Insulin-Like Growth Factor-1 (IGF-1) and Its Monitoring in Medical Diagnostic and in Sports
Abstract
1. Introduction
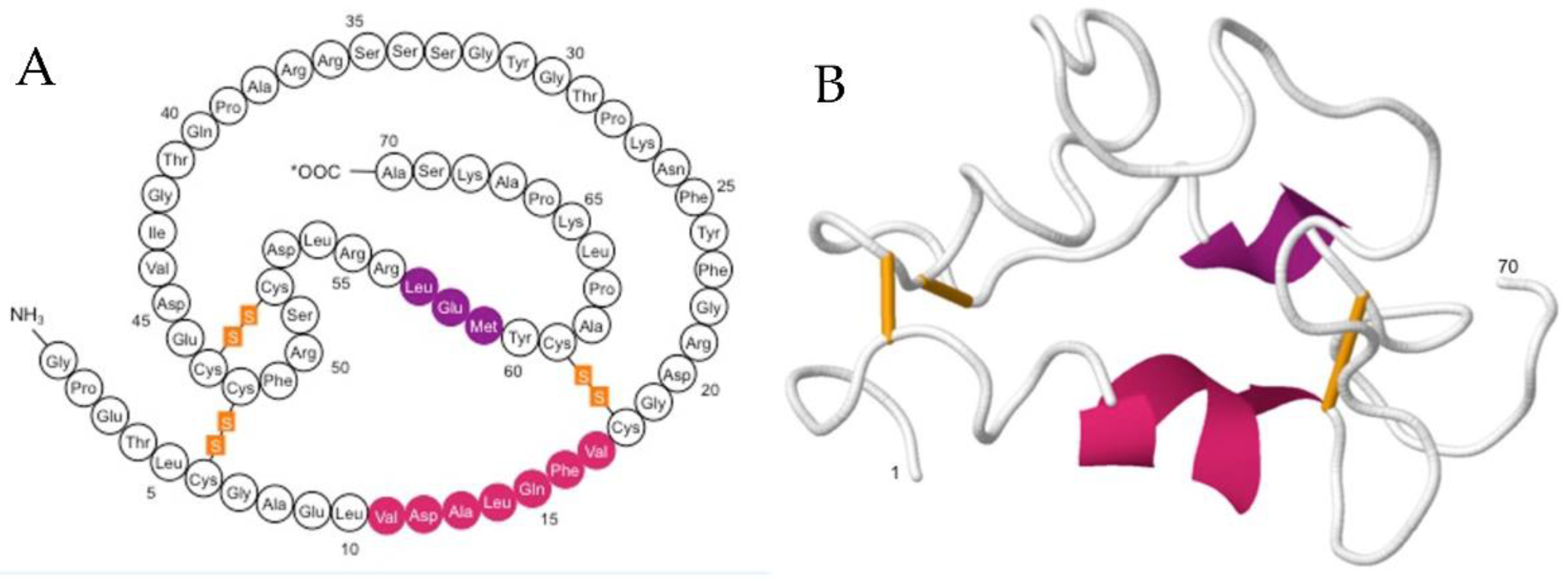
1.1. IGF-1: Structure, Function and Mode of Action
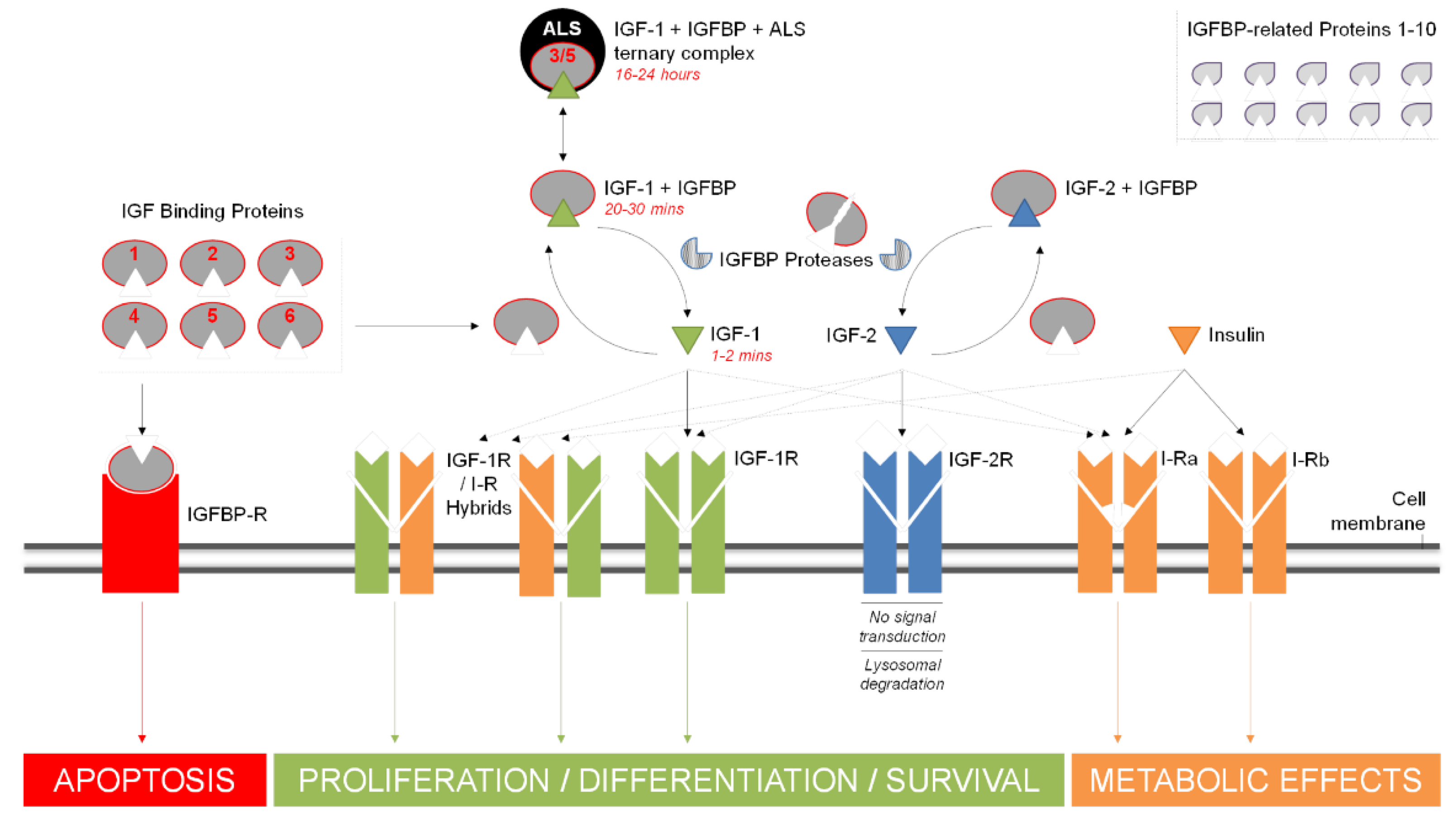
1.2. Growth Factors and IGFBPs

2. IGF-1 as a Marker in Medical Conditions and Disease
3. IGF-1 as a Performance-Enhancing Drug (PED): Doping in Sports
4. Challenges of Monitoring IGF-1 Levels: Complications and Shortfalls of Existing Assays
5. Conclusions
Funding
Conflicts of Interest
References
- Gusscott, S.; Jenkins, C.E.; Lam, S.H.; Giambra, V.; Pollak, M.; Weng, A.P. IGF1R derived PI3K/AKT signaling maintains growth in a subset of human T-cell acute lymphoblastic leukemias. PLoS ONE 2016, 11, e0161158. [Google Scholar] [CrossRef]
- Yakar, S.; Liu, J.L.I.; Stannard, B.; Butler, A.; Accili, D.; Sauer, B.; LeRoith, D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. USA 1999, 96, 7324–7329. [Google Scholar] [CrossRef]
- Sjögren, K.; Liu, J.L.; Blad, K.; Skrtic, S.; Vidal, O.; Wallenius, V.; LeRoith, D.; Törnell, I.; Isaksson, O.G.P.; Jansson, J.; et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 7088–7092. [Google Scholar] [CrossRef] [PubMed]
- Baserga, R. The IGF-I receptor in cancer research. Exp. Cell Res. 1999, 253, 1–6. [Google Scholar] [CrossRef]
- Annibalini, G.; Contarelli, S.; De Santi, M.; Saltarelli, R.; Di Patria, L.; Guescini, M.; Villarini, A.; Brandi, G.; Stocchi, V.; Barbieri, E. The intrinsically disordered E-domains regulate the IGF-1 prohormones stability, subcellular localisation and secretion. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, L.A.; Brisson, B.K.; Lei, H.; Barton, E.R. The insulin-like growth factor (IGF)-I E-peptides modulate cell entry of the mature IGF-I protein. Mol. Biol. Cell 2009, 20, 3810–3817. [Google Scholar] [CrossRef] [PubMed]
- Hede, M.S.; Salimova, E.; Piszczek, A.; Perlas, E.; Winn, N.; Nastasi, T.; Rosenthal, N. E-Peptides Control Bioavailability of IGF-1. PLoS ONE 2012, 7, e51152. [Google Scholar] [CrossRef]
- Goldspink, G. Muscle growth and muscle function: A molecular biological perspective. Res. Vet. Sci. 1996, 60, 193–204. [Google Scholar] [CrossRef]
- Yang, S.; Alnaqeeb, M.; Simpson, H.; Goldspink, G. Cloning and characterization of an IGF-1 isoform expressed in skeletal muscle subjected to stretch. J. Muscle Res. Cell Motil. 1996, 17, 487–495. [Google Scholar] [CrossRef]
- Yang, S.; Alnaqeeb, M.; Simpson, H.; Goldspink, G. Changes in muscle fibre type, muscle mass and IGF-I gene expression in rabbit skeletal muscle subjected to stretch. J. Anat. 1997, 190, 613–622. [Google Scholar] [CrossRef]
- McKoy, G.; Ashley, W.; Mander, J.; Yu Yang, S.; Williams, N.; Russell, B.; Goldspink, G. Expression of insulin growth factor-1 splice variants and structural genes in rabbit skeletal muscle induced by stretch and stimulation. J. Physiol. 1999, 516, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Burniston, J.G.; Chester, N.; Clark, W.A.; Tan, L.-B.; Goldspink, D.F. Dose-dependent apoptotic and necrotic myocyte death induced by the β2-adrenergic receptor agonist, clenbuterol. Muscle Nerve 2005, 32, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Dłużniewska, J.; Sarnowska, A.; Beręsewicz, M.; Johnson, I.; Srai, S.K.S.; Ramesh, B.; Goldspink, G.; Górecki, D.C.; Zabłocka, B. A strong neuroprotective effect of the autonomous C-terminal peptide of IGF-1 Ec (MGF) in brain ischemia. FASEB J. 2005, 19, 1896–1898. [Google Scholar] [CrossRef]
- Carpenter, V.; Matthews, K.; Devlin, G.; Stuart, S.; Jensen, J.; Conaglen, J.; Jeanplong, F.; Goldspink, P.; Yang, S.Y.; Goldspink, G.; et al. Mechano-Growth Factor Reduces Loss of Cardiac Function in Acute Myocardial Infarction. Heart Lung Circ. 2008, 17, 33–39. [Google Scholar] [CrossRef]
