rabbit mAb | 30 | Rabbit | 1:1000 | |
| Phospho_Smad2 (Ser465/467)/Smad3 (Ser423/425) (D27F4) | 52, 60 | Rabbit | 1:1000 | |
| Smad2/3 Antibody | 52, 60 | Rabbit | 1:1000 | |
| β-Actin (8H10D10) Mouse mAb | 45 | Mouse | 1:2500 | |
| Secondary antibodies | ||||
| Anti-Rabbit IgG HRP | - | Goat | 1:1000 | Cell Signaling Technology, Danvers, Massachusetts, United States |
| Anti-Mouse IgG HRP | - | Goat | 1:1000 |
| Primary Antibodies | Origin | Dilution | Source |
|---|---|---|---|
| Anti-Fibronectin antibody (ab2413) | Rabbit | 1 μg/mL | Abcam |
| N-Cadherin (D4R1H) XP Rabbit mAb | Rabbit | 1:200 | Cell Signaling Technology, Danvers, Massachusetts, United States |
| E-Cadherin (4A2) Mouse mAb | Mouse | 1:200 | |
| Secondary antibodies | |||
| Alexa Fluor 488 anti-mouse IgG | Goat | 1:1000 | Cell Signaling Technology, Danvers, Massachusetts, United States |
| Alexa Fluor 488 anti-rabbit IgG | Goat | 1:1000 |
| Cell Line | Pseudevernia furfuracea (L.) Zopf Extract | Physodic Acid | ||
|---|---|---|---|---|
| IC10 (μM) | IC50 (μM) | IC10 (μM) | IC50 (μM) | |
| MCF-10A | 22.10 ± 1.82 | 45.53 ± 0.11 | 59.25 ± 1.36 | 79.88 ± 0.93 |
| BJ-5ta | 26.60 ± 1.81 | 72.22 ± 9.11 | 46.35 ± 0.39 | 83.25 ± 1.02 |
| HUVECs | 35.79 ± 1.40 | 92.70 ± 4.32 | 60.62 ± 3.30 | 92.76 ± 3.62 |
| Cell Line | Pseudevernia furfuracea (L.) Zopf Extract | Physodic Acid | ||
|---|---|---|---|---|
| IC10 (μM) | IC50 (μM) | IC10 (μM) | IC50 (μM) | |
| MCF-10A | 38.72 ± 2.25 | 83.80 ± 3.48 | 63.63 ± 3.40 | 155.00 ± 7.40 |
| BJ-5ta | 37.49 ± 0.82 | 116.82 ± 1.82 | 62.88 ± 0.90 | 164.26 ± 4.50 |
| HUVECs | 59.84 ± 4.50 | 110.52 ± 2.10 | 89.53 ± 3.21 | 124.16 ± 5.33 |
| Treatment | Time (h) | Control | TGF-β | PSE | Phy | PSE/TGF-β | Phy/TGF-β |
|---|---|---|---|---|---|---|---|
| Sub G0 | 24 | 1.03 ± 0.50 | 1.07 ± 0.22 | 2.33 ± 0.99 | 0.86 ± 0.12 | 1.46 ± 0.45 | 1.10 ± 0.07 |
| 48 | 1.04 ± 0.25 | 0.94 ± 0.16 | 1.03 ± 0.40 | 2.54 ± 0.04 | 0.61 ± 0.23 | 1.82 ± 0.24 | |
| 72 | 0.77 ± 0.00 | 1.22 ± 0.07 * | 1.94 ± 0.79 | 2.06 ± 0.35 | 1.14 ± 0.38 | 2.77 ± 0.94 | |
| G1 | 24 | 76.75 ± 3.88 | 74.55 ± 1.59 | 78.45 ± 4.37 | 73.25 ± 0.37 | 79.00 ± 0.33 | 73.45 ± 0.69 |
| 48 | 87.10 ± 3.76 | 83.05 ± 2.74 | 87.20 ± 4.49 | 88.15 ± 0.53 | 81.80 ± 1.55 | 89.05 ± 1.18Δ | |
| 72 | 87.60 ± 2.78 | 81.35 ± 0.37 | 84.90 ± 1.47 | 88.50 ± 1.14 | 79.40 ± 1.06 | 89.75 ± 0.86Δ | |
| S | 24 | 7.45 ± 2.90 | 11.15 ± 0.69 | 9.38 ± 0.84 | 12.05 ± 0.86 | 9.62 ± 0.72 | 12.20 ± 2.69 |
| 48 | 4.72 ± 1.29 | 7.39 ± 0.88 | 6.87 ± 2.88 | 4.23 ± 0.48 | 8.10 ± 2.69 | 3.36 ± 0.27 | |
| 72 | 4.63 ± 1.23 | 6.61 ± 0.74 | 6.19 ± 1.27 | 5.45 ± 2.26 | 8.46 ± 0.19 | 3.14 ± 0.52 | |
| G2/M | 24 | 14.80 ± 1.47 | 13.25 ± 2.49 | 9.84 ± 4.54 | 13.85 ± 0.61 | 9.95 ± 0.61 | 13.25 ± 2.08 |
| 48 | 7.12 ± 2.22 | 8.63 ± 1.69 | 4.90 ± 1.20 | 5.06 ± 1.00 | 9.50 ± 0.90 | 5.78 ± 1.16 | |
| 72 | 7.00 ± 1.58 | 10.85 ± 0.45 | 6.95 ± 0.57 | 3.99 ± 0.80 | 11.00 ± 0.49 | 4.34 ± 1.27Δ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrova, K.; Kello, M.; Kuruc, T.; Backorova, M.; Petrovova, E.; Vilkova, M.; Goga, M.; Rucova, D.; Backor, M.; Mojzis, J. Potential Effect of Pseudevernia furfuracea (L.) Zopf Extract and Metabolite Physodic Acid on Tumour Microenvironment Modulation in MCF-10A Cells. Biomolecules 2021, 11, 420. https://doi.org/10.3390/biom11030420
Petrova K, Kello M, Kuruc T, Backorova M, Petrovova E, Vilkova M, Goga M, Rucova D, Backor M, Mojzis J. Potential Effect of Pseudevernia furfuracea (L.) Zopf Extract and Metabolite Physodic Acid on Tumour Microenvironment Modulation in MCF-10A Cells. Biomolecules. 2021; 11(3):420. https://doi.org/10.3390/biom11030420
Chicago/Turabian StylePetrova, Klaudia, Martin Kello, Tomas Kuruc, Miriam Backorova, Eva Petrovova, Maria Vilkova, Michal Goga, Dajana Rucova, Martin Backor, and Jan Mojzis. 2021. "Potential Effect of Pseudevernia furfuracea (L.) Zopf Extract and Metabolite Physodic Acid on Tumour Microenvironment Modulation in MCF-10A Cells" Biomolecules 11, no. 3: 420. https://doi.org/10.3390/biom11030420
APA StylePetrova, K., Kello, M., Kuruc, T., Backorova, M., Petrovova, E., Vilkova, M., Goga, M., Rucova, D., Backor, M., & Mojzis, J. (2021). Potential Effect of Pseudevernia furfuracea (L.) Zopf Extract and Metabolite Physodic Acid on Tumour Microenvironment Modulation in MCF-10A Cells. Biomolecules, 11(3), 420. https://doi.org/10.3390/biom11030420









