Spermine and Spermidine Priming against Botrytis cinerea Modulates ROS Dynamics and Metabolism in Arabidopsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Plant Treatments
2.3. B. cinerea Cultivation and Infection
2.4. Polyamine Extraction and Quantification by GC–MS
2.5. H2O2 Extraction and Quantification
2.6. Antioxidant Enzyme Extraction and Activity Measurements
2.7. Soluble Sugar Extraction and Quantification
2.8. Enzyme Extraction and Nitrate Reductase Activity Measurement
2.9. Amino Acid Extraction and Quantification
2.10. Experimental Setup
2.11. Graphical Preparation and Statistical Analysis
3. Results
3.1. High Concentrations of Polyamines Induce Cell Death in Arabidopsis Source Leaves
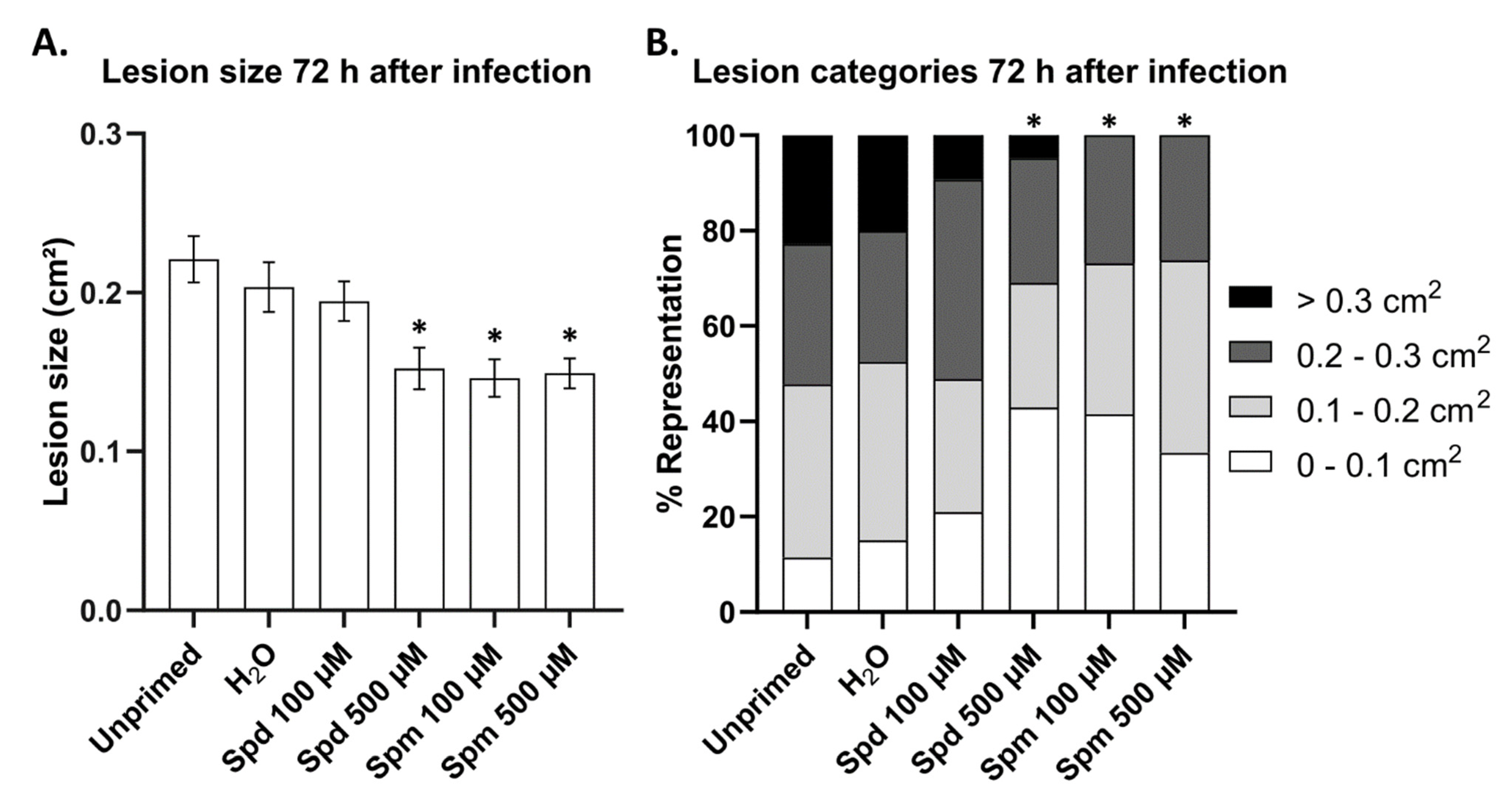
3.2. Polyamines Prime Arabidopsis Resistance Against B. cinerea Infection
3.3. Exogenous Polyamine Treatment Enhanced Endogenous Polyamine Levels
- they can be imported through PA transporters and contribute to endogenous PA levels;
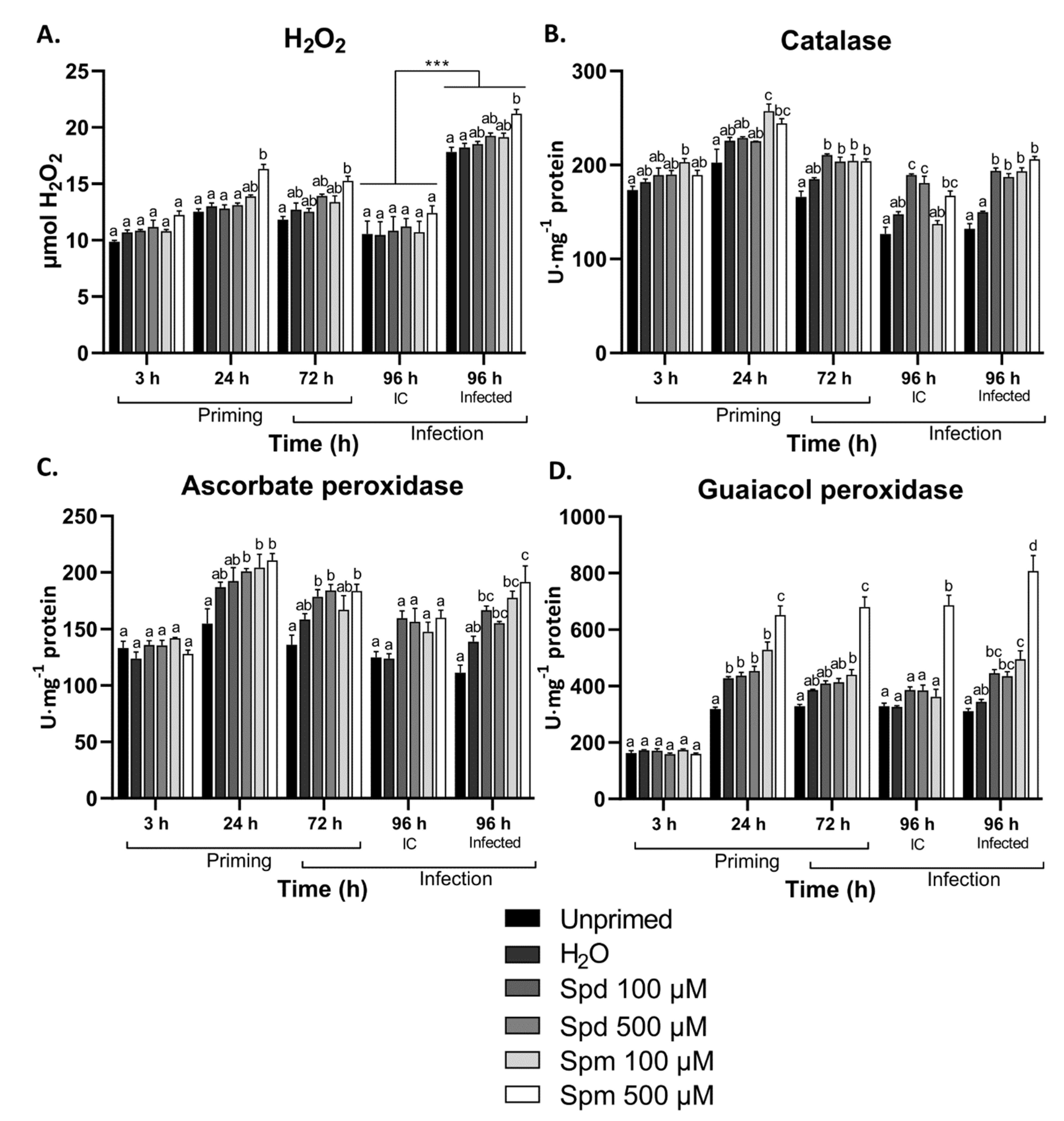
3.4. Exogenous Polyamines Induced H2O2 Scavenging Enzymes in Arabidopsis
3.5. Polyamines Induce Temporal Sugar Accumulation after Treatment and B. cinerea Infection
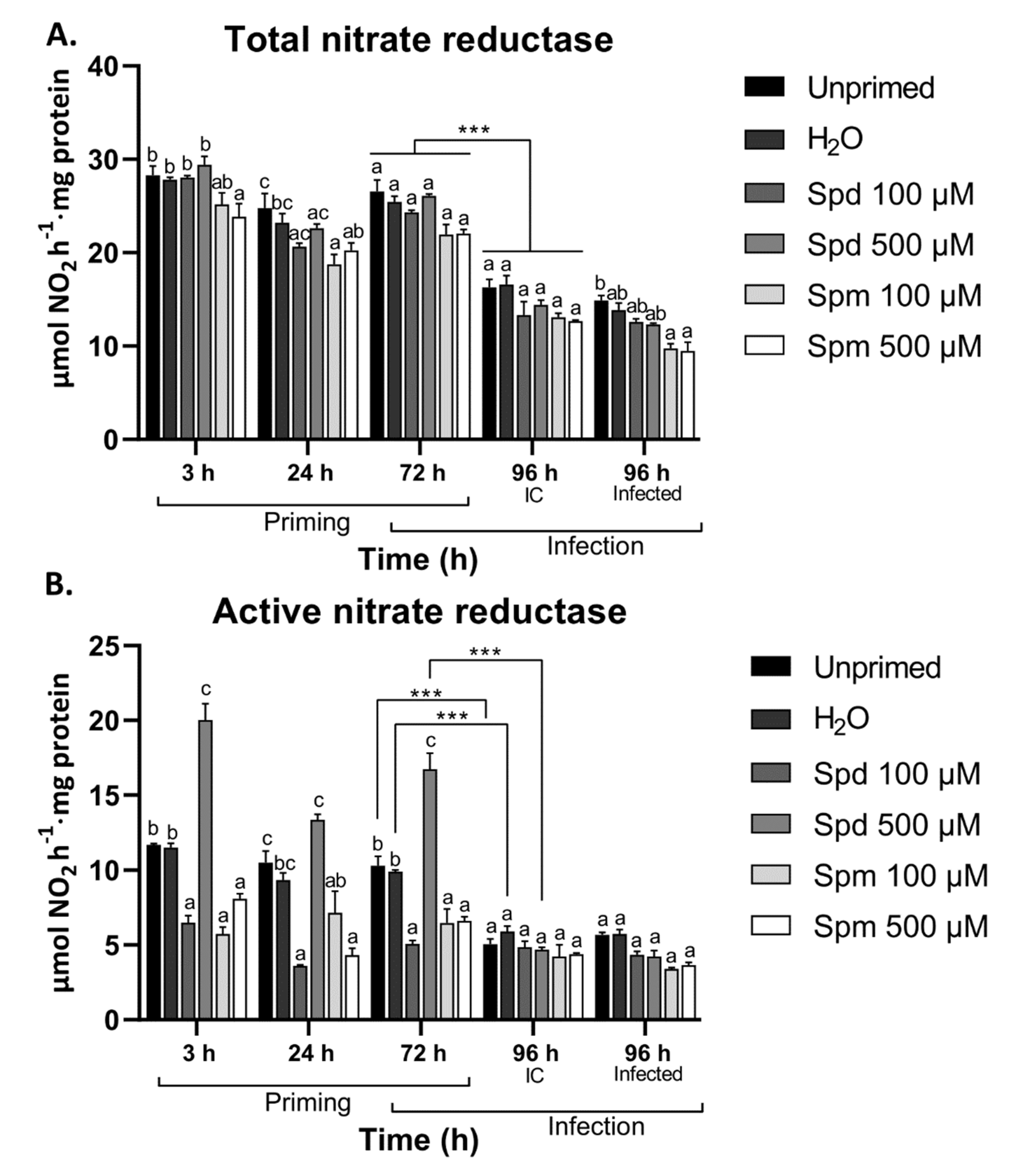
3.6. Polyamines Differentially Modulated Nitrate Reductase Activity in Arabidopsis Leaves
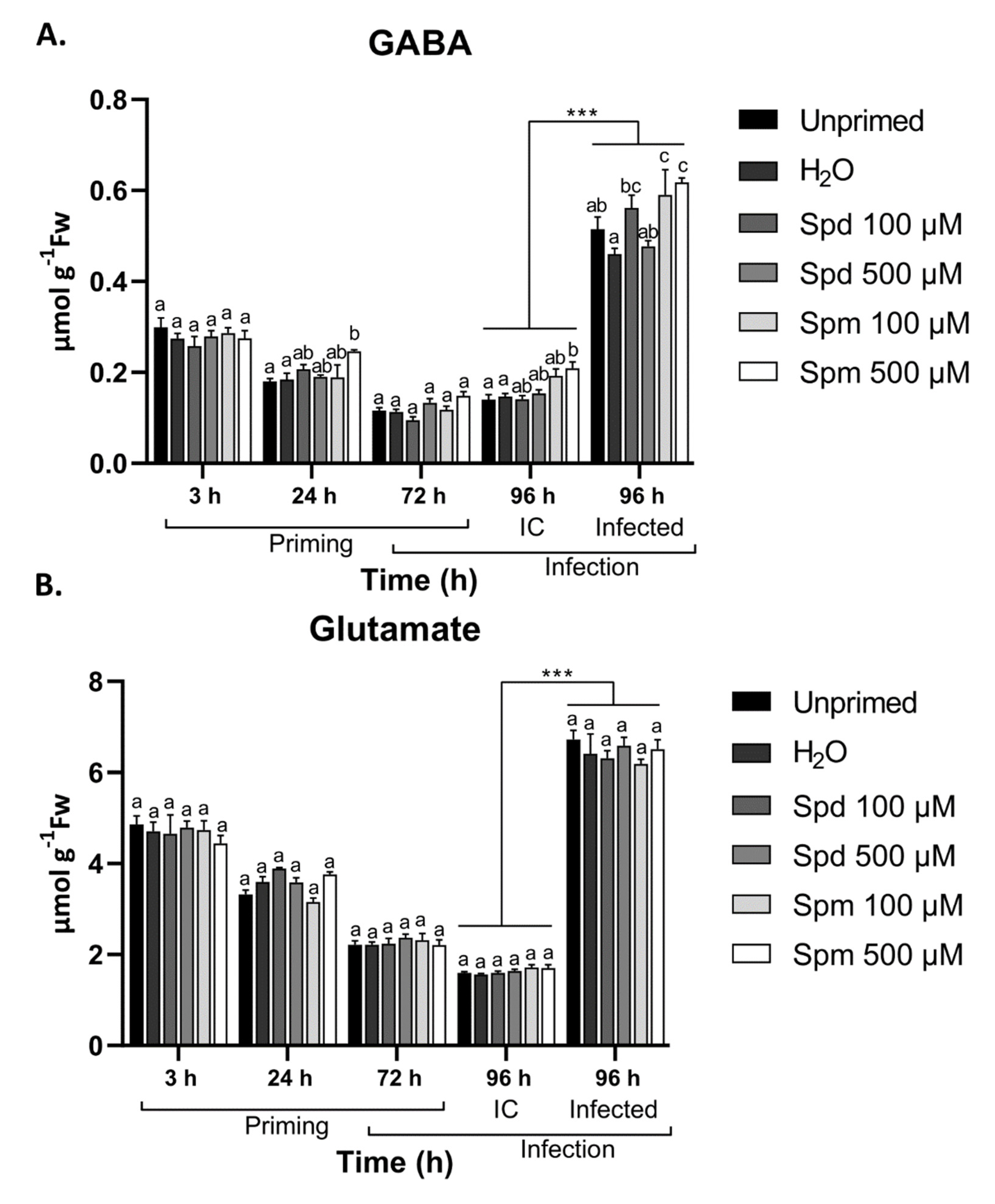
3.7. Exogenous Spm But Not Spd Induce GABA Accumulation after Treatment and B. cinerea Infection
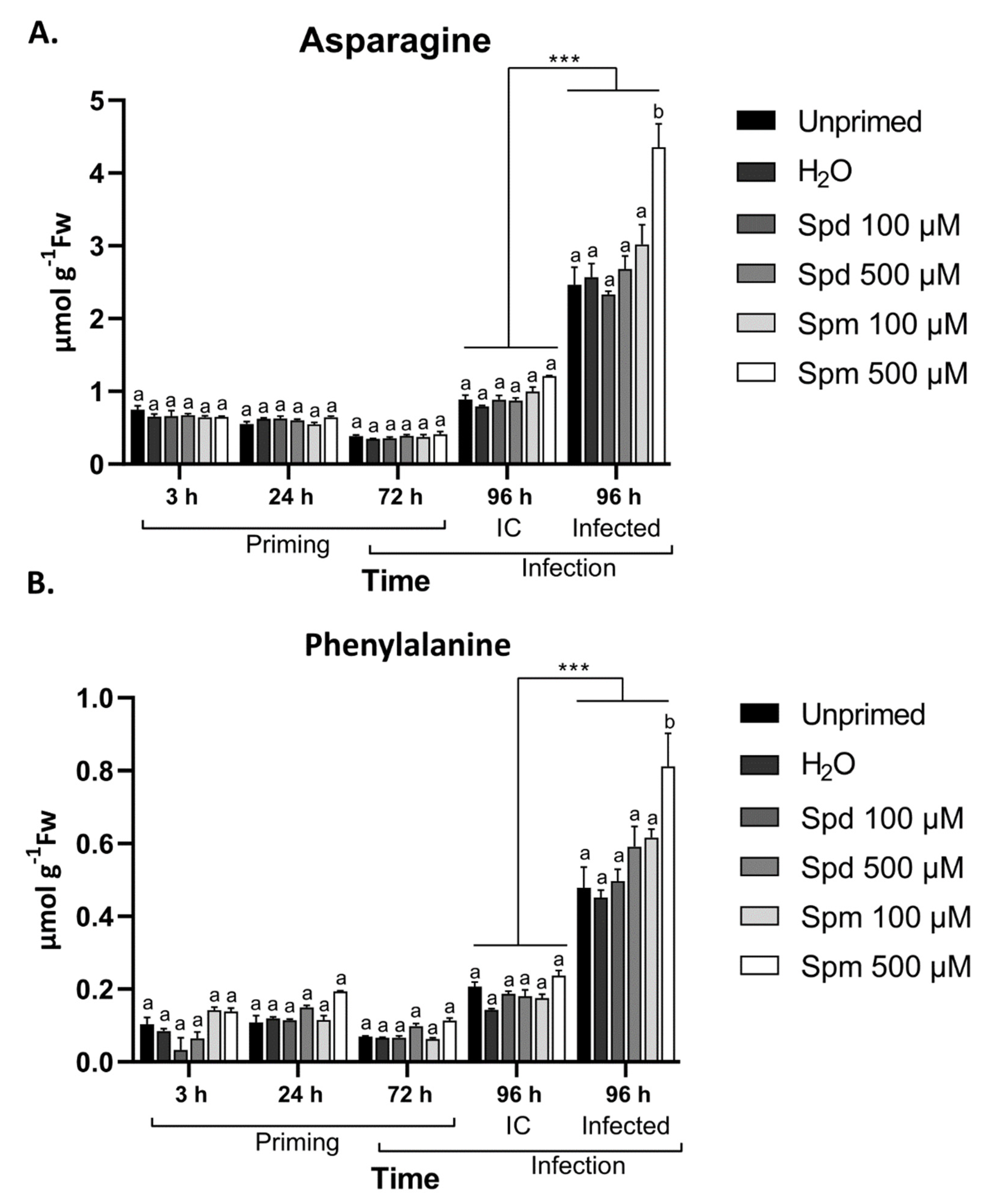
3.8. Defence-Related Amino Acids Accumulate in Spm-Treated Plants after Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, Z.; Jia, D.; Liu, T. Polyamine Oxidases Play Various Roles in Plant Development and Abiotic Stress Tolerance. Plants 2019, 8, 184. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Pandey, S.; Ranade, S.A.; Nagar, P.K.; Kumar, N. Role of polyamines and ethylene as modulators of plant senescence. J. Biosci. 2000, 25, 291–299. [Google Scholar] [CrossRef]
- Seifi, H.S.; Shelp, B.J. Spermine differentially refines plant defense responses against biotic and abiotic stresses. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Romero, F.M.; Maiale, S.J.; Rossi, F.R.; Marina, M.; Ruíz, O.A.; Gárriz, A. Polyamine metabolism responses to biotic and abiotic stress. Methods Mol. Biol. 2018, 1694, 37–49. [Google Scholar]
- Tsaniklidis, G.; Pappi, P.; Tsafouros, A.; Charova, S.N.; Nikoloudakis, N.; Roussos, P.A.; Paschalidis, K.A.; Delis, C. Polyamine homeostasis in tomato biotic/abiotic stress cross-tolerance. Gene 2020, 727, 144230. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Kitashiba, H.; Wang, J.; Ban, Y.; Moriguchi, T. Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechnol. 2007, 24, 117–126. [Google Scholar] [CrossRef]
