Beneficial Effect of Tempol, a Membrane-Permeable Radical Scavenger, on Inflammation and Osteoarthritis in In Vitro Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Cytotoxicity Assay
2.3. Griess Nitrite Assay
2.4. MMP1 and MMP3 Activity Evaluation
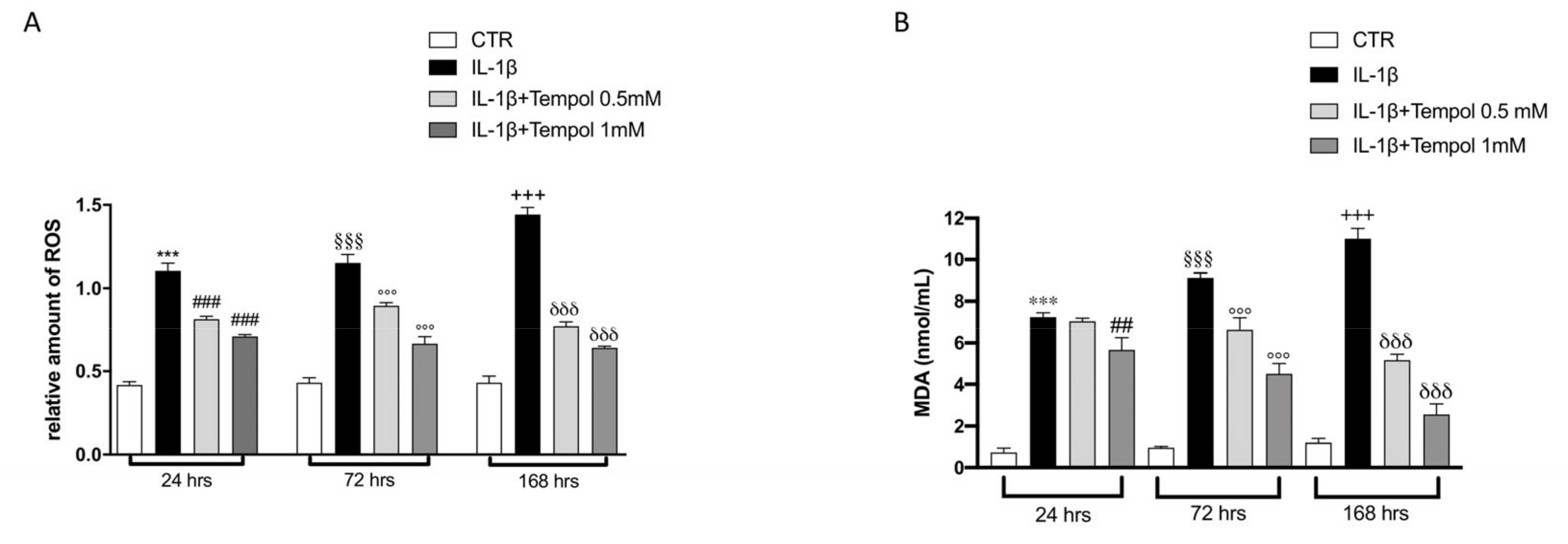
2.5. ROS Assay
2.6. Determination of Malondialdehyde (MDA) Levels
2.7. Quantitative Realtime PCR (qRT-PCR)
2.8. Materials
2.9. Statistical Analysis
3. Results
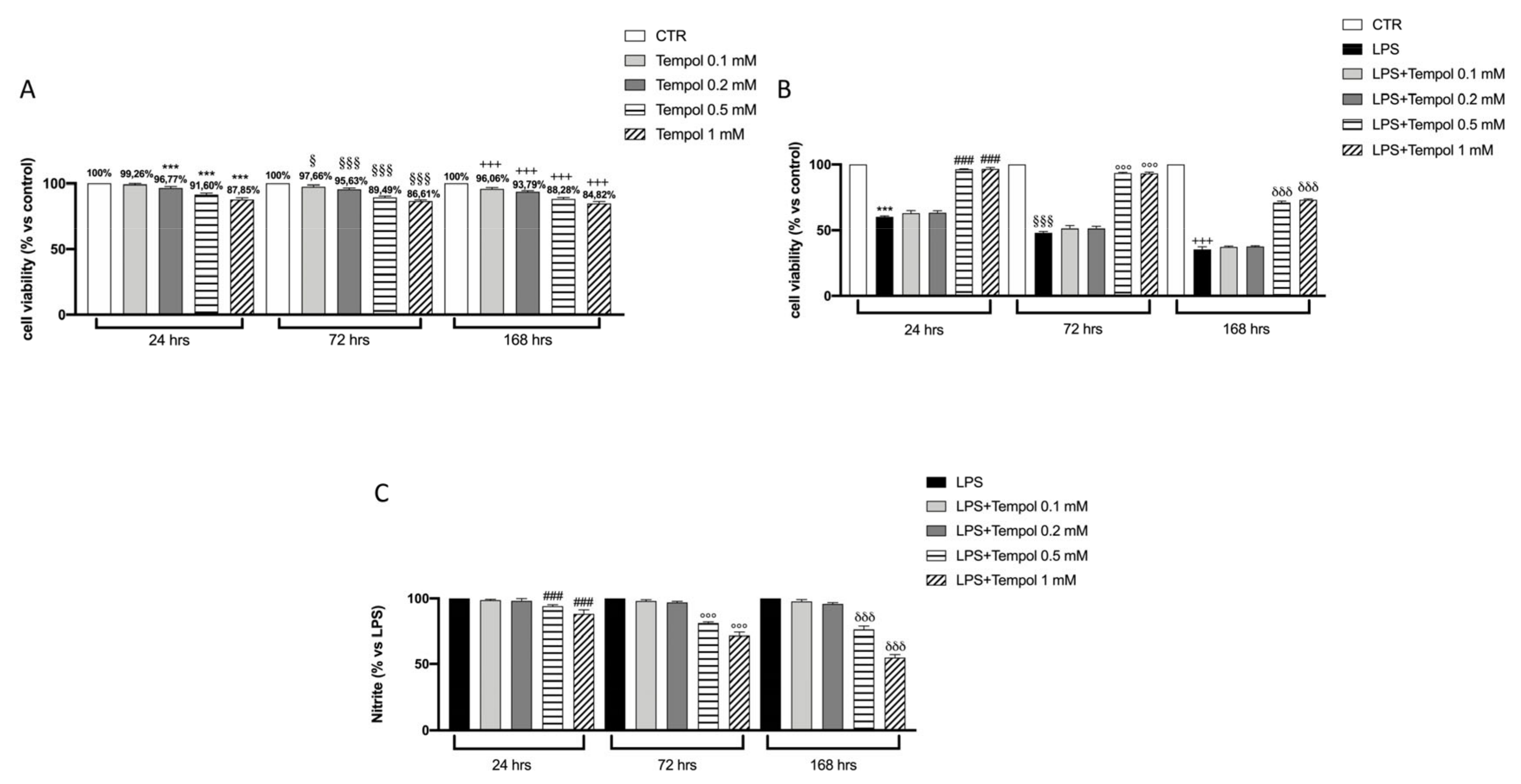
3.1. Effects of Tempol on Macrophages Cell Viability
3.2. Effects of Tempol on LPS-Induced NO Production in J774 Cells
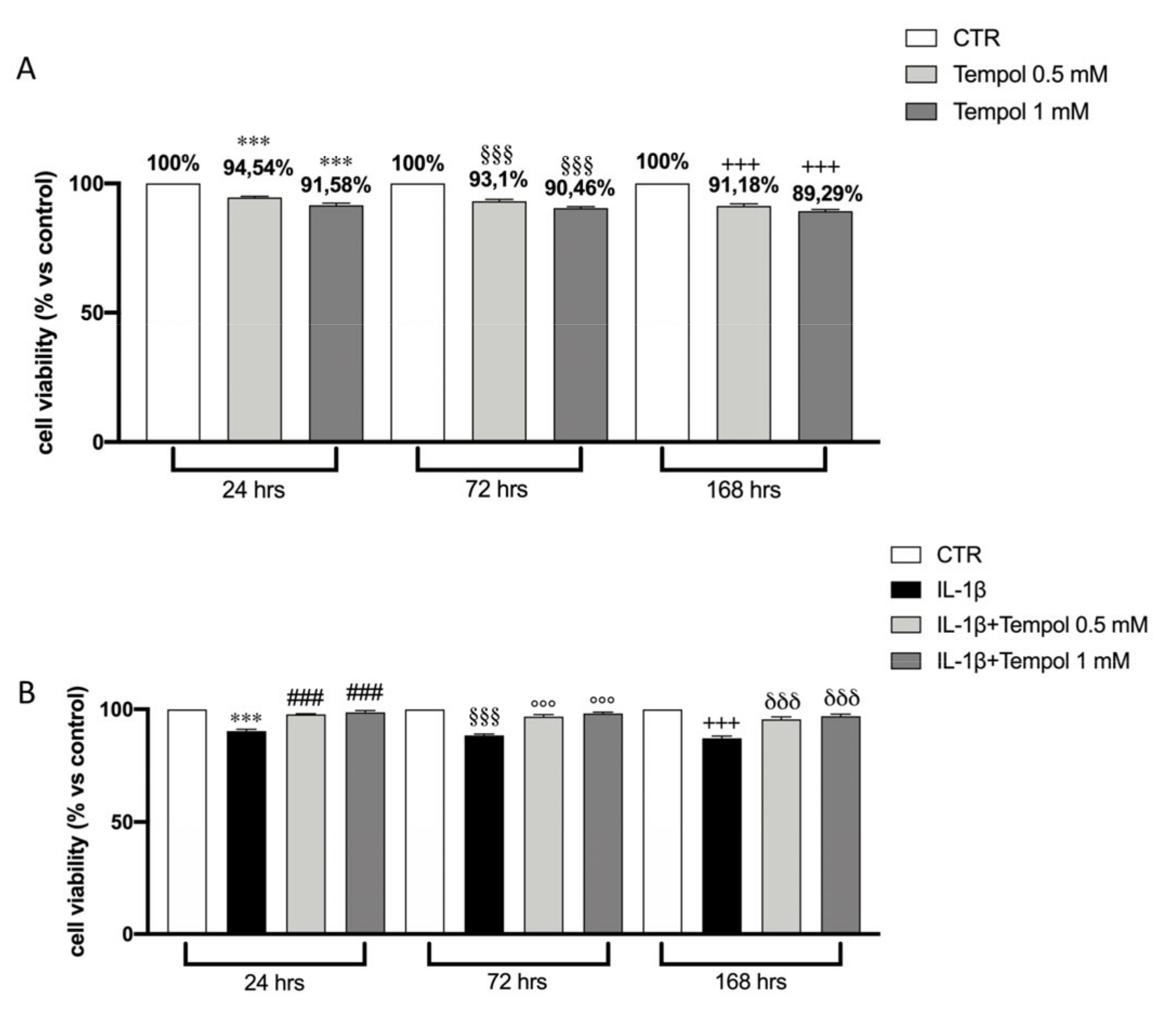
3.3. Effects of te3mpol on CC Cell Viability
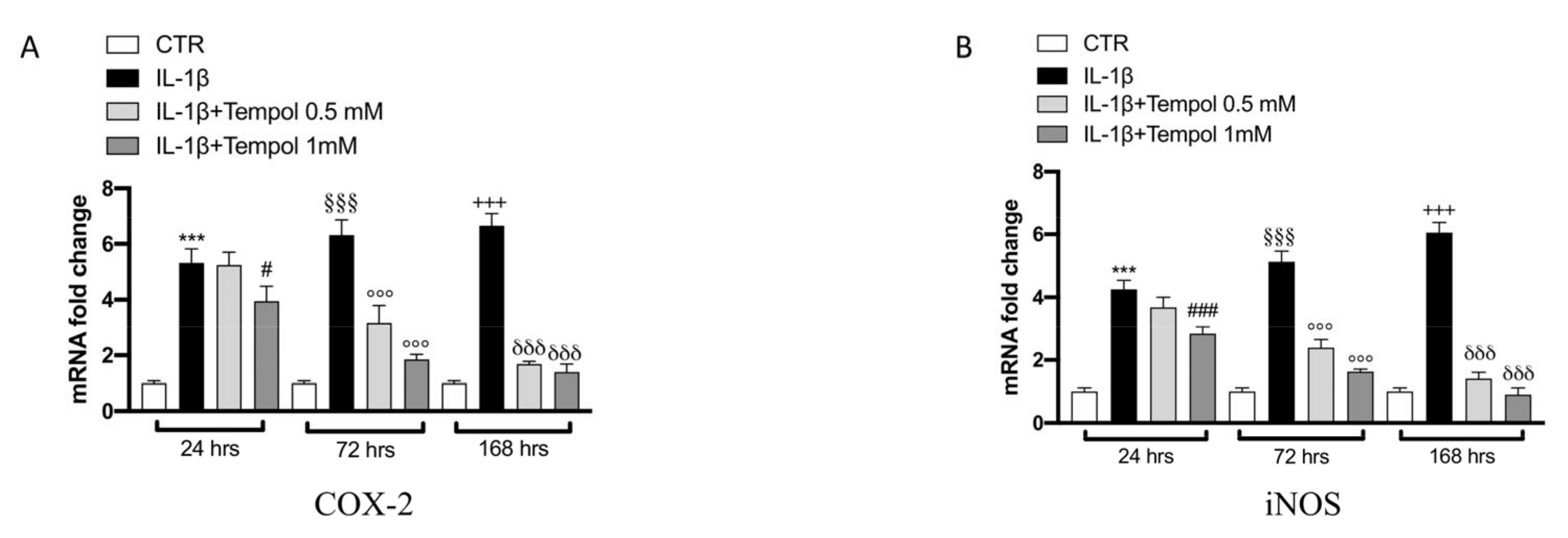
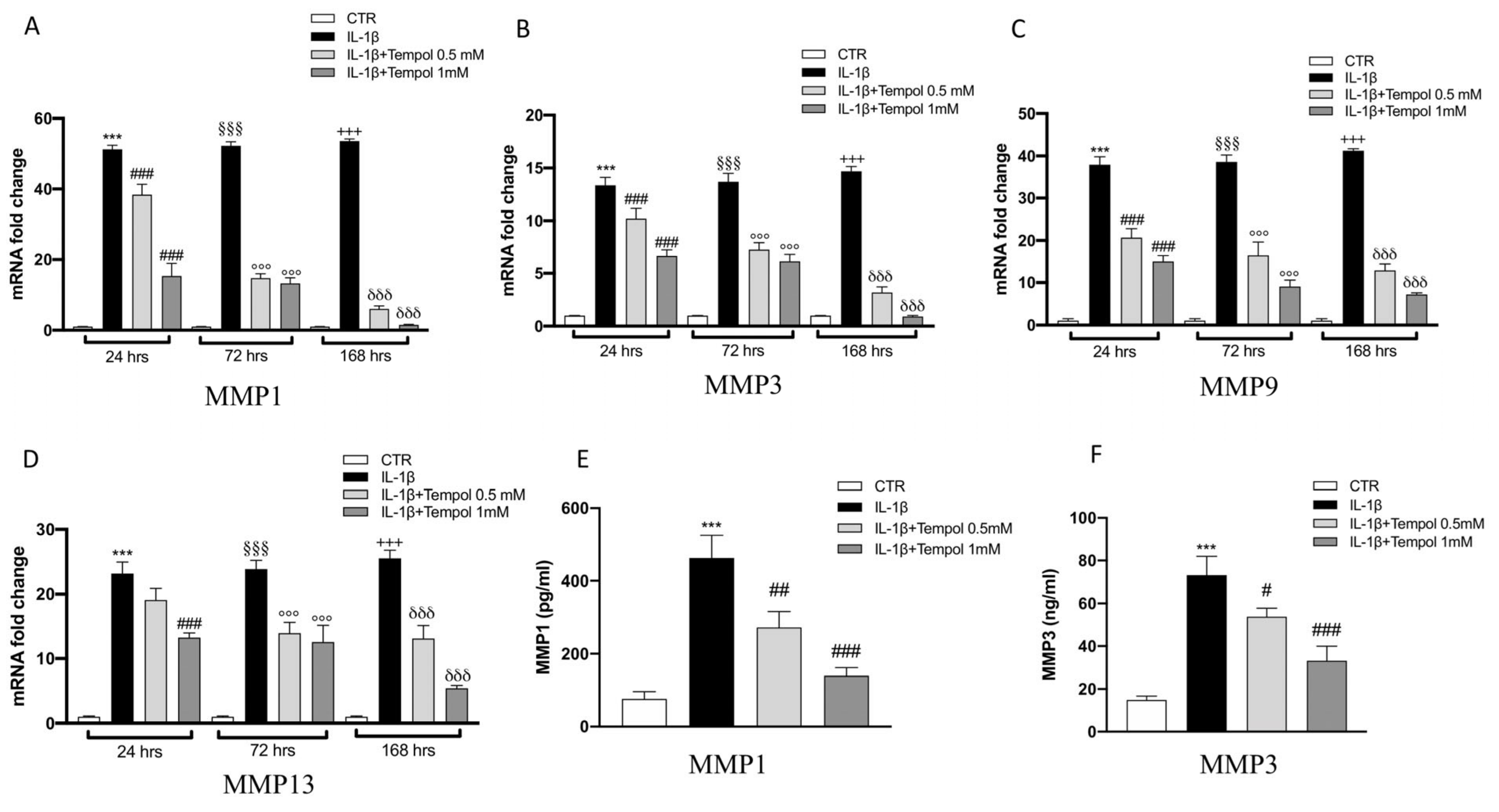
3.4. Effects of Tempol on IL-1β-Induced Inflammatory me3diators and MMPs
3.5. Antioxidant Effects of Tempol on Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, D.; Ramos, E.; Branco, J. Osteoarthritis. Acta Med. Port. 2015, 28, 99–106. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Berenbaum, F. Osteoarthritis year in review 2019: Epidemiology and therapy. Osteoarthr. Cartil. 2020, 28, 242–248. [Google Scholar] [CrossRef]
- Taruc-Uy, R.L.; Lynch, S.A. Diagnosis and treatment of osteoarthritis. Prim. Care 2013, 40, 821–836. [Google Scholar] [CrossRef]
