Adipocytokines in Untreated Newly Diagnosed Rheumatoid Arthritis: Association with Circulating Chemokines and Markers of Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Clinical and Biochemical Assessment
2.3. Assessment of Adipocytokines
2.4. Assessment of Chemokines
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics and Chemokine Measurement
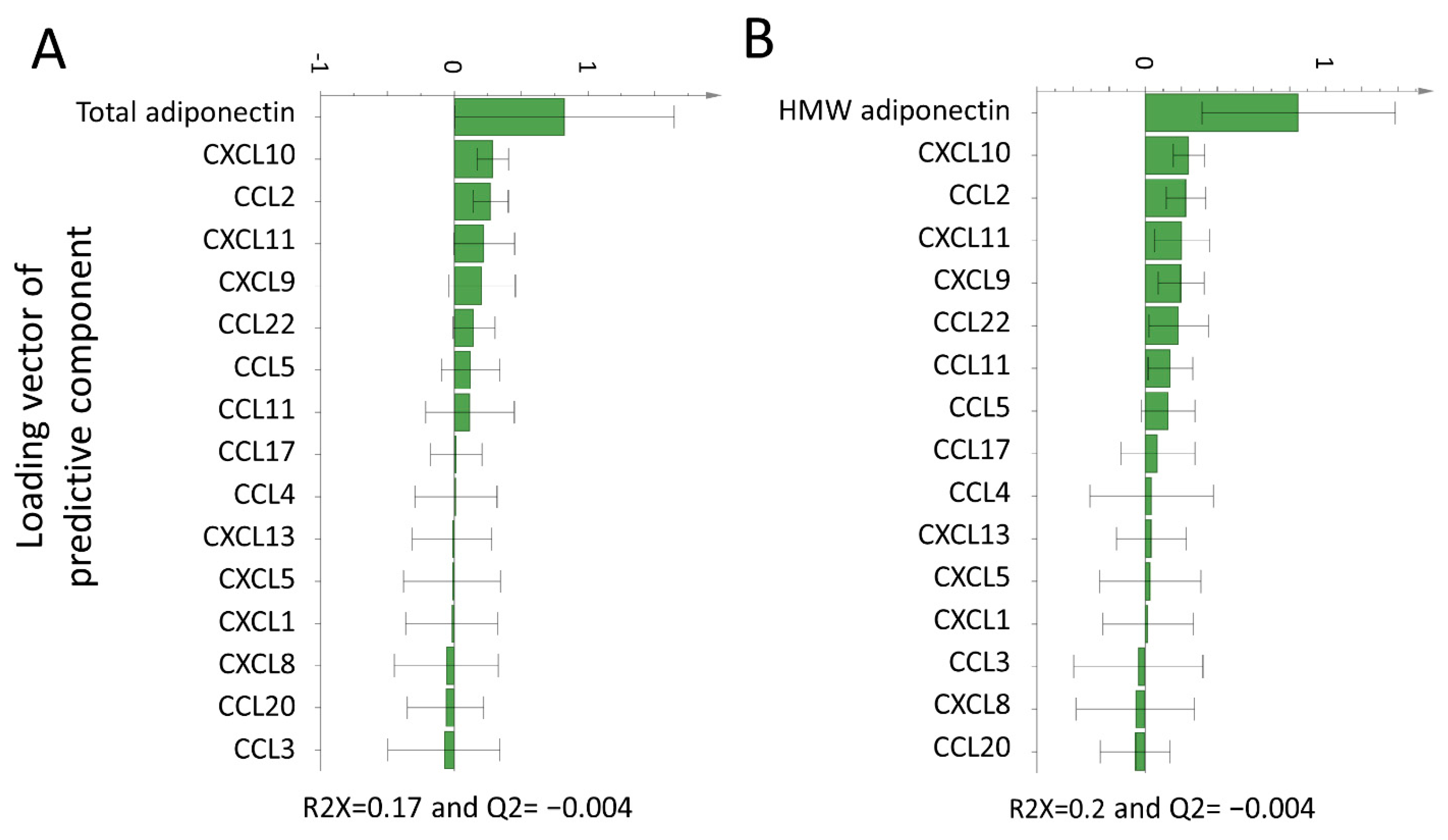
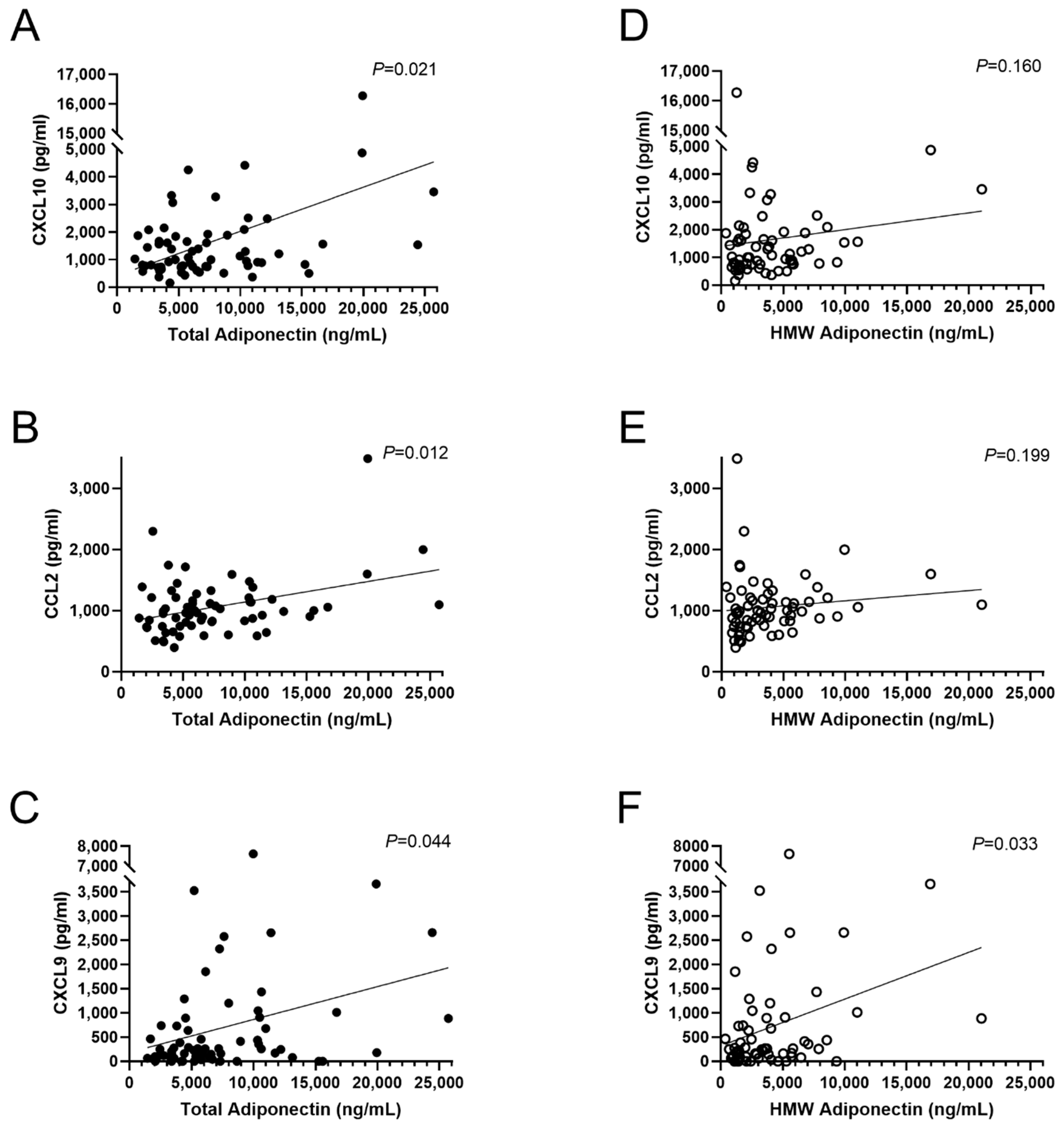
3.2. Association of Adipocytokines with Plasma Chemokines
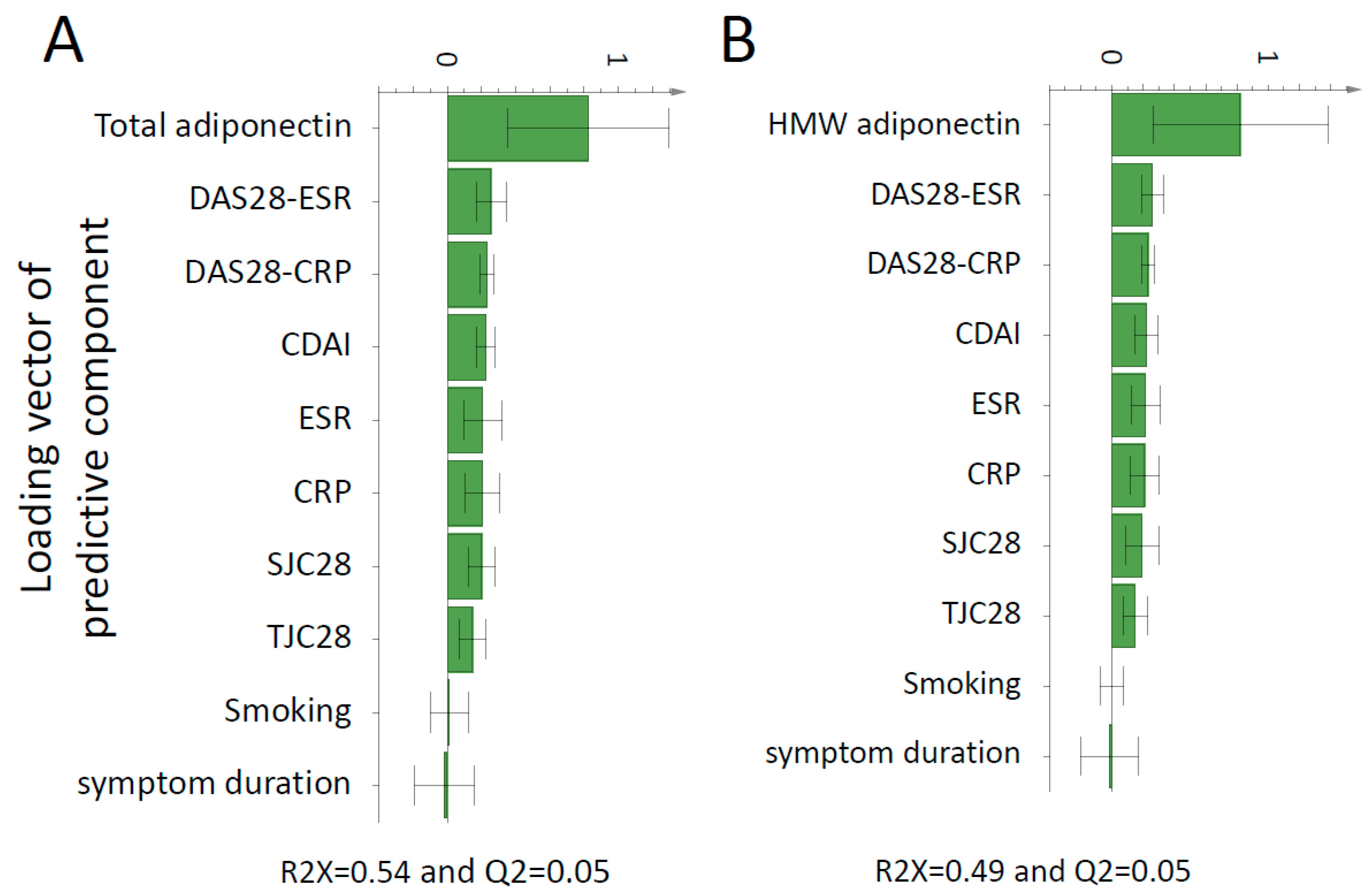
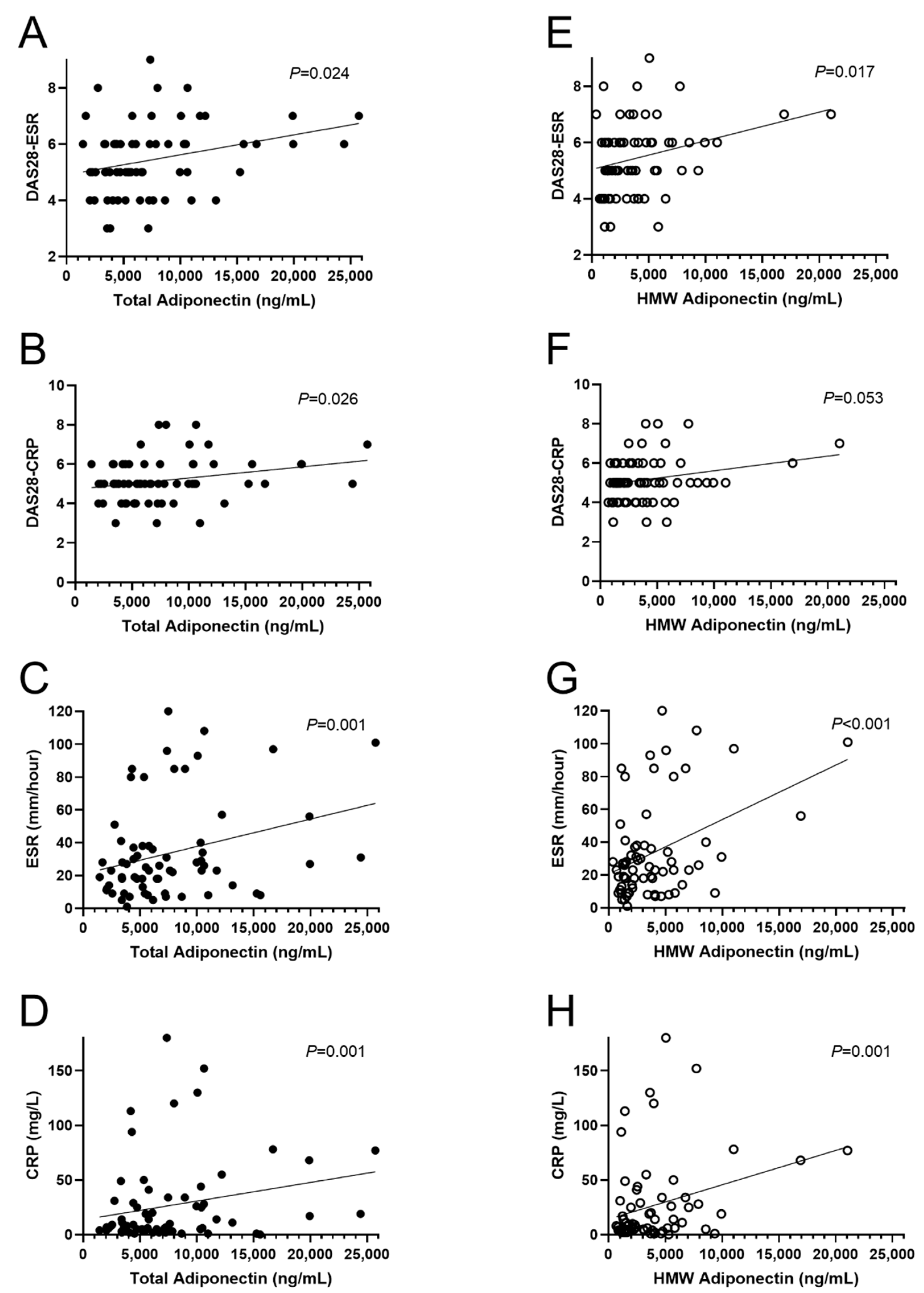
3.3. Association of Adipocytokines with Inflammation and Disease Activity Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carrion, M.; Frommer, K.W.; Perez-Garcia, S.; Muller-Ladner, U.; Gomariz, R.P.; Neumann, E. The Adipokine Network in Rheumatic Joint Diseases. Int. J. Mol. Sci. 2019, 20, 4091. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, I.J.; Liu, S.C.; Huang, C.C.; Kuo, S.J.; Tsai, C.H.; Tang, C.H. Associations between Adipokines in Arthritic Disease and Implications for Obesity. Int. J. Mol. Sci. 2019, 20, 1505. [Google Scholar] [CrossRef] [PubMed]
- Szumilas, K.; Szumilas, P.; Sluczanowska-Glabowska, S.; Zgutka, K.; Pawlik, A. Role of Adiponectin in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 8265. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.; Lago, R.; Gomez, R.; Lago, F.; Dieguez, C.; Gomez-Reino, J.J.; Gualillo, O. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2006, 65, 1198–1201. [Google Scholar] [CrossRef]
- Schaffler, A.; Ehling, A.; Neumann, E.; Herfarth, H.; Tarner, I.; Scholmerich, J.; Muller-Ladner, U.; Gay, S. Adipocytokines in synovial fluid. JAMA 2003, 290, 1709–1710. [Google Scholar] [CrossRef]
