Microwave-Assisted Rapid Green Synthesis of Gold Nanoparticles Using Seed Extract of Trachyspermum ammi: ROS Mediated Biofilm Inhibition and Anticancer Activity
Abstract
1. Introduction
2. Material and Methods
2.1. Synthesis and Characterization of Gold Nanoparticles (AuNPs) Using T. ammi Seed Extract
2.2. Evaluation of Minimum Inhibitory Concentration (MIC)
2.3. Biofilm Inhibition Activity
2.4. Extraction and Quantification of Exopolysaccharides (EPS)
2.5. Swarming Motility Assay
2.6. MATH Assay
2.7. Disruption of Mature (Preformed) Biofilm
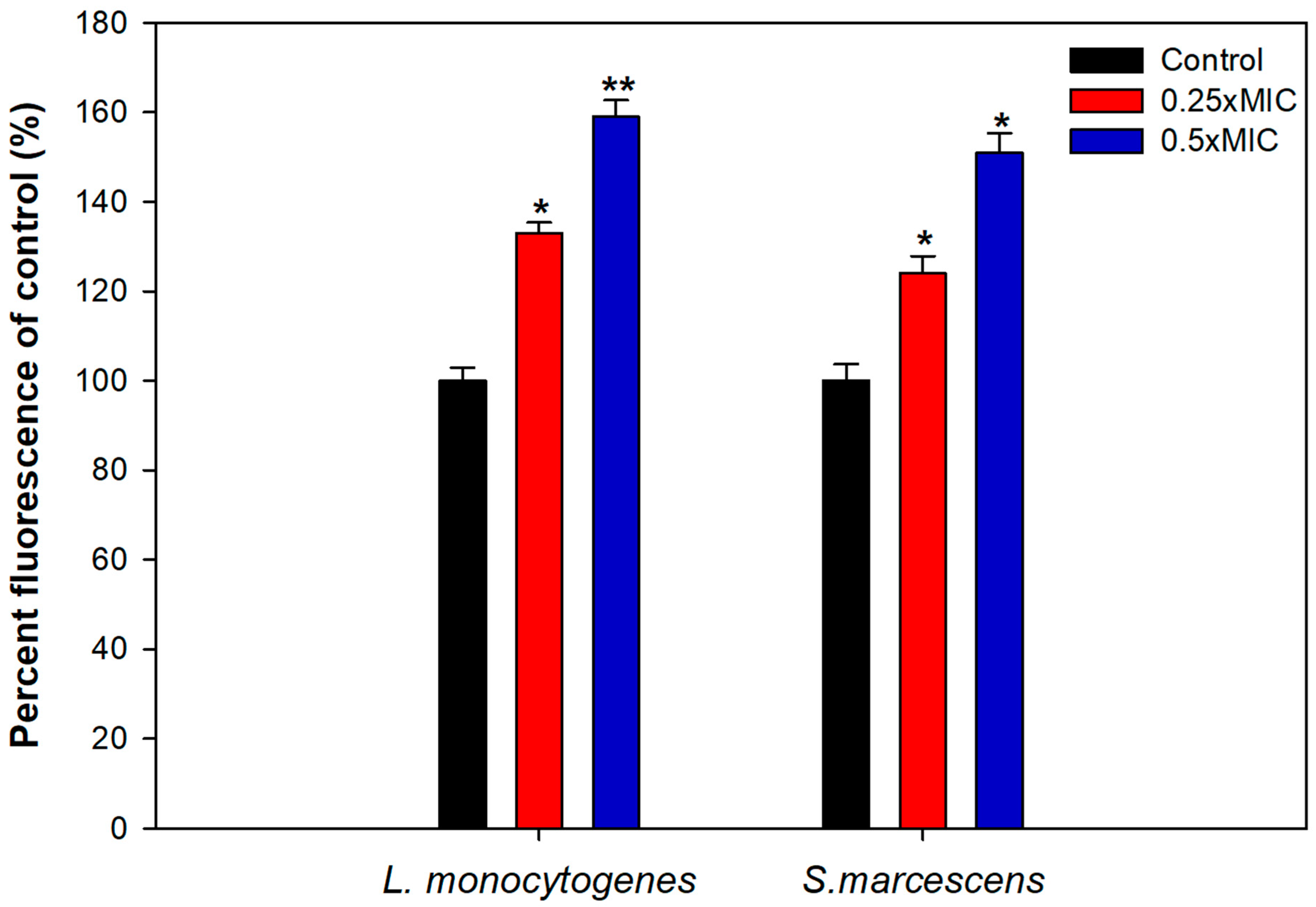
2.8. ROS Generation
2.9. Cell Viability Assay/MTT Assay
2.10. Protein Determination (Bradford Assay)
2.11. Measurement of GSH Levels in HepG2 Cancer Cell LINES
2.12. Estimation of Lipid Peroxidation in HepG2 Cancer Cell Lines
3. Results and Discussion
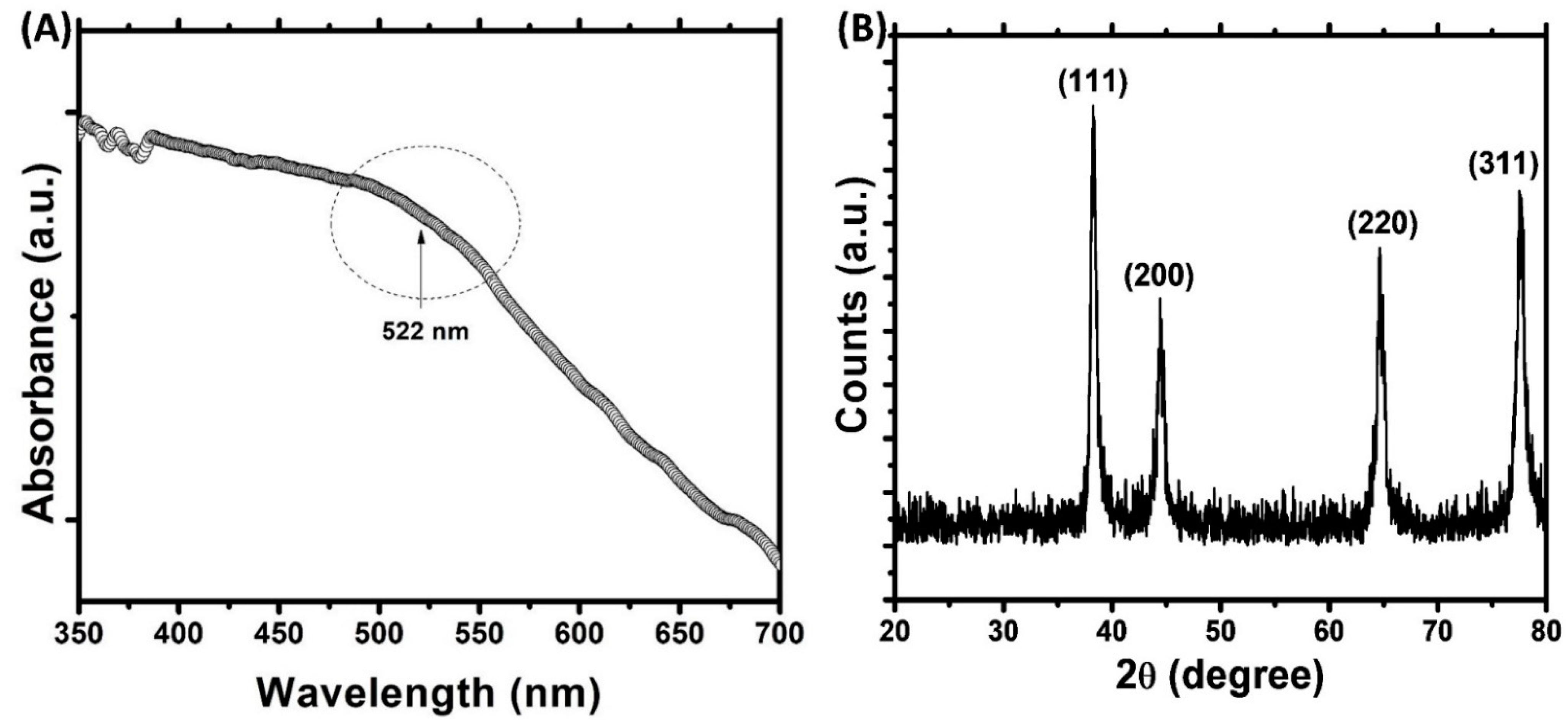
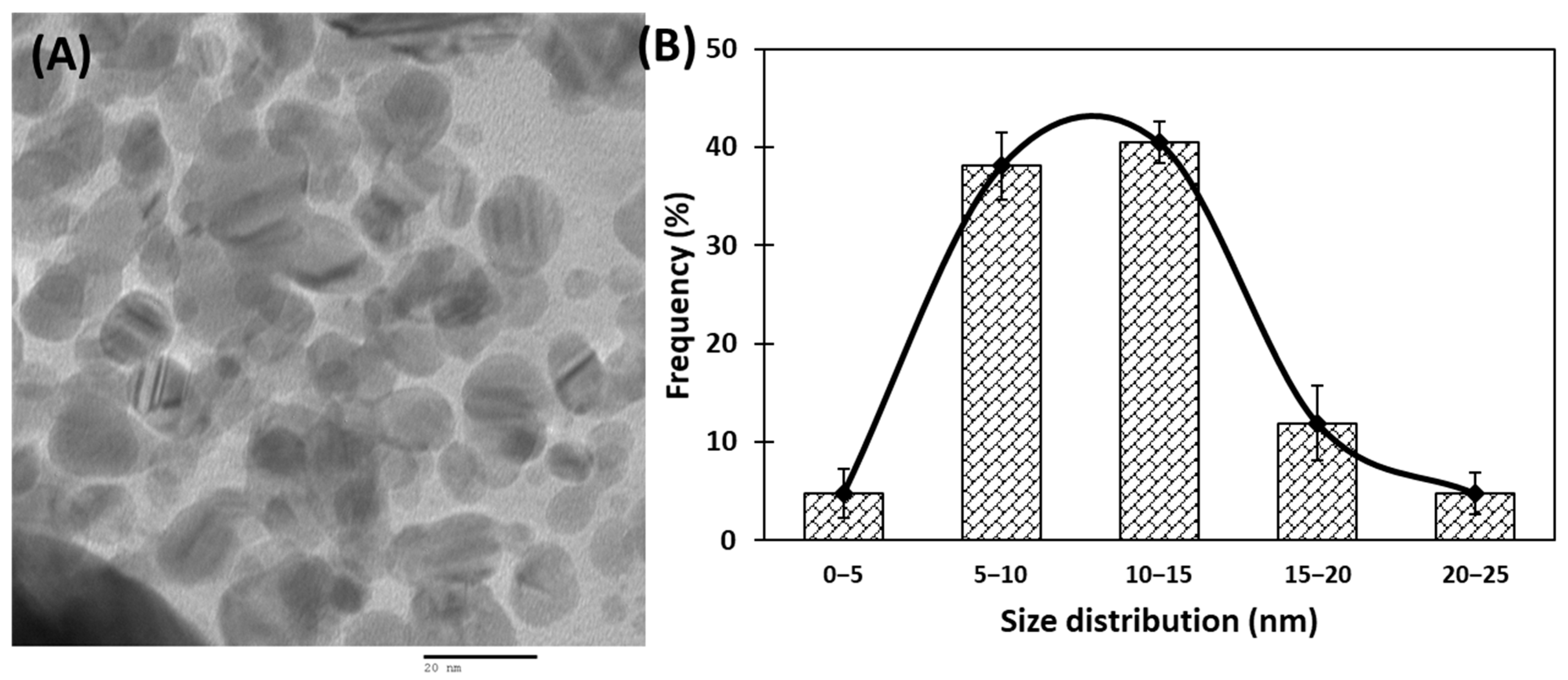
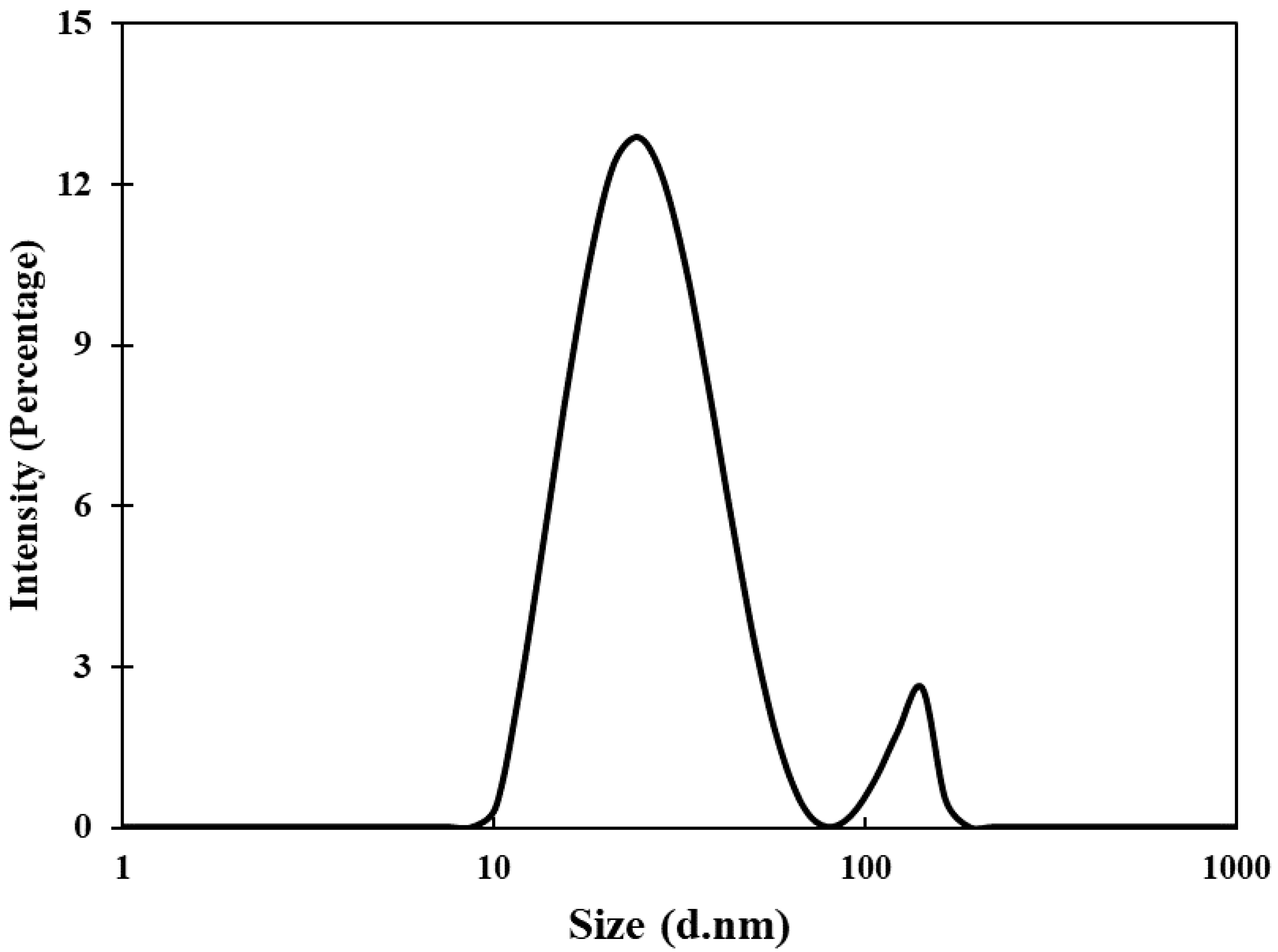
3.1. Synthesis and Characterization of AuNPs
3.2. Minimum Inhibitory Concentration (MIC)
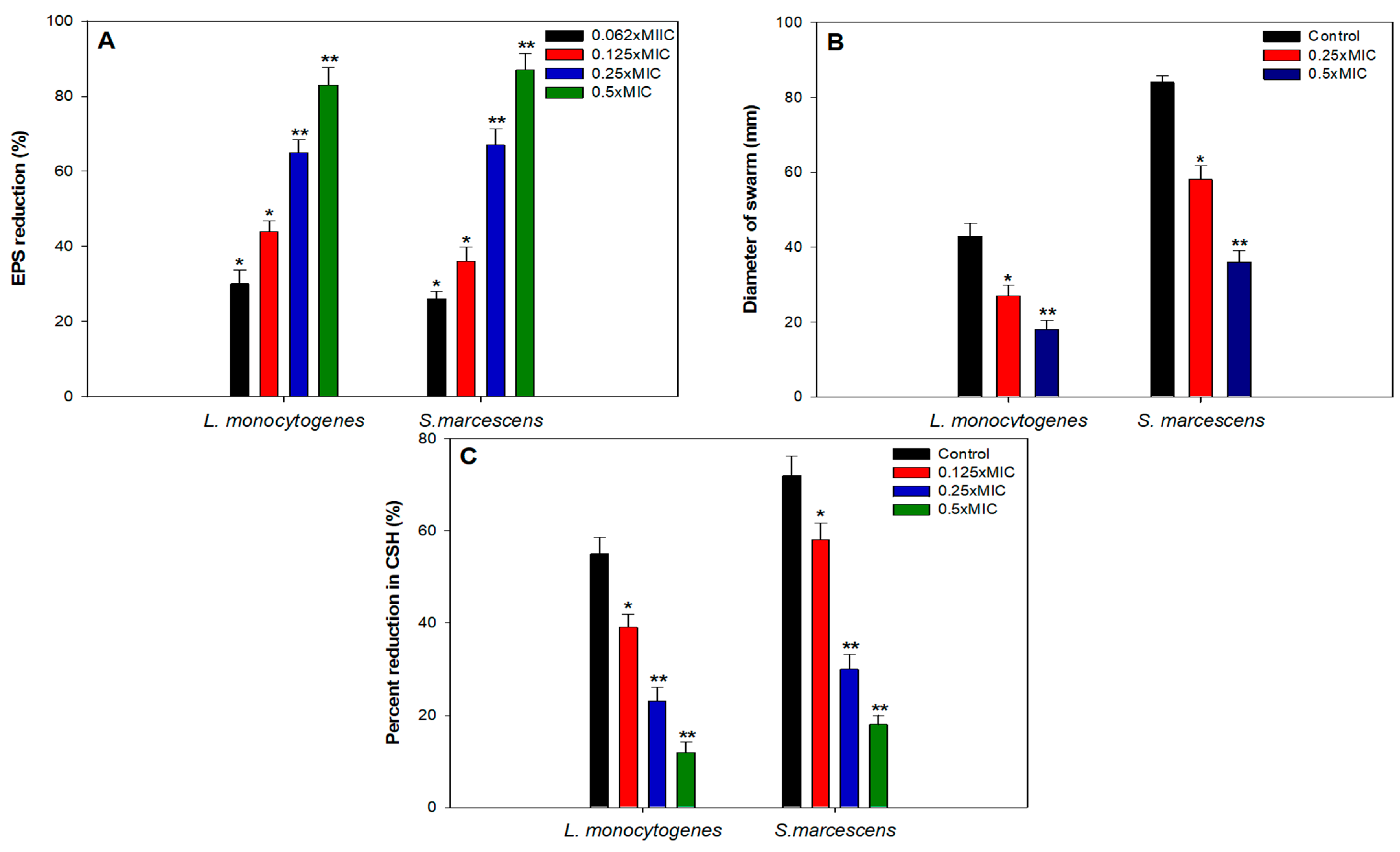
3.3. Biofilm Inhibition Studies
3.4. Effect of Sub-MICs of TA-AuNPs Biofilm Related Virulence Traits
EPS
3.5. Swarming Motility
3.6. Cell Surface Hydrophobicity (CSH)
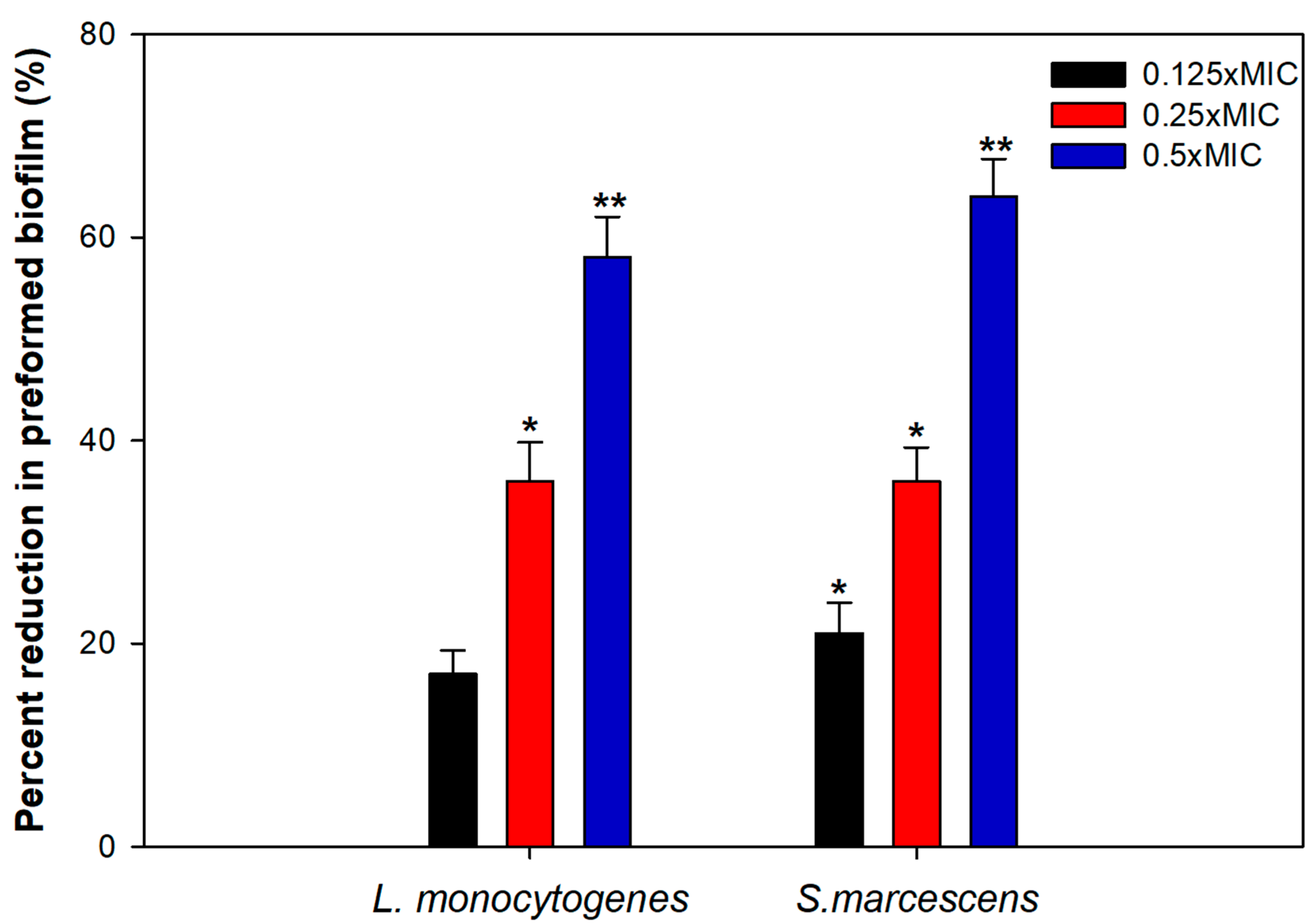
3.7. Effect on Preformed Biofilms
3.8. Mechanism of Biofilm Inhibition
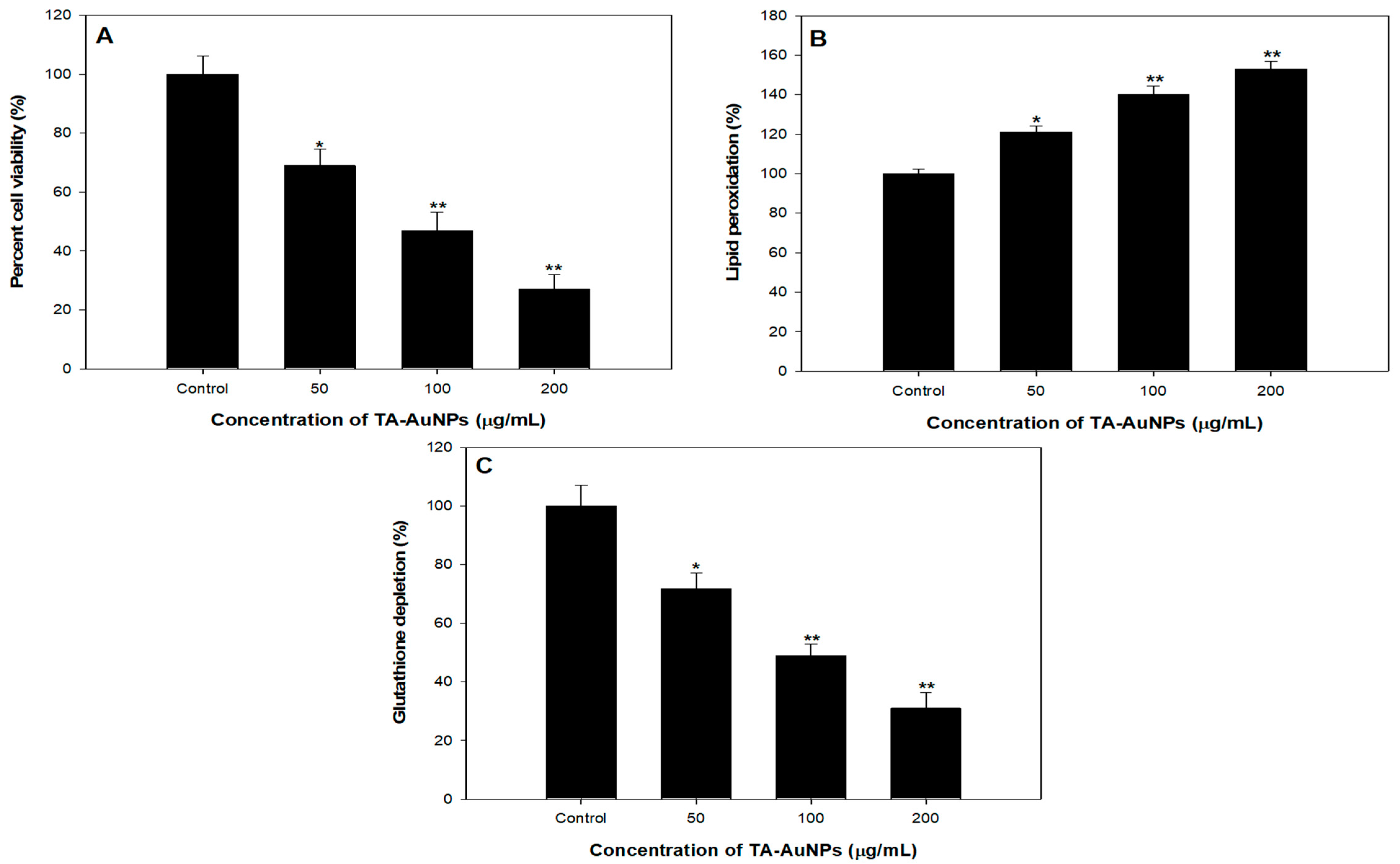
4. TA-AuNPs Inhibits Growth and Viability of HepG2 Cancer Cells
5. Effect of TA-AuNPs Treatment on Lipid Peroxidation in HepG2 Cancer Cells
6. TA-AuNPs Treatment Leads to Depletion of Intracellular Glutathione (GSH) of HepG2 Cancer Cells
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andujar, B.C.; Tung, L.D.; Thanh, N.T.K. Synthesis of nanoparticles for biomedical applications. Annu. Rep. Sect. A Inorg. Chem. 2010, 106, 553. [Google Scholar] [CrossRef]
- Qamar, S.A.; Asgher, M.; Khalid, N.; Sadaf, M. Nanobiotechnology in health sciences: Current applications and future perspectives. Biocatal. Agric. Biotechnol. 2019, 22. [Google Scholar] [CrossRef]
- Kubik, B.K.; Sugisaka, M. From molecular biology to nanotechnology and nanomedicine. Biosystems 2002, 65, 123–138. [Google Scholar] [CrossRef]