- Mow, V.C.; Holmes, M.H.; Lai, W.M. Fluid transport and mechanical properties of articular cartilage: A review. J. Biomech. 1984, 17, 377–394. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Torres, R.T.; Duarte, J.A.; Goncalves, R.S. Non-Pharmacological and Non-Surgical Interventions for Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Acta Reumatol. Port. 2019, 44, 173–217. [Google Scholar] [PubMed]
- Richter, K.; Muller-Ladner, U.; Dischereit, G.; Uwe, L. Potentials and Limits of Physiotherapy in Osteoarthritis. Curr. Rheumatol. Rev. 2018, 14, 117–122. [Google Scholar] [CrossRef]
- Rutjes, A.W.; Nuesch, E.; Sterchi, R.; Kalichman, L.; Hendriks, E.; Osiri, M.; Brosseau, L.; Reichenbach, S.; Juni, P. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef]
- Shen, J.; Abu-Amer, Y.; O’Keefe, R.J.; McAlinden, A. Inflammation and epigenetic regulation in osteoarthritis. Connect. Tissue Res. 2017, 58, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Altindag, O.; Erel, O.; Aksoy, N.; Selek, S.; Celik, H.; Karaoglanoglu, M. Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol. Int. 2007, 27, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F. The Role of Aging in the Development of Osteoarthritis. Trans. Am. Clin. Climatol. Assoc. 2017, 128, 44–54. [Google Scholar] [PubMed]
- Ansari, M.Y.; Khan, N.M.; Ahmad, I.; Haqqi, T.M. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthr. Cartil. 2018, 26, 1087–1097. [Google Scholar] [CrossRef]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef]