- Rodríguez-Kessler, M.; Ruiz, O.A.; Maiale, S.; Ruiz-Herrera, J.; Jiménez-Bremont, J.F. Polyamine metabolism in maize tumors induced by Ustilago maydis. Plant Physiol. Biochem. 2008, 46, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Jasso-Robles, F.I.; Jiménez-Bremont, J.F.; Becerra-Flora, A.; Juárez-Montiel, M.; Gonzalez, M.E.; Pieckenstain, F.L.; García de la Cruz, R.F.; Rodríguez-Kessler, M. Inhibition of polyamine oxidase activity affects tumor development during the maize-Ustilago maydis interaction. Plant Physiol. Biochem. 2016, 102, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, R.; Minocha, R.; Lebar, M.D.; Rajasekaran, K.; Long, S.; Carter-Wientjes, C.; Minocha, S.; Cary, J.W. Contribution of maize polyamine and amino acid metabolism toward resistance against Aspergillus flavus infection and aflatoxin production. Front. Plant Sci. 2019, 10, 692. [Google Scholar] [CrossRef]
- Walters, D.R. Polyamines and plant disease. Phytochemistry 2003, 64, 97–107. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, B.S.; Hwang, B.K. Pepper arginine decarboxylase is required for polyamine and γ-aminobutyric acid signaling in cell death and defense response. Plant Physiol. 2013, 162, 2067–2083. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Bremont, J.F.; Marina, M.; de la Luz Guerrero-González, M.; Rossi, F.R.; Sánchez-Rangel, D.; Rodríguez-Kessler, M.; Ruiz, O.A.; Gárriz, A. Physiological and molecular implications of plant polyamine metabolism during biotic interactions. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Cowley, T.; Walters, D.R. Polyamine metabolism in barley reacting hypersensitively to the powdery mildew fungus Blumeria graminis f. sp. hordei. Plant Cell Environ. 2002, 25, 461–468. [Google Scholar] [CrossRef]
- Broetto, F.; Marchese, J.; Leonardo, M.; Regina, M. Fungal elicitor-mediated changes in polyamine content, phenylalanine ammonia-lyase and peroxidase activities in bean cell culture. Gen. Appl. Plant Physiol. 2005, 31, 235–246. [Google Scholar]
- Marina, M.; Maiale, S.J.; Rossi, F.R.; Romero, M.F.; Rivas, E.I.; Gárriz, A.; Ruiz, O.A.; Pieckenstain, F.L. Apoplastic polyamine oxidation plays different roles in local responses of tobacco to infection by the necrotrophic fungus Sclerotinia sclerotiorum and the biotrophic bacterium Pseudomonas viridiflava. Plant Physiol. 2008, 147, 2164–2178. [Google Scholar] [CrossRef]
- Angelini, R.; Tisi, A.; Rea, G.; Chen, M.M.; Botta, M.; Federico, R.; Cona, A. Involvement of polyamine oxidase in wound healing. Plant Physiol. 2008, 146, 162–177. [Google Scholar] [CrossRef]