- Qais, F.A.; Samreen; Ahmad, I. Green Synthesis of Metal Nanoparticles: Characterization and their Antibacterial Efficacy. In Antibacterial Drug Discovery to Combat MDR; Springer: Singapore, 2019; pp. 635–680. [Google Scholar]
- Zhang, D.; Ma, X.; Gu, Y.; Huang, H.; Zhang, G. Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer. Front. Chem. 2020, 8. [Google Scholar] [CrossRef]
- Joerger, R.; Klaus, T.; Granqvist, C.G. Biologically Produced Silver-Carbon Composite Materials for Optically Functional Thin-Film Coatings. Adv. Mater. 2000, 12, 407–409. [Google Scholar] [CrossRef]
- Chauhan, R.P.; Charu, G.; Dhan, P. Methodological advancements in green nanotechnology and their applications in biological synthesis of herbal nanoparticles. Int. J. Bioassays 2012, 1, 6–10. [Google Scholar]
- Ingale, A.G. Biogenic Synthesis of Nanoparticles and Potential Applications: An Eco-Friendly Approach. J. Nanomed. Nanotechnol. 2013, 04. [Google Scholar] [CrossRef]
- Mukherjee, S.; Patra, C.R. Biologically synthesized metal nanoparticles: Recent advancement and future perspectives in cancer theranostics. Futur. Sci. OA 2017, 3, FSO203. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnology 2018, 16. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Green, L.A.W. Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Rauwel, P.; Rauwel, E. Emerging Trends in Nanoparticle Synthesis Using Plant Extracts for Biomedical Applications. Glob. J. Nanomedicine 2017, 1, 555562. [Google Scholar] [CrossRef]
- Husain, F.M.; Khan, M.S.; Siddiqui, S.; Khan, A.; Arshad, M.; Alyousef, A.A.; Rahman, M.; Al-Shabib, N.A.; Ahmad, I. Nanoparticles as new emerging antibacterials: Potentials and limitations. Antibact. Drug Discov. Combat MDR 2019, 561–579. [Google Scholar] [CrossRef]
- Shakoor, S.; Mills, P.J.A.; Hasan, R. Antibiotic-Resistant Enteric Infections. Infect. Dis. Clin. N. Am. 2019, 33, 1105–1123. [Google Scholar] [CrossRef] [PubMed]
- Andleeb, S.; Majid, M.; Sardar, S. Environmental and public health effects of antibiotics and AMR/ARGs. In Antibiotics and Antimicrobial Resistance Genes in the Environment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 269–291. [Google Scholar]