- Afonso, V.; Champy, R.; Mitrovic, D.; Collin, P.; Lomri, A. Reactive oxygen species and superoxide dismutases: Role in joint diseases. Joint Bone Spine 2007, 74, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Carlo, M.D., Jr.; Loeser, R.F. Increased oxidative stress with aging reduces chondrocyte survival: Correlation with intracellular glutathione levels. Arthritis Rheum. 2003, 48, 3419–3430. [Google Scholar] [CrossRef]
- Collins, J.A.; Wood, S.T.; Nelson, K.J.; Rowe, M.A.; Carlson, C.S.; Chubinskaya, S.; Poole, L.B.; Furdui, C.M.; Loeser, R.F. Oxidative Stress Promotes Peroxiredoxin Hyperoxidation and Attenuates Pro-survival Signaling in Aging Chondrocytes. J. Biol. Chem. 2016, 291, 6641–6654. [Google Scholar] [CrossRef]
- Lahiani, A.; Hidmi, A.; Katzhendler, J.; Yavin, E.; Lazarovici, P. Novel Synthetic PEGylated Conjugate of alpha-Lipoic Acid and Tempol Reduces Cell Death in a Neuronal PC12 Clonal Line Subjected to Ischemia. ACS Chem. Neurosci. 2016, 7, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Phungphong, S.; Kijtawornrat, A.; Wattanapermpool, J.; Bupha-Intr, T. Improvement in cardiac function of ovariectomized rats by antioxidant tempol. Free Radic. Biol. Med. 2020, 160, 239–245. [Google Scholar] [CrossRef]
- Silva, D.A.D.; Correia, T.M.L.; Pereira, R.; da Silva, R.A.A.; Augusto, O.; Queiroz, R.F. Tempol reduces inflammation and oxidative damage in cigarette smoke-exposed mice by decreasing neutrophil infiltration and activating the Nrf2 pathway. Chem. Biol. Interact. 2020, 329, 109210. [Google Scholar] [CrossRef]
