Diethylstilbestrol Modifies the Structure of Model Membranes and Is Localized Close to the First Carbons of the Fatty Acyl Chains
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Differential Scanning Calorimetry (DSC)
2.4. X-Ray Diffraction
2.5. 31P-NMR
2.6. 1H-NOESY-MAS-NMR
2.7. Molecular Dynamics Simulations
3. Results
3.1. DSC Study of the Interaction of DES with DMPC
3.2. DSC Study of the Interaction of DES with DEPE
3.3. X-Ray Diffraction
3.4. Changes in Phase of DEPE as Observed by SAXD and 31P-NMR
3.5. 1H-NOESY-MAS NMR
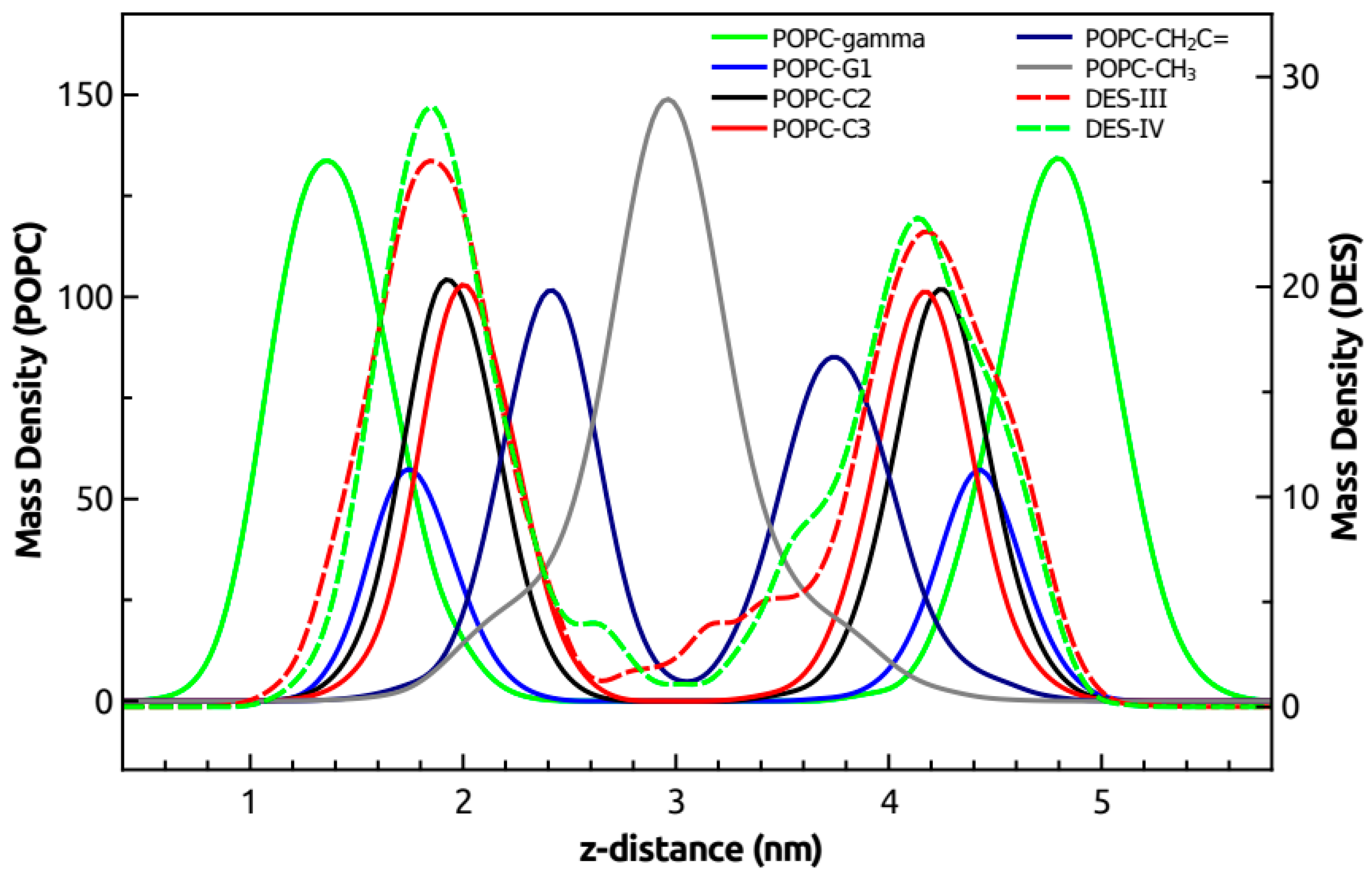
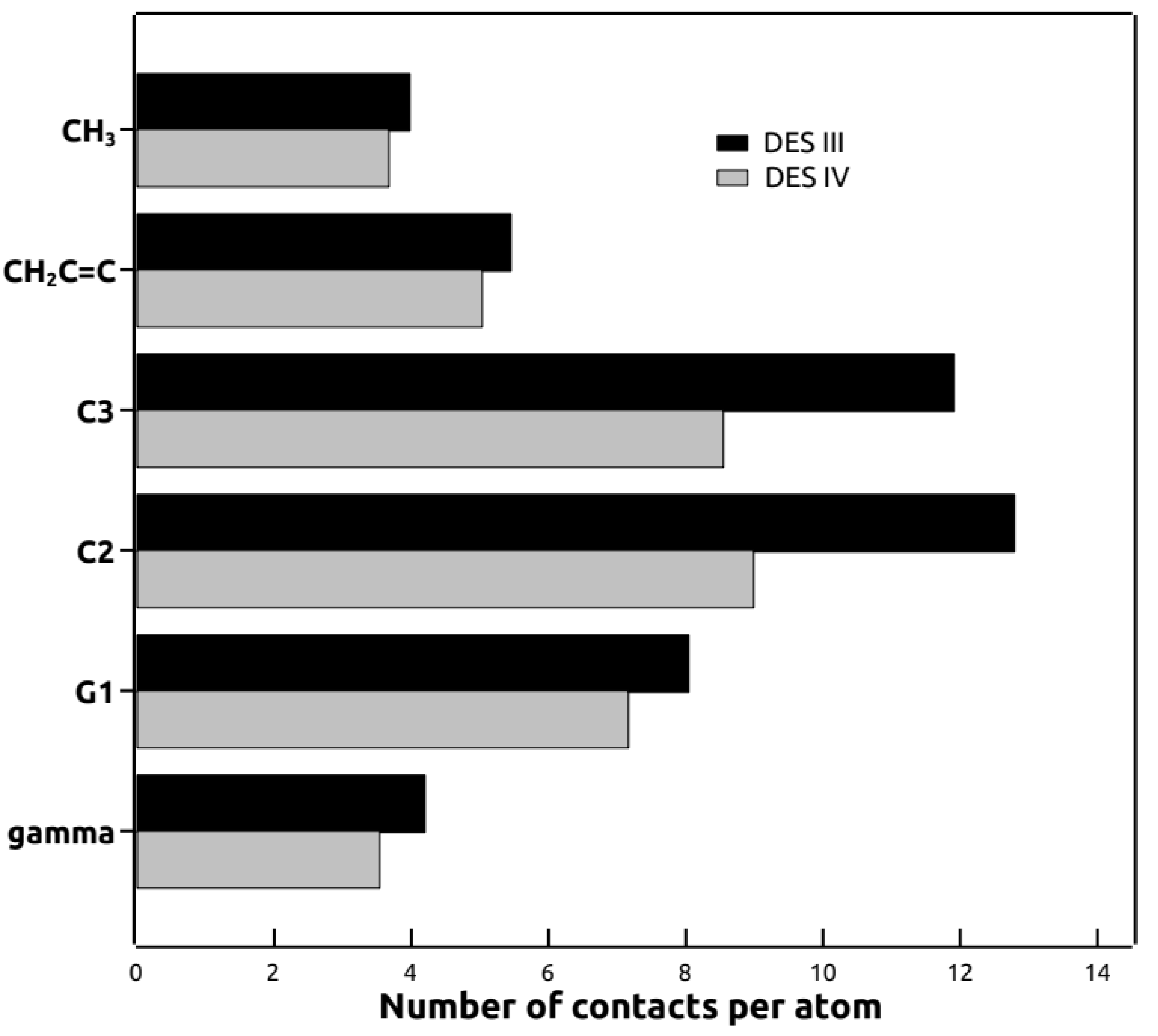
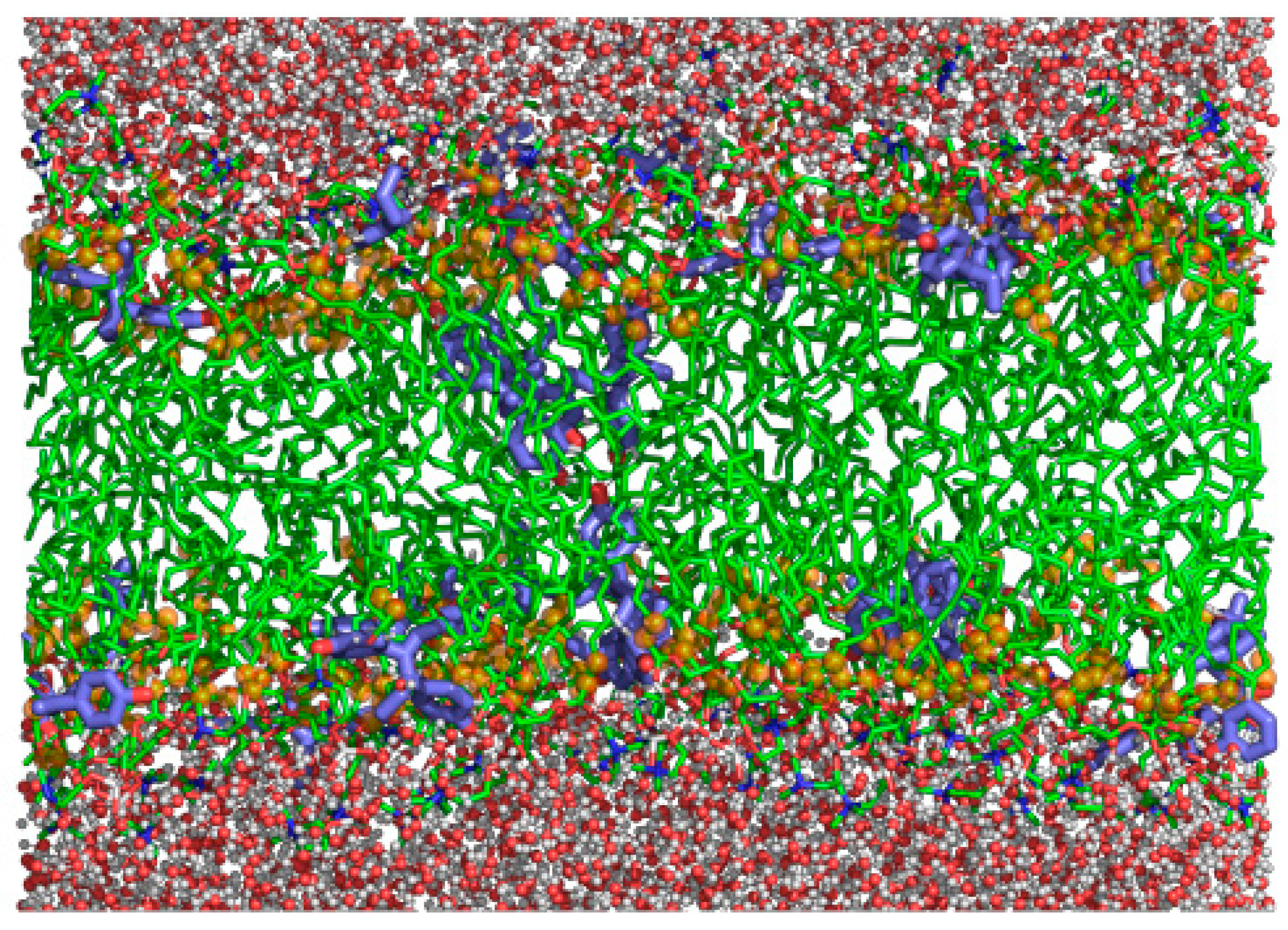
3.6. Molecular Dynamics Simulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tournaire, M.; Epelboin, S.; Devouche, E.; Viot, G.; Le Bidois, J.; Cabau, A.; Dunbavand, A.; Levadou, A. Adverse health effects in children of women exposed in utero to diethylstilbestrol (DES). Therapie 2016, 71, 395–404. [Google Scholar] [CrossRef]
- Al Jishi, T.; Sergi, C. Current perspective of diethylstilbestrol (DES) exposure in mothers and offspring. Reprod. Toxicol 2017, 71, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.L. Diethylstilbestrol: Potential health risks for women exposed in utero and their offspring. Jaapa Off. J. Am. Acad. Physician Assist. 2017, 30, 49–52. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Savastano, S.; Colao, A. Obesogenic endocrine disruptors and obesity: Myths and truths. Arch. Toxicol. 