- Rea, G.; Metoui, O.; Infantino, A.; Federico, R.; Angelini, R. Copper amine oxidase expression in defense responses to wounding and Ascochyta rabiei invasion. Plant Physiol. 2002, 128, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Nambeesan, S.; AbuQamar, S.; Laluk, K.; Mattoo, A.K.; Mickelbart, M.V.; Ferruzzi, M.G.; Mengiste, T.; Handa, A.K. Polyamines attenuate ethylene-mediated defense responses to abrogate resistance to Botrytis cinerea in tomato. Plant Physiol. 2012, 158, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Seifi, H.S.; Zarei, A.; Hsiang, T.; Shelp, B.J. Spermine is a potent plant defense activator against gray mold disease on Solanum lycopersicum, Phaseolus vulgaris, and Arabidopsis thaliana. Phytopathology 2019, 109, 1367–1377. [Google Scholar] [CrossRef]
- Sagor, G.H.M.; Cong, R.Z.; Berberich, T.; Takahashi, H.; Takahashi, Y.; Kusano, T. Spermine signaling in defense reaction against avirulent viral pathogen in Arabidopsis thaliana. Plant Signal. Behav. 2009, 4, 316–318. [Google Scholar] [CrossRef]
- Mitsuya, Y.; Takahashi, Y.; Berberich, T.; Miyazaki, A.; Matsumura, H.; Takahashi, H.; Terauchi, R.; Kusano, T. Spermine signaling plays a significant role in the defense response of Arabidopsis thaliana to cucumber mosaic virus. J. Plant Physiol. 2009, 166, 626–643. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, A.K.; Sobieszczuk-Nowicka, E. Polyamine as signaling molecules and leaf senescence. In Senescence Signalling and Control in Plants; Elsevier: Amsterdam, The Netherlands, 2018; pp. 125–138. ISBN 9780128131879. [Google Scholar]
- Karatan, E.; Duncan, T.R.; Watnick, P.I. NspS, a predicted polyamine sensor, mediates activation of Vibrio cholerae biofilm formation by norspermidine. J. Bacteriol. 2005, 187, 7434–7443. [Google Scholar] [CrossRef]
- Zarza, X.; Shabala, L.; Fujita, M.; Shabala, S.; Haring, M.A.; Tiburcio, A.F.; Munnik, T. Extracellular spermine triggers a rapid intracellular phosphatidic acid response in arabidopsis, involving pldδ activation and stimulating ion flux. Front. Plant Sci. 2019, 10, 601. [Google Scholar] [CrossRef] [PubMed]
- Tarkowski, Ł.P.; Signorelli, S.; Höfte, M. γ-Aminobutyric acid and related amino acids in plant immune responses: Emerging mechanisms of action. Plant Cell Environ. 2020, 43. [Google Scholar] [CrossRef] [PubMed]
- Hatmi, S.; Gruau, C.; Trotel-Aziz, P.; Villaume, S.; Rabenoelina, F.; Baillieul, F.; Eullaffroy, P.; Clément, C.; Ferchichi, A.; Aziz, A. Drought stress tolerance in grapevine involves activation of polyamine oxidation contributing to improved immune response and low susceptibility to Botrytis cinerea. J. Exp. Bot. 2015, 66, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Tajti, J.; Hamow, K.Á.; Majláth, I.; Gierczik, K.; Németh, E.; Janda, T.; Pál, M. Polyamine-induced hormonal changes in Eds5 and Sid2 mutant arabidopsis plants. Int. J. Mol. Sci. 2019, 20, 5746. [Google Scholar] [CrossRef]