- Lopez, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold Spring Harb. Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilm Formation: A Clinically Relevant Microbiological Process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Schachter, B. Slimy business—the biotechnology of biofilms. Nat. Biotechnol. 2003, 21, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Rodrigues, C.F. Biomaterial-Related Infections. J. Clin. Med. 2020, 9, 722. [Google Scholar] [CrossRef] [PubMed]
- Lasa, I.; Pozo, J.L.; Penadés, J.R.; Leiva, J. Biofilms bacterianos e infección. An. Sist. Sanit. Navar. 2005, 28, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Farland, L.V. Normal flora: Diversity and functions. Microb. Ecol. Health Dis. 2000, 12, 193–207. [Google Scholar] [CrossRef]
- Oliver, S.P.; Jayarao, B.M.; Almeida, R.A. Foodborne Pathogens in Milk and the Dairy Farm Environment: Food Safety and Public Health Implications. Foodborne Pathog. Dis. 2005, 2, 115–129. [Google Scholar] [CrossRef]
- Raposo, A.; Pérez, E.; Faria, C.T.; Ferrús, M.A.; Carrascosa, C. Food Spoilage by Pseudomonas spp.-An Overview. In Foodborne Pathogens and Antibiotic Resistance; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 41–71. [Google Scholar]
- Gram, L.; Ravn, L.; Rasch, M.; Bruhn, J.B.; Christensen, A.B.; Givskov, M. Food spoilage—Interactions between food spoilage bacteria. Int. J. Food Microbiol. 2002, 78, 79–97. [Google Scholar] [CrossRef]
- Priyanka, B.; Patil, R.; Dwarakanath, S. A review on detection methods used for foodborne pathogens. Indian J. Med. Res. 2016, 144, 327. [Google Scholar] [CrossRef]
- Smith, J.; Fratamico, P.M.; Uhlich, G. Molecular mechanisms involved in biofilm formation by food-associated bacteria. In Biofilms in the Food and Beverage Industries; Elsevier: Cambridge, UK, 2009; pp. 42–98. [Google Scholar]
- Assefa, G.A.; Mesfin, A.A.; Akele, L.M.; Alemu, K.A.; Gangapuram, B.R.; Guttena, V.; Alle, M. Microwave-Assisted Green Synthesis of Gold Nanoparticles Using Olibanum Gum (Boswellia serrate) and its Catalytic Reduction of 4-Nitrophenol and Hexacyanoferrate (III) by Sodium Borohydride. J. Clust. Sci. 2017, 28, 917–935. [Google Scholar] [CrossRef]
- Qais, F.A.; Shafiq, A.; Khan, H.M.; Husain, F.M.; Khan, R.A.; Alenazi, B.; Alsalme, A.; Ahmad, I. Antibacterial effect of silver nanoparticles synthesized using Murraya koenigii (L.) against multidrug-resistant pathogens. Bioinorg. Chem. Appl. 2019, 2019. [Google Scholar] [CrossRef]