2017, 91, 3469–3475. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.L.; Carafoli, E. Ion motive ATPases. I. Ubiquity, properties, and significance to cell function. Trends Biochem. Sci. 1987, 12, 146–150. [Google Scholar] [CrossRef]
- McEnery, M.W.; Pedersen, P.L. Diethylstilbestrol. A novel F0-directed probe of the mitochondrial proton ATPase. J. Biol. Chem. 1986, 261, 1745–1752. [Google Scholar] [CrossRef]
- McEnery, M.W.; Hullihen, J.; Pedersen, P.L. F0 “proton channel” of rat liver mitochondria. Rapid purification of a functional complex and a study of its interaction with the unique probe diethylstilbestrol. J. Biol. Chem. 1989, 264, 12029–12036. [Google Scholar] [CrossRef]
- Martinez-Azorin, F.; Teruel, J.A.; Fernandez-Belda, F.; Gomez-Fernandez, J.C. Effect of diethylstilbestrol and related compounds on the Ca(2+)-transporting ATPase of sarcoplasmic reticulum. J. Biol. Chem. 1992, 267, 11923–11929. [Google Scholar] [CrossRef]
- Nanjappa, M.K.; Medrano, T.I.; Mesa, A.M.; Ortega, M.T.; Caldo, P.D.; Mao, J.; Kinkade, J.A.; Levin, E.R.; Rosenfeld, C.S.; Cooke, P.S. Mice lacking membrane estrogen receptor 1 are protected from reproductive pathologies resulting from developmental estrogen exposuredagger. Biol. Reprod. 2019, 101, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, C.J.F.; Van Gent, C.M.; Pries, C. A rapid and sensitive sub-micro phosphorus determination. Anal. Chim. Acta. 1961, 24, 203–204. [Google Scholar] [CrossRef]
