Zebrafish Tools for Deciphering Habenular Network-Linked Mental Disorders
Abstract
Simple Summary
Abstract
1. Introduction
2. The Zebrafish Habenular Network and Tools for Manipulation
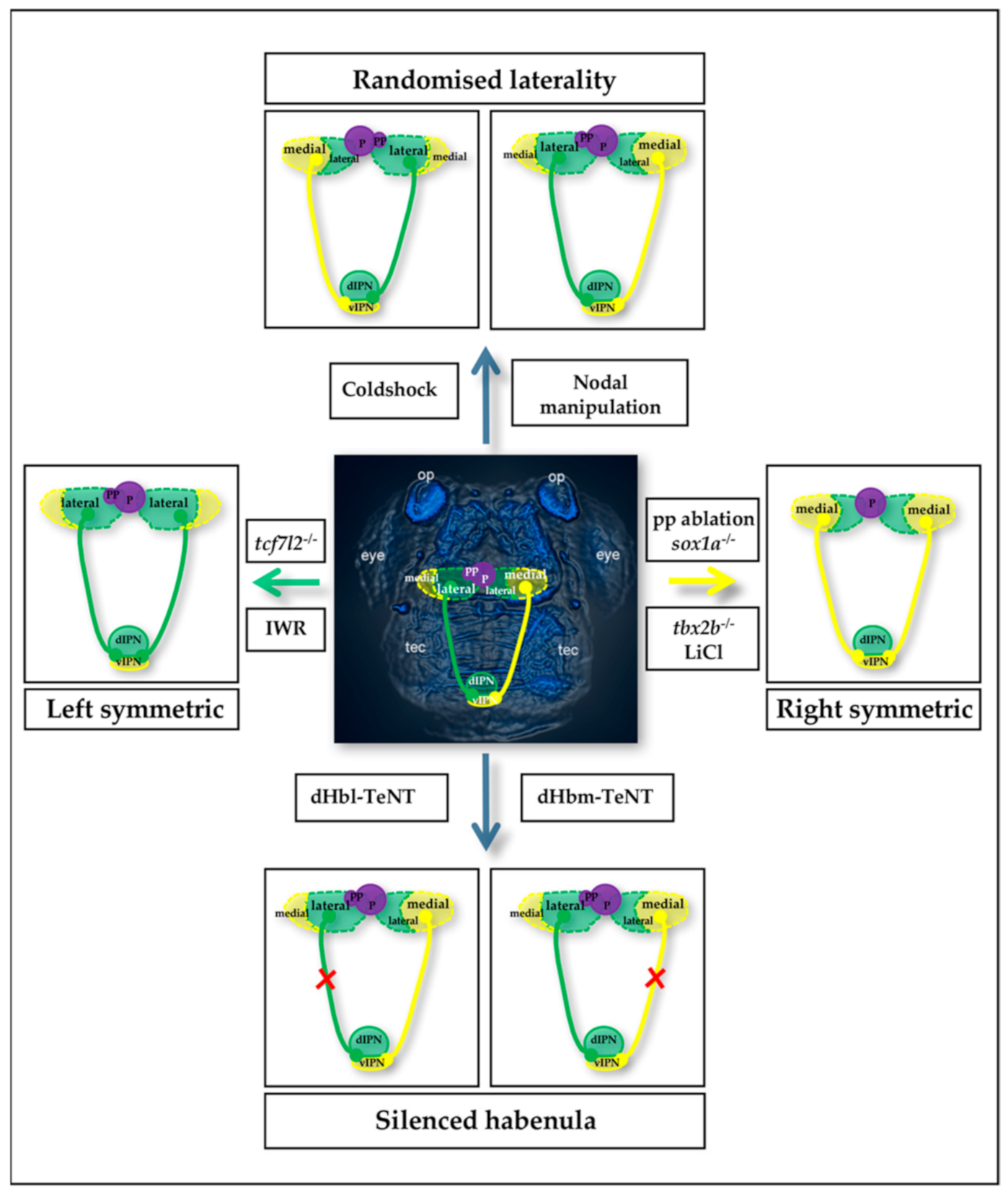
2.1. The Zebrafish Habenular Circuit
2.2. Zebrafish Habenular Network Manipulations
3. Behavioral Tests in Zebrafish to Study Human Habenula Linked Disorders
3.1. Behavioral Tests for Anxiety
3.2. Behavioral Paradigms for Depression
3.3. Social Behavior Paradigm
4. Critical Review and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sutherland, R.J. The dorsal diencephalic conduction system: A review of the anatomy and functions of the habenular complex. Neurosci. Biobehav. Rev. 1982, 6, 1–13. [Google Scholar] [CrossRef]
- BBianco, I.H.; Carl, M.; Russell, C.; Clarke, J.D.; Wilson, S.W. Brain asymmetry is encoded at the level of axon terminal morphology. Neural Dev. 2008, 3, 9. [Google Scholar] [CrossRef]
- Beretta, C.A.; Dross, N.; Guiterrez-Triana, J.A.; Ryu, S.; Carl, M. Habenula Circuit Development: Past, Present, and Future. Front. Behav. Neurosci. 2012, 6, 51. [Google Scholar] [CrossRef]
- Okamoto, H.; Agetsuma, M.; Aizawa, H. Genetic dissection of the zebrafish habenula, a possible switching board for selection of behavioral strategy to cope with fear and anxiety. Dev. Neurobiol. 2012, 72, 386–394. [Google Scholar] [CrossRef]
- McLaughlin, I.; Dani, J.A.; De Biasi, M. The medial habenula and interpeduncular nucleus circuitry is critical in addiction, anxiety, and mood regulation. J. Neurochem. 2017, 142, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, H.; Kobayashi, M.; Tanaka, S.; Fukai, T.; Okamoto, H. Molecular Characterization of the Subnuclei in Rat Habenula. J. Comp. Neurol. 2012, 520, 4051–4066. [Google Scholar] [CrossRef]
- Benekareddy, M.; Stachniak, T.J.; Bruns, A.; Knoflach, F.; Von Kienlin, M.; Künnecke, B.; Ghosh, A. Identification of a Corticohabenular Circuit Regulating Socially Directed Behavior. Biol. Psychiatry 2018, 83, 607–617. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, Y.; Ni, Z.; Dong, Y.; Cai, G.; Foncelle, A.; Ma, S.; Sang, K.; Tang, S.; Li, Y.; et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nat. Cell Biol. 2018, 554, 323–327. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, Y.; Sang, K.; Dong, Y.; Ni, Z.; Ma, S.; Hu, H. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nat. Cell Biol. 2018, 554, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Cui, Y.; Yang, Y. Circuits and functions of the lateral habenula in health and in disease. Nat. Rev. Neurosci. 2020, 21, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Caldecott-Hazard, S.; Mazziotta, J.; Phelps, M. Cerebral Correlates 14C-2-Deoxyglucose. J. Neurosci. 1988, 8, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Smith, K.; Cowen, P.; Friston, K.; Dolan, R. Covariation of Activity in Habenula and Dorsal Raphé Nuclei Following Tryptophan Depletion. NeuroImage 1999, 10, 163–172. [Google Scholar] [CrossRef]
- Mirrione, M.M.; Schulz, D.; Lapidus, K.A.B.; Zhang, S.; Goodman, W.; Henn, F.A. Increased metabolic activity in the septum and habenula during stress is linked to subsequent expression of learned helplessness behavior. Front. Hum. Neurosci. 2014, 8, 29. [Google Scholar] [CrossRef]
- Schmidt, F.M.; Schindler, S.; Adamidis, M.; Strauß, M.; Tränkner, A.; Trampel, R.; Walter, M.; Hegerl, U.; Turner, R.; Geyer, S.; et al. Habenula volume increases with disease severity in unmedicated major depressive disorder as revealed by 7T MRI. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 107–115. [Google Scholar] [CrossRef]
- Kiening, K.; Sartorius, A. A new translational target for deep brain stimulation to treat depression. EMBO Mol. Med. 2013, 5, 1151–1153. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, A.; Kiening, K.L.; Kirsch, P.; Von Gall, C.C.; Haberkorn, U.; Unterberg, A.W.; Henn, F.A.; Meyer-Lindenberg, A. Remission of Major Depression Under Deep Brain Stimulation of the Lateral Habenula in a Therapy-Refractory Patient. Biol. Psychiatry 2010, 67, e9–e11. [Google Scholar] [CrossRef]
- Sartorius, A.; Henn, F.A. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med. Hypotheses 2007, 69, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Lax, E.; Dikshtein, Y.; Abraham, L.; Flaumenhaft, Y.; Sudai, E.; Ben-Tzion, M.; Yadid, G. Electrical stimulation of the lateral habenula produces an inhibitory effect on sucrose self-administration. Neuropharmacology 2011, 60, 381–387. [Google Scholar] [CrossRef]
- Yadid, G.; Gispan, I.; Lax, E. Lateral habenula deep brain stimulation for personalized treatment of drug addiction. Front. Hum. Neurosci. 2013, 7, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Ingason, A.; Djurovic, S.; Melle, I.; Fenger, M.; Gustafsson, O.; Jakobsen, K.D.; Rasmussen, H.B.; Tosato, S.; Rietschel, M.; et al. At-Risk Variant in TCF7L2 for Type II Diabetes Increases Risk of Schizophrenia. Biol. Psychiatry 2011, 70, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.Q.; Cuccaro, M.L.; Jaworski, J.M.; Haynes, C.S.; Stephan, D.A.; Parod, J.; Abramson, R.K.; Wright, H.H.; Gilbert, J.R.; Haines, J.L.; et al. Dissecting the locus heterogeneity of autism: Significant linkage to chromosome 12q14. Mol. Psychiatry 2006, 12, 376–384. [Google Scholar] [CrossRef]