- Kandalla, P.K.; Goldspink, G.; Butler-Browne, G.; Mouly, V. Mechano Growth Factor E peptide (MGF-E), derived from an isoform of IGF-1, activates human muscle progenitor cells and induces an increase in their fusion potential at different ages. Mech. Ageing Dev. 2011, 132, 154–162. [Google Scholar] [CrossRef]
- Armakolas, A.; Philippou, A.; Panteleakou, Z.; Nezos, A.; Sourla, A.; Petraki, C.; Koutsilieris, M. Preferential expression of IGF-1Ec (MGF) transcript in cancerous tissues of human prostate: Evidence for a novel and autonomous growth factor activity of MGF E peptide in human prostate cancer cells. Prostate 2010, 70, 1233–1242. [Google Scholar] [CrossRef]
- Milingos, D.S.; Philippou, A.; Armakolas, A.; Papageorgiou, E.; Sourla, A.; Protopapas, A.; Liapi, A.; Antsaklis, A.; Mastrominas, M.; Koutsilieris, K. Insulinlike growth factor-1Ec (MGF) expression in eutopic and ectopic endometrium: Characterization of the MGF E-peptide actions in vitro. Mol. Med. 2011, 17, 21–28. [Google Scholar] [CrossRef]
- Philippou, A.; Armakolas, A.; Panteleakou, Z.; Pissimissis, N.; Nezos, A.; Theos, A.; Kaparelou, M.; Armakolas, N.; Pneumaticos, S.G.; Koutsilieris, M. IGF1Ec expression in MG-63 human osteoblast-like osteosarcoma cells. Anticancer Res. 2011, 31, 4259–4265. [Google Scholar]
- Jones, J.I.; Clemmons, D.R. Insulin-like growth factors and their binding proteins: Biological actions. Endocr. Rev. 1995, 16, 3–34. [Google Scholar] [CrossRef]
- Jehle, P.M.; Schulten, K.; Schulz, W.; Jehle, D.R.; Stracke, S.; Manfras, B.; Boehm, B.O.; Baylink, D.J.; Mohan, S. Serum levels of insulin-like growth factor (IGF)-I and IGF binding protein (IGFBP)-1 to-6 and their relationship to bone metabolism in osteoporosis patients. Eur. J. Intern. Med. 2003, 14, 32–38. [Google Scholar] [CrossRef]
- Baxter, R.C. Characterization of the Acid-Labile Subunit of the Growth Hormone-Dependent Insulin-Like Growth Factor Binding Protein Complex. J. Clin. Endocrinol. Metab. 1988, 67, 265–272. [Google Scholar] [CrossRef]
- Durai, R.; Yang, S.Y.; Seifalian, A.M.; Goldspink, G.; Winslet, M.C. Role of insulin-like growth factor binding protein-4 in prevention of colon cancer. World J. Surg. Oncol. 2007, 5, 1–8. [Google Scholar] [CrossRef]
- Shibata, Y.; Tsukazaki, T.; Hirata, K.; Xin, C.; Yamaguchi, A. Role of a new member of IGFBP superfamily, IGFBP-rP10, in proliferation and differentiation of osteoblastic cells. Biochem. Biophys. Res. Commun. 2004, 325, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Hwa, V.; Oh, Y.; Rosenfeld, R.G. The Insulin-Like Growth Factor-Binding Protein (IGFBP) Superfamily. Endocr. Rev. 1999, 20, 761–787. [Google Scholar] [CrossRef]
- Rodgers, B.D.; Roalson, E.H.; Thompson, C. Phylogenetic analysis of the insulin-like growth factor binding protein (IGFBP) and IGFBP-related protein gene families. Gen. Comp. Endocrinol. 2008, 155, 201–207. [Google Scholar] [CrossRef]
- López-Bermejo, A.; Buckway, C.K.; Devi, G.R.; Hwa, V.; Plymate, S.R.; Oh, Y.; Rosenfeld, R.G. Characterization of Insulin-Like Growth Factor-Binding Protein-Related Proteins (IGFBP-rPs) 1, 2, and 3 in Human Prostate Epithelial Cells: Potential Roles for IGFBP-rP1 and 2 in Senescence of the Prostatic Epithelium*. Endocrinology 2000, 141, 4072–4080. [Google Scholar] [CrossRef] [PubMed]
- Deshayes, K.; Schaffer, M.L.; Skelton, N.J.; Nakamura, G.R.; Kadkhodayan, S.; Sidhu, S.S. Rapid identification of small binding motifs with high-throughput phage display: Discovery of peptidic antagonists of IGF-1 function. Chem. Biol. 2002, 9, 495–505. [Google Scholar] [CrossRef]