- Husain, F.M.; Ansari, A.A.; Khan, A.; Ahmad, N.; Albadri, A.; Albalawi, T.H. Mitigation of acyl-homoserine lactone (AHL) based bacterial quorum sensing, virulence functions, and biofilm formation by yttrium oxide core/shell nanospheres: Novel approach to combat drug resistance. Sci. Rep. 2019, 9, 18476. [Google Scholar] [CrossRef]
- Zubair, M.; Husain, F.M.; Qais, F.A.; Alam, P.; Ahmad, I.; Albalawi, T.; Ahmad, N.; Alam, M.; Baig, M.H.; Dong, J.J.; et al. Bio-fabrication of titanium oxide nanoparticles from Ochradenus arabicus to obliterate biofilms of drug-resistant Staphylococcus aureus and Pseudomonas aeruginosa isolated from diabetic foot infections. Appl. Nanosci. 2021. [Google Scholar] [CrossRef]
- Qais, F.A.; Khan, M.S.; Ahmad, I. Broad-spectrum quorum sensing and biofilm inhibition by green tea against gram-negative pathogenic bacteria: Deciphering the role of phytocompounds through molecular modelling. Microb. Pathog. 2019, 126, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Sivaranjani, M.; Gowrishankar, S.; Kamaladevi, A.; Pandian, S.K.; Balamurugan, K.; Ravi, A.V. Morin inhibits biofilm production and reduces the virulence of Listeria monocytogenes—An in vitro and in vivo approach. Int. J. Food Microbiol. 2016, 237, 73–82. [Google Scholar] [CrossRef]
- Qais, F.A.; Shafiq, A.; Ahmad, I.; Husain, F.M.; Khan, R.A.; Hassan, I. Green synthesis of silver nanoparticles using Carum copticum: Assessment of its quorum sensing and biofilm inhibitory potential against gram negative bacterial pathogens. Microb. Pathog. 2020, 144. [Google Scholar] [CrossRef]
- Hasan, I.; Qais, F.A.; Husain, F.M.; Khan, R.A.; Alsalme, A.; Alenazi, B.; Usman, M.; Jaafar, M.H.; Ahmad, I. Eco-friendly green synthesis of dextrin based poly (methyl methacrylate) grafted silver nanocomposites and their antibacterial and antibiofilm efficacy against multi-drug resistance pathogens. J. Clean. Prod. 2019, 230, 1148–1155. [Google Scholar] [CrossRef]
- Qais, F.A.; Samreen; Ahmad, I. Broad-spectrum inhibitory effect of green synthesised silver nanoparticles from Withania somnifera (L.) on microbial growth, biofilm and respiration: A putative mechanistic approach. IET Nanobiotechnology 2018, 12, 325–335. [Google Scholar] [CrossRef]