- Iossifov, I.; O’Roak, B.J.; Sanders, S.J.; Ronemus, M.; Krumm, N.; Levy, D.; Stessman, H.A.; Witherspoon, K.T.; Vives, L.; Patterson, K.E.; et al. The contribution of de novo coding mutations to autism spectrum disorder. Nat. Cell Biol. 2014, 515, 216–221. [Google Scholar] [CrossRef]
- Wang, T.; Hoekzema, K.; Vecchio, D.; Wu, H.; Sulovari, A.; Coe, B.P.; Gillentine, M.A.; Wilfert, A.B.; Perez-Jurado, L.A.; Kvarnung, M.; et al. Large-scale targeted sequencing identifies risk genes for neurodevelopmental disorders. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Hüsken, U.; Stickney, H.L.; Gestri, G.; Bianco, I.H.; Faro, A.; Young, R.M.; Roussigne, M.; Hawkins, T.A.; Beretta, C.A.; Brinkmann, I.; et al. Tcf7l2 Is Required for Left-Right Asymmetric Differentiation of Habenular Neurons. Curr. Biol. 2014, 24, 2217–2227. [Google Scholar] [CrossRef]
- Guglielmi, L.; Bühler, A.; Moro, E.; Argenton, F.; Poggi, L.; Carl, M. Temporal control of Wnt signaling is required for habenular neuron diversity and brain asymmetry. Development 2020, 147, dev182865. [Google Scholar] [CrossRef] [PubMed]
- Lipiec, M.A.; Bem, J.; Koziński, K.; Chakraborty, C.; Urban-Ciećko, J.; Zajkowski, T.; Dąbrowski, M.; Szewczyk Łukasz, M.; Toval, A.; Ferran, J.L.; et al. TCF7L2 regulates postmitotic differentiation programmes and excitability patterns in the thalamus. Development 2020, 147, dev190181. [Google Scholar] [CrossRef]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-VanderWeele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef]
- Upthegrove, R.; Marwaha, S.; Birchwood, M. Depression and Schizophrenia: Cause, Consequence, or Trans-diagnostic Issue? Schizophr. Bull. 2016, 43, 240–244. [Google Scholar] [CrossRef]
- Panula, P.; Sallinen, V.; Sundvik, M.; Kolehmainen, J.; Torkko, V.; Tiittula, A.; Moshnyakov, M.; Podlasz, P. Modulatory Neurotransmitter Systems and Behavior: Towards Zebrafish Models of Neurodegenerative Diseases. Zebrafish 2006, 3, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Amo, R.; Aizawa, H.; Takahoko, M.; Kobayashi, M.; Takahashi, R.; Aoki, T.; Okamoto, H. Identification of the Zebrafish Ventral Habenula As a Homolog of the Mammalian Lateral Habenula. J. Neurosci. 2010, 30, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Beretta, C.A.; Dross, N.; Bankhead, P.; Carl, M. The ventral habenulae of zebrafish develop in prosomere 2 dependent on Tcf7l2 function. Neural Dev. 2013, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.S.; Roberson, S.; Akitake, C.M.; Fortuno, L.; Gamse, J.; Moens, C.; Halpern, M.E. Distinct requirements for Wntless in habenular development. Dev. Biol. 2015, 406, 117–128. [Google Scholar] [CrossRef]
- Hsieh, J.-C.; Kodjabachian, L.; Rebbert, M.L.; Rattner, A.; Smallwood, P.M.; Samos, C.H.; Nusse, R.; Dawid, I.B.; Nathans, J. A new secreted protein that binds to Wnt proteins and inhibits their activites. Nat. Cell Biol. 1999, 398, 431–436. [Google Scholar] [CrossRef]
- Poggi, L.; Casarosa, S.; Carl, M. An Eye on the Wnt Inhibitory Factor Wif1. Front. Cell Dev. Biol. 2018, 6, 167. [Google Scholar] [CrossRef]
- Mattes, B.; Weber, S.; Peres, J.; Chen, Q.; Davidson, G.; Houart, C.; Scholpp, S. Wnt3 and Wnt3a are required for induction of the mid-diencephalic organizer in the caudal forebrain. Neural Dev. 2012, 7, 12. [Google Scholar] [CrossRef]
- Aizawa, H.; Goto, M.; Sato, T.; Okamoto, H. Temporally Regulated Asymmetric Neurogenesis Causes Left-Right Difference in the Zebrafish Habenular Structures. Dev. Cell 2007, 12, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Roussigné, M.; Bianco, I.H.; Wilson, S.W.; Blader, P. Nodal signalling imposes left-right asymmetry upon neurogenesis in the habenular nuclei. Development 2009, 136, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Roussigne, M.; Blader, P.; Wilson, S.W. The Zebrafish Epithalamus Clears a Path through the Complexity of Brain Lateralization. Dev. Neurobiol. 2011, 72, 269–281. [Google Scholar]
- Roussigné, M.; Wei, L.; Tsingos, E.; Kuchling, F.; Alkobtawi, M.; Tsalavouta, M.; Wittbrodt, J.; Carl, M.; Blader, P.; Wilson, S.W. Left/right asymmetric collective migration of parapineal cells is mediated by focal FGF signaling activity in leading cells. Proc. Natl. Acad. Sci. USA 2018, 115, E9812–E9821. [Google Scholar] [CrossRef]
- Regan, J.C.; Concha, M.L.; Roussigne, M.; Russell, C.; Wilson, S.W. An Fgf8-Dependent Bistable Cell Migratory Event Establishes CNS Asymmetry. Neuron 2009, 61, 27–34. [Google Scholar] [CrossRef]
- Gamse, J.T.; Thisse, C.; Thisse, B.; Halpern, M.E. The parapineal mediates left-right asymmetry in the zebrafish diencephalon. Development 2003, 130, 1059–1068. [Google Scholar] [CrossRef]
- Concha, M.L.; Russell, C.; Regan, J.C.; Tawk, M.; Sidi, S.; Gilmour, D.T.; Kapsimali, M.; Sumoy, L.; Goldstone, K.; Amaya, E.; et al. Local Tissue Interactions across the Dorsal Midline of the Forebrain Establish CNS Laterality. Neuron 2003, 39, 423–438. [Google Scholar] [CrossRef]