- Wang, M.; Li, K.; Zou, Z.; Li, L.; Zhu, L.; Wang, Q.; Gao, W.; Wang, Y.; Huang, W.; Liu, R.; et al. Piperidine nitroxide Tempol enhances cisplatin-induced apoptosis in ovarian cancer cells. Oncol. Lett. 2018, 16, 4847–4854. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Pearlman, A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol. Rev. 2008, 60, 418–469. [Google Scholar] [CrossRef] [PubMed]
- Dornas, W.C.; Silva, M.; Tavares, R.; de Lima, W.G.; dos Santos, R.C.; Pedrosa, M.L.; Silva, M.E. Efficacy of the superoxide dismutase mimetic tempol in animal hypertension models: A meta-analysis. J. Hypertens. 2015, 33, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Wang, H.; Han, B.; Zhang, L.; Guo, J. Tempol Attenuates Neuropathic Pain by Inhibiting Nitric Oxide Production. Anal Cell Pathol. 2019, 2019, 8253850. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; McDonald, M.C.; Mazzon, E.; Dugo, L.; Lepore, V.; Fonti, M.T.; Ciccolo, A.; Terranova, M.L.; Caputi, A.P.; Thiemermann, C. Tempol, a membrane-permeable radical scavenger, reduces dinitrobenzene sulfonic acid-induced colitis. Eur. J. Pharmacol. 2000, 406, 127–137. [Google Scholar] [CrossRef]
- Calabrese, G.; Dolcimascolo, A.; Caruso, G.; Forte, S. miR-19a Is Involved In Progression And Malignancy Of Anaplastic Thyroid Cancer Cells. OncoTargets Ther. 2019, 12, 9571–9583. [Google Scholar] [CrossRef] [PubMed]
