Deeper Insights on Alchornea cordifolia (Schumach. & Thonn.) Müll.Arg Extracts: Chemical Profiles, Biological Abilities, Network Analysis and Molecular Docking
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Preparation of Extracts
2.2. Profile of Bioactive Compounds
2.3. Determination of Antioxidant and Enzyme Inhibitory Effects
2.4. Cell Culture
2.5. Assessment of Cell Viability and Selectivity
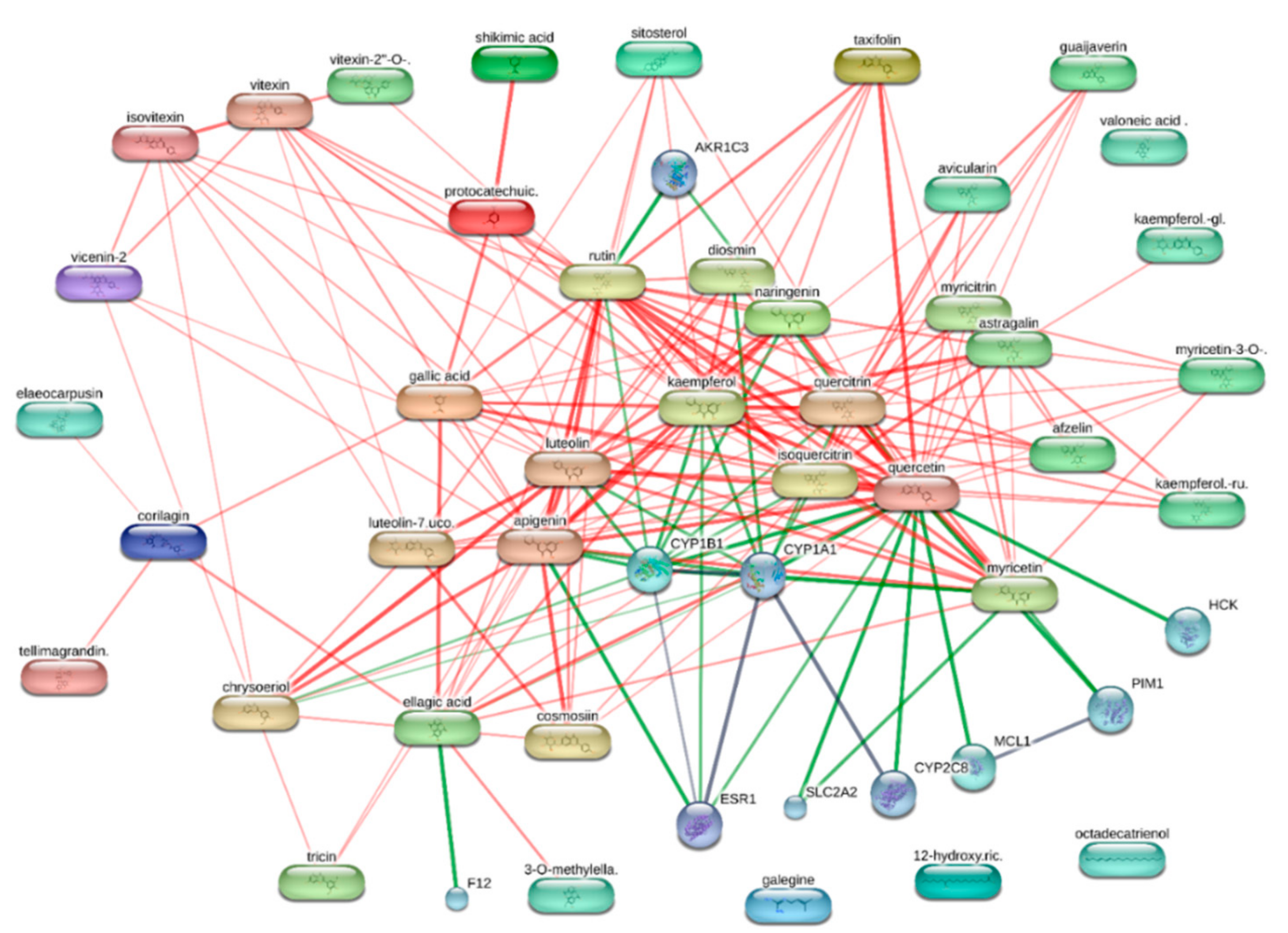
2.6. Bioinformatics
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manga, H.M.; Brkic, D.; Marie, D.; Quetin-Leclercq, J. In vivo anti-inflammatory activity of Alchornea cordifolia (Schumach. & Thonn.) Müll. Arg.(Euphorbiaceae). J. Ethnopharmacol. 2004, 92, 209–214. [Google Scholar]
- Ansah, C.; Oppong, E.; Woode, E. Subacute oral toxicity assessment of Alchornea cordifolia (Schumach and Thonn) Müll Arg (Euphorbiaceae) extract in rats. Trop. J. Pharm. Res. 2011, 10, 587–594. [Google Scholar] [CrossRef]
- Bayor, M.; Ansah, C.; Duwiejua, M.; Abaitey, A. Alchornea Cordifolia (Euphorbiaceae), the Major Constituent of Antiasthmatic Herbal Formulations in Ghana, Stimulates β-adrenoceptors. J. Ghana Sci. Assoc. 2008, 10, 1–11. [Google Scholar] [CrossRef]
- Osei Akoto, C.; Acheampong, A.; Boakye, Y.D.; Akwata, D.; Okine, M. In vitro anthelminthic, antimicrobial and antioxidant activities and FTIR analysis of extracts of Alchornea cordifolia leaves. J. Pharmacogn. Phytochem. 2019, 8, 2432–2442. [Google Scholar]
- Agbor, G.A.; Léopold, T.; Jeanne, N.Y. The antidiarrhoeal activity of Alchornea cordifolia leaf extract. Phytother. Res. 2004, 18, 873–876. [Google Scholar] [CrossRef]
- Mavar-Manga, H.; Haddad, M.; Pieters, L.; Baccelli, C.; Penge, A.; Quetin-Leclercq, J. Anti-inflammatory compounds from leaves and root bark of Alchornea cordifolia (Schumach. & Thonn.) Müll. Arg. J. Ethnopharmacol. 2008, 115, 25–29. [Google Scholar] [PubMed]
- Mohammed, R.; Ibrahim, S.; Atawodi, S.; Eze, E.; Suleiman, J.; Malgwi, I. Anti-diabetic and haematological effects of n-butanol fraction of Alchornea cordifolia leaf extract in streptozotocin-induced diabetic wistar rats. Sci. J. Biol. Sci. 2013, 2, 45–53. [Google Scholar]
- Jacob, J.; Olaleye, M.; Olugbuyiro, J. Hepatoprotective effect of Alchornea cordifolia leaf on liver damage in albino rats. Int. J. Appl. Sci. Biotechnol. 2014, 2, 217–221. [Google Scholar] [CrossRef]
- Odimegwu, D.C.; Okoye, F.B.C.; Nworu, S.C.; Esimone, C.C. Anti-respiratory syncytial virus activities of leaf extracts of Alchornea cordifolia and Alchornea floribunda. Afr. J. Pharm. Pharmacol. 2018, 12, 97–105. [Google Scholar]
- Ajali, U. Antibacterial activity of Alchornea cordifolia stem bark. Fitoterapia 2000, 71, 436–438. [Google Scholar] [CrossRef]
- Noundou, X.S.; Krause, R.; Van Vuuren, S.; Ndinteh, D.T.; Olivier, D. Antibacterial effects of Alchornea cordifolia (Schumach. and Thonn.) Müll. Arg extracts and compounds on gastrointestinal, skin, respiratory and urinary tract pathogens. J. Ethnopharmacol. 2016, 179, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Adeshina, G.O.; Onaolapo, J.A.; Ehinmidu, J.O.; Odama, L.E. Phytochemical and antimicrobial studies of the ethyl acetate extract of Alchornea cordifolia leaf found in Abuja, Nigeria. J. Med. Plants Res. 2010, 4, 649–658. [Google Scholar]