- Afsar, T.; Trembley, J.H.; Salomon, C.E.; Razak, S.; Khan, M.R.; Ahmed, K. Growth inhibition and apoptosis in cancer cells induced by polyphenolic compounds of Acacia hydaspica: Involvement of multiple signal transduction pathways. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Okuno, S.; Sato, H.; Matsumura, K.K.; Tamba, M.; Wang, H.; Sohda, S.; Hamada, H.; Yoshikawa, H.; Kondo, T.; Bannai, S. Role of cystine transport in intracellular glutathione level and cisplatin resistance in human ovarian cancer cell lines. Br. J. Cancer 2003, 88, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.N.; Riyazuddin; Babji, P.; Ahmad, N.; Khan, R.A.; Hassan, I.; Shahzad, S.A.; Husain, F.M. Green synthesis and structural classification of Acacia nilotica mediated-silver doped titanium oxide (Ag/TiO2) spherical nanoparticles: Assessment of its antimicrobial and anticancer activity. Saudi J. Biol. Sci. 2019, 26, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Rajkumari, J.; Busi, S.; Vasu, A.C.; Reddy, P. Facile green synthesis of baicalein fabricated gold nanoparticles and their antibiofilm activity against Pseudomonas aeruginosa PAO1. Microb. Pathog. 2017, 107, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Bhatnagar, A.; Tripathi, S.K. Size Tunable Green Synthesis of Silver Nanoparticles Using Trachyspermum Ammi (Ajwain) and Their Effect on a B Cell Line. J. Nanoeng. Nanomanufacturing 2013, 3, 154–161. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Khan, S.A.; Shahid, S.; Lee, C.S. Green Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Clerodendrum inerme; Characterization, Antimicrobial, and Antioxidant Activities. Biomolecules 2020, 10, 835. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Varadan, S.; Anthony, K.J.P.; Murugan, M.; Raja, A.; Gurunathan, S. Antimicrobial and anticoagulation activity of silver nanoparticles synthesized from the culture supernatant of Pseudomonas aeruginosa. J. Ind. Eng. Chem. 2013, 19, 1299–1303. [Google Scholar] [CrossRef]