- Migita, K.; Maeda, Y.; Miyashita, T.; Kimura, H.; Nakamura, M.; Ishibashi, H.; Eguchi, K. The serum levels of resistin in rheumatoid arthritis patients. Clin. Exp. Rheumatol. 2006, 24, 698–701. [Google Scholar]
- Bokarewa, M.; Bokarew, D.; Hultgren, O.; Tarkowski, A. Leptin consumption in the inflamed joints of patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 952–956. [Google Scholar] [CrossRef]
- Seven, A.; Guzel, S.; Aslan, M.; Hamuryudan, V. Serum and synovial fluid leptin levels and markers of inflammation in rheumatoid arthritis. Rheumatol. Int. 2009, 29, 743–747. [Google Scholar] [CrossRef]
- Johansson, L.; Pratesi, F.; Brink, M.; Arlestig, L.; D’Amato, C.; Bartaloni, D.; Migliorini, P.; Rantapaa-Dahlqvist, S. Antibodies directed against endogenous and exogenous citrullinated antigens pre-date the onset of rheumatoid arthritis. Arthritis Res. Ther. 2016, 18, 127. [Google Scholar] [CrossRef]
- Kokkonen, H.; Soderstrom, I.; Rocklov, J.; Hallmans, G.; Lejon, K.; Rantapaa Dahlqvist, S. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010, 62, 383–391. [Google Scholar] [CrossRef]
- Maglio, C.; Zhang, Y.; Peltonen, M.; Andersson-Assarsson, J.; Svensson, P.A.; Herder, C.; Rudin, A.; Carlsson, L. Bariatric surgery and the incidence of rheumatoid arthriti—A Swedish Obese Subjects study. Rheumatology (Oxford) 2020, 59, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Elemam, N.M.; Hannawi, S.; Maghazachi, A.A. Role of Chemokines and Chemokine Receptors in Rheumatoid Arthritis. Immunotargets Ther. 2020, 9, 43–56. [Google Scholar] [CrossRef]
- Pandya, J.M.; Lundell, A.C.; Andersson, K.; Nordstrom, I.; Theander, E.; Rudin, A. Blood chemokine profile in untreated early rheumatoid arthritis: CXCL10 as a disease activity marker. Arthritis Res. Ther. 2017, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- van Hooij, A.; Boeters, D.M.; Tjon Kon Fat, E.M.; van den Eeden, S.J.F.; Corstjens, P.; van der Helm-van Mil, A.H.M.; Geluk, A. Longitudinal IP-10 Serum Levels Are Associated with the Course of Disease Activity and Remission in Patients with Rheumatoid Arthritis. Clin. Vaccine Immunol. 2017, 24. [Google Scholar] [CrossRef]