- Joseph, N.; Sorel, N.E.M.; Kasali, F.M.; Emmanuel, M.M. Phytochemical screening and antibacterial properties from extract of Alchornea cordifolia (Schumach. &Thonn.) Müll. Arg. J. Pharmacogn. Phytochem. 2015, 4, 176–180. [Google Scholar]
- Boniface, P.K.; Ferreira, S.B.; Kaiser, C.R. Recent trends in phytochemistry, ethnobotany and pharmacological significance of Alchornea cordifolia (Schumach. & Thonn.) Muell. Arg. J. Ethnopharmacol. 2016, 191, 216–244. [Google Scholar]
- Okoye, F.; Osadebe, P.; Nworu, C.; Okoye, N.; Omeje, E.; Esimone, C. Topical anti-inflammatory constituents of lipophilic leaf fractions of Alchornea floribunda and Alchornea cordifolia. Nat. Prod. Res. 2011, 25, 1941–1949. [Google Scholar] [CrossRef]
- Onocha, P.A.; Oloyede, G.K.; Olasunkanmi, G.S. Chemical composition, brine shrimp toxicity and free-radical scavenging activity of leaf essential oil of Acalypha Ornata (Hochst). Adv. Environ. Biol. 2011, 5, 188–193. [Google Scholar]
- Essien, E.E.; Newby, J.S.; Walker, T.M.; Setzer, W.N.; Ekundayo, O. Characterization and antimicrobial activity of volatile constituents from fresh fruits of Alchornea cordifolia and Canthium subcordatum. Medicines 2016, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Lamikanra, A.; Ogundaini, A.O.; Ogungbamila, F.O. Antibacterial constituents of Alchornea cordifolia leaves. Phytother. Res. 1990, 4, 198–200. [Google Scholar] [CrossRef]
- Ogungbamila, F.O.; Samuelsson, G. Smooth muscle relaxing flavonoids from Alchornea cordifolia. Acta Pharm. Nord. 1990, 2, 421. [Google Scholar]
- Alves, A.C.; Prista, L.N. Gentisic acid as a possible precursor in the biosynthesis of yohimbine. An. Fac. Farm. Porto 1962, 22, 27–33. [Google Scholar]
- Mavar-Manga, H.; Chapon, D.; Hoet, S.; Block, S.; De Pauw-Gillet, M.-C.; Quetin-Leclercq, J. N1, N2, N3-Trisisopentenyl guanidine and N1, N2-diisopentenyl guanidine, two cytotoxic alkaloids from Alchornea cordifolia (Schumach. & Thonn.) Müll. Arg.(Euphorbiaceae) root barks. Nat. Prod. Commun. 2006, 1, 1934578X0600101205. [Google Scholar]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Katanić Stanković, J.S.; Ceylan, R.; Zengin, G.; Matić, S.; Jurić, T.; Diuzheva, A.; Jeko, J.; Cziáky, Z.; Aktumsek, A. Multiple biological activities of two Onosma species (O. sericea and O. stenoloba) and HPLC-MS/MS characterization of their phytochemical composition. Ind. Crops Prod. 2020, 144, 112053. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Neves, V.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.; Varela, J.; Barreira, L.; Custódio, L. In vitro antioxidant and anti-inflammatory properties of Limonium algarvense flowers’ infusions and decoctions: A comparison with green tea (Camellia sinensis). Food Chem. 2016, 200, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Chiavaroli, A.; Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Brunetti, L.; Petrucci, M.; Politi, M.; Menghini, L.; Leone, S. Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago virga-aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinosa Extracts. Antibiotics 2020, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Sinan, K.I.; Zengin, G.; Zheleva-Dimitrova, D.; Etienne, O.K.; Fawzi Mahomoodally, M.; Bouyahya, A.; Lobine, D.; Chiavaroli, A.; Ferrante, C.; Menghini, L. Qualitative Phytochemical Fingerprint and Network Pharmacology Investigation of Achyranthes aspera Linn. Extracts. Molecules 2020, 25, 1973. [Google Scholar] [CrossRef]