- Adesso, S.; Paterniti, I.; Cuzzocrea, S.; Fujioka, M.; Autore, G.; Magnus, T.; Pinto, A.; Marzocco, S. AST-120 Reduces Neuroinflammation Induced by Indoxyl Sulfate in Glial Cells. J. Clin. Med. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Tiemeier, G.L.; Wang, G.; Dumas, S.J.; Sol, W.; Avramut, M.C.; Karakach, T.; Orlova, V.V.; van den Berg, C.W.; Mummery, C.L.; Carmeliet, P.; et al. Closing the Mitochondrial Permeability Transition Pore in hiPSC-Derived Endothelial Cells Induces Glycocalyx Formation and Functional Maturation. Stem Cell Rep. 2019, 13, 803–816. [Google Scholar] [CrossRef]
- Meng, X.; Wang, M.; Sun, G.; Ye, J.; Zhou, Y.; Dong, X.; Wang, T.; Lu, S.; Sun, X. Attenuation of Abeta25-35-induced parallel autophagic and apoptotic cell death by gypenoside XVII through the estrogen receptor-dependent activation of Nrf2/ARE pathways. Toxicol. Appl. Pharmacol. 2014, 279, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Vicari, L.; Calabrese, G.; Forte, S.; Giuffrida, R.; Colarossi, C.; Parrinello, N.L.; Memeo, L. Potential Role of Activating Transcription Factor 5 during Osteogenesis. Stem Cells Int. 2016, 2016, 5282185. [Google Scholar] [CrossRef]
- Geyer, M.; Schonfeld, C. Novel Insights into the Pathogenesis of Osteoarthritis. Curr. Rheumatol. Rev. 2018, 14, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Conaghan, P.G.; Cook, A.D.; Hamilton, J.A.; Tak, P.P. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat. Rev. Rheumatol. 