- Lee, X.K.; Shameli, K.; Miyake, M.; Kuwano, N.; Khairudin, A.N.B.; Mohamad, S.E.; Yew, Y.P. Green Synthesis of Gold Nanoparticles Using Aqueous Extract of Garcinia mangostana Fruit Peels. J. Nanomater. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnology 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F.C.; George, A.T. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Beshay, M.; Mokkapati, V.R.S.S.; Garnaes, J.; Olsson, M.E.; Sultan, A.; Mackevica, A.; Mateiu, R.V.; Lütken, H.; et al. Anti-biofilm effects of gold and silver nanoparticles synthesized by the Rhodiola rosea rhizome extracts. Artif. Cells Nanomed. Biotechnol. 2018, 46, S886–S899. [Google Scholar] [CrossRef] [PubMed]
- Uru, C.; Chopo, E.G.; Tommassen, J.; Mainar, J.R.C. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2021, 10, 3. [Google Scholar] [CrossRef]
- Shabib, N.A.; Husain, F.M.; Nadeem, M.; Khan, M.S.; Qurainy, F.; Alyousef, A.A.; Arshad, M.; Khan, A.; Khan, J.M.; Alam, P.; et al. Bio-inspired facile fabrication of silver nanoparticles from in vitro grown shoots of Tamarix nilotica: Explication of its potential in impeding growth and biofilms of Listeria monocytogenes and assessment of wound healing ability. RSC Adv. 2020, 10, 30139–30149. [Google Scholar] [CrossRef]
- Husain, F.M.; Ahmad, I.; Baig, M.H.; Khan, M.S.; Khan, M.S.; Hassan, I.; Shabib, N.A. Broad-spectrum inhibition of AHL-regulated virulence factors and biofilms by sub-inhibitory concentrations of ceftazidime. RSC Adv. 2016, 6. [Google Scholar] [CrossRef]
- Shabib, N.A.; Husain, F.M.; Ahmad, N.; Qais, F.A.; Khan, A.; Khan, A.; Khan, M.S.; Khan, J.M.; Shahzad, S.A.; Ahmad, I. Facile Synthesis of Tin Oxide Hollow Nanoflowers Interfering with Quorum Sensing-Regulated Functions and Bacterial Biofilms. J. Nanomater. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Shabib, N.A.; Husain, F.M.; Ahmed, F.; Khan, R.A.; Khan, M.S.; Ansari, F.A.; Alam, M.Z.; Ahmed, M.A.; Khan, M.S.; Baig, M.H.; et al. Low temperature synthesis of superparamagnetic iron oxide (Fe3O4) nanoparticles and their ROS mediated inhibition of biofilm formed by food-associated bacteria. Front. Microbiol. 2018, 9, 2567. [Google Scholar] [CrossRef]
- Lemon, K.P.; Higgins, D.E.; Kolter, R. Flagellar Motility Is Critical for Listeria monocytogenes Biofilm Formation. J. Bacteriol. 2007, 189, 4418–4424. [Google Scholar] [CrossRef]