- Vasileva, L.V.; Savova, M.S.; Amirova, K.M.; Balcheva-Sivenova, Z.; Ferrante, C.; Orlando, G.; Wabitsch, M.; Georgiev, M.I. Caffeic and Chlorogenic Acids Synergistically Activate Browning Program in Human Adipocytes: Implications of AMPK- and PPAR-Mediated Pathways. Int. J. Mol. Sci. 2020, 21, 9740. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A comparative review on the extraction, antioxidant content and antioxidant potential of different parts of walnut (Juglans regia L.) fruit and tree. Molecules 2019, 24, 2133. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Barchan, A.; Bakkali, M.; Arakrak, A.; Pagán, R.; Laglaoui, A. The effects of solvents polarity on the phenolic contents and antioxidant activity of three Mentha species extracts. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 399–412. [Google Scholar]
- Kadioglu, O.; Nass, J.; Saeed, M.E.; Schuler, B.; Efferth, T. Kaempferol is an anti-inflammatory compound with activity towards NF-κB pathway proteins. Anticancer Res. 2015, 35, 2645–2650. [Google Scholar]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar]
- Verdam, M.C.D.S.; Guilhon-Simplicio, F.; Andrade, K.C.D.; Fernandes, K.L.M.; Machado, T.M.; da Silva, F.M.A.; Souza, M.P.d.; Koolen, H.H.F.; Paula, C.D.S.; Hirota, B.C.K. Analgesic, anti-inflammatory, and antioxidant activities of Byrsonima duckeana WR Anderson (Malpighiaceae). Sci. World J. 2017, 2017, 8367042. [Google Scholar] [CrossRef] [PubMed]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-H.; Zhang, N.; Meng, Y.-Y.; Feng, H.; Yang, J.-J.; Li, W.-J.; Chen, S.; Wu, H.-M.; Deng, W.; Tang, Q.-Z. Myricetin Alleviates Pathological Cardiac Hypertrophy via TRAF6/TAK1/MAPK and Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 6304058. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ren, F.; Li, B.; Song, Z.; Chen, P.; Ouyang, L. Ellagic acid exerts antitumor effects via the PI3K signaling pathway in endometrial cancer. J. Cancer 2019, 10, 3303. [Google Scholar] [CrossRef]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a dietary anti-inflammatory agent: Current therapeutic standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Huang, D. Dietary antioxidants and health promotion. Antioxidants 2018, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Kouakou-Siransy, G.; Sahpaz, S.; Irié Nguessan, G.; Datté, J.Y.; Brou, J.K.; Gressier, B.; Bailleul, F. Effects of Alchornea cordifolia on elastase and superoxide anion produced by human neutrophils. Pharm. Biol. 2010, 48, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Bhebhe, M.; Füller, T.N.; Chipurura, B.; Muchuweti, M. Effect of solvent type on total phenolic content and free radical scavenging activity of black tea and herbal infusions. Food Anal. Methods 2016, 9, 1060–1067. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Juraimi, A.S.; Tayebi-Meigooni, A. Comparative evaluation of different extraction techniques and solvents for the assay of phytochemicals and antioxidant activity of hashemi rice bran. Molecules 2015, 20, 10822–10838. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Effects of solvent type on phenolics and flavonoids content and antioxidant activities in two varieties of young ginger (Zingiber officinale Roscoe) extracts. J. Med. Plants Res. 2011, 5, 1147–1154. [Google Scholar]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Al-Rifai, A.; Aqel, A.; Al-Warhi, T.; Wabaidur, S.M.; Al-Othman, Z.A.; Badjah-Hadj-Ahmed, A.Y. Antibacterial, Antioxidant Activity of Ethanolic Plant Extracts of Some Convolvulus Species and Their DART-ToF-MS Profiling. Evid. Based Complement. Alternat. Med. 2017, 2017, 5694305. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Copeland, R.A.; Harpel, M.R.; Tummino, P.J. Targeting enzyme inhibitors in drug discovery. Expert Opin. Ther. Targets 2007, 11, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Holzgrabe, U.; Kapková, P.; Alptüzün, V.; Scheiber, J.; Kugelmann, E. Targeting acetylcholinesterase to treat neurodegeneration. Expert Opin. Ther. Targets 2007, 11, 161–179. [Google Scholar] [CrossRef]
- Ahmed, F.; Ghalib, R.M.; Sasikala, P.; Ahmed, K.K. Cholinesterase inhibitors from botanicals. Pharmacogn. Rev. 2013, 7, 121–130. [Google Scholar] [CrossRef]
- Amir Rawa, M.S.; Hassan, Z.; Murugaiyah, V.; Nogawa, T.; Wahab, H.A. Anti-cholinesterase potential of diverse botanical families from Malaysia: Evaluation of crude extracts and fractions from liquid-liquid extraction and acid-base fractionation. J. Ethnopharmacol. 2019, 245, 112160. [Google Scholar] [CrossRef]
- De Freitas, M.M.; Fontes, P.R.; Souza, P.M.; William Fagg, C.; Guerra, E.N.S.; de Medeiros Nóbrega, Y.K.; Silveira, D.; Fonseca-Bazzo, Y.; Simeoni, L.A.; Homem-de-Mello, M.; et al. Extracts of Morus nigra L. Leaves Standardized in Chlorogenic Acid, Rutin and Isoquercitrin: Tyrosinase Inhibition and Cytotoxicity. PLoS ONE 2016, 11, e0163130. [Google Scholar] [CrossRef]
- Bonesi, M.; Xiao, J.; Tundis, R.; Aiello, F.; Sicari, V.; Loizzo, M.R. Advances in the Tyrosinase Inhibitors from Plant Source. Curr. Med. Chem. 2019, 26, 3279–3299. [Google Scholar] [CrossRef]
- Yu, Q.; Fan, L.; Duan, Z. Five individual polyphenols as tyrosinase inhibitors: Inhibitory activity, synergistic effect, action mechanism, and molecular docking. Food Chem. 2019, 297, 124910. [Google Scholar] [CrossRef]
- Özer, Ö.; Mutlu, B.; Kivçak, B. Antityrosinase Activity of Some Plant Extracts and Formulations Containing Ellagic Acid. Pharm. Biol. 2008, 45, 519–524. [Google Scholar] [CrossRef]
- Solimine, J.; Garo, E.; Wedler, J.; Rusanov, K.; Fertig, O.; Hamburger, M.; Atanassov, I.; Butterweck, V. Tyrosinase inhibitory constituents from a polyphenol enriched fraction of rose oil distillation wastewater. Fitoterapia 2016, 108, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Chipiti, T.; Ibrahim, M.A.; Singh, M.; Islam, M.S. In vitro α-amylase and α-glucosidase inhibitory effects and cytotoxic activity of Albizia antunesiana extracts. Pharmacogn. Mag. 2015, 11, S231–S236. [Google Scholar] [CrossRef] [PubMed]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Silveira, D. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef] [PubMed]
- Ali Asgar, M. Anti-diabetic potential of phenolic compounds: A review. Int. J. Food Prop. 2013, 16, 91–103. [Google Scholar] [CrossRef]