- Garric, L.; Ronsin, B.; Roussigné, M.; Booton, S.; Gamse, J.T.; Dufourcq, P.; Blader, P. Pitx2c ensures habenular asymmetry by restricting parapineal cell number. Development 2014, 141, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Lekk, I.; Duboc, V.; Faro, A.; Nicolaou, S.; Blader, P.; Wilson, S.W. Sox1a mediates the ability of the parapineal to impart habenular left-right asymmetry. eLife 2019, 8, 47376. [Google Scholar] [CrossRef]
- Ahumada-Galleguillos, P.; Lemus, C.G.; Díaz, E.; Osorio-Reich, M.; Härtel, S.; Concha, M.L. Directional asymmetry in the volume of the human habenula. Brain Struct. Funct. 2016, 222, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, H.; Zhu, M. Toward an understanding of the habenula’s various roles in human depression. Psychiatry Clin. Neurosci. 2019, 73, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Ichijo, H.; Hamada, M.; Takahashi, S.; Kobayashi, M.; Nagai, T.; Toyama, T.; Kawaguchi, M. Lateralization, maturation, and anteroposterior topography in the lateral habenula revealed by ZIF268/EGR1 immunoreactivity and labeling history of neuronal activity. Neurosci. Res. 2015, 95, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Dreosti, E.; Llopis, N.V.; Carl, M.; Yaksi, E.; Wilson, S.W. Left-Right Asymmetry Is Required for the Habenulae to Respond to Both Visual and Olfactory Stimuli. Curr. Biol. 2014, 24, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Facchin, L.; Duboué, E.R.; Halpern, M.E. Disruption of Epithalamic Left-Right Asymmetry Increases Anxiety in Zebrafish. J. Neurosci. 2015, 35, 15847–15859. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Shekhar, K.; Regev, A.; Schier, A.F. Comprehensive Identification and Spatial Mapping of Habenular Neuronal Types Using Single-Cell RNA-Seq. Curr. Biol. 2018, 28, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Gamse, J.T.; Kuan, Y.-S.; Macurak, M.; Brösamle, C.; Thisse, B.; Thisse, C.; Halpern, M.E. Directional asymmetry of the zebrafish epithalamus guides dorsoventral innervation of the midbrain target. Development 2005, 132, 4869–4881. [Google Scholar] [CrossRef]
- Beretta, C.A.; Dross, N.; Guglielmi, L.; Bankhead, P.; Soulika, M.; Gutierrez-Triana, J.A.; Paolini, A.; Poggi, L.; Falk, J.; Ryu, S.; et al. Early Commissural Diencephalic Neurons Control Habenular Axon Extension and Targeting. Curr. Biol. 2017, 27, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, H.; Bianco, I.H.; Hamaoka, T.; Miyashita, T.; Uemura, O.; Concha, M.L.; Russell, C.; Wilson, S.W.; Okamoto, H. Laterotopic Representation of Left-Right Information onto the Dorso-Ventral Axis of a Zebrafish Midbrain Target Nucleus. Curr. Biol. 2005, 15, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Morley, B.J. The Interpeduncular Nucleus. Int. Rev. Neurobiol. 1986, 28, 157–182. [Google Scholar] [CrossRef] [PubMed]
- Agetsuma, M.; Aizawa, H.; Aoki, T.; Nakayama, R.; Takahoko, M.; Goto, M.; Sassa, T.; Amo, R.; Shiraki, T.; Kawakami, K.; et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat. Neurosci. 2010, 13, 1354–1356. [Google Scholar] [CrossRef]
- Hong, E.; Santhakumar, K.; Akitake, C.A.; Ahn, S.J.; Thisse, C.; Thisse, B.; Wyart, C.; Mangin, J.-M.; Halpern, M.E. Cholinergic left-right asymmetry in the habenulo-interpeduncular pathway. Proc. Natl. Acad. Sci. USA 2013, 110, 21171–21176. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.-S.; Yu, H.-H.; Moens, C.; Halpern, M.E.; Strutt, D. Neuropilin asymmetry mediates a left-right difference in habenular connectivity. Development 2007, 134, 857–865. [Google Scholar] [CrossRef][Green Version]
- Carl, M.; Bianco, I.H.; Bajoghli, B.; Aghaallaei, N.; Czerny, T.; Wilson, S.W. Wnt/Axin1/β-Catenin Signaling Regulates Asymmetric Nodal Activation, Elaboration, and Concordance of CNS Asymmetries. Neuron 2007, 55, 393–405. [Google Scholar] [CrossRef]
- Concha, M.L.; Burdine, R.D.; Russell, C.; Schier, A.F.; Wilson, S.W. A Nodal Signaling Pathway Regulates the Laterality of Neuroanatomical Asymmetries in the Zebrafish Forebrain Have Shed Little Light on the Development of CNS Asym-Metry (Reviewed in Capdevilla et al., 2000). The Nodal Pathway Is One of Several Signaling P. Neuron 2000, 28, 399–409. [Google Scholar] [CrossRef]
- Long, S.; Ahmad, N.; Rebagliati, M. The zebrafish nodal-related gene southpaw is required for visceral and diencephalic left-right asymmetry. Development 2003, 130, 2303–2316. [Google Scholar] [CrossRef]
- Duboc, V.; Dufourcq, P.; Blader, P.; Roussigné, M. Asymmetry of the Brain: Development and Implications. Annu. Rev. Genet. 2015, 49, 647–672. [Google Scholar] [CrossRef]
- Chou, M.-Y.; Amo, R.; Kinoshita, M.; Cherng, B.-W.; Shimazaki, H.; Agetsuma, M.; Shiraki, T.; Aoki, T.; Takahoko, M.; Yamazaki, M.; et al. Social conflict resolution regulated by two dorsal habenular subregions in zebrafish. Science 2016, 352, 87–90. [Google Scholar] [CrossRef]
- Cherng, B.-W.; Islam, T.; Torigoe, M.; Tsuboi, T.; Okamoto, H. The Dorsal Lateral Habenula-Interpeduncular Nucleus Pathway Is Essential for Left-Right-Dependent Decision Making in Zebrafish. Cell Rep. 2020, 32, 108143. [Google Scholar] [CrossRef] [PubMed]