- Soo, P.C.; Horng, Y.T.; Chang, Y.L.; Tsai, W.W.; Jeng, W.Y.; Lu, C.C.; Lai, H.C. ManA is regulated by RssAB signaling and promotes motility in Serratia marcescens. Res. Microbiol. 2014, 165, 21–29. [Google Scholar] [CrossRef]
- Gowrishankar, S.; Pandian, S.K.; Balasubramaniam, B.; Balamurugan, K. Quorum quelling efficacy of marine cyclic dipeptide -cyclo(L-leucyl-L-prolyl) against the uropathogen Serratia marcescens. Food Chem. Toxicol. 2019, 123, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Shabib, N.A.; Husain, F.M.; Ahmed, F.; Khan, R.A.; Ahmad, I.; Alsharaeh, E.; Khan, M.S.; Hussain, A.; Rehman, M.T.; Yusuf, M.; et al. Biogenic synthesis of Zinc oxide nanostructures from Nigella sativa seed: Prospective role as food packaging material inhibiting broad-spectrum quorum sensing and biofilm. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Khan, M.F.; Husain, F.M.; Zia, Q.; Ahmad, E.; Jamal, A.; Alaidarous, M.; Banawas, S.; Alam, M.M.; Alshehri, B.A.; Jameel, M.; et al. Anti-quorum Sensing and Anti-biofilm Activity of Zinc Oxide Nanospikes. ACS Omega 2020, 5, 32203–32215. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.M.; Hasan, I.; Qais, F.A.; Khan, R.A.; Alam, P.; Alsalme, A. Fabrication of Zinc Oxide-Xanthan Gum Nanocomposite via Green Route: Attenuation of Quorum Sensing Regulated Virulence Functions and Mitigation of Biofilm in Gram-Negative Bacterial Pathogens. Coatings 2020, 10, 1190. [Google Scholar] [CrossRef]
- Lewis, O.F.; Mubarak, A.D.; Nithya, C.; Priyanka, R.; Gopinath, V.; Alharbi, N.S.; Thajuddin, N. One pot synthesis and anti-biofilm potential of copper nanoparticles (CuNPs) against clinical strains of Pseudomonas aeruginosa. Biofouling 2015, 31, 379–391. [Google Scholar] [CrossRef]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef]
- Aljaafari, A.; Ahmed, F.; Husain, F.M. Bio-Inspired Facile Synthesis of Graphene-Based Nanocomposites: Elucidation of Antimicrobial and Biofilm Inhibitory Potential against Foodborne Pathogenic Bacteria. Coatings 2020, 10, 1171. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Qayyum, S.; Khan, A.U. Antibiofilm efficacy of green synthesized graphene oxide-silver nanocomposite using Lagerstroemia speciosa floral extract: A comparative study on inhibition of gram-positive and gram-negative biofilms. Microb. Pathog. 2017, 103, 167–177. [Google Scholar] [CrossRef]