- Xiao, J.; Ni, X.; Kai, G.; Chen, X. Advance in dietary polyphenols as aldose reductases inhibitors: Structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2015, 55, 16–31. [Google Scholar] [CrossRef]
- Kuete, V.; Efferth, T. African flora has the potential to fight multidrug resistance of cancer. Biomed. Res. Int. 2015, 2015, 914813. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Tchinda, C.F.; Mambe, F.T.; Beng, V.P.; Efferth, T. Cytotoxicity of methanol extracts of 10 Cameroonian medicinal plants towards multi-factorial drug-resistant cancer cell lines. BMC Complement. Altern. Med. 2016, 16, 267. [Google Scholar] [CrossRef]
- Poh, A.R.; Dwyer, A.R.; Eissmann, M.F.; Chand, A.L.; Baloyan, D.; Boon, L.; Murrey, M.W.; Whitehead, L.; O’Brien, M.; Lowell, C.A. Inhibition of the SRC kinase HCK impairs STAT3-dependent gastric tumor growth in mice. Cancer Immunol. Res. 2020, 8, 428–435. [Google Scholar] [CrossRef]
- Shen, M.; Cao, J.; Shi, H. Effects of estrogen and estrogen receptors on transcriptomes of HepG2 Cells: A preliminary study using RNA sequencing. Int. J. Endocrinol. 2018, 2018, 5789127. [Google Scholar] [CrossRef]
- Wang, Z.; Ying, C.; Zhang, A.; Xu, H.; Jiang, Y.; Lou, M. HCK promotes glioblastoma progression by TGFβ signaling. Biosci. Rep. 2020, 40, BSR20200975. [Google Scholar] [CrossRef]
- Yang, H.; He, K.; Dong, W.; Fang, J.; Zhong, S.; Tang, L.; Long, L. PIM-1 may function as an oncogene in cervical cancer via activating the EGFR signaling. Int. J. Biol. Markers 2020, 35, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Suliman, S.; Yagi, S.; Elbashir, A.A.; Mohammed, I.; Mohammed, A.; Ak, G.; Zengin, G.; Orlando, G.; Ferrante, C. Phenolic profile, enzyme inhibition and antioxidant activities and bioinformatics analysis of leaf and stem bark of Ficus sycomorus L. Process Biochem. 2020, 101, 169–178. [Google Scholar] [CrossRef]
- Corni, G.; Brighenti, V.; Pellati, F.; Morlock, G.E. Effect-directed analysis of bioactive compounds in Cannabis sativa L. by high-performance thin-layer chromatography. J. Chromatogr. 2020, 1629, 461511. [Google Scholar] [CrossRef]
- Orlando, G.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Carradori, S.; Di Simone, S.; Ciferri, M.C.; Zengin, G.; Ak, G. Water Extract from Inflorescences of Industrial Hemp Futura 75 Variety as a Source of Anti-Inflammatory, Anti-Proliferative and Antimycotic Agents: Results from In Silico, In Vitro and Ex Vivo Studies. Antioxidants 2020, 9, 437. [Google Scholar] [CrossRef] [PubMed]



| Extracts | Total Phenolic Content | Total Flavonoid Content |
|---|---|---|
| (mg GAE/g Extract) | (mg RE/g Extract) | |
| EA | 120.38 ± 9.31 c | 9.66 ± 0.51 c |
| MeOH | 208.38 ± 0.41 b | 57.18 ± 0.94 a |
| Infusion | 213.12 ± 1.32 a | 46.30 ± 0.58 b |
| No. | Name | Formula | Rt | [M + H]+ | [M − H]− | Ethyl Acetate | Methanol | Water |
|---|---|---|---|---|---|---|---|---|
| 1 | Shikimic acid | C7H10O5 | 1,27 | 17,304,500 | + | + | + | |
| 2 1 | Gallic acid (3,4,5-Trihydroxybenzoic acid) | C7H6O5 | 2,60 | 16,901,370 | + | + | + | |
| 3 | Protocatechuic acid (3,4-Dihydroxybenzoic acid) | C7H6O4 | 5,43 | + | + | + | ||
| 4 | Galegine (Isopentenyl guanidine) | C6H13N3 | 6,10 | 12,811,878 | − | + | + | |
| 5 | Unidentified alkaloid 1 | C9H9NO5 | 8,98 | 21,205,590 | − | + | − | |