- Pabst, G.; Rappolt, M.; Amenitsch, H.; Laggner, P. Structural information from multilamellar liposomes at full hydration: Full q-range fitting with high quality x-ray data. Phys. Rev. E 2000, 62, 4000–4009. [Google Scholar] [CrossRef] [PubMed]
- Pabst, G.; Koschuch, R.; Pozo-Navas, B.; Rappolt, M.; Lohner, K.; Laggner, P. Structural analysis of weakly ordered membrane stacks. Appl. Cryst. 2003, 36, 1378–1388. [Google Scholar] [CrossRef]
- Pabst, G.B.R.L. Global properties of biomimetic membranes: Perspectives on molecular features. Biophys. Rev. Lett. 2006, 1, 57–84. [Google Scholar] [CrossRef]
- Huster, D.; Arnold, K.; Gawrisch, K. Investigation of Lipid Organization in Biological Membranes by Two-Dimensional Nuclear Overhauser Enhancement Spectroscopy. J. Phys. Chem. B 1999, 103, 243–251. [Google Scholar] [CrossRef]
- Siarheyeva, A.; Lopez, J.J.; Glaubitz, C. Localization of multidrug transporter substrates within model membranes. Biochemistry 2006, 45, 6203–6211. [Google Scholar] [CrossRef] [PubMed]
- Holte, L.L.; Gawrisch, K. Determining ethanol distribution in phospholipid multilayers with MAS-NOESY spectra. Biochemistry 1997, 36, 4669–4674. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic. Acids. Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.; Hess, B.D.; Van Der Spoel, D.; Lindahl, E. GROMACS User Manual Version 5.0.7. 2015. Available online: www.Gromacs.Org (accessed on 19 September 2020).