- Snelson, C.D.; Santhakumar, K.; Halpern, M.E.; Gamse, J.T. Tbx2b is required for the development of the parapineal organ. Development 2008, 135, 1693–1702. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muncan, V.; Faro, A.; Haramis, A.G.; Hurlstone, A.F.L.; Wienholds, E.; Van Es, J.; Korving, J.; Begthel, H.; Zivkovic, D.; Clevers, H. T-cell factor 4 (Tcf7l2) maintains proliferative compartments in zebrafish intestine. EMBO Rep. 2007, 8, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Asnani, A.; Peterson, R.T. The zebrafish as a tool to identify novel therapies for human cardiovascular disease. Dis. Model. Mech. 2014, 7, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.O.; Millington, M.E.; Combe, F.J.; Brennan, C.H. Development and implementation of a three-choice serial reaction time task for zebrafish (Danio rerio). Behav. Brain Res. 2012, 227, 73–80. [Google Scholar] [CrossRef]
- Khan, K.M.; Collier, A.D.; Meshalkina, D.A.; Kysil, E.V.; Khatsko, S.L.; Kolesnikova, T.; Morzherin, Y.Y.; Warnick, J.E.; Kalueff, A.V.; Echevarria, D.J. Zebrafish models in neuropsychopharmacology and CNS drug discovery. Br. J. Pharmacol. 2017, 174, 1925–1944. [Google Scholar] [CrossRef]
- Luca, R.M.; Gerlai, R. In search of optimal fear inducing stimuli: Differential behavioral responses to computer animated images in zebrafish. Behav. Brain Res. 2012, 226, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Cachat, J.; Canavello, P.; Elegante, M.; Bartels, B.; Hart, P.; Bergner, C.; Egan, R.; Duncan, A.; Tien, D.; Chung, A.; et al. Modeling withdrawal syndrome in zebrafish. Behav. Brain Res. 2010, 208, 371–376. [Google Scholar] [CrossRef]
- Canzian, J.; Fontana, B.D.; Quadros, V.A.; Rosemberg, D.B. Conspecific alarm substance differently alters group behavior of zebrafish populations: Putative involvement of cholinergic and purinergic signaling in anxiety- and fear-like responses. Behav. Brain Res. 2017, 320, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Krause, J.; Butlin, R.K.; Peuhkuri, N.; Pritchard, V.L. The social organization of fish shoals: A test of the predictive power of laboratory experiments for the field. Biol. Rev. 2007, 75, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.J.; Norton, W.H. Using zebrafish to uncover the genetic and neural basis of aggression, a frequent comorbid symptom of psychiatric disorders. Behav. Brain Res. 2015, 276, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Engeszer, R.E.; Wang, G.; Ryan, M.J.; Parichy, D.M. Sex-specific perceptual spaces for a vertebrate basal social aggregative behavior. Proc. Natl. Acad. Sci. USA 2008, 105, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Dreosti, E.; Elopes, G.; Kampff, A.R.; Wilson, S.W. Development of social behavior in young zebrafish. Front. Neural Circuits 2015, 9, 39. [Google Scholar] [CrossRef]
- Cachat, J.; Stewart, A.; Grossman, L.; Gaikwad, S.; Kadri, F.; Chung, K.M.; Wu, N.; Wong, K.; Roy, S.; Suciu, C.; et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc. 2010, 5, 1786–1799. [Google Scholar] [CrossRef]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef]
- Blaser, R.E.; Rosemberg, D.B. Measures of Anxiety in Zebrafish (Danio rerio): Dissociation of Black/White Preference and Novel Tank Test. PLoS ONE 2012, 7, e36931. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Elegante, M.; Bartels, B.; Elkhayat, S.; Tien, D.; Roy, S.; Goodspeed, J.; Suciu, C.; Tan, J.; Grimes, C.; et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio). Behav. Brain Res. 2010, 208, 450–457. [Google Scholar] [CrossRef]
- Jesuthasan, S. Fear, anxiety, and control in the zebrafish. Dev. Neurobiol. 2012, 72, 395–403. [Google Scholar] [CrossRef]
- Champagne, D.L.; Hoefnagels, C.C.; De Kloet, R.E.; Richardson, M.K. Translating rodent behavioral repertoire to zebrafish (Danio rerio): Relevance for stress research. Behav. Brain Res. 2010, 214, 332–342. [Google Scholar] [CrossRef]
- López-Patiño, M.A.; Yu, L.; Cabral, H.; Zhdanova, I.V. Anxiogenic effects of cocaine withdrawal in zebrafish. Physiol. Behav. 2008, 93, 160–171. [Google Scholar] [CrossRef]
- Peitsaro, N.; Kaslin, J.; Anichtchik, O.V.; Panula, P. Modulation of the histaminergic system and behaviour by α-fluoromethylhistidine in zebrafish. J. Neurochem. 2004, 86, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Cianca, V.; Bartolini, T.; Porfiri, M.; Macrì, S. A Robotics-Based Behavioral Paradigm to Measure Anxiety-Related Responses in Zebrafish. PLoS ONE 2013, 8, e69661. [Google Scholar] [CrossRef]
- Ahmed, O.; Séguin, D.; Gerlai, R. An automated predator avoidance task in zebrafish. Behav. Brain Res. 2011, 216, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Jesuthasan, S.J.; Mathuru, A.S. The Alarm Response in Zebrafish: Innate Fear in a Vertebrate Genetic Model. J. Neurogenet. 2008, 22, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Speedie, N.; Gerlai, R. Alarm substance induced behavioral responses in zebrafish (Danio rerio). Behav. Brain Res. 2008, 188, 168–177. [Google Scholar] [CrossRef]