| 6 | Unidentified ellagic acid derivative | C21H10O13 | 11,31 | 46,900,432 | + | + | + | |
| 7 | Taxifolin-O-hexoside | C21H22O12 | 13,86 | 46,510,331 | − | + | + | |
| 8 | Putranjivain A | C46H36O31 | 15,71 | 108,311,623 | + | + | + | |
| 9 | Brevifolincarboxylic acid or isomer | C13H8O8 | 17,06 | 29,101,410 | + | + | + | |
| 10 | Procyanidin A isomer 1 | C30H24O12 | 17,20 | 57,511,896 | − | + | − | |
| 11 | Potentillin or isomer | C41H28O26 | 17,30 | 93,507,906 | + | + | + | |
| 12 | Sanguisorbic acid dilactone | C21H10O13 | 17,36 | 46,900,432 | − | + | − | |
| 13 | Elaeocarpusin | C47H34O32 | 17,59 | 110,909,550 | + | + | − | |
| 14 | Procyanidin A isomer 2 | C30H24O12 | 17,65 | 57,511,896 | + | + | + | |
| 15 | Valoneic acid dilactone | C21H10O13 | 17,70 | 46,900,432 | + | + | + | |
| 16 | Potentillin or isomer | C41H28O26 | 17,73 | 93,507,906 | + | + | + | |
| 17 | Corilagin or isomer | C27H22O18 | 17,89 | 63,307,279 | + | + | + | |
| 18 | Unidentified alkaloid 2 | C13H10N2O3 | 18,07 | 24,206,914 | − | + | − | |
| 19 | Unidentified ellagic acid derivative | C21H10O13 | 18,53 | 46,900,432 | + | + | + | |
| 20 | Vicenin-2 (Apigenin-6,8-di-C-glucoside) | C27H30O15 | 19,33 | 59,516,630 | − | + | + | |
| 21 | Procyanidin A isomer 3 | C30H24O12 | 19,45 | 57,511,896 | + | + | − | |
| 22 1 | Taxifolin (Dihydroquercetin) | C15H12O7 | 19,84 | 30,305,048 | + | + | + | |
| 23 | Procyanidin A isomer 4 | C30H24O12 | 20,18 | 57,511,896 | + | + | + | |
| 24 | Ellagic acid-4-O-glucoside | C20H16O13 | 20,37 | 46,305,127 | − | + | + | |
| 25 | Tellimagrandin I or isomer | C34H26O22 | 20,44 | 78,508,375 | − | + | + | |
| 26 | Quercetin-O-hexosylhexoside | C27H30O17 | 20,63 | 62,514,048 | + | + | + | |
| 27 | Myricetin-3’-O-glucoside | C21H20O13 | 21,36 | 47,908,257 | + | + | + | |
| 28 | Myricetin-O-rhamnosylhexoside isomer 1 | C27H30O17 | 21,47 | 62,514,048 | + | + | + | |
| 29 | Unidentified hexahydroxydiphenoylhexose derivative | C34H26O22 | 21,61 | 78,508,375 | + | + | + | |
| 30 | Procyanidin A isomer 5 | C30H24O12 | 21,68 | 57,511,896 | + | + | − | |
| 31 1 | Vitexin (Apigenin-8-C-glucoside) | C21H20O10 | 21,79 | 43,311,347 | + | + | + | |
| 32 1 | Vitexin-2’’-O-rhamnoside | C27H30O14 | 22,11 | 57,917,139 | + | + | + | |
| 33 | Taxifolin-O-pentoside | C20H20O11 | 22,37 | 43,509,274 | + | + | + | |
| 34 | Apigenin-C-hexoside-O-pentoside | C26H28O14 | 22,40 | 56,515,574 | + | + | + | |
| 35 | Myricitrin (Myricetin-3-O-rhamnoside) | C21H20O12 | 22,47 | 46,308,765 | + | + | + | |
| 36 | Isovitexin (Apigenin-6-C-glucoside) | C21H20O10 | 22,72 | 43,311,347 | + | + | + | |
| 37 | Luteolin-7-O-glucoside (Cynaroside) | C21H20O11 | 22,81 | 44,709,274 | − | + | + | |
| 38 | Luteolin-O-rhamnosylhexoside isomer 1 | C27H30O15 | 22,84 | 59,315,065 | + | + | + | |
| 39 | Isovitexin-2’’-O-rhamnoside | C27H30O14 | 23,03 | 57,917,139 | + | + | + | |
| 40 | N1,N2-Diisopentenyl guanidine | C11H21N3 | 23,07 | 19,618,138 | + | + | + | |
| 41 | Hyperoside (Quercetin-3-O-galactoside) | C21H20O12 | 23,18 | 46,308,765 | + | + | + | |
| 42 | Ellagic acid-O-pentoside | C19H14O12 | 23,28 | 43,304,071 | − | + | + | |
| 43 1 | Isoquercitrin (Quercetin-3-O-glucoside) | C21H20O12 | 23,40 | 46,308,765 | + | + | + | |