- Christine PBurren Wilson, E.M.; Hwa, V.; Oh, Y.; Rosenfeld, R.G. Binding Properties and Distribution of Insulin-Like Growth Factor Binding Protein-Related Protein 3 (IGFBP-rP3/NovH), an Additional Member of the IGFBP Superfamily 1. J. Clin. Endocrinol. Metab. 1999, 84, 1096–1103. [Google Scholar] [CrossRef]
- Baxter, R.C. IGF binding proteins in cancer: Mechanistic and clinical insights. Nat. Rev. Cancer 2014, 14, 329–341. [Google Scholar] [CrossRef]
- Gonda, Y.; Sakurai, H.; Hirata, Y.; Tabata, H.; Ajioka, I.; Nakajima, K. Expression profiles of Insulin-like growth factor binding protein-like 1 in the developing mouse forebrain. Gene Expr. Patterns 2007, 7, 431–440. [Google Scholar] [CrossRef]
- Twigg, S.M.; Baxter, R.C. Insulin-like growth factor (IGF)-binding protein 5 forms an alternative ternary complex with IGFs and the acid-labile subunit. J. Biol. Chem. 1998, 273, 6074–6079. [Google Scholar] [CrossRef]
- Soos, M.A.; Field, C.E.; Siddle, K. Purified hybrid insulin/insulin-like growth factor-I receptors bind insulin-like growth factor-I, but not insulin, with high affinity. Biochem. J. 1993, 290, 419–426. [Google Scholar] [CrossRef]
- Eigenbrot, C.; Ultsch, M.; Lipari, M.T.; Moran, P.; Lin, S.J.; Ganesan, R.; Quan, C.; Tom, J.; Sandoval, W.; Van Lookeren, C.; et al. Structural and functional analysis of HtrA1 and its subdomains. Structure 2012, 20, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Sitar, T.; Popowicz, G.M.; Siwanowicz, I.; Huber, R.; Holak, T.A. Structural basis for the inhibition of insulin-like growth factors by insulin-like growth factor-binding proteins. Proc. Natl. Acad. Sci. USA 2006, 103, 13028–13033. [Google Scholar] [CrossRef] [PubMed]
- Menting, J.G.; Lawrence, C.F.; Kong, G.K.W.; Margetts, M.B.; Ward, C.W.; Lawrence, M.C. Structural Congruency of Ligand Binding to the Insulin and Insulin/Type 1 Insulin-like Growth Factor Hybrid Receptors. Structure 2015, 23, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Vasques, G.A.; Andrade, N.L.M.; Correa, F.A.; Jorge, A.A.L. Update on new GH-IGF axis genetic defects. Arch. Endocrinol. Metab. 2019, 63, 608–617. [Google Scholar] [CrossRef]
- Pfäffle, R.; Kiess, W. Gh and igf-1 replacement in children. Handb. Exp. Pharmacol. 2020, 261, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.; Sarfstein, R.; Nagaraj, K.; Laron, Z. Laron Syndrome Research Paves the Way for New Insights in Oncological Investigation. Cells 2020, 9, 2446. [Google Scholar] [CrossRef]
- Puche, J.E.; Castilla-Cortázar, I. Human conditions of insulin-like growth factor-I (IGF-I) deficiency. J. Transl. Med. 2012, 10, 224. [Google Scholar] [CrossRef]
- Laron, Z.; Werner, H. Laron syndrome—A historical perspective. Rev. Endocr. Metab. Disord. 2020, 2020, 192. [Google Scholar] [CrossRef]
- Woods, K.A.; Camacho-Hübner, C.; Savage, M.O.; Clark, A.J.L. Intrauterine Growth Retardation and Postnatal Growth Failure Associated with Deletion of the Insulin-Like Growth Factor I Gene. N. Engl. J. Med. 1996, 335, 1363–1367. [Google Scholar] [CrossRef]
- Solomon-Zemler, R.; Basel-Vanagaite, L.; Steier, D.; Yakar, S.; Mel, E.; Phillip, M.; Bazak, L.; Bercovich, D.; Werner, H.; De Vries, L. A novel heterozygous IGF-1 receptor mutation associated with hypoglycemia. Endocr. Connect. 2017, 6, 395–403. [Google Scholar] [CrossRef]
- Domené, H.M.; Bengolea, S.V.; Martínez, A.S.; Ropelato, M.G.; Pennisi, P.; Scaglia, P.; Heinrich, J.J.; Jasper, H.G. Deficiency of the Circulating Insulin-like Growth Factor System Associated with Inactivation of the Acid-Labile Subunit Gene. N. Engl. J. Med. 2004, 350, 570–577. [Google Scholar] [CrossRef]