- Norton, W.; Bally-Cuif, L. Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci. 2010, 11, 90. [Google Scholar] [CrossRef]
- Maximino, C.; De Brito, T.M.; Colmanetti, R.; Pontes, A.A.A.; De Castro, H.M.; De Lacerda, R.I.T.; Morato, S.; Gouveia, A., Jr. Parametric analyses of anxiety in zebrafish scototaxis. Behav. Brain Res. 2010, 210, 1–7. [Google Scholar] [CrossRef]
- Blaser, R.; Peñalosa, Y. Stimuli affecting zebrafish (Danio rerio) behavior in the light/dark preference test. Physiol. Behav. 2011, 104, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.P.; Neuhauss, S.C. Behavioral Neurobiology: How Larval Fish Orient towards the Light. Curr. Biol. 2010, 20, R159–R161. [Google Scholar] [CrossRef]
- Burgess, H.A.; Schoch, H.; Granato, M. Distinct Retinal Pathways Drive Spatial Orientation Behaviors in Zebrafish Navigation. Curr. Biol. 2010, 20, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Schnörr, S.; Steenbergen, P.; Richardson, M.; Champagne, D. Measuring thigmotaxis in larval zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar] [CrossRef]
- Duboué, E.R.; Hong, E.; Eldred, K.C.; Halpern, M.E. Left Habenular Activity Attenuates Fear Responses in Larval Zebrafish. Curr. Biol. 2017, 27, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Mathuru, A.S.; Teh, C.; Kibat, C.; Korzh, V.; Penney, T.B.; Jesuthasan, S. The Habenula Prevents Helpless Behavior in Larval Zebrafish. Curr. Biol. 2010, 20, 2211–2216. [Google Scholar] [CrossRef]
- Maaswinkel, H.; Le, X.; He, L.; Zhu, L.; Weng, W. Dissociating the effects of habituation, black walls, buspirone and ethanol on anxiety-like behavioral responses in shoaling zebrafish. A 3D approach to social behavior. Pharmacol. Biochem. Behav. 2013, 108, 16–27. [Google Scholar] [CrossRef]
- Gerlai, R. Social behavior of zebrafish: From synthetic images to biological mechanisms of shoaling. J. Neurosci. Methods 2014, 234, 59–65. [Google Scholar] [CrossRef]
- Engeszer, R.E.; Da Barbiano, L.A.; Ryan, M.J.; Parichy, D.M. Timing and plasticity of shoaling behaviour in the zebrafish, Danio rerio. Anim. Behav. 2007, 74, 1269–1275. [Google Scholar] [CrossRef]
- Eddins, D.; Cerutti, D.; Williams, P.; Linney, E.; Levin, E.D. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: Comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol. Teratol. 2010, 32, 99–108. [Google Scholar] [CrossRef]
- Burgess, H.A.; Granato, M. Modulation of locomotor activity in larval zebrafish during light adaptation. J. Exp. Biol. 2007, 210, 2526–2539. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.A.; Johnson, S.L.; Granato, M. Unidirectional startle responses and disrupted left-right co-ordination of motor behaviors inrobo3mutant zebrafish. Genes Brain Behav. 2009, 8, 500–511. [Google Scholar] [CrossRef]
- Colwill, R.M.; Creton, R. Imaging escape and avoidance behavior in zebrafish larvae. Rev. Neurosci. 2011, 22, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Zeddies, D.G. Development of the acoustically evoked behavioral response in zebrafish to pure tones. J. Exp. Biol. 2005, 208, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Nakajo, H.; Tsuboi, T.; Okamoto, H. The behavioral paradigm to induce repeated social defeats in zebrafish. Neurosci. Res. 2020, 161, 24–32. [Google Scholar] [CrossRef]
- Piato, Â.L.; Capiotti, K.M.; Tamborski, A.R.; Oses, J.P.; Barcellos, L.J.; Bogo, M.R.; Lara, D.R.; Vianna, M.R.; Bonan, C.D. Unpredictable chronic stress model in zebrafish (Danio rerio): Behavioral and physiological responses. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2011, 35, 561–567. [Google Scholar] [CrossRef]
- Chakravarty, S.; Reddy, B.R.; Sudhakar, S.R.; Saxena, S.; Das, T.; Meghah, V.; Swamy, C.V.B.; Kumar, A.; Idris, M.M. Chronic Unpredictable Stress (CUS)-Induced Anxiety and Related Mood Disorders in a Zebrafish Model: Altered Brain Proteome Profile Implicates Mitochondrial Dysfunction. PLoS ONE 2013, 8, e63302. [Google Scholar] [CrossRef]
- Golla, A.; Østby, H.; Kermen, F. Chronic unpredictable stress induces anxiety-like behaviors in young zebrafish. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Song, C.; Liu, B.-P.; Zhang, Y.-P.; Peng, Z.; Wang, J.; Collier, A.D.; Echevarria, D.J.; Savelieva, K.V.; Lawrence, R.F.; Rex, C.S.; et al. Modeling consequences of prolonged strong unpredictable stress in zebrafish: Complex effects on behavior and physiology. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2018, 81, 384–394. [Google Scholar] [CrossRef]
- Andalman, A.S.; Burns, V.M.; Lovett-Barron, M.; Broxton, M.; Poole, B.; Yang, S.J.; Grosenick, L.; Lerner, T.N.; Chen, R.; Benster, T.; et al. Neuronal Dynamics Regulating Brain and Behavioral State Transitions. Cell 2019, 177, 970–985. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.J. Neurocognitive mechanisms of anxiety: An integrative account. Trends Cogn. Sci. 2007, 11, 307–316. [Google Scholar] [CrossRef]
- Epidemiology of anxiety disorders in the 21st century. Dialog. Clin. Neurosci. 2015, 17, 327–335. [CrossRef]