2019, 15, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Liu-Bryan, R.; Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R. Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 79–85. [Google Scholar] [CrossRef]
- Griffin, T.M.; Scanzello, C.R. Innate inflammation and synovial macrophages in osteoarthritis pathophysiology. Clin. Exp. Rheumatol. 2019, 37 Suppl 120, 57–63. [Google Scholar]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Xue, E.X.; Lin, J.P.; Zhang, Y.; Sheng, S.R.; Liu, H.X.; Zhou, Y.L.; Xu, H. Pterostilbene inhibits inflammation and ROS production in chondrocytes by activating Nrf2 pathway. Oncotarget 2017, 8, 41988–42000. [Google Scholar] [CrossRef]
- Shateri, H.; Ranjbar, A.; Kheiripour, N.; Ghasemi, H.; Pourfarjam, Y.; Habibitabar, E.; Gholami, H.; Moridi, H. Tempol improves oxidant/antioxidant parameters in testicular tissues of diabetic rats. Life Sci. 2019, 221, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhao, Y.; Li, X.; Xu, L.; Jiang, F.; Hou, W.; Dong, L.; Cao, J. Effects of Antioxidant Tempol on Systematic Inflammation and Endothelial Apoptosis in Emphysematous Rats Exposed to Intermittent Hypoxia. Yonsei Med. J. 2018, 59, 1079–1087. [Google Scholar] [CrossRef]