- Zhang, Y.; Peltonen, M.; Andersson-Assarsson, J.C.; Svensson, P.A.; Herder, C.; Rudin, A.; Carlsson, L.; Maglio, C. Elevated adiponectin predicts the development of rheumatoid arthritis in subjects with obesity. Scand. J. Rheumatol. 2020, 49, 452–460. [Google Scholar] [CrossRef]
- Zhang, Y.; Aldridge, J.; Vasileiadis, G.K.; Edebo, H.; Ekwall, A.-K.H.; Lundell, A.-C.; Rudin, A.; Maglio, C. Recombinant Adiponectin Induces the Production of Pro-Inflammatory Chemokines and Cytokines in Circulating Mononuclear Cells and Fibroblast-Like Synoviocytes From Non-Inflamed Subjects. Front. Immunol. 2021, 11, 569883. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Bae, S.C. Circulating adiponectin and visfatin levels in rheumatoid arthritis and their correlation with disease activity: A meta-analysis. Int. J. Rheum. Dis. 2018, 21, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Schraw, T.; Wang, Z.V.; Halberg, N.; Hawkins, M.; Scherer, P.E. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology 2008, 149, 2270–2282. [Google Scholar] [CrossRef]
- Lei, Y.; Li, X.; Gao, Z.; Liu, Y.; Zhang, B.; Xia, L.; Lu, J.; Shen, H. Association Between Adiponectin and Clinical Manifestations in Rheumatoid Arthritis. J. Interferon. Cytokine Res. 2020, 40, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Senolt, L.; Housa, D.; Vernerova, Z.; Jirasek, T.; Svobodova, R.; Veigl, D.; Anderlova, K.; Muller-Ladner, U.; Pavelka, K.; Haluzik, M. Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Ann. Rheum. Dis. 2007, 66, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.; Nasef, S.; Elsammak, M.Y.; Abduljaleel, P.M.; Musalam, D.; Ahmed, M.M.; Osman, I. Resistin, an Adipokine, Its Relation to Inflammation in Systemic Lupus Erythematosus and Rheumatoid Arthritis. Middle East. J. Intern. Med. 2014, 7, 3–9. [Google Scholar] [CrossRef]