- Stewart, A.; Wu, N.; Cachat, J.; Hart, P.; Gaikwad, S.; Wong, K.; Utterback, E.; Gilder, T.; Kyzar, E.; Newman, A.; et al. Pharmacological modulation of anxiety-like phenotypes in adult zebrafish behavioral models. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2011, 35, 1421–1431. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a Comprehensive Catalog of Zebrafish Behavior 1.0 and Beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef]
- Maximino, C.; Da Silva, A.W.B.; Araújo, J.; Lima, M.G.; Miranda, V.; Puty, B.; Benzecry, R.; Picanço-Diniz, D.L.W.; Gouveia, A., Jr.; Oliveira, K.R.M.; et al. Fingerprinting of Psychoactive Drugs in Zebrafish Anxiety-Like Behaviors. PLoS ONE 2014, 9, e103943. [Google Scholar] [CrossRef]
- Bourin, M.; Hascoët, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Patterns of avoidance behaviours in the light/dark preference test in young juvenile zebrafish: A pharmacological study. Behav. Brain Res. 2011, 222, 15–25. [Google Scholar] [CrossRef]
- Lau, B.Y.B.; Mathur, P.; Gould, G.G.; Guo, S. Identification of a brain center whose activity discriminates a choice behavior in zebrafish. Proc. Natl. Acad. Sci. USA 2011, 108, 2581–2586. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R.; Fernandes, Y.; Pereira, T. Zebrafish (Danio rerio) responds to the animated image of a predator: Towards the development of an automated aversive task. Behav. Brain Res. 2009, 201, 318–324. [Google Scholar] [CrossRef]
- Bencan, Z.; Sledge, D.; Levin, E.D. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol. Biochem. Behav. 2010, 94, 75–80. [Google Scholar] [CrossRef]
- MacPhail, R.; Brooks, J.; Hunter, D.; Padnos, B.; Irons, T.; Padilla, S. Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. NeuroToxicology 2009, 30, 52–58. [Google Scholar] [CrossRef]
- Hamilton, T.J.; Morrill, A.; Lucas, K.; Gallup, J.; Harris, M.; Healey, M.; Pitman, T.; Schalomon, M.; Digweed, S.; Tresguerres, M. Establishing zebrafish as a model to study the anxiolytic effects of scopolamine. Sci. Rep. 2017, 7, 15081. [Google Scholar] [CrossRef] [PubMed]
- Dlugos, C.A.; Rabin, R.A. Ethanol effects on three strains of zebrafish: Model system for genetic investigations. Pharmacol. Biochem. Behav. 2003, 74, 471–480. [Google Scholar] [CrossRef]
- Grossman, L.; Utterback, E.; Stewart, A.; Gaikwad, S.; Chung, K.M.; Suciu, C.; Wong, K.; Elegante, M.; Elkhayat, S.; Tan, J.; et al. Characterization of behavioral and endocrine effects of LSD on zebrafish. Behav. Brain Res. 2010, 214, 277–284. [Google Scholar] [CrossRef]
- Seibt, K.J.; Oliveira, R.D.L.; Zimmermann, F.F.; Capiotti, K.M.; Bogo, M.R.; Ghisleni, G.; Bonan, C.D. Antipsychotic drugs prevent the motor hyperactivity induced by psychotomimetic MK-801 in zebrafish (Danio rerio). Behav. Brain Res. 2010, 214, 417–422. [Google Scholar] [CrossRef]
- Sackerman, J.; Donegan, J.J.; Cunningham, C.S.; Nguyen, N.N.; Lawless, K.; Long, A.; Benno, R.H.; Gould, G.G. Zebrafish Behavior in Novel Environments: Effects of Acute Exposure to Anxiolytic Compounds and Choice of Danio rerio Line. Int. J. Comp. Psychol. 2010, 23, 43–61. [Google Scholar]
- Stewart, A.; Wong, K.; Cachat, J.; Gaikwad, S.; Kyzar, E.; Wu, N.; Hart, P.; Piet, V.; Utterback, E.; Elegante, M.; et al. Zebrafish models to study drug abuse-related phenotypes. Rev. Neurosci. 2011, 22, 95–105. [Google Scholar] [CrossRef]
- Echevarria, D.J.; Toms, C.N.; Jouandot, D.J. Alcohol-induced behavior change in zebrafish models. Rev. Neurosci. 2011, 22, 85–93. [Google Scholar] [CrossRef]
- Miller, N.; Greene, K.; Dydinski, A.; Gerlai, R. Effects of nicotine and alcohol on zebrafish (Danio rerio) shoaling. Behav. Brain Res. 2013, 240, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Y.; Gerlai, R. Long-Term Behavioral Changes in Response to Early Developmental Exposure to Ethanol in Zebrafish. Alcohol. Clin. Exp. Res. 2009, 33, 601–609. [Google Scholar] [CrossRef]
- Lockwood, B.; Bjerke, S.; Kobayashi, K.; Guo, S. Acute effects of alcohol on larval zebrafish: A genetic system for large-scale screening. Pharmacol. Biochem. Behav. 2004, 77, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R.; Lahav, M.; Guo, S.; Rosenthal, A. Drinks like a fish: Zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol. Biochem. Behav. 2000, 67, 773–782. [Google Scholar] [CrossRef]
- Mathuru, A.S.; Jesuthasan, S. The medial habenula as a regulator of anxiety in adult zebrafish. Front. Neural Circuits 2013, 7, 99. [Google Scholar] [CrossRef]
- Burgess, H.A.; Granato, M. Sensorimotor Gating in Larval Zebrafish. J. Neurosci. 2007, 27, 4984–4994. [Google Scholar] [CrossRef]
- Castellanos, F.X.; Fine, E.J.; Kaysen, D.; Marsh, W.L.; Rapoport, J.L.; Hallett, M. Sensorimotor gating in boys with Tourette’s syndrome and ADHD: Preliminary results. Biol. Psychiatry 1996, 39, 33–41. [Google Scholar] [CrossRef]
- Braff, D.L.; Geyer, M.A.; Swerdlow, N.R. Human studies of prepulse inhibition of startle: Normal subjects, patient groups, and pharmacological studies. Psychopharmacology 2001, 156, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V.; Soni, W.; Sharma, T. Normalization of information processing deficits in schizophrenia with clozapine. Am. J. Psychiatry 1999, 156, 1046–1051. [Google Scholar]