- Janse van Rensburg, H.C.; Van den Ende, W. Priming with γ-aminobutyric acid against Botrytis cinerea reshuffles metabolism and reactive oxygen species: Dissecting signalling and metabolism. Antioxidants 2020, 9, 1174. [Google Scholar] [CrossRef]
- El Amrani, A.; Couée, I.; Berthomé, R.; Ramel, F.; Gouesbet, G.; Sulmon, C. Involvement of polyamines in sucrose-induced tolerance to atrazine-mediated chemical stress in Arabidopsis thaliana. J. Plant Physiol. 2019, 238, 1–11. [Google Scholar] [CrossRef]
- Ren, M.; Venglat, P.; Qiu, S.; Feng, L.; Cao, Y.; Wang, E.; Xiang, D.; Wang, J.; Alexander, D.; Chalivendra, S.; et al. Target of rapamycin signaling regulates metabolism, growth, and life Span in Arabidopsis. Plant Cell 2012, 24, 4850–4874. [Google Scholar] [CrossRef]
- Mattoo, A.K.; Sobolev, A.P.; Neelam, A.; Goyal, R.K.; Handa, A.K.; Segre, A.L. Nuclear magnetic resonance spectroscopy-based metabolite profiling of transgenic tomato fruit engineered to accumulate spermidine and spermine reveals enhanced anabolic and nitrogen-carbon interactions. Plant Physiol. 2006, 142, 1759–1770. [Google Scholar] [CrossRef]
- Handa, A.K.; Mattoo, A.K. Differential and functional interactions emphasize the multiple roles of polyamines in plants. Plant Physiol. Biochem. 2010, 48, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Athwal, G.S.; Huber, S.C. Divalent cations and polyamines bind to loop 8 of 14-3-3 proteins, modulating their interaction with phosphorylated nitrate reductase. Plant J. 2002, 29, 119–129. [Google Scholar] [CrossRef]
- Rosales, E.P.; Iannone, M.F.; Groppa, M.D.; Benavides, M.P. Polyamines modulate nitrate reductase activity in wheat leaves: Involvement of nitric oxide. Amino Acids 2012, 42, 857–865. [Google Scholar] [CrossRef]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant Sci. 2017, 22, 163–174. [Google Scholar] [CrossRef]
- Mur, L.A.J.; Simpson, C.; Kumari, A.; Gupta, A.K.; Gupta, K.J. Moving nitrogen to the centre of plant defence against pathogens. Ann. Bot. 2017, 119, 703–709. [Google Scholar] [CrossRef]
- De Cremer, K.; Mathys, J.; Vos, C.; Froenicke, L.; Michelmore, R.W.; Cammue, B.P.A.; De Coninck, B. RNAseq-based transcriptome analysis of Lactuca sativa infected by the fungal necrotroph Botrytis cinerea. Plant Cell Environ. 2013, 36, 1992–2007. [Google Scholar] [CrossRef] [PubMed]
- Janse van Rensburg, H.C.; Takács, Z.; Freynschlag, F.; Toksoy Öner, E.; Jonak, C.; Van den Ende, W. Fructans Prime ROS Dynamics and Botrytis cinerea Resistance in Arabidopsis. Antioxidants 2020, 9, 805. [Google Scholar] [CrossRef]