| 44 1 | Rutin (Quercetin-3-O-rutinoside) | C27H30O16 | 23,46 | 61,116,122 | + | + | + | |
| 45 | Luteolin-O-rhamnosylhexoside isomer 2 | C27H30O15 | 23,48 | 59,315,065 | + | + | + | |
| 46 | Eschweilenol C (Ellagic acid-4-O-rhamnoside) | C20H16O12 | 23,57 | 44,705,636 | + | + | + | |
| 47 | Reinutrin (Quercetin-3-O-xyloside) | C20H18O11 | 23,70 | 43,307,709 | − | + | − | |
| 48 | Ellagic acid | C14H6O8 | 23,84 | 30,099,845 | + | + | + | |
| 49 | Avicularin (Quercetin-3-O-arabinofuranoside) | C20H18O11 | 24,02 | 43,307,709 | + | + | + | |
| 50 | Mallotusinin or isomer | C41H26O25 | 24,20 | 91,706,850 | + | + | + | |
| 51 | Apigenin-O-rhamnosylhexoside isomer 1 | C27H30O14 | 24,37 | 57,715,574 | − | + | + | |
| 52 1 | Cosmosiin (Apigenin-7-O-glucoside) | C21H20O10 | 24,46 | 43,311,347 | + | + | + | |
| 53 | Myricetin-O-galloylrhamnoside | C28H24O16 | 24,57 | 61,509,861 | + | + | + | |
| 54 | Kaempferol-7-O-glucoside | C21H20O11 | 24,66 | 44,709,274 | − | + | + | |
| 55 1 | Myricetin (3,3’,4’,5,5’,7-Hexahydroxyflavone) | C15H10O8 | 24,70 | 31,702,974 | + | + | + | |
| 56 | Chrysoeriol-O-hexoside | C22H22O11 | 24,73 | 46,110,839 | + | + | + | |
| 57 | Guaijaverin (Quercetin-3-O-arabinoside) | C20H18O11 | 24,74 | 43,307,709 | + | + | + | |
| 58 | Tricin-7-O-glucoside | C23H24O12 | 24,77 | 49,111,896 | − | + | + | |
| 59 | Apigenin-O-rhamnosylhexoside isomer 2 | C27H30O14 | 24,89 | 57,715,574 | − | + | + | |
| 60 1 | Diosmin (Diosmetin-7-O-rutinoside) | C28H32O15 | 24,96 | 60,918,195 | + | + | + | |
| 61 1 | Quercitrin (Quercetin-3-O-rhamnoside) | C21H20O11 | 24,97 | 44,709,274 | + | + | + | |
| 62 | Astragalin (Kaempferol-3-O-glucoside) | C21H20O11 | 25,18 | 44,709,274 | − | + | + | |
| 63 | Unidentified ellagic acid derivative | C21H10O12 | 25,31 | 45,300,940 | + | + | + | |
| 64 | Kaempferol-3-O-rutinoside (Nicotiflorin) | C27H30O15 | 25,34 | 59,315,065 | + | + | + | |
| 65 | Kaempferol-O-pentoside | C20H18O10 | 25,40 | 41,708,218 | + | + | + | |
| 66 | 3-O-Methylellagic acid | C15H8O8 | 26,26 | 31,501,410 | + | + | + | |
| 67 | Afzelin (Kaempferol-3-O-rhamnoside) | C21H20O10 | 26,92 | 43,109,782 | + | + | + | |
| 68 1 | Quercetin (3,3’,4’,5,7-Pentahydroxyflavone) | C15H10O7 | 27,51 | 30,103,483 | + | + | + | |
| 69 1 | Naringenin (4’,5,7-Trihydroxyflavanone) | C15H12O5 | 27,71 | 27,106,065 | + | + | + | |
| 70 1 | Luteolin (3’,4’,5,7-Tetrahydroxyflavone) | C15H10O6 | 28,38 | 28,503,991 | + | + | + | |
| 71 | 3,3’-Di-O-methylellagic acid | C16H10O8 | 28,45 | 32,902,975 | + | + | + | |
| 72 | Dihydroxy-methoxy(iso)flavone-O-hexoside | C22H22O10 | 28,59 | 44,712,913 | + | + | + | |
| 73 1 | Kaempferol (3,4’,5,7-Tetrahydroxyflavone) | C15H10O6 | 29,87 | 28,503,991 | + | + | + | |
| 74 1 | Apigenin (4’,5,7-Trihydroxyflavone) | C15H10O5 | 30,23 | 26,904,500 | + | + | + | |
| 75 1 | Tricin (3’,5’-Dimethoxy-4’,5,7-trihydroxyflavone) | C17H14O7 | 30,41 | 32,906,613 | + | + | + | |
| 76 | Chrysoeriol (3’-Methoxy-4’,5,7-trihydroxyflavone) | C16H12O6 | 30,46 | 29,905,556 | + | + | + | |
| 77 | N1,N2,N3-Triisopentenyl guanidine | C16H29N3 | 30,82 | 26,424,398 | + | + | + | |
| 78 | 3,3’,4-Tri-O-methylellagic acid | C17H12O8 | 30,84 | 34,304,540 | + | + | + | |
| 79 | 3,3’,4,4’-Tetra-O-methylellagic acid | C18H14O8 | 32,67 | 35,907,670 | + | + | + | |