- Poyrazoğlu, Ş.; Hwa, V.; Baş, F.; Dauber, A.; Rosenfeld, R.; Darendeliler, F. A novel homozygous mutation of the acid-labile subunit (IGFALS) gene in a male adolescent. JCRPE J. Clin. Res. Pediatric Endocrinol. 2019, 11, 432–438. [Google Scholar] [CrossRef]
- Tanimoto, K.; Hizuka, N.; Fukuda, I.; Takano, K.; Hanafusa, T. The influence of age on the GH-IGF1 axis in patients with acromegaly. Eur. J. Endocrinol. 2008, 159, 375–379. [Google Scholar] [CrossRef]
- Muller, A.F.; Kopchick, J.J.; Flyvbjerg, A.; Van der Lely, A.J. Growth hormone receptor antagonists. J. Clin. Endocrinol. Metab. 2004, 89, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Ambrosio, M.R.; Beck Peccoz, P.; Bogazzi, F.; Cannavo’, S.; De Marinis, L.; De Menis, E.; Grottoli, S.; Pivonello, R. Use of Pegvisomant in acromegaly. An Italian Society of Endocrinology guideline. J. Endocrinol. Investig. 2014, 37, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Steuerman, R.; Shevah, O.; Laron, Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur. J. Endocrinol. 2011, 164, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, P.F.; Msaouel, P.; Koutsilieris, M. The role of the insulin-like growth factor-1 system in breast cancer. Mol. Cancer 2015, 14, 43. [Google Scholar] [CrossRef]
- Crudden, C.; Girnita, A.; Girnita, L. Targeting the IGF-1R: The tale of the tortoise and the hare. Front. Endocrinol. 2015, 6. [Google Scholar] [CrossRef]
- Major, J.M.; Laughlin, G.A.; Kritz-Silverstein, D.; Wingard, D.L.; Barrett-Connor, E. Insulin-like growth factor-I and cancer mortality in older men. J. Clin. Endocrinol. Metab. 2010, 95, 1054–1059. [Google Scholar] [CrossRef]
- Atzori, F.; Traina, T.A.; Ionta, M.T.; Massidda, B. Targeting insulin-like growth factor type 1 receptor in cancer therapy. Target. Oncol. 2009, 4, 255–266. [Google Scholar] [CrossRef]
- López-Calderero, I.; Chávez, E.S.; García-Carbonero, R. The insulin-like growth factor pathway as a target for cancer therapy. Clin. Transl. Oncol. 2010, 12, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.; Petnga, W.; Macaulay, V.M.; Weyer-Czernilofsky, U.; Bogenrieder, T. Insulin-Like Growth Factor (IGF) Pathway Targeting in Cancer: Role of the IGF Axis and Opportunities for Future Combination Studies. Target. Oncol. 2017, 12, 571–597. [Google Scholar] [CrossRef] [PubMed]
- Osher, E.; Macaulay, V.M. Therapeutic Targeting of the IGF Axis. Cells 2019, 8, 895. [Google Scholar] [CrossRef]
- Baserga, R. The decline and fall of the IGF-I receptor. J. Cell. Physiol. 2013, 228, 675–679. [Google Scholar] [CrossRef]
- De Groot, S.; Gelderblom, H.; Fiocco, M.; Vmgbovée, J.; Van der Hoeven, J.J.M.; Pijl, H.; Kroep, J.R. Serum levels of IGF-1 and IGF-BP3 are associated with event-free survival in adult ewing sarcoma patients treated with chemotherapy. Oncotargets Ther. 2017, 10, 2963–2970. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crown, J.; Sablin, M.-P.; Cortés, J.; Bergh, J.; Im, S.-A.; Lu, Y.S.; Martínez, N.; Neven, P.; Lee, K.S.; Morales, S.; et al. Abstract P6-21-01, Xentuzumab (BI 836845), an insulin-like growth factor (IGF)-neutralizing antibody (Ab), combined with exemestane and everolimus in hormone receptor-positive (HR+) locally advanced/metastatic breast cancer (LA/mBC): Randomized phase 2 re. Cancer Res. 2019, 79, P6-21-01. [Google Scholar] [CrossRef]
- Van Maldegem, A.M.; Bovée, J.V.M.G.; Peterse, E.F.P.; Hogendoorn, P.C.W.; Gelderblom, H. Ewing sarcoma: The clinical relevance of the insulin-like growth factor 1 and the poly-ADP-ribose-polymerase pathway. Eur. J. Cancer 2016, 53, 171–180. [Google Scholar] [CrossRef]