- Oner, S.Y.; Volkan, O.; Oner, C.; Mengi, A.; Direskeneli, H.; Tasan, D.A. Serum leptin levels do not correlate with disease activity in rheumatoid arthritis. Acta Reumatol. Port. 2015, 40, 50–54. [Google Scholar]
- Lee, Y.H.; Bae, S.C. Circulating leptin level in rheumatoid arthritis and its correlation with disease activity: A meta-analysis. Z. Rheumatol. 2016, 75, 1021–1027. [Google Scholar] [CrossRef]
- Kononoff, A.; Vuolteenaho, K.; Hamalainen, M.; Kautiainen, H.; Elfving, P.; Savolainen, E.; Arstila, L.; Niinisalo, H.; Rutanen, J.; Marjoniemi, O.; et al. Metabolic Syndrome, Disease Activity, and Adipokines in Patients with Newly Diagnosed Inflammatory Joint Diseases. J. Clin. Rheumatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Laurberg, T.B.; Frystyk, J.; Ellingsen, T.; Hansen, I.T.; Jorgensen, A.; Tarp, U.; Hetland, M.L.; Horslev-Petersen, K.; Hornung, N.; Poulsen, J.H.; et al. Plasma adiponectin in patients with active, early, and chronic rheumatoid arthritis who are steroid- and disease-modifying antirheumatic drug-naive compared with patients with osteoarthritis and controls. J. Rheumatol. 2009, 36, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Toussirot, E. Mini-Review: The Contribution of Adipokines to Joint Inflammation in Inflammatory Rheumatic Diseases. Front. Endocrinol. (Lausanne) 2020, 11, 606560. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Ma, Y.; Wu, M.; Hu, X.; Han, R.; Yuan, Y.; Wang, M.; Chen, M.; Jiang, S.; et al. Serum levels of leptin, adiponectin and resistin in patients with ankylosing spondylitis: A systematic review and meta-analysis. Int. Immunopharmacol. 2017, 52, 310–317. [Google Scholar] [CrossRef]