- Cheeseman, J.M. Hydrogen peroxide concentrations in leaves under natural conditions. J. Exp. Bot. 2006, 57, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhao, X.; Wu, J.; Hu, M.; Xia, S. The Benefits of Exogenous NO: Enhancing Arabidopsis to Resist Botrytis cinerea. Am. J. Plant Sci. 2011, 2, 511–519. [Google Scholar] [CrossRef]
- Gil-ad, N.L.; Bar-Nun, N.; Noy, T.; Mayer, A.M. Enzymes of Botrytis cinerea capable of breaking down hydrogen peroxide. FEMS Microbiol. Lett. 2000, 190, 121–126. [Google Scholar] [CrossRef][Green Version]
- Jiménez, A.; Hernández, J.A.; Del Río, L.A.; Sevilla, F. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 1997, 114, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta-Mancera, H.A.; López-Delgado, H.; Loza-Tavera, H.; Mora-Herrera, M.; Trevilla-García, C.; Vargas-Suárez, M.; Ougham, H. Cytokinin promotes catalase and ascorbate peroxidase activities and preserves the chloroplast integrity during dark-senescence. J. Plant Physiol. 2007, 164, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Małolepsza, U.; Urbanek, H. The oxidants and antioxidant enzymes in tomato leaves treated with o-hydroxyethylorutin and infected with Botrytis cinerea. Eur. J. Plant Pathol. 2000, 106, 657–665. [Google Scholar] [CrossRef]
- Svara, A.; Tarkowski, Ł.P.; Janse van Rensburg, H.C.; Deleye, E.; Vaerten, J.; De Storme, N.; Keulemans, W.; Van den Ende, W. Sweet Immunity: The Effect of Exogenous Fructans on the Susceptibility of Apple (Malus × domestica Borkh.) to Venturia inaequalis. Int. J. Mol. Sci. 2020, 21, 5885. [Google Scholar] [CrossRef] [PubMed]
- Scheible, W.R.; Lauerer, M.; Schulze, E.D.; Caboche, M.; Stitt, M. Accumulation of nitrate in the shoot acts as a signal to regulate shoot-root allocation in tobacco. Plant J. 1997, 11, 671–691. [Google Scholar] [CrossRef]
- Yoda, H.; Yamaguchi, Y.; Sano, H. Induction of hypersensitive cell death by hydrogen peroxide produced through polyamine degradation in tobacco plants. Plant Physiol. 2003, 132, 1973–1981. [Google Scholar] [CrossRef]
- Park, J.; Gu, Y.; Lee, Y.; Yang, Z.; Lee, Y. Phosphatidic Acid Induces Leaf Cell Death in Arabidopsis by Activating the Rho-Related Small G Protein GTPase-Mediated Pathway of Reactive Oxygen Species Generation. Plant Physiol. 2004, 134, 129–136. [Google Scholar] [CrossRef]
- Pastor, V.; Luna, E.; Ton, J.; Cerezo, M.; García-Agustín, P.; Flors, V. Fine tuning of reactive oxygen species homeostasis regulates primed immune responses in Arabidopsis. Mol. Plant-Microbe Interact. 2013, 26, 1334–1344. [Google Scholar] [CrossRef]
- Yoshioka, H.; Bouteau, F.; Kawano, T. Discovery of oxidative burst in the field of plant immunity: Looking back at the early pioneering works and towards the future development. Plant Signal. Behav. 2008, 3, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Shinozaki, K. Identification of polyamine transporters in plants: Paraquat transport provides crucial clues. Plant Cell Physiol. 2014, 55, 855–861. [Google Scholar] [CrossRef]