- Furlanetto, R.W.; DiCarlo, J.N. Somatomedin-C Receptors and Growth Effects in Human Breast Cells Maintained in Long-Term Tissue Culture. Cancer Res. 1984, 44, 2122–2128. [Google Scholar]
- Peyra, J.P.; Bonneterre, J.; Hecquet, B.; Vennin, P.; Louchez, M.M.; Fournier, C.; Lefebvre, J.; Demaille, A. Plasma insulin-like growth factor-1 (IGF-1) concentrations in human breast cancer. Eur. J. Cancer 1993, 29, 492–497. [Google Scholar] [CrossRef]
- Hankinson, S.E.; Willett, W.C.; Colditz, G.A.; Hunter, D.J.; Michaud, D.S.; Deroo, B.; Rosner, B.; Speizer, F.E.; Pollak, M. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 1998, 351, 1393–1396. [Google Scholar] [CrossRef]
- The Endogenous Hormones and Breast Cancer Collaborative Group. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: Pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010, 11, 530–542. [CrossRef]
- Murphy, N.; Knuppel, A.; Papadimitriou, N.; Martin, R.M.; Tsilidis, K.K.; Smith-Byrne, K.; Fensom, G.; Perez-Cornago, A.; Travis, R.C.; Key, T.J.; et al. Insulin-like growth factor-1, insulin-like growth factor-binding protein-3, and breast cancer risk: Observational and Mendelian randomization analyses with ∼430,000 women. Ann. Oncol. 2020, 31, 641–649. [Google Scholar] [CrossRef]
- Stattin, P.; Rinaldi, S.; Biessy, C.; Stenman, U.H.; Hallmans, G.; Kaaks, R. High levels of circulating insulin-like growth factor-I increase prostate cancer risk: A prospective study in a population-based nonscreened cohort. J. Clin. Oncol. 2004, 22, 3104–3112. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Stampfer, M.J.; Giovannucci, E.; Gann, P.H.; Ma, J.; Wilkinson, P.; Hennekens, C.H.; Pollak, M. Plasma insulin-like growth factor-I and prostate cancer risk: A prospective study. Science 1998, 279, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Lacey, J.V.; Hsing, A.W.; Fillmore, C.M.; Hoffman, S.; Helzlsouer, K.J.; Comstock, G.W. Null association between insulin-like growth factors, insulin-like growth factor-binding proteins, and prostate cancer in a prospective study. Cancer Epidemiol. Biomark. Prev. 2001, 10, 1101–1102. [Google Scholar]
- Woodson, K.; Tangrea, J.A.; Pollak, M.; Copeland, T.D.; Taylor, P.R.; Virtamo, J.; Albanes, D. Serum insulin-like growth factor I: Tumor marker or etiologic factor? A prospective study of prostate cancer among Finnish men. Cancer Res. 2003, 63, 3991–3994. [Google Scholar] [PubMed]
- Chen, C.; Lewis, S.K.; Voigt, L.; Fitzpatrick, A.; Plymate, S.R.; Weiss, N.S. Prostate carcinoma incidence in relation to prediagnostic circulating levels of insulin-like growth factor I, insulin-like growth factor binding protein 3, and insulin. Cancer 2005, 103, 76–84. [Google Scholar] [CrossRef]
- Meyer, F.; Galan, P.; Douville, P.; Bairati, I.; Kegle, P.; Bertrais, S.; Czernichow, S.; Hercberg, S. A prospective study of the insulin-like growth factor axis in relation with prostate cancer in the SU.VI.MAX trial. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2269–2272. [Google Scholar] [CrossRef][Green Version]
- Rowlands, M.A.; Gunnell, D.; Harris, R.; Vatten, L.J.; Holly, J.M.P.; Martin, R.M. Circulating insulin-like growth factor peptides and prostate cancer risk: A systematic review and meta-analysis. Int. J. Cancer 2009, 124, 2416–2429. [Google Scholar] [CrossRef]
- Travis, R.; Watts, E.; Fensom, G.; Perez-Cornago, A.; Knuppel, A.; Allen, N.; Gunter, M.; Martin, R.; Byrne, K.S.; Murphy, N.; et al. Serum Hormones and Prostate Cancer Incidence and Mortality in UK Biobank [abstract]. NCRI Cancer Conference. 2019. Available online: https://abstracts.ncri.org.uk/abstract/serum-hormones-and-prostate-cancer-incidence-and-mortality-in-uk-biobank/ (accessed on 17 January 2021).