- Afjal, M.A.; Abdi, S.H.; Sharma, S.; Ahmad, S.; Fatima, M.; Dabeer, S.; Akhter, J.; Raisuddin, S. Anti-inflammatory role of tempol (4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl) in nephroprotection. Hum Exp Toxicol 2019, 38, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.I.; Argyle, D.J.; Clements, D.N. In vitro models for the study of osteoarthritis. Vet. J. 2016, 209, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Huang, Z.; Yu, X.; Zhou, L.; Pei, F. Clinical implications of macrophage dysfunction in the development of osteoarthritis of the knee. Cytokine Growth Factor Rev. 2019, 46, 36–44. [Google Scholar] [CrossRef]
- Cai, S.; Ming, B.; Ye, C.; Lin, S.; Hu, P.; Tang, J.; Zheng, F.; Dong, L. Similar Transition Processes in Synovial Fibroblasts from Rheumatoid Arthritis and Osteoarthritis: A Single-Cell Study. J. Immunol. Res. 2019, 2019, 4080735. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Zeng, G.Q.; Chen, A.B.; Li, W.; Song, J.H.; Gao, C.Y. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet. Mol. Res. 2015, 14, 14811–14822. [Google Scholar] [CrossRef]
- Rose, B.J.; Kooyman, D.L. A Tale of Two Joints: The Role of Matrix Metalloproteases in Cartilage Biology. Dis. Markers 2016, 2016, 4895050. [Google Scholar] [CrossRef]

| Homo Sapiens Gene | Forward | Reverse |
|---|---|---|
| COX2 | TGCAGTGAGCGTCAGGAG | CAAGGATTTGCTGTATGGCT |
| IL-1ß | GAAGTACCTGAGCTCGCCATGGAA | CGTGCAGTTCAGTGATCGTACAGG |
| IL-6 | CAAATTCGGTACATCCTC | CTGGCTTGTTCCTCACTA |
| iNOS | TCACCTACCACACCCGAGA | CGCTGGCATTCCGCACAA |
| TNF-α | CAAGCCTGTAGCCCATGTTGT | CCAAAGTAGACCTGCCCAGAC |
| MMP1 | CGACTCTAGAAACACAAGAGCAAGA | AAGGTTAGCTTACTGTCACACGCTT |