- Oostenbrink, C.; Soares, T.A.; van der Vegt, N.F.; van Gunsteren, W.F. Validation of the 53A6 GROMOS force field. Eur. Biophys. J. 2005, 34, 273–284. [Google Scholar] [CrossRef]
- Oostenbrink, C.; Villa, A.; Mark, A.E.; van Gunsteren, W.F. A biomolecular force field based on the free enthalpy of hydration and solvation: The GROMOS force-field parameter sets 53A5 and 53A6. J. Comput. Chem. 2004, 25, 1656–1676. [Google Scholar] [CrossRef]
- Poger, D.; Mark, A.E. On the Validation of Molecular Dynamics Simulations of Saturated and cis-Monounsaturated Phosphatidylcholine Lipid Bilayers: A Comparison with Experiment. J. Chem. Theory. Comput. 2010, 6, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Poger, D.; Van Gunsteren, W.F.; Mark, A.E. A new force field for simulating phosphatidylcholine bilayers. J. Comput. Chem. 2010, 31, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Koziara, K.B.; Stroet, M.; Malde, A.K.; Mark, A.E. Testing and validation of the Automated Topology Builder (ATB) version 2.0: Prediction of hydration free enthalpies. J. Comput. -Aided Mol. Des. 2014, 28, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An Automated Force Field Topology Builder (ATB) and Repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef]
- Martinez, L.; Andrade, R.; Birgin, E.G.; Martinez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular Dynamics with Coupling to an External Bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle Mesh Ewald: An N•log(N) Method for Ewald Sums in Large Systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A Linear Constraint Solver for Molecular Simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic Transitions in Single Crystals: A New Molecular Dynamics Method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Epand, R.M.; Lester, D.S. The role of membrane biophysical properties in the regulation of protein kinase C activity. Trends Pharm. Sci. 1990, 11, 317–320. [Google Scholar] [CrossRef]