- Dikbas, O.; Tosun, M.; Bes, C.; Tonuk, S.B.; Aksehirli, O.Y.; Soy, M. Serum levels of visfatin, resistin and adiponectin in patients with psoriatic arthritis and associations with disease severity. Int. J. Rheum. Dis. 2016, 19, 672–677. [Google Scholar] [CrossRef]
- Maeda, N.; Funahashi, T.; Matsuzawa, Y.; Shimomura, I. Adiponectin, a unique adipocyte-derived factor beyond hormones. Atherosclerosis 2020, 292, 1–9. [Google Scholar] [CrossRef]
- Isidori, A.M.; Strollo, F.; More, M.; Caprio, M.; Aversa, A.; Moretti, C.; Frajese, G.; Riondino, G.; Fabbri, A. Leptin and aging: Correlation with endocrine changes in male and female healthy adult populations of different body weights. J. Clin. Endocrinol. Metab. 2000, 85, 1954–1962. [Google Scholar] [CrossRef]
- Acquarone, E.; Monacelli, F.; Borghi, R.; Nencioni, A.; Odetti, P. Resistin: A reappraisal. Mech. Ageing Dev. 2019, 178, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Klein-Wieringa, I.R.; Andersen, S.N.; Herb-van Toorn, L.; Kwekkeboom, J.C.; van der Helm-van Mil, A.H.; Meulenbelt, I.; Huizinga, T.W.; Kloppenburg, M.; Toes, R.E.; Ioan-Facsinay, A. Are baseline high molecular weight adiponectin levels associated with radiographic progression in rheumatoid arthritis and osteoarthritis? J. Rheumatol. 2014, 41, 853–857. [Google Scholar] [CrossRef]
- Hoffman, E.; Rahat, M.A.; Feld, J.; Elias, M.; Rosner, I.; Kaly, L.; Lavie, I.; Gazitt, T.; Zisman, D. Effects of Tocilizumab, an Anti-Interleukin-6 Receptor Antibody, on Serum Lipid and Adipokine Levels in Patients with Rheumatoid Arthritis. Int. J. Mol. Sci. 2019, 20, 4633. [Google Scholar] [CrossRef]