- Bolouri Moghaddam, M.R.; Van den Ende, W. Sweet immunity in the plant circadian regulatory network. J. Exp. Bot. 2013, 64, 1439–1449. [Google Scholar] [CrossRef]
- Janse van Rensburg, H.C.; Van den Ende, W.; Signorelli, S. Autophagy in Plants: Both a Puppet and a Puppet Master of Sugars. Front. Plant Sci. 2019, 10, 14. [Google Scholar] [CrossRef]
- Rolland, F.; Sheen, J. Sugar sensing and signalling networks in plants. Biochem. Soc. Trans. 2005, 33, 269–271. [Google Scholar] [CrossRef]
- Paschalidis, K.; Tsaniklidis, G.; Wang, B.Q.; Delis, C.; Trantas, E.; Loulakakis, K.; Makky, M.; Sarris, P.F.; Ververidis, F.; Liu, J.H. The interplay among polyamines and nitrogen in plant stress responses. Plants 2019, 8, 315. [Google Scholar] [CrossRef]
- Ikeda, Y.; Koizumi, N.; Kusano, T.; Sano, H. Specific binding of a 14-3-3 protein to autophosphorylated WPK4, an SNF1-related wheat protein kinase, and to WPK4-phosphorylated nitrate reductase. J. Biol. Chem. 2000, 275, 31695–31700. [Google Scholar] [CrossRef]
- Sugden, C.; Donaghy, P.G.; Halford, N.G.; Hardie, D.G. Two SNF1-Related Protein Kinases from Spinach Leaf Phosphorylate and Inactivate 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase, Nitrate Reductase, and Sucrose Phosphate Synthase in Vitro. Plant Physiol. 2002, 120, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Seifi, H.S.; Curvers, K.; De Vleesschauwer, D.; Delaere, I.; Aziz, A.; Höfte, M. Concurrent overactivation of the cytosolic glutamine synthetase and the GABA shunt in the ABA-deficient sitiens mutant of tomato leads to resistance against Botrytis cinerea. New Phytol. 2013, 199, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. γ-Aminobutyric acid (GABA) signalling in plants. Cell. Mol. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef]
- Zeier, J. New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Environ. 2013, 36, 2085–2103. [Google Scholar] [CrossRef]
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Bio-Molecules with Diverse Functions in Plant and Human Health and Disease. Front. Chem. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Betti, L.; Scaramagli, S.; Biondi, S.; Torrigiani, P. Polyamine metabolism is upregulated in response to tobacco mosaic virus in hypersensitive, but not in susceptible, tobacco. New Phytol. 2001, 149, 301–309. [Google Scholar] [CrossRef]