- Luzzati, V. X-ray diffraction studies of water lipid systems. In Biological Membranes; Chapman, D., Ed.; Academic Press: London, UK, 1968; pp. 71–123. [Google Scholar]
- Rand, R.P.; Tinker, D.O.; Fast, P.G. Polymorphism of phosphatidylethanolamines from two natural sources. Chem. Phys. Lipids 1971, 6, 333–342. [Google Scholar] [CrossRef]
- Janiak, M.J.; Small, D.M.; Shipley, G.G. Nature of the Thermal pretransition of synthetic phospholipids: Dimyristolyl- and dipalmitoyllecithin. Biochemistry 1976, 15, 4575–4580. [Google Scholar] [CrossRef] [PubMed]
- Harlos, K.; Eibl, H. Influence of calcium on phosphatidylglycerol. Two separate lamellar structures. Biochemistry 1980, 19, 895–899. [Google Scholar] [CrossRef]
- Tardieu, A.; Luzzati, V.; Reman, F.C. Structure and polymorphism of the hydrocarbon chains of lipids: A study of lecithin-water phases. J. Mol. Biol. 1973, 75, 711–733. [Google Scholar] [CrossRef]
- Cullis, P.R.; de Kruijff, B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim. Biophys. Acta. 1979, 559, 399–420. [Google Scholar] [CrossRef]
- Inamori, Y.; Kubo, M.; Ogawa, M.; Moriwaki, M.; Tsujibo, H.; Baba, K.; Kozawa, M. The biological activities of diethylstilbestrol and its derivatives. Chem. Pharm. Bull. 1985, 33, 4478–4483. [Google Scholar] [CrossRef] [PubMed]
- Ausili, A.; Martinez-Valera, P.; Torrecillas, A.; Gomez-Murcia, V.; de Godos, A.M.; Corbalan-Garcia, S.; Teruel, J.A.; Gomez Fernandez, J.C. Anticancer Agent Edelfosine Exhibits a High Affinity for Cholesterol and Disorganizes Liquid-Ordered Membrane Structures. Langmuir 2018, 34, 8333–8346. [Google Scholar] [CrossRef]
- Oliva, A.; Teruel, J.A.; Aranda, F.J.; Ortiz, A. Effect of a dirhamnolipid biosurfactant on the structure and phase behaviour of dimyristoylphosphatidylserine model membranes. Colloids Surf. B. 2020, 185, 110576. [Google Scholar] [CrossRef]