- Han, B.K.; Kuzin, I.; Gaughan, J.P.; Olsen, N.J.; Bottaro, A. Baseline CXCL10 and CXCL13 levels are predictive biomarkers for tumor necrosis factor inhibitor therapy in patients with moderate to severe rheumatoid arthritis: A pilot, prospective study. Arthritis Res. Ther. 2016, 18, 93. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Kageyama, Y.; Kobayashi, H.; Kato, N.; Tsujimura, K.; Koide, Y. Etanercept treatment reduces the serum levels of interleukin-15 and interferon-gamma inducible protein-10 in patients with rheumatoid arthritis. Rheumatol. Int. 2010, 30, 725–730. [Google Scholar] [CrossRef]
- Eriksson, C.; Rantapaa-Dahlqvist, S.; Sundqvist, K.G. Changes in chemokines and their receptors in blood during treatment with the TNF inhibitor infliximab in patients with rheumatoid arthritis. Scand. J. Rheumatol. 2013, 42, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Engvall, I.L.; Tengstrand, B.; Brismar, K.; Hafstrom, I. Infliximab therapy increases body fat mass in early rheumatoid arthritis independently of changes in disease activity and levels of leptin and adiponectin: A randomised study over 21 months. Arthritis Res. Ther. 2010, 12, R197. [Google Scholar] [CrossRef] [PubMed]
- Yasar Bilge, N.S.; Kasifoglu, N.; Kasifoglu, T.; Sahin, F.; Gonullu, E.; Korkmaz, C. The role of methotrexate and low-dose prednisolone on adiponectine levels and insulin resistance in patients with rheumatoid arthritis naive to disease-modifying antirheumatic drugs. Int. J. Rheum. Dis. 2016, 19, 665–671. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Vanheule, V.; Janssens, R.; Struyf, S.; Proost, P. Overview of the Mechanisms that May Contribute to the Non-Redundant Activities of Interferon-Inducible CXC Chemokine Receptor 3 Ligands. Front. Immunol. 2017, 8, 1970. [Google Scholar] [CrossRef]