- Banono, N.S.; Esguerra, C.V. Pharmacological Validation of the Prepulse Inhibition of Startle Response in Larval Zebrafish using a Commercial Automated System and Software. J. Vis. Exp. 2020, 161, e61423. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M. Commentary: Nature-nurture interplay in emotional disorders. J. Child Psychol. Psychiatry 2003, 44, 934–944. [Google Scholar] [CrossRef]
- Venzala, E.; García-García, A.; Elizalde, N.; Tordera, R. Social vs. environmental stress models of depression from a behavioural and neurochemical approach. Eur. Neuropsychopharmacol. 2013, 23, 697–708. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Borsini, F.; Meli, A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology 1988, 94, 147–160. [Google Scholar] [CrossRef]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Katz, R.J.; Roth, K.A.; Carroll, B.J. Acute and chronic stress effects on open field activity in the rat: Implications for a model of depression. Neurosci. Biobehav. Rev. 1981, 5, 247–251. [Google Scholar] [CrossRef]
- Willner, P. Chronic Mild Stress (CMS) Revisited: Consistency and Behavioural-Neurobiological Concordance in the Effects of CMS. Neuropsychobiology 2005, 52, 90–110. [Google Scholar] [CrossRef]
- Berton, O.; McClung, C.A.; Dileone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rios, M.; et al. Essential Role of BDNF in the Mesolimbic Dopamine Pathway in Social Defeat Stress. Science 2006, 311, 864–868. [Google Scholar] [CrossRef]
- Marcon, M.; Herrmann, A.P.; Mocelin, R.; Rambo, C.L.; Koakoski, G.; Abreu, M.S.; Conterato, G.M.M.; Kist, L.W.; Bogo, M.R.; Zanatta, L.; et al. Prevention of unpredictable chronic stress-related phenomena in zebrafish exposed to bromazepam, fluoxetine and nortriptyline. Psychopharmacology 2016, 233, 3815–3824. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Han, M.-H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; LaPlant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular Adaptations Underlying Susceptibility and Resistance to Social Defeat in Brain Reward Regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Amo, R.; Fredes, F.; Kinoshita, M.; Aoki, R.; Aizawa, H.; Agetsuma, M.; Aoki, T.; Shiraki, T.; Kakinuma, H.; Matsuda, M.; et al. The Habenulo-Raphe Serotonergic Circuit Encodes an Aversive Expectation Value Essential for Adaptive Active Avoidance of Danger. Neuron 2014, 84, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Piriz, J.; Mirrione, M.M.; Chung, C.; Proulx, C.D.; Schulz, D.; Henn, F.; Malinow, R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nat. Cell Biol. 2011, 470, 535–539. [Google Scholar] [CrossRef]
- Oliveira, R.F.; Silva, J.F.; Simões, J.M. Fighting Zebrafish: Characterization of Aggressive Behavior and Winner–Loser Effects. Zebrafish 2011, 8, 73–81. [Google Scholar] [CrossRef]
- Abril-De-Abreu, R.; Cruz, A.S.; Oliveira, R.F. Social dominance modulates eavesdropping in zebrafish. R. Soc. Open Sci. 2015, 2, 150220. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, G.; Lysiak, N. Kin recognition and inbreeding avoidance in zebrafish, Danio rerio, is based on phenotype matching. Anim. Behav. 2006, 71, 1371–1377. [Google Scholar] [CrossRef]
- Hinz, F.I.; Aizenberg, M.; Tushev, G.; Schuman, E.M. Protein Synthesis-Dependent Associative Long-Term Memory in Larval Zebrafish. J. Neurosci. 2013, 33, 15382–15387. [Google Scholar] [CrossRef]
- Miller, N.; Gerlai, R. Quantification of shoaling behaviour in zebrafish (Danio rerio). Behav. Brain Res. 2007, 184, 157–166. [Google Scholar] [CrossRef]
- Rehnberg, B.G.; Smith, R.J.F. The Influence of Alarm Substance and Shoal Size on the Behaviour of Zebra Danios, Brachydanio Rerio (Cyprinidae). J. Fish Biol. 1988, 33, 155–163. [Google Scholar] [CrossRef]
- Maaswinkel, H.; Zhu, L.; Weng, W. Assessing Social Engagement in Heterogeneous Groups of Zebrafish: A New Paradigm for Autism-Like Behavioral Responses. PLoS ONE 2013, 8, e75955. [Google Scholar] [CrossRef] [PubMed]
- Kyzar, E.J.; Kalueff, A.V. Exploring Hallucinogen Pharmacology and Psychedelic Medicine with Zebrafish Models. Zebrafish 2016, 13, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Ellis, L.D.; Soanes, K.H. A larval zebrafish model of bipolar disorder as a screening platform for neuro-therapeutics. Behav. Brain Res. 2012, 233, 450–457. [Google Scholar] [CrossRef]
- Fore, S.; Yaksi, E. Habenula: A Role in Brain State Transitions during Coping Behavior. Curr. Biol. 2019, 29, R692–R694. [Google Scholar] [CrossRef]
- Maximino, C.; De Brito, T.M.; Batista, A.W.D.S.; Herculano, A.M.; Morato, S.; Gouveia, A. Measuring anxiety in zebrafish: A critical review. Behav. Brain Res. 2010, 214, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Dahlbom, S.J.; Backström, T.; Lundstedt-Enkel, K.; Winberg, S. Aggression and monoamines: Effects of sex and social rank in zebrafish (Danio rerio). Behav. Brain Res. 2012, 228, 333–338. [Google Scholar] [CrossRef]
- Riecher-Rössler, A. Sex and gender differences in mental disorders. Lancet Psychiatry 2017, 4, 8–9. [Google Scholar] [CrossRef]