- Epand, R.M. The relationship between the effects of drugs on bilayer stability and on protein kinase C activity. Chem. Biol. Interact. 1987, 63, 239–247. [Google Scholar] [CrossRef]
- Epand, R.M.; Epand, R.F.; Lancaster, C.R. Modulation of the bilayer to hexagonal phase transition of phosphatidylethanolamines by acylglycerols. Biochim. Biophys. Acta 1988, 945, 161–166. [Google Scholar] [CrossRef]
- Ausili, A.; Clemente, J.; Pons-Belda, O.D.; de Godos, A.; Corbalan-Garcia, S.; Torrecillas, A.; Teruel, J.A.; Gomez-Fernandez, J.C. Interaction of Vitamin K1 and Vitamin K2 with Dimyristoylphosphatidylcholine and Their Location in the Membrane. Langmuir 2020, 36, 1062–1073. [Google Scholar] [CrossRef]
- Ortiz, A.; Aranda, F.J. The influence of vitamin K1 on the structure and phase behaviour of model membrane systems. Biochim. Biophys. Acta. 1999, 1418, 206–220. [Google Scholar] [CrossRef]
- Sanchez-Migallon, M.P.; Aranda, F.J.; Gomez-Fernandez, J.C. Interaction between alpha-tocopherol and heteroacid phosphatidylcholines with different amounts of unsaturation. Biochim. Biophys. Acta 1996, 1279, 251–258. [Google Scholar] [CrossRef]
- Epand, R.M.; Robinson, K.S.; Andrews, M.E.; Epand, R.F. Dependence of the bilayer to hexagonal phase transition on amphiphile chain length. Biochemistry 1989, 28, 9398–9402. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M.; Bryszewska, M. Modulation of the bilayer to hexagonal phase transition and solvation of phosphatidylethanolamines in aqueous salt solutions. Biochemistry 1988, 27, 8776–8779. [Google Scholar] [CrossRef]
- Scheidt, H.A.; Huster, D. The interaction of small molecules with phospholipid membranes studied by 1H NOESY NMR under magic-angle spinning. Acta Pharmacol. Sin. 2008, 29, 35–49. [Google Scholar] [CrossRef]
- Torrecillas, A.; Schneider, M.; Fernandez-Martinez, A.M.; Ausili, A.; de Godos, A.M.; Corbalan-Garcia, S.; Gomez-Fernandez, J.C. Capsaicin Fluidifies the Membrane and Localizes Itself near the Lipid-Water Interface. Acs. Chem. Neurosci. 2015, 6, 1741–1750. [Google Scholar] [CrossRef]