| 80 | Dihydroxy-methoxy(iso)flavone | C16H12O5 | 34,42 | 28,507,630 | + | + | + | |
| 81 | Octadecatrienol | C18H32O | 45,71 | 26,525,314 | + | + | − | |
| 82 | 2-Hydroxystearic acid | C18H36O3 | 47,01 | 29,925,863 | + | + | − | |
| 83 | β-Sitosterol | C29H50O | 49,56 | 41,539,400 | + | + | − | |
| 84 | Myricetin-O-rhamnosylhexoside isomer 2 | C27H30O17 | 22,02 | 62,514,048 | − | − | + |
| Extracts | DPPH | ABTS | CUPRAC | FRAP | PPB | MCA |
|---|---|---|---|---|---|---|
| (mg TE/g Extract) | (mmol TE/g) | (mg EDTAE/g) | ||||
| EA | 188.94 ± 0.15 c | 357.98 ± 0.76 c | 454.25 ± 7.69 c | 201.66 ± 6.00 c | 4.04 ± 0.10 c | 21.56 ± 0.55 b |
| MeOH | 500.38 ± 1.28 a | 900.64 ± 0.69 a | 1277.66 ± 2.98 b | 655.19 ± 16.00 b | 5.76 ± 0.51 b | 24.78 ± 1.18 a |
| Infusion | 490.94 ± 0.55 b | 839.30 ± 18.71 b | 1476.64 ± 1.08 a | 822.04 ± 6.54 a | 6.01 ± 0.10 a | 22.44 ± 0.82 b |
| Extracts | AChE | BChE | Tyrosinase | α-Amylase | α-Glucosidase |
|---|---|---|---|---|---|
| (mg GALAE/g) | (mg KAE/g) | (mmol ACAE/g) | |||
| EA | 4.47 ± 0.05 a | 5.81 ± 0.31 b | 119.11 ± 0.67 b | 1.19 ± 0.01 a | na |
| MeOH | 4.56 ± 0.06 a | 7.79 ± 0.21 a | 131.01 ± 0.84 a | 1.03 ± 0.06 b | na |
| Infusion | 2.02 ± 0.05 b | na | 59.53 ± 0.34 c | 0.21 ± 0.01 c | 6.82 ± 0.02 |
| Extracts | HepG2 | SE | B16 4A5 | SE | S17 |
|---|---|---|---|---|---|
| DMSO 0.5% | 101 ± 7 a | 88 ± 2 a | 79 ± 5 a | ||
| EA | 85 ± 4 b | 0.7 | 46 ± 1 c | 1.3 | 62 ± 3 b |
| MeOH | 17 ± 1 c | 1.2 | 88 ± 6 a | 0.2 | 21 ± 1 d |
| Infusion | 14 ± 1 d | 1.9 | 64 ± 1 b | 0.4 | 26 ± 1 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinan, K.I.; Ak, G.; Etienne, O.K.; Jekő, J.; Cziáky, Z.; Gupcsó, K.; João Rodrigues, M.; Custodio, L.; Mahomoodally, M.F.; Sharmeen, J.B.; et al. Deeper Insights on Alchornea cordifolia (Schumach. & Thonn.) Müll.Arg Extracts: Chemical Profiles, Biological Abilities, Network Analysis and Molecular Docking. Biomolecules 2021, 11, 219. https://doi.org/10.3390/biom11020219
Sinan KI, Ak G, Etienne OK, Jekő J, Cziáky Z, Gupcsó K, João Rodrigues M, Custodio L, Mahomoodally MF, Sharmeen JB, et al. Deeper Insights on Alchornea cordifolia (Schumach. & Thonn.) Müll.Arg Extracts: Chemical Profiles, Biological Abilities, Network Analysis and Molecular Docking. Biomolecules. 2021; 11(2):219. https://doi.org/10.3390/biom11020219
Chicago/Turabian StyleSinan, Kouadio Ibrahime, Gunes Ak, Ouattara Katinan Etienne, József Jekő, Zoltán Cziáky, Katalin Gupcsó, Maria João Rodrigues, Luisa Custodio, Mohamad Fawzi Mahomoodally, Jugreet B. Sharmeen, and et al. 2021. "Deeper Insights on Alchornea cordifolia (Schumach. & Thonn.) Müll.Arg Extracts: Chemical Profiles, Biological Abilities, Network Analysis and Molecular Docking" Biomolecules 11, no. 2: 219. https://doi.org/10.3390/biom11020219
APA StyleSinan, K. I., Ak, G., Etienne, O. K., Jekő, J., Cziáky, Z., Gupcsó, K., João Rodrigues, M., Custodio, L., Mahomoodally, M. F., Sharmeen, J. B., Brunetti, L., Leone, S., Recinella, L., Chiavaroli, A., Orlando, G., Menghini, L., Tacchini, M., Ferrante, C., & Zengin, G. (2021). Deeper Insights on Alchornea cordifolia (Schumach. & Thonn.) Müll.Arg Extracts: Chemical Profiles, Biological Abilities, Network Analysis and Molecular Docking. Biomolecules, 11(2), 219. https://doi.org/10.3390/biom11020219
