- Mastrandrea, L.D.; Wactawski-Wende, J.; Donahue, R.P.; Hovey, K.M.; Clark, A.; Quattrin, T. Young women with type 1 diabetes have lower bone mineral density that persists over time. Diabetes Care 2008, 31, 1729–1735. [Google Scholar] [CrossRef]
- Van Sickle, B.J.; Simmons, J.; Hall, R.; Raines, M.; Ness, K.; Spagnoli, A. Increased circulating IL-8 is associated with reduced IGF-1 and related to poor metabolic control in adolescents with type 1 diabetes mellitus. Cytokine 2009, 48, 290–294. [Google Scholar] [CrossRef]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; De Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of st-elevation myocardial infarction: A report of the American college of cardiology foundation/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2013, 61, 78–140. [Google Scholar] [CrossRef]
- Cubbon, R.M.; Kearney, M.T.; Wheatcroft, S.B. Endothelial IGF-1 Receptor Signalling in Diabetes and Insulin Resistance. Trends Endocrinol. Metab. 2016, 27, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Brea, A.; Mosquera, D.; Martín, E.; Arizti, A.; Cordero, J.L.; Ros, E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: A case-control study. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.A.M.J.L.; Stolk, R.P.; Pols, H.A.P.; Grobbee, D.E.; Lamberts, S.W.J. Serum total IGF-I, free IGF-I, and IGFBP-1 levels in an elderly population: Relation to cardiovascular risk factors and disease. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Juul, A.; Scheike, T.; Davidsen, M.; Gyllenborg, J.; Jørgensen, T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: A population-based case-control study. Circulation 2002, 106, 939–944. [Google Scholar] [CrossRef]
- Laughlin, G.A.; Barrett-Connor, E.; Criqui, M.H.; Kritz-Silverstein, D. The Prospective Association of Serum Insulin-Like Growth Factor I (IGF-I) and IGF-Binding Protein-1 Levels with All Cause and Cardiovascular Disease Mortality in Older Adults: The Rancho Bernardo Study. J. Clini. Endocrinol. Metab. 2004, 89, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Page, J.H.; Ma, J.; Pollak, M.; Manson, J.A.E.; Hankinson, S.E. Plasma insulinlike growth factor 1 and binding-protein 3 and risk of myocardial infarction in women: A prospective study. Clin. Chem. 2008, 54, 1682–1688. [Google Scholar] [CrossRef]
- Ichikawa, T.; Nakao, K.; Hamasaki, K.; Furukawa, R.; Tsuruta, S.; Ueda, Y.; Taura, N.; Shibata, H.; Fujimoto, M.; Toriyama, K.; et al. Role of growth hormone, insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 in development of non-alcoholic fatty liver disease. Hepatol. Int. 2007, 1, 287–294. [Google Scholar] [CrossRef] [PubMed]
- De Groof, F.; Joosten, K.F.M.M.; Janssen, J.A.M.J.L.; De Kleijn, E.D.; Hazelzet, J.A.; Hop, W.C.J.J.; Uitterlinden, P.; Van Doorn, J.; Hokken-Koelega, A.C.S. Acute stress response in children with meningococcal sepsis: Important differences in the growth hormone/insulin-like growth factor I axis between nonsurvivors and survivors. J. Clin. Endocrinol. Metab. 2002, 87, 3118–3124. [Google Scholar] [CrossRef]
- Ashare, A.; Nymon, A.B.; Doerschug, K.C.; Morrison, J.M.; Monick, M.M.; Hunninghake, G.W. Insulin-like growth factor-1 improves survival in sepsis via enhanced hepatic bacterial clearance. Am. J. Respir. Crit. Care Med. 2008, 178, 149–157. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, S.; Li, Y.; Gao, F.; Cao, Y.; Zhao, X.; Gao, G.; Li, L. Effects of early administration of insulin-like growth factor-1 on cognitive function in septic encephalopathy. Neuropsychiatr. Dis. Treat. 2019, 15, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Doper Turned Informer, Conte Offers to Help Clean up Sport|Reuters. Available online: https://www.reuters.com/article/us-olympics-rio-doping-conte/doper-turned-informer-conte-offers-to-help-clean-up-sport-idUKKCN10J1EZ (accessed on 17 January 2021).
- A Miami Clinic Supplies Drugs to Sports’ Biggest Names|Miami New Times. Available online: https://www.miaminewtimes.com/news/a-miami-clinic-supplies-drugs-to-sports-biggest-names-6396907 (accessed on 17 January 2021).
- Michael Rasmussen Confesses to 12 Years of Doping|Cyclingnews. Available online: https://www.cyclingnews.com/news/michael-rasmussen-confesses-to-12-years-of-doping/ (accessed on 17 January 2021).