- Ausili, A.; de Godos, A.M.; Torrecillas, A.; Aranda, F.J.; Corbalan-Garcia, S.; Gomez-Fernandez, J.C. The vertical location of alpha-tocopherol in phosphatidylcholine membranes is not altered as a function of the degree of unsaturation of the fatty acyl chains. Phys. Chem. Chem. Phys. 2017, 19, 6731–6742. [Google Scholar] [CrossRef]
- Ausili, A.; Torrecillas, A.; de Godos, A.M.; Corbalan-Garcia, S.; Gomez-Fernandez, J.C. Phenolic Group of alpha-Tocopherol Anchors at the Lipid-Water Interface of Fully Saturated Membranes. Langmuir 2018, 34, 3336–3348. [Google Scholar] [CrossRef] [PubMed]
- Ausili, A.; Gomez-Murcia, V.; Candel, A.M.; Beltran, A.; Torrecillas, A.; He, L.; Jiang, Y.; Zhang, S.; Teruel, J.A.; Gomez-Fernandez, J.C. A comparison of the location in membranes of curcumin and curcumin-derived bivalent compounds with potential neuroprotective capacity for Alzheimer’s disease. Colloids Surf. B Biointerfaces 2020, 199, 111525. [Google Scholar] [CrossRef]
- Vogel, A.; Scheidt, H.A.; Feller, S.E.; Metso, J.; Badeau, R.M.; Tikkanen, M.J.; Wahala, K.; Jauhiainen, M.; Huster, D. The orientation and dynamics of estradiol and estradiol oleate in lipid membranes and HDL disc models. Biophys. J. 2014, 107, 114–125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Biruss, B.; Dietl, R.; Valenta, C. The influence of selected steroid hormones on the physicochemical behaviour of DPPC liposomes. Chem. Phys. Lipids 2007, 148, 84–90. [Google Scholar] [CrossRef] [PubMed]
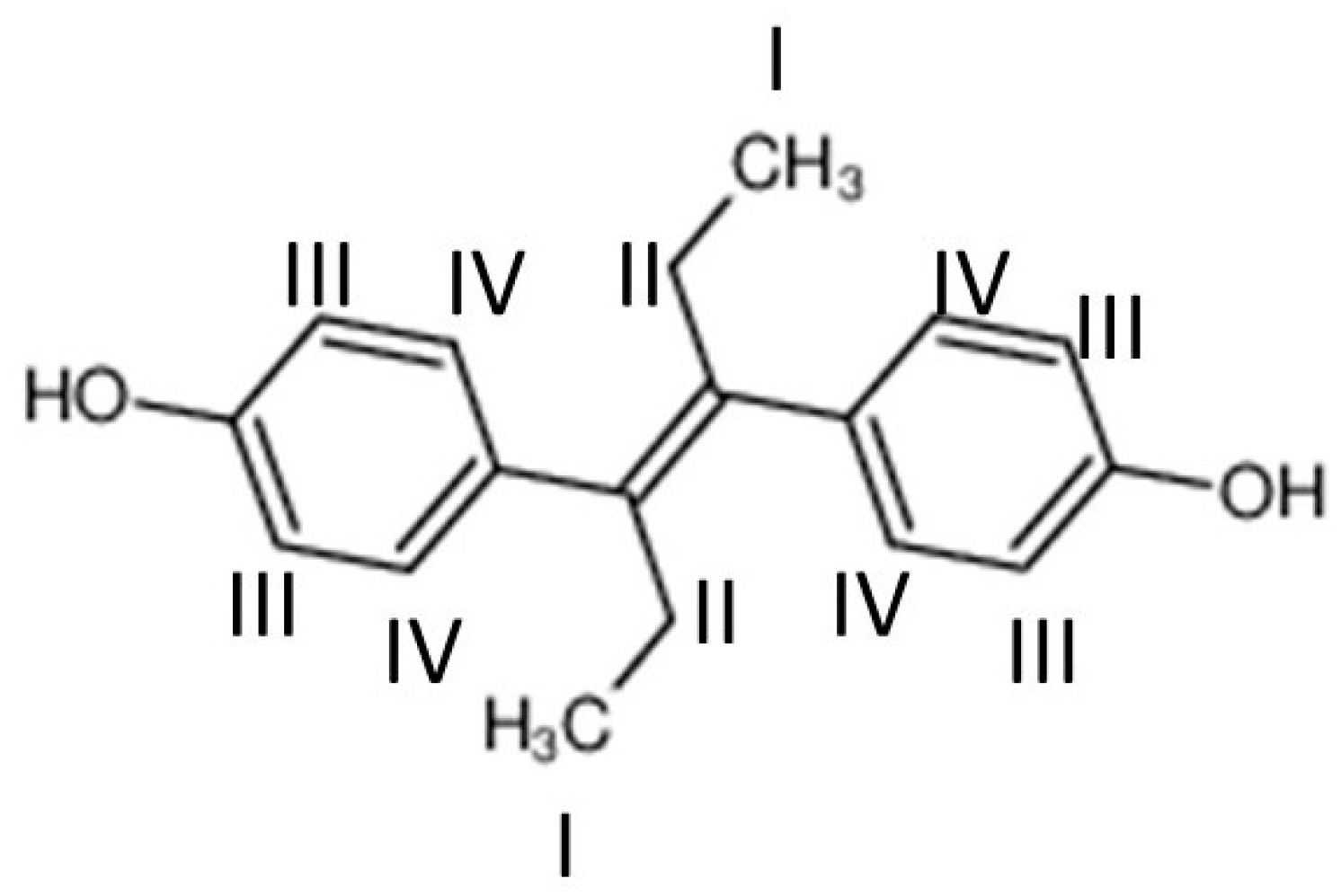
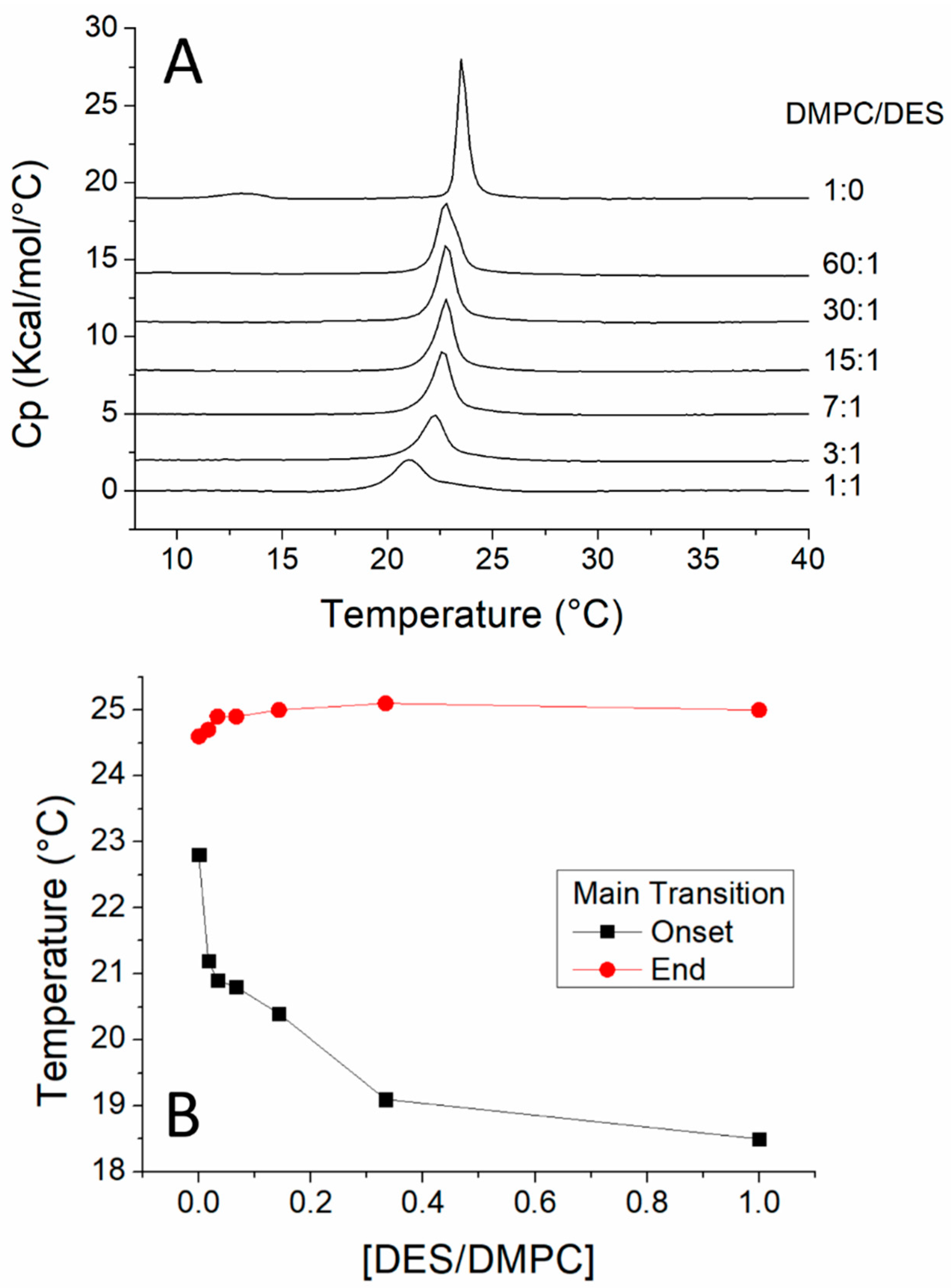
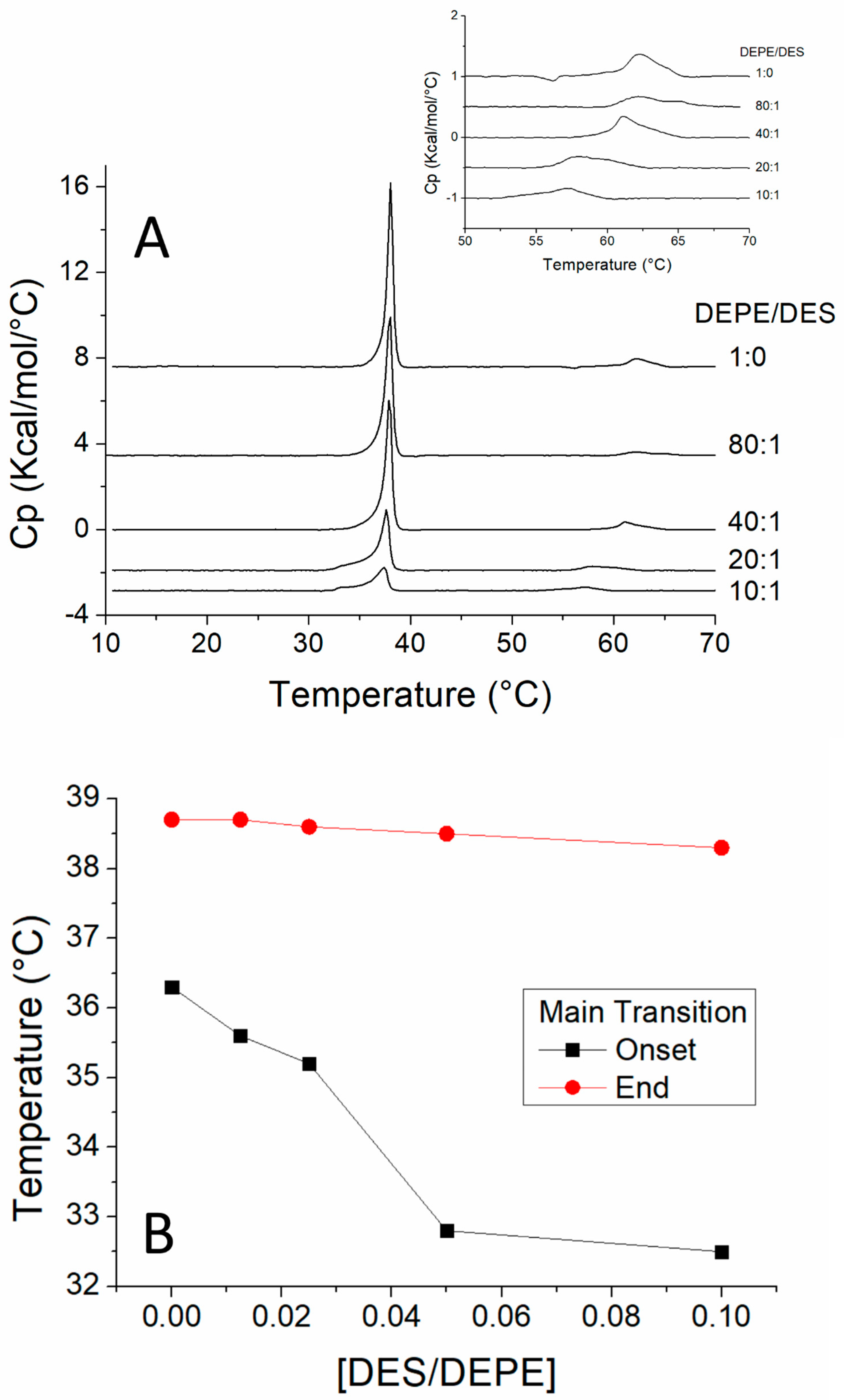

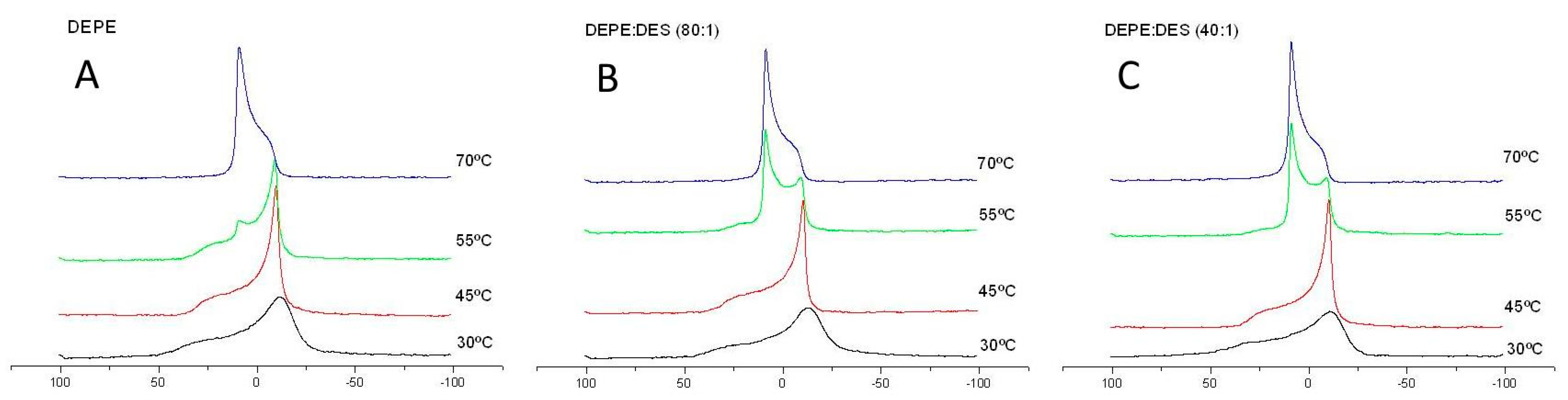

| Molar Ratio | DMPC/DES |
|---|---|
| ΔH (kcal/mol) | |
| 1:0 | 6.5812 |
| 60:1 | 6.3835 |
| 30:1 | 6.3749 |
| 15:1 | 6.2677 |
| 7:1 | 6.198 |
| 3:1 | 5.4762 |
| 1:1 | 4.775 |
| 1:0 | 7.1094 |
| 80:1 | 6.8634 |
| 40:1 | 6.5521 |
| 20:1 | 4.5866 |