- Arkema, E.V.; Lu, B.; Malspeis, S.; Karlson, E.W.; Costenbader, K.H. Monocyte chemotactic protein-1 elevation prior to the onset of rheumatoid arthritis among women. Biomark. Med. 2015, 9, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Warr, G.; Loy, J.; Bravo, R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J. Exp. Med. 1997, 186, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.; Barnetche, T.; Morel, J.; Combe, B.; Daien, C. Association of Body Mass Index Categories with Disease Activity and Radiographic Joint Damage in Rheumatoid Arthritis: A Systematic Review and Metaanalysis. J. Rheumatol 2015, 42, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Ogura, T.; Hirata, A.; Takenaka, S.; Mizushina, K.; Fujisawa, Y.; Katagiri, T.; Hayashi, N.; Kameda, H. Global assessments of disease activity are age-dependent determinant factors of clinical remission in rheumatoid arthritis. Semin. Arthritis Rheum. 2017, 47, 310–314. [Google Scholar] [CrossRef]
- Forslind, K.; Hafstrom, I.; Ahlmen, M.; Svensson, B.; Group, B.S. Sex: A major predictor of remission in early rheumatoid arthritis? Ann. Rheum. Dis. 2007, 66, 46–52. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.A.; Garcia-Unzueta, M.T.; Gonzalez-Juanatey, C.; Miranda-Filloy, J.A.; Vazquez-Rodriguez, T.R.; De Matias, J.M.; Martin, J.; Dessein, P.H.; Llorca, J. Anti-TNF-alpha therapy modulates resistin in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2008, 26, 311–316. [Google Scholar]
| Characteristic | Early RA (no = 70) |
|---|---|
| Women, no (%) | 47 (69) |
| Age, yr | 55 (42–64) |
| BMI, kg/m2 | 25 (23–28) |
| CRP, mg/L | 9 (4–31) |
| ESR, mm/hour | 24 (12–38) |
| SJC28 | 9 (5–12) |
| TJC28 | 9 (4–13) |
| DAS28-CRP | 5 (4–6) |
| DAS28-ESR | 5 (5–6) |
| CDAI | 28 (22–38) |
| ACPA+, no (%) | 57 (81%) |
| RF+, no (%) | 48 (69%) |
| Symptom Duration (months) | 5 (3–8) |
| Smoking, no (%) * | 15 (22) |
| Protein | Early RA/(no = 70) |
|---|---|
| Total Adiponectin (mg/L) | 6.0 (4.0–10.3) |
| HMW Adiponectin (mg/L) | 3.0 (1.5–5.2) |
| Leptin (ng/mL) | 13.2 (4.7–35.3) |