| MMP3 | GAACAATGGACAAAGGATACAACA | TTCTTCAAAAACAGCATCAATCTT |
| MMP9 | CACTGTCCACCCCTCAGAGC | GCCACTTGTCGGCGATAAGG |
| MMP13 | GTGGTGTGGGAAGTATCATCA | GCATCTGGAGTAACCGTATTG |
| GAPDH | GGAGAAGGCTGGGGCTCAT | TGGGTGGCAGTGATGGCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, G.; Ardizzone, A.; Campolo, M.; Conoci, S.; Esposito, E.; Paterniti, I. Beneficial Effect of Tempol, a Membrane-Permeable Radical Scavenger, on Inflammation and Osteoarthritis in In Vitro Models. Biomolecules 2021, 11, 352. https://doi.org/10.3390/biom11030352
Calabrese G, Ardizzone A, Campolo M, Conoci S, Esposito E, Paterniti I. Beneficial Effect of Tempol, a Membrane-Permeable Radical Scavenger, on Inflammation and Osteoarthritis in In Vitro Models. Biomolecules. 2021; 11(3):352. https://doi.org/10.3390/biom11030352
Chicago/Turabian StyleCalabrese, Giovanna, Alessio Ardizzone, Michela Campolo, Sabrina Conoci, Emanuela Esposito, and Irene Paterniti. 2021. "Beneficial Effect of Tempol, a Membrane-Permeable Radical Scavenger, on Inflammation and Osteoarthritis in In Vitro Models" Biomolecules 11, no. 3: 352. https://doi.org/10.3390/biom11030352
APA StyleCalabrese, G., Ardizzone, A., Campolo, M., Conoci, S., Esposito, E., & Paterniti, I. (2021). Beneficial Effect of Tempol, a Membrane-Permeable Radical Scavenger, on Inflammation and Osteoarthritis in In Vitro Models. Biomolecules, 11(3), 352. https://doi.org/10.3390/biom11030352