| 10:1 | 2.1381 |
| d (Å) | zH (Å) | σC (Å) | dHH (Å) | dB (Å) | dw (Å) | |
|---|---|---|---|---|---|---|
| DMPC (8 °C) | 64.0 ± 0.2 | 19.5 ± 0.2 | 5.4 ± 0.2 | 39.0 ± 0.3 | 51.0 ± 1.2 | 13.0 ± 1.4 |
| DMPC/DES (15:1) 8 °C | 70.9 ± 0.2 | 19.2 ± 0.1 | 5.9 ± 0.2 | 38.4 ± 0.2 | 50.4 ± 1.2 | 20.5 ± 1.3 |
| DMPC/DES (7:1) 8 °C | 72.8 ± 0.2 | 19.1 ± 0.2 | 6.1 ± 0.3 | 38.2 ± 0.3 | 50.2 ± 1.3 | 22.6 ± 1.5 |
| DMPC (30 °C) | 64.6 ± 0.2 | 18.0 ± 0.2 | 6.3 ± 0.2 | 36.0 ± 0.3 | 48.0 ± 1.2 | 16.6 ± 1.4 |
| DMPC/DES (15:1) (30 °C) | 65.6 ± 0.1 | 17.5 ± 0.2 | 6.4 ± 0.2 | 35.0 ± 0.3 | 47.0 ± 1.2 | 18.6 ± 1.3 |
| DMPC/DES (7:1) (30 °C) | 66.7 ± 0.1 | 17.5 ± 0.1 | 6.4 ± 0.1 | 35.0 ± 0.2 | 47.0 ± 1.1 | 19.7 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ausili, A.; Rodríguez-González, I.; Torrecillas, A.; Teruel, J.A.; Gómez-Fernández, J.C. Diethylstilbestrol Modifies the Structure of Model Membranes and Is Localized Close to the First Carbons of the Fatty Acyl Chains. Biomolecules 2021, 11, 220. https://doi.org/10.3390/biom11020220
Ausili A, Rodríguez-González I, Torrecillas A, Teruel JA, Gómez-Fernández JC. Diethylstilbestrol Modifies the Structure of Model Membranes and Is Localized Close to the First Carbons of the Fatty Acyl Chains. Biomolecules. 2021; 11(2):220. https://doi.org/10.3390/biom11020220
Chicago/Turabian StyleAusili, Alessio, Inés Rodríguez-González, Alejandro Torrecillas, José A. Teruel, and Juan C. Gómez-Fernández. 2021. "Diethylstilbestrol Modifies the Structure of Model Membranes and Is Localized Close to the First Carbons of the Fatty Acyl Chains" Biomolecules 11, no. 2: 220. https://doi.org/10.3390/biom11020220
APA StyleAusili, A., Rodríguez-González, I., Torrecillas, A., Teruel, J. A., & Gómez-Fernández, J. C. (2021). Diethylstilbestrol Modifies the Structure of Model Membranes and Is Localized Close to the First Carbons of the Fatty Acyl Chains. Biomolecules, 11(2), 220. https://doi.org/10.3390/biom11020220