- Yoda, H.; Hiroi, Y.; Sano, H. Polyamine oxidase is one of the key elements for oxidative burst to induce programmed cell death in tobacco cultured cells. Plant Physiol. 2006, 142, 193–206. [Google Scholar] [CrossRef]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006, 11, 80–88. [Google Scholar] [CrossRef]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Gupta, K.; Sengupta, A.; Chakraborty, M.; Gupta, B. Hydrogen peroxide and polyamines act as double edged swords in plant abiotic stress responses. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Morkunas, I.; Ratajczak, L. The role of sugar signaling in plant defense responses against fungal pathogens. Acta Physiol. Plant 2014, 36, 1607–1619. [Google Scholar] [CrossRef]
- Bolouri-Moghaddam, M.R.; Le Roy, K.; Xiang, L.; Rolland, F.; Van den Ende, W. Sugar signalling and antioxidant network connections in plant cells. FEBS J. 2010, 277, 2022–2037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Primavesi, L.F.; Jhurreea, D.; Andralojc, P.J.; Mitchell, R.A.C.; Powers, S.J.; Schluepmann, H.; Delatte, T.; Wingler, A.; Paul, M.J. Inhibition of SNF1-related protein kinasel activity and regulation of metabolic pathways by trehalose-6-phosphate1. Plant Physiol. 2009, 149, 1860–1871. [Google Scholar] [CrossRef]
- Hulsmans, S.; Rodriguez, M.; De Coninck, B.; Rolland, F. The SnRK1 Energy Sensor in Plant Biotic Interactions. Trends Plant Sci. 2016, 21, 648–661. [Google Scholar] [CrossRef]
- Bachmann, M.; McMichael, R.W.; Huber, J.L.; Kaiser, W.M.; Huber, S.C. Partial Purification and Characterization of a Calcium-Dependent Protein Kinase and an Inhibitor Protein Required for Inactivation of Spinach Leaf Nitrate Reductase. Plant Physiol. 1995, 108, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Mungre, S.M. The inhibition of nitrate reductase induction by spermine in excised radish cotyledons. Phytochemistry 1986, 25, 1563–1565. [Google Scholar] [CrossRef]
- Rojas, C.M.; Senthil-Kumar, M.; Tzin, V.; Mysore, K.S. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front. Plant Sci. 2014, 5, 17. [Google Scholar] [CrossRef]
- Häusler, R.E.; Ludewig, F.; Krueger, S. Amino acids—A life between metabolism and signaling. Plant Sci. 2014, 229, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Jin, L.; Cai, Y.; Huang, Y.; Zheng, X.; Yu, T. L-Glutamate treatment enhances disease resistance of tomato fruit by inducing the expression of glutamate receptors and the accumulation of amino acids. Food Chem. 2019, 293, 263–270. [Google Scholar] [CrossRef]
- Pottosin, I.; Velarde-Buendía, A.M.; Bose, J.; Fuglsang, A.T.; Shabala, S. Polyamines cause plasma membrane depolarization, activate Ca2+, and modulate H-ATPase pump activity in pea roots. J. Exp. Bot. 2014, 65, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Michaeli, S.; Fromm, H. Closing the loop on the GABA shunt in plants: Are GABA metabolism and signaling entwined? Front. Plant Sci. 2015, 6, 419. [Google Scholar] [CrossRef]