| Resistin (ng/mL) * | 10.1 (7.8–13.4) |
| CXCL8/IL-8 | 489 (162–1001) |
| CXCL10/IP-10 | 1024 (748–1859) |
| CCL17/TARC | 301 (215–516) |
| CCL2/MCP-1 | 960 (746–1195) |
| CCL5/RANTES | 17,538 (13,354–22,134) |
| CCL3/MIP-1α * | 29 (14–56) |
| CXCL9/MIG * | 245 (89–813) |
| CXCL5/ENA-78 | 547 (311–762) |
| CCL20/MIP-3α * | 25 (2–74) |
| CXCL1/GROα * | 214 (147–341) |
| CXCL11/I-TAC | 985 (736–1446) |
| CCL4/MIP-1β * | 44 (28–72) |
| CCL22/MDC | 466 (356–620) |
| CXCL13/BCL | 490 (205–990) |
| CCL11/Eotaxin | 732 (628–1056) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileiadis, G.K.; Lundell, A.-C.; Zhang, Y.; Andersson, K.; Gjertsson, I.; Rudin, A.; Maglio, C. Adipocytokines in Untreated Newly Diagnosed Rheumatoid Arthritis: Association with Circulating Chemokines and Markers of Inflammation. Biomolecules 2021, 11, 325. https://doi.org/10.3390/biom11020325
Vasileiadis GK, Lundell A-C, Zhang Y, Andersson K, Gjertsson I, Rudin A, Maglio C. Adipocytokines in Untreated Newly Diagnosed Rheumatoid Arthritis: Association with Circulating Chemokines and Markers of Inflammation. Biomolecules. 2021; 11(2):325. https://doi.org/10.3390/biom11020325
Chicago/Turabian StyleVasileiadis, Georgios K., Anna-Carin Lundell, Yuan Zhang, Kerstin Andersson, Inger Gjertsson, Anna Rudin, and Cristina Maglio. 2021. "Adipocytokines in Untreated Newly Diagnosed Rheumatoid Arthritis: Association with Circulating Chemokines and Markers of Inflammation" Biomolecules 11, no. 2: 325. https://doi.org/10.3390/biom11020325
APA StyleVasileiadis, G. K., Lundell, A.-C., Zhang, Y., Andersson, K., Gjertsson, I., Rudin, A., & Maglio, C. (2021). Adipocytokines in Untreated Newly Diagnosed Rheumatoid Arthritis: Association with Circulating Chemokines and Markers of Inflammation. Biomolecules, 11(2), 325. https://doi.org/10.3390/biom11020325