- Gupta, S.; Afaq, F.; Mukhtar, H. Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene 2002, 21, 3727–3738. [Google Scholar] [CrossRef]
- Adhami, V.M.; Ahmad, N.; Mukhtar, H. Molecular Targets for Green Tea in Prostate Cancer Prevention. J. Nutr. 2003, 133. [Google Scholar] [CrossRef]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of Glutathione in Cancer Progression and Chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lash, L.; Putt, D.; Jankovich, A. Glutathione Levels and Susceptibility to Chemically Induced Injury in Two Human Prostate Cancer Cell Lines. Molecules 2015, 20, 10399–10414. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perveen, K.; Husain, F.M.; Qais, F.A.; Khan, A.; Razak, S.; Afsar, T.; Alam, P.; Almajwal, A.M.; Abulmeaty, M.M.A. Microwave-Assisted Rapid Green Synthesis of Gold Nanoparticles Using Seed Extract of Trachyspermum ammi: ROS Mediated Biofilm Inhibition and Anticancer Activity. Biomolecules 2021, 11, 197. https://doi.org/10.3390/biom11020197
Perveen K, Husain FM, Qais FA, Khan A, Razak S, Afsar T, Alam P, Almajwal AM, Abulmeaty MMA. Microwave-Assisted Rapid Green Synthesis of Gold Nanoparticles Using Seed Extract of Trachyspermum ammi: ROS Mediated Biofilm Inhibition and Anticancer Activity. Biomolecules. 2021; 11(2):197. https://doi.org/10.3390/biom11020197
Chicago/Turabian StylePerveen, Kahkashan, Fohad Mabood Husain, Faizan Abul Qais, Altaf Khan, Suhail Razak, Tayyaba Afsar, Pravej Alam, Ali M. Almajwal, and Mahmoud M. A. Abulmeaty. 2021. "Microwave-Assisted Rapid Green Synthesis of Gold Nanoparticles Using Seed Extract of Trachyspermum ammi: ROS Mediated Biofilm Inhibition and Anticancer Activity" Biomolecules 11, no. 2: 197. https://doi.org/10.3390/biom11020197
APA StylePerveen, K., Husain, F. M., Qais, F. A., Khan, A., Razak, S., Afsar, T., Alam, P., Almajwal, A. M., & Abulmeaty, M. M. A. (2021). Microwave-Assisted Rapid Green Synthesis of Gold Nanoparticles Using Seed Extract of Trachyspermum ammi: ROS Mediated Biofilm Inhibition and Anticancer Activity. Biomolecules, 11(2), 197. https://doi.org/10.3390/biom11020197