- Wittbrodt, J.; Shima, A.; Schartl, M. Medaka—A model organism from the far east. Nat. Rev. Genet. 2002, 3, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Herpin, A.; Schartl, M. Molecular mechanisms of sex determination and evolution of the Y-chromosome: Insights from the medakafish (Oryzias latipes). Mol. Cell. Endocrinol. 2009, 306, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Signore, I.A.; Guerrero, N.; Loosli, F.; Colombo, A.; Villalón, A.; Wittbrodt, J.; Concha, M.L. Zebrafish and medaka: Model organisms for a comparative developmental approach of brain asymmetry. Philos. Trans. R. Soc. B Biol. Sci. 2008, 364, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Bajoghli, B.; Aghaallaei, N.; Soroldoni, D.; Czerny, T. The roles of Groucho/Tle in left–right asymmetry and Kupffer’s vesicle organogenesis. Dev. Biol. 2007, 303, 347–361. [Google Scholar] [CrossRef] [PubMed]
| Human Disorder | Zebrafish Test | Observed Behavior/Phenotype | Stage | Rodent Test | Refs |
|---|---|---|---|---|---|
| Anxiety disorder | Novel tank test | Diving and reduced exploration behavior, thigmotaxis, erratic swimming and freezing. | Adult | Open field test, elevated plus maze | [76,77,78,79,80] |
| Anxiety disorder | Open field test | Thigmotaxis, hyperactive swimming, erratic swimming, freezing | Adult | Open field test | [81,82,83] |
| Anxiety disorder | Aversive cue exposure (shadow, predator, alarm substance) | Erratic movements, freezing post aversive cue | Adult | Predator, predator image, odor exposure | [76,84,85,86,87,88] |
| Anxiety disorder | Light–dark box | Avoidance of bright area in adults (scototaxis) and dark area in larval fish | Adult, larva | Light–dark box | [89,90,91,92] |
| Anxiety disorder | Sudden light-to-darkness transition | Increased thigmotaxis | Larva | [93] | |
| Anxiety disorder | Electric shock assay | Increased freezing bouts post single, unconditioned shock | Larva | [94] | |
| Anxiety disorder | Cued fear-conditioning tasks | Reduced flight behavior to conditioned aversive stimulus | Adult, larva | Cued fear-conditioning tasks | [53,95] |
| Anxiety disorder, substance abuse, withdrawal | Shoaling test | Increased shoal density, smaller distance between individual fish, scototaxis, thigmotaxis | Adult | Social behavior test | [96,97,98] |
| Anxiety disorder, schizophrenia | Startle test | Reduced escape response to unexpected and/or aversive cues, impaired prepulse inhibition (PPI) | Adult, larva | Startle test | [99,100,101,102,103] |
| Depression like state | Repeated social defeat in fights | Loss of motivation to fight and win | Adult | Social defeat model | [104] |
| Depression like state | Unpredictable chronic stress (UCS) | Elevated anxiety levels, disturbed social interaction | Adult, larva | Unpredictable chronic mild stress (UCMS) | [105,106,107] |
| Depression like state | Prolonged unpredictable strong chronic stress (PUCS) | Anxiety-like and motor retardation-like behaviors | Adult | Unpredictable chronic stress (UCS) | [108] |
| Depression like state | Behavioral challenge (BC) assays, (e.g., inescapable aversive cue) | Initial escape response followed by reduced overall locomotive activity | Larva | Inescapable shock | [109] |
| Human Disorder | Compound | Observed Behavior | Stages | Refs |
|---|---|---|---|---|
| Anxiety disorder, depression | GABA-ergic: ethanol, chlordiazepoxide, diazepam | Anxiolytic in anxiety paradigms | Adult, larva | [77,79,82,83,116,119,121,122,123,124,125] |
| Serotonergic: buspirone, citalopram, desipramine, fluoxetine, ketamine, LSD, olanzapine | ||||
| Cholinergic: nicotine, scopolamine | ||||
| Histaminergic: α-fluoromethylhistidine | ||||
| Glutamatergic: MK-801 | ||||
| Adenosinergic: caffeine | Anxiogenic in anxiety paradigms | |||
| GABA-ergic: FG-7142 | ||||
| Substance abuse and withdrawal | Alcohol, benzodiazepines, barbiturates, opioids (e.g., cocaine, morphine), psychostimulants (e.g., LSD), nicotine | Erratic behavior, freezing, reduced diving and explorative behavior, shoaling, increased aggressive behavior | Adult, larva | [70,126,127,128] |
| Substance abuse, Fetal alcohol syndrome (FAS) | Alcohol | Hyperlocomotion at lower doses and hypolocomotion at higher doses, longtime changes in social behavior in FAS model | Adult, larva | [129,130,131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bühler, A.; Carl, M. Zebrafish Tools for Deciphering Habenular Network-Linked Mental Disorders. Biomolecules 2021, 11, 324. https://doi.org/10.3390/biom11020324
Bühler A, Carl M. Zebrafish Tools for Deciphering Habenular Network-Linked Mental Disorders. Biomolecules. 2021; 11(2):324. https://doi.org/10.3390/biom11020324
Chicago/Turabian StyleBühler, Anja, and Matthias Carl. 2021. "Zebrafish Tools for Deciphering Habenular Network-Linked Mental Disorders" Biomolecules 11, no. 2: 324. https://doi.org/10.3390/biom11020324
APA StyleBühler, A., & Carl, M. (2021). Zebrafish Tools for Deciphering Habenular Network-Linked Mental Disorders. Biomolecules, 11(2), 324. https://doi.org/10.3390/biom11020324