- Fintini, D.; Brufani, C.; Cappa, M. Profile of mecasermin for the long-term treatment of growth failure in children and adolescents with severe primary IGF-1 deficiency. Ther. Clin. Risk Manag. 2009, 5, 553–559. [Google Scholar] [CrossRef]
- Kemp, S.F. Insulin-like growth factor-i deficiency in children with growth hormone insensitivity: Current and future treatment options. BioDrugs 2009, 23, 155–163. [Google Scholar] [CrossRef]
- Kemp, S.F. Mecasermin rinfabate. Drugs Today 2007, 43, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, A.L. Mecasermin (recombinant human insulin-like growth factor I). Adv. Ther. 2009, 26, 40–54. [Google Scholar] [CrossRef]
- Tomas, F.M.; Lemmey, A.B.; Read, L.C.; Ballard, F.J. Superior potency of infused IGF-I analogue which bind poorly to IGF-binding proteins is maintained when administered by injection. J. Endocrinol. 1996, 150, 77–84. [Google Scholar] [CrossRef]
- Ballard, F.J.; Wallace, J.C.; Francis, G.L.; Read, L.C.; Tomas, F.M. Des(1-3)IGF-I: A truncated form of insulin-like growth Factor-I. Int. J. Biochem. Cell Biol. 1996, 28, 1085–1087. [Google Scholar] [CrossRef]
- Tomas, F.M.; Knowles, S.E.; Owens, P.C.; Chandler, C.S.; Francis, G.L.; Read, L.C.; Ballard, F.J. Insulin-like growth factor-I (IGF-I) and especially IGF-I variants are anabolic in dexamethasone-treated rats. Biochem. J. 1992, 282, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ernst, S.; Simon, P. A quantitative approach for assessing significant improvements in elite sprint performance: Has IGF-1 entered the arena? Drug Test. Anal. 2013, 5, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Guha, N.; Dashwood, A.; Thomas, N.J.; Skingle, A.J.; Sönksen, P.H. Holt RIG. IGF-I abuse in sport. Curr. Drug Abus. Rev. 2009, 2, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Cox, H.D.; Eichner, D. Detection of human insulin-like growth factor-1 in deer antler velvet supplements. Rapid Commun. Mass Spectrom. 2013, 27, 2170–2178. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.R.; Davies, B.; Grace, F.M.; Evans, P.J.; Baker, J.S. Exercise, Science and Designer Doping: Traditional and Emerging Trends. J. Steroids Horm. Sci. 2012, 3, 1. [Google Scholar] [CrossRef]
- Wallace, J.D.; Cuneo, R.C.; Lundberg, P.A.; Rosén, T.; Jørgensen, J.O.L.; Longobardi, S.; Keay, N.; Sacca, L.; Christiansen, J.S.; Bengtsson, B.A.; et al. Responses of Markers of Bone and Collagen Turnover to Exercise, Growth Hormone (GH) Administration, and GH Withdrawal in Trained Adult Males1. J. Clin. Endocrinol. Metab. 2000, 85, 124–133. [Google Scholar] [CrossRef]
- Sönksen, P. The International Olympic Committee (IOC) and GH-2000. Growth Horm. IGF Res. 2009, 19, 341–345. [Google Scholar] [CrossRef]
- Holt, R.I.G.; Bassett, E.E.; Erotokritou-Mulligan, I.; McHugh, C.; Cowan, D.; Bartlett, C.; Sönksen, P.H. Moving one step closer to catching the GH cheats: The GH-2004 experience. Growth Horm. IGF Res. 2009, 19, 346–351. [Google Scholar] [CrossRef]
- Powrie, J.K.; Bassett, E.E.; Rosen, T.; Jørgensen, J.O.; Napoli, R.; Sacca, L.; Christiansen, J.S.; Bengtsson, B.A.; Sönksen, P.H. Detection of growth hormone abuse in sport. Growth Horm. IGF Res. 2007, 17, 220–226. [Google Scholar] [CrossRef]
- Erotokritou-Mulligan, I.; Guha, N.; Stow, M.; Bassett, E.E.; Bartlett, C.; Cowan, D.A.; Sönksen, P.H.; Holt, R.I.G. The development of decision limits for the implementation of the GH-2000 detection methodology using current commercial insulin-like growth factor-I and amino-terminal pro-peptide of type III collagen assays. Growth Horm. IGF Res. 2012, 22, 53–58. [Google Scholar] [CrossRef]
- Holt, R.I.G.; Erotokritou-Mulligan, I.; Bartlett, C.; Sonksen, P. The GH-2004 project: The response of IGF1 and type III pro-collagen to the administration of exogenous GH in non-Caucasian amateur athletes Review of control of body composition European. J. Endocrinol. 2010, 163, 45–54. [Google Scholar] [CrossRef]
- Guha, N.; Erotokritou-Mulligan, I.; Bartlett, C.; Nevitt, S.P.; Francis, M.; Bassett, E.E.; Cowan, D.A.; Sönksen, P.H.; Holt, R.I.G. Biochemical Markers of Insulin-Like Growth Factor-I Misuse in Athletes: The Response of Serum IGF-I, Procollagen Type III Amino-Terminal Propeptide, and the GH-2000 Score to the Administration of rhIGF-I/rhIGF Binding Protein-3 Complex. J. Clin. Endocrinol. Metab. 2014, 99, 2259–2268. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Evans, M.J.; Livesey, J.H.; Ellis, M.J.; Yandle, T.G. Effect of anticoagulants and storage temperatures on stability of plasma and serum hormones. Clin. Biochem. 2001, 34, 107–112. [Google Scholar] [CrossRef]