- Oliva, M.; Hatan, E.; Kumar, V.; Galsurker, O.; Nisim-Levi, A.; Ovadia, R.; Galili, G.; Lewinsohn, E.; Elad, Y.; Alkan, N.; et al. Increased phenylalanine levels in plant leaves reduces susceptibility to Botrytis cinerea. Plant Sci. 2020, 290, 110289. [Google Scholar] [CrossRef]
- Kumar, V.; Hatan, E.; Bar, E.; Davidovich-Rikanati, R.; Doron-Faigenboim, A.; Spitzer-Rimon, B.; Elad, Y.; Alkan, N.; Lewinsohn, E.; Oren-Shamir, M. Phenylalanine increases chrysanthemum flower immunity against Botrytis cinerea attack. Plant J. 2020, 104, 226–240. [Google Scholar] [CrossRef]
- Seifi, H.S.; De Vleesschauwer, D.; Aziz, A.; Höfte, M. Modulating plant primary amino acid metabolism as a necrotrophic virulence strategy: The immune-regulatory role of asparagine synthetase in Botrytis cinerea-tomato interaction. Plant Signal. Behav. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Devaux, C.; Baldet, P.; Joubès, J.; Dieuaide-Noubhani, M.; Just, D.; Chevalier, C.; Raymond, P. Physiological, biochemical and molecular analysis of sugar-starvation responses in tomato roots. J. Exp. Bot. 2003, 54, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Zarza, X.; Van Wijk, R.; Shabala, L.; Hunkeler, A.; Lefebvre, M.; Rodriguez-Villalón, A.; Shabala, S.; Tiburcio, A.F.; Heilmann, I.; Munnik, T. Lipid kinases PIP5K7 and PIP5K9 are required for polyamine-triggered K+ efflux in Arabidopsis roots. Plant J. 2020, 104, 416–432. [Google Scholar] [CrossRef] [PubMed]
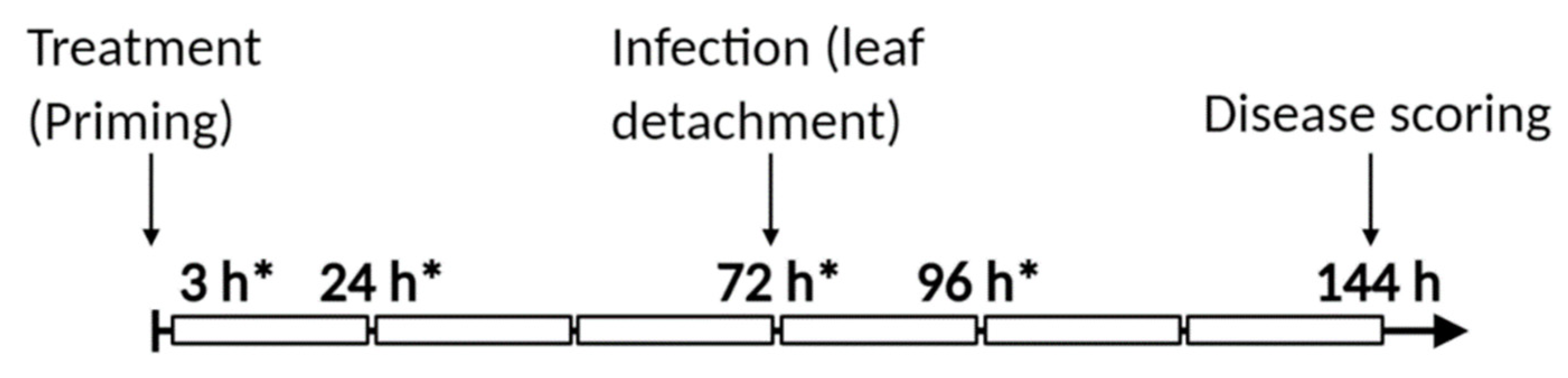


| Treatment | 48 h | 96 h | 120 h |
|---|---|---|---|
| Control | - | - | - |
| Spermidine 100 µM | - | - | - |
| Spermidine 500 µM | - | - | - |
| Spermidine 1 mM | - | 2.75 ± 0.57 | 4.5 ± 0.57 |
| Spermine 100 µM | - | - | - |
| Spermine 500 µM | - | - | - |
| Spermine 1 mM | 3 ± 0.70 | 5.2 ± 0.47 | 6.8 ± 1.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janse van Rensburg, H.C.; Limami, A.M.; Van den Ende, W. Spermine and Spermidine Priming against Botrytis cinerea Modulates ROS Dynamics and Metabolism in Arabidopsis. Biomolecules 2021, 11, 223. https://doi.org/10.3390/biom11020223
Janse van Rensburg HC, Limami AM, Van den Ende W. Spermine and Spermidine Priming against Botrytis cinerea Modulates ROS Dynamics and Metabolism in Arabidopsis. Biomolecules. 2021; 11(2):223. https://doi.org/10.3390/biom11020223
Chicago/Turabian StyleJanse van Rensburg, Henry Christopher, Anis M. Limami, and Wim Van den Ende. 2021. "Spermine and Spermidine Priming against Botrytis cinerea Modulates ROS Dynamics and Metabolism in Arabidopsis" Biomolecules 11, no. 2: 223. https://doi.org/10.3390/biom11020223
APA StyleJanse van Rensburg, H. C., Limami, A. M., & Van den Ende, W. (2021). Spermine and Spermidine Priming against Botrytis cinerea Modulates ROS Dynamics and Metabolism in Arabidopsis. Biomolecules, 11(2), 223. https://doi.org/10.3390/biom11020223