- Hartog, H.; Van der Graaf, W.T.A.; Wesseling, J.; Van der Veer, E.; Boezen, H.M. Measurement of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 after delayed separation of whole blood samples. Clin. Biochem. 2008, 41, 636–639. [Google Scholar] [CrossRef]
- Elmlinger, M.W.; Zwirner, M.; Kühnel, W. Stability of insulin-like growth factor (IGF)-I and IGF binding protein (IGFBP)-3 measured by the IMMULITE® automated chemiluminescence assay system in different blood specimens. Clin. Lab. 2005, 51, 145–152. [Google Scholar]
- Yu, H.; Mistry, J.; Nicar, M.J.; Khosravi, M.J.; Diamandis, A.; Van Doorn, J.; Juul, A. Insulin-like growth factors (IGF-I, free IGF-I, and IGF-II) and insulin- like growth factor binding proteins (IGFBP-2, IGFBP-3, IGFBP-6, and ALS) in blood circulation. J. Clin. Lab. Anal. 1999, 13, 166–172. [Google Scholar] [CrossRef]
- Baxter, R.C.; Martin, J.L. Radioimmunoassay of growth hormone-dependent insulinlike growth factor binding protein in human plasma. J. Clin. Investig. 1986, 78, 1504–1512. [Google Scholar] [CrossRef]
- Frystyk, J.; Freda, P.; Clemmons, D.R. The current status of IGF-I assays—A 2009 update. Growth Horm. Igf Res. 2010, 20, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, D.R. IGF-I assays: Current assay methodologies and their limitations. Pituitary 2007, 10, 121–128. [Google Scholar] [CrossRef]
- Bidlingmaier, M.; Freda, P.U. Measurement of human growth hormone by immunoassays: Current status, unsolved problems and clinical consequences. Growth Horm. IGF Res. 2010, 20, 19–25. [Google Scholar] [CrossRef]
- Burns, C.; Rigsby, P.; Moore, M.; Rafferty, B. The First International Standard for Insulin-like Growth Factor-1 (IGF-1) for immunoassay: Preparation and calibration in an international collaborative study. Growth Horm. Igf Res. 2009, 19, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Quarmby, V.; Quan, C. How much insulin-like growth factor-I (IGF-I) circulates? Impact of standardization on IGF-I assay accuracy. Dev. Biol. Stand. 1999, 97, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Krebs, A.; Wallaschofski, H.; Spilcke-Liss, E.; Kohlmann, T.; Brabant, G.; Völzke, H.; Nauck, M. Five commercially available insulin-like growth factor I (IGF-I) assays in comparison to the former Nichols Advantage IGF-I in a growth hormone treated population. Clin. Chem. Lab. Med. 2008, 46, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Brabant, G.; Wallaschofski, H. Normal levels of serum IGF-I: Determinants and validity of current reference ranges. Pituitary 2007, 10, 129–133. [Google Scholar] [CrossRef]
- Khosravi, J.; Diamandi, A.; Bodani, U.; Khaja, N.; Krishna, R.G. Pitfalls of immunoassay and sample for IGF-I: Comparison of different assay methodologies using various fresh and stored serum samples. Clin. Biochem. 2005, 38, 659–666. [Google Scholar] [CrossRef]
- Clemmons, D.R. Consensus statement on the standardization and evaluation of growth hormone and insulin-like growth factor assays. Clin. Chem. 2011, 57, 555–559. [Google Scholar] [CrossRef]
| Hormones and Pro-Hormones | IGF Receptors | IGF Binding Proteins (IGFBPs) | IGFBP Related Proteins (IGFBP-rPs) 1 |
|---|---|---|---|
| IGF-1 (P05019, | IGF-1R (P08069) 2 | IGFBP-1 (P08833) | IGFBP-rP1/GFBP-7 (Q16270) |
| and Ea, Eb, Ec | IGF-2R (P11717) 2 | IGFBP-2 (P18065) | IGFBP-rP2/IGFBP-8 (P29279) |
| pos. 119–195) 3 | I-R (P06213) 2 | IGFBP-3 (P17936) | IGFBP-rP3/IGFBP-9 (P48745) |
| IGF-2 (P01344) | ________________________ | IGFBP-4 (P22692) | IGFBP-rP4/IGFBP-10 (O00622) |
| Insulin (P01308) | IGFBPR [29] | IGFBP-5 (P24593) | IGFBP-rP5/L56/HtrA (Q92743) |
| _______________________ | IGFBP-6 (P24592) | IGFBP-rP6/ESM-1 (Q9NQ30) | |
| IGF-1 LR3 4 | _______________________________ | IGFBP-rP7/WISP-2 (O76076) | |
| des(1–3)IGF-1 5 | IGFBPL1 (Q8WX77) 8 | IGFBP-rP8/WISP-1 (O95388) | |
| Mecasermin 6 | _______________________________ | IGFBP-rP9/WISP-3 (O95389) | |
| Mecasermin rinfabate 7 | ALS (P35858) 9 | IGFBP-rP10/KAZALD1 (Q96I82) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailes, J.; Soloviev, M. Insulin-Like Growth Factor-1 (IGF-1) and Its Monitoring in Medical Diagnostic and in Sports. Biomolecules 2021, 11, 217. https://doi.org/10.3390/biom11020217
Bailes J, Soloviev M. Insulin-Like Growth Factor-1 (IGF-1) and Its Monitoring in Medical Diagnostic and in Sports. Biomolecules. 2021; 11(2):217. https://doi.org/10.3390/biom11020217
Chicago/Turabian StyleBailes, Julian, and Mikhail Soloviev. 2021. "Insulin-Like Growth Factor-1 (IGF-1) and Its Monitoring in Medical Diagnostic and in Sports" Biomolecules 11, no. 2: 217. https://doi.org/10.3390/biom11020217
APA StyleBailes, J., & Soloviev, M. (2021). Insulin-Like Growth Factor-1 (IGF-1) and Its Monitoring in Medical Diagnostic and in Sports. Biomolecules, 11(2), 217. https://doi.org/10.3390/biom11020